Abstract
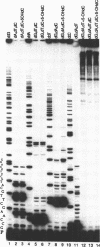
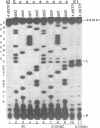
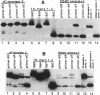
Two major stable oxidation products of 2'-deoxycytidine are 2'-deoxy-5-hydroxycytidine (5-OHdC) and 2'-deoxy-5-hydroxyuridine (5-OHdU). In order to study the in vitro incorporation of 5-OHdC and 5-OHdU into DNA by DNA polymerase, and to check the base pairing specificity of these modified bases, 5-OHdCTP and 5-OHdUTP were synthesized. Incorporation studies showed that 5-OHdCTP can replace dCTP, and to a much lesser extent dTTP, as a substrate for Escherichia coli DNA polymerase I Klenow fragment (exonuclease free). However, 5-OHdUTP can only be incorporated into DNA in place of dTTP. To study the specificity of nucleotide incorporation opposite 5-hydroxypyrimidines in template DNA, 18- and 45-member oligodeoxyribonucleotides, containing an internal 5-OHdC or 5-OHdU in two different sequence contexts, were used. Translesion synthesis past 5-OHdC and 5-OHdU in both oligonucleotides occurred, but pauses both opposite, and one nucleotide prior to, the modified base in the template were observed. The specificity of nucleotide incorporation opposite 5-OHdC and 5-OHdU in the template was sequence context dependent. In one sequence context, dG was the predominant nucleotide incorporated opposite 5-OHdC with dA incorporation also observed; in this sequence context, dA was the principal nucleotide incorporated opposite 5-OHdU. However in a second sequence context, dC was the predominant base incorporated opposite 5-OHdC. In that same sequence context, dC was also the predominant nucleotide incorporated opposite 5-OHdU. These data suggest that the 5-hydroxypyrimidines have the potential to be premutagenic lesions leading to C-->T transitions and C-->G transversions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Ayaki H., Higo K., Yamamoto O. Specificity of ionizing radiation-induced mutagenesis in the lac region of single-stranded phage M13 mp10 DNA. Nucleic Acids Res. 1986 Jun 25;14(12):5013–5018. doi: 10.1093/nar/14.12.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimer L. H. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3(4):188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Dizdaroglu M., Simic M. G. Radiation-induced crosslinking of cytosine. Radiat Res. 1984 Oct;100(1):41–46. [PubMed] [Google Scholar]
- Eaton M. A., Hutchinson D. W. Poly(5-hydroxycytidylic acid) . Biochim Biophys Acta. 1973 Sep 7;319(3):281–287. doi: 10.1016/0005-2787(73)90167-6. [DOI] [PubMed] [Google Scholar]
- Fuciarelli A. F., Wegher B. J., Blakely W. F., Dizdaroglu M. Yields of radiation-induced base products in DNA: effects of DNA conformation and gassing conditions. Int J Radiat Biol. 1990 Sep;58(3):397–415. doi: 10.1080/09553009014551761. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Rietveld K., Aaron C. S. gamma-Ray induced mutational spectrum in the lacI gene of Escherichia coli: comparison of induced and spontaneous spectra at the molecular level. Mutat Res. 1980 Jan;69(1):1–12. doi: 10.1016/0027-5107(80)90171-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Hatahet Z., Purmal A. A., Wallace S. S. A novel method for site specific introduction of single model oxidative DNA lesions into oligodeoxyribonucleotides. Nucleic Acids Res. 1993 Apr 11;21(7):1563–1568. doi: 10.1093/nar/21.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand G. G., McCluskey A. H., Abbott K. A., Revich G. G., Beattie K. L. Misincorporation during DNA synthesis, analyzed by gel electrophoresis. Nucleic Acids Res. 1984 Apr 11;12(7):3155–3171. doi: 10.1093/nar/12.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes E. C., Keyse S. M., Pidoux M., Tyrrell R. M. The spectrum of mutations generated by passage of a hydrogen peroxide damaged shuttle vector plasmid through a mammalian host. Nucleic Acids Res. 1989 Oct 25;17(20):8301–8312. doi: 10.1093/nar/17.20.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschel R. C., Behrman E. J. Oxidation of nucleic acid bases by potassium peroxodisulfate in alkaline aqueous solution. J Org Chem. 1974 Jul 12;39(14):1983–1989. doi: 10.1021/jo00928a001. [DOI] [PubMed] [Google Scholar]
- Pacifici R. E., Davies K. J. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology. 1991;37(1-3):166–180. doi: 10.1159/000213257. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L. Spontaneous mutation in the Escherichia coli lacI gene. Genetics. 1991 Oct;129(2):317–326. doi: 10.1093/genetics/129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8(6):583–599. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- Tchou J., Grollman A. P. Repair of DNA containing the oxidatively-damaged base, 8-oxoguanine. Mutat Res. 1993 May;299(3-4):277–287. doi: 10.1016/0165-1218(93)90104-l. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stein J., Hutchinson F. Changes in DNA base sequence induced by gamma-ray mutagenesis of lambda phage and prophage. Genetics. 1988 Apr;118(4):551–560. doi: 10.1093/genetics/118.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkeshelashvili L. K., McBride T., Spence K., Loeb L. A. Mutation spectrum of copper-induced DNA damage. J Biol Chem. 1991 Apr 5;266(10):6401–6406. [PubMed] [Google Scholar]
- Téoule R. Radiation-induced DNA damage and its repair. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Apr;51(4):573–589. doi: 10.1080/09553008414552111. [DOI] [PubMed] [Google Scholar]
- Wagner J. R., Hu C. C., Ames B. N. Endogenous oxidative damage of deoxycytidine in DNA. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3380–3384. doi: 10.1073/pnas.89.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Sikpi M. O., Preston R. J., Mitra S., Jaberaboansari A. Mutations induced by ionizing radiation in a plasmid replicated in human cells. I. Similar, nonrandom distribution of mutations in unirradiated and X-irradiated DNA. Radiat Res. 1991 Aug;127(2):190–201. [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]