Abstract
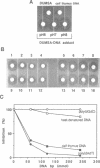
Intact drugs with spirocyclopropylhexadienone moieties can be regenerated from the covalent DNA adducts induced by antitumor antibiotics duocarmycin (DUM) A, SA and some DUMA analogues in neutral aqueous solution. We detected the reversible nature of DUMs by determination of the antimicrobial activity and cytotoxicity of DUM-DNA adducts. All of the adducts selectively inhibited the growth of a sensitive strain of Bacillus but not that of the wild type strain, a property of parent DUM and its analogues. Most of the DNA adducts were also cytotoxic to HeLa S3. These results suggested that active drugs can be released from their covalent DNA adducts under these biological assay conditions. Regeneration of intact drugs was quantitatively analyzed by HPLC and the amount of free drug released from DNA adducts revealed that the rate and efficiency of this reversal were dependent on structural variables among the drugs. The differences in rates of reversibility were correlated with the biological activity of DUMs. The effect of pH, temperature and salt concentration on the regeneration of drugs from their DNA adducts suggest a catalytic role of double-helical DNA on the reversal pathway.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anin M. F., Gaucheron F., Leng M. Lability of monofunctional cis-platinum adducts: role of DNA double helix. Nucleic Acids Res. 1992 Sep 25;20(18):4825–4830. doi: 10.1093/nar/20.18.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowy-Borowski H., Lipman R., Chowdary D., Tomasz M. Duplex oligodeoxyribonucleotides cross-linked by mitomycin C at a single site: synthesis, properties, and cross-link reversibility. Biochemistry. 1990 Mar 27;29(12):2992–2999. doi: 10.1021/bi00464a015. [DOI] [PubMed] [Google Scholar]
- D'Arpa P., Liu L. F. Topoisomerase-targeting antitumor drugs. Biochim Biophys Acta. 1989 Dec 17;989(2):163–177. doi: 10.1016/0304-419x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Gaucheron F., Malinge J. M., Blacker A. J., Lehn J. M., Leng M. Possible catalytic activity of DNA in the reaction between the antitumor drug cis-diamminedichloroplatinum(II) and the intercalator N-methyl-2,7-diazapyrenium. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3516–3519. doi: 10.1073/pnas.88.9.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura M., Muroi K., Asano K., Kawamoto I., Tomita F., Morimoto M., Nakano H. DC89-A1, a new antitumor antibiotic from Streptomyces. J Antibiot (Tokyo) 1988 Sep;41(9):1285–1288. doi: 10.7164/antibiotics.41.1285. [DOI] [PubMed] [Google Scholar]
- Ichimura M., Ogawa T., Katsumata S., Takahashi K., Takahashi I., Nakano H. Duocarmycins, new antitumor antibiotics produced by Streptomyces; producing organisms and improved production. J Antibiot (Tokyo) 1991 Oct;44(10):1045–1053. doi: 10.7164/antibiotics.44.1045. [DOI] [PubMed] [Google Scholar]
- Ichimura M., Ogawa T., Takahashi K., Kobayashi E., Kawamoto I., Yasuzawa T., Takahashi I., Nakano H. Duocarmycin SA, a new antitumor antibiotic from Streptomyces sp. J Antibiot (Tokyo) 1990 Aug;43(8):1037–1038. doi: 10.7164/antibiotics.43.1037. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Ichimura M., Katsumata S., Morimoto M., Takahashi K. New antitumor antibiotics, duocarmycins B1 and B2. J Antibiot (Tokyo) 1989 Aug;42(8):1299–1301. doi: 10.7164/antibiotics.42.1299. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Nakanishi S., Kobayashi E., Nakano H., Suzuki K., Tamaoki T. Hypericin and pseudohypericin specifically inhibit protein kinase C: possible relation to their antiretroviral activity. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1207–1212. doi: 10.1016/0006-291x(89)92730-7. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Takahashi K., Ichimura M., Morimoto M., Asano K., Kawamoto I., Tomita F., Nakano H. Duocarmycin A, a new antitumor antibiotic from Streptomyces. J Antibiot (Tokyo) 1988 Dec;41(12):1915–1917. doi: 10.7164/antibiotics.41.1915. [DOI] [PubMed] [Google Scholar]
- Warpehoski M. A., Harper D. E., Mitchell M. A., Monroe T. J. Reversibility of the covalent reaction of CC-1065 and analogues with DNA. Biochemistry. 1992 Mar 10;31(9):2502–2508. doi: 10.1021/bi00124a009. [DOI] [PubMed] [Google Scholar]
- Yasuzawa T., Iida T., Muroi K., Ichimura M., Takahashi K., Sano H. Structures of duocarmycins, novel antitumor antibiotics produced by Streptomyces sp. Chem Pharm Bull (Tokyo) 1988 Sep;36(9):3728–3731. doi: 10.1248/cpb.36.3728. [DOI] [PubMed] [Google Scholar]
- Yasuzawa T., Saitoh Y., Ichimura M., Takahashi I., Sano H. Structure of duocarmycin SA, a potent antitumor antibiotic. J Antibiot (Tokyo) 1991 Apr;44(4):445–447. doi: 10.7164/antibiotics.44.445. [DOI] [PubMed] [Google Scholar]