Abstract
Different locomotor and postural demands are met partly due to the varying properties and proportions of the muscle fibre types within the skeletal muscles. Such data are therefore important in understanding the subtle relationships between morphology, function and behaviour. The triceps surae muscle group is of particular interest when studying our closest living relatives, the non-human great apes, as they lack a significant external Achilles tendon, crucial to running locomotion in humans and other cursorial species. The aim of this study, therefore, was to determine the proportions of type I (slow) and type II (fast) fibres throughout these muscles in chimpanzees and orangutans using immunohistochemistry. The orangutan had a higher proportion of type I fibres in all muscles compared with the chimpanzees, related to their slower, more controlled movements in their arboreal habitat. The higher proportion of type II fibres in the chimpanzees likely reflects a compromise between their need for controlled mobility when arboreal, and greater speed and power when terrestrial. Overall, the proportion of slow fibres was greater in the soleus muscle compared with the gastrocnemius muscles, and there was some evidence of proximal to distal and medial to lateral variations within some muscles. This study has shown that not only do orangutans and chimpanzees have very different muscle fibre populations that reflect their locomotor repertoires, but it also shows how the proportion of fibre types provides an additional mechanism by which the performance of a muscle can be modulated to suit the needs of a species.
Keywords: gastrocnemius, hominoids, primate, soleus
Introduction
Different locomotor and postural demands are met, partly due to variations in gross anatomy [e.g. muscles present, points of origin and insertion, fibre lengths, pennation angles and physiological cross-sectional areas (PCSA); see Close, 1972; Alexander & Vernon, 1975; Eng et al. 2008], and partly due to variations in the physiological properties of the individual muscle fibres (reviewed by Pette & Staron, 1990) in the musculoskeletal system. Sarcomeres are the smallest functional units of muscles, containing the two proteins, actin and myosin, among others, that interact to enable a muscle to contract. The speed of muscle fibre contraction is largely determined by the myosin heavy chain isoform (MHC), leading to the distinction of two fibre types: fast and slow. Further analysis of the fibre's metabolic pathway based on myosin ATPase histochemistry reveals two types of pathway, either aerobic/oxidative or anaerobic/glycolytic. Combining these properties results in muscle fibres being defined as either fast (type IIa and IIb), also known as fast oxidative glycolytic and fast glycolytic fibres, respectively, or slow (type I), also known as slow oxidative fibres. Further subtypes have been identified but, functionally, the distinction of fast and slow is best defined (Punkt, 2002). Type I fibres are fatigue-resistant and slow-contracting, whereas type II fibres are more fatigable, but fast-contracting. The proportion of these two fibre types throughout a muscle belly in combination with different macro-architecture influences the function of a muscle.
While gross muscle architecture can be used to estimate functional properties, such as force production and muscle excursion and velocity (e.g. Thorpe et al. 1999; Payne et al. 2006; Eng et al. 2008; Channon et al. 2009), details of the fibre type distribution within muscles can provide further insight into the contractile properties, such as maximum velocity and time to fatigue of different muscles and muscle regions, allowing more detailed relationships between form and function to be established (e.g. Sickles & Pinkstaff, 1981a,b; Moritz et al. 2007; Schmidt & Schilling, 2007; Eng et al. 2008). For example, muscles with a high proportion of slow fibres tend to be predominantly used in anti-gravity or postural roles, whereas muscles with a high proportion of fast fibres are important for powerful, propulsive movements, such as jumping, in addition to quick responses and counterbalancing movements, such as those needed during dynamic stabilisation (e.g. Sickles & Pinkstaff, 1981b; Rome et al. 1988; Schilling, 2009). In addition to a muscle having an overall proportion of fast and slow fibres, particular fibre types may be segregated into specific regions (reviewed by Kernell, 1998). Functionally, such fibre type regionalisation has been linked to the maintenance of joint stabilisation, energy-saving mechanisms and improving muscle efficiency (see Kernell, 1998; von Mering & Fischer, 1999).
Muscle physiology and gross architecture have been studied in some detail in non-human primates (for more recent examples, see apes: Thorpe et al. 1999; Vereecke et al. 2005; Carlson, 2006; Payne et al. 2006; Oishi et al. 2008; Channon et al. 2009, 2010; Michilsens et al. 2010; other primates: Kikuchi, 2009); however, to date, we are not aware of any detailed study of the fibre type distribution in non-human ape hindlimb muscles, although Kimura (2002) has looked at the orangutan psoas major muscle. The ability to relate form to function in non-human apes is crucial for developing our understanding of how subtle variations in morphology may relate to their positional behaviour repertoire.
The focus of this study was the triceps surae muscle group. This group is composed of two major muscles: superficially the gastrocnemius muscle with its two heads (lateralis and medialis) and, lying deep to these, the soleus muscle. In both humans and non-human apes, the gastrocnemius muscle originates on the femur, whereas the soleus muscle originates from both the fibula and tibia in Homo but predominantly from the fibula in non-human apes, with both inserting onto the calcaneus via the Achilles tendon (see Swindler & Wood, 1973). In addition to these two main muscles, the small plantaris muscle may be present, although it is absent in ∼10% of humans, ∼39% of chimpanzees and ∼95% of orangutans (Langdon, 1990). The gastrocnemius muscle is biarticular, responsible for flexing the knee and plantarflexing the foot, whereas the soleus muscle only exerts a plantar-flexor moment about the ankle (Alexander & Vernon, 1975).
The triceps surae group in chimpanzees and orangutans is characterised by long fibres and small PCSAs when compared with humans, reflecting adaptations for relatively low force generation over a large range of joint displacements, beneficial for moving in an arboreal habitat (Thorpe et al. 1999; Payne et al. 2006). The triceps surae group of the non-human great apes is particularly interesting because, compared with humans, gibbons and most cursorial species, the length of the external Achilles tendon is substantially shorter (Swindler & Wood, 1973; J.P. Myatt, personal observation). During locomotion the forces produced by muscle contraction are directed through the tendons to the point of attachment on the bone and, by varying the distance of the attachment point from the joint centre of rotation, the speed with which the distal elements move can be modulated (Lieber, 2002; Benjamin et al. 2008). In humans, and other cursorial species, the Achilles tendon also functions as a mechanical energy store during certain forms of locomotion, such as running, where it acts as a spring, returning energy with every step (Alexander, 1991, 1992, 2002).
While we have long understood that the Achilles tendon plays a role in lowering the energetic cost of running in humans (Alexander, 1991, 1992), more recently, Maganaris & Paul (2002) have shown that it can also return energy during walking, albeit to a lesser extent. Muscle, however, has a major advantage over tendon in its ability to provide active control of limb movement and position, and is therefore well-suited to take account of changes in substrate properties, such as irregularities, discontinuities and material characteristics, by controlled contractions, unlike tendon, which is limited to passive extension (Alexander, 2002; Roberts, 2002). The shorter length of the external Achilles tendon in non-human great apes might suggest that adaptations for such control, which is particularly important for large-bodied arboreal primates, outweigh the benefits of energy return that would enable the use of jumping and running behaviours, as used by smaller primates in the same habitat. Whilst this makes sense for orangutans that are largely arboreal, chimpanzees move both terrestrially and arboreally, and when terrestrial undertake both fast dominance displays and long-distance patrols throughout their range (Goodall, 1990; Thorpe et al. 2002). This suggests that the requirement for faster, terrestrial locomotion should select for energetic efficiency, i.e. a longer Achilles’ tendon, but the arboreal requirements should limit the length of the Achilles’ tendon in preference for muscular control. As chimpanzees do indeed have a small Achilles tendon, we would therefore predict that chimpanzees possess some form of compromise morphology at the micro-architecture level to aid in the reduction of terrestrial travel costs despite macro-adaptations for arboreality.
Fibre type proportion in various muscles of the triceps surae group have been studied in a range of species (e.g. human: Gollnick et al. 1974; Edgerton et al. 1975; cat: Burke et al. 1971; dog: Armstrong et al. 1982; cheetah: Williams et al. 1997) and under a range of conditions (e.g. bed-rest in humans: Ohira et al. 2000; spaceflight: Fitts et al. 2001; loading: Demirel et al. 1999; and aging: Deschenes, 2004). In general, these studies have found that most mammals have between 70% and 100% type I fibres in their soleus muscle, for example cat (100%), rat (84%), guinea pig (100%), bush baby (87%), slow loris (72%; Ariano et al. 1973) and long-tailed macaque (∼94%; Acosta & Roy, 1987). On average the gastrocnemius muscle has between 4% and 51% type I fibres, for example rat (4%), cat (25%; Ariano et al. 1973), cheetah (∼40%), dog (∼50%; Armstrong et al. 1982) and long-tailed macaque (∼23%; Acosta & Roy, 1987). As in other mammals, human studies have found that the soleus muscle contains the highest proportion of type I fibres in this muscle group, on average, between 70% and 80% type I fibres, compared with the gastrocnemius muscle, which contains between 57% and 64% type I fibres (Edstrom & Nystrom, 1969; Gollnick et al. 1974; Edgerton, 1975; Dahmane et al. 2005). The high proportion of type I fibres in the soleus muscle reflects the functional partitioning of this synergistic muscle group, resulting in the deeper, soleus muscle taking on the postural role during activities and slow locomotion, and the more superficial gastrocnemius muscle producing the propulsive forces for rapid and powerful plantarflexion (see Smith et al. 1977; Walmsley et al. 1978; Spector et al. 1980). We would therefore anticipate both chimpanzees and orangutans to have a high proportion of type I fibres in their soleus muscle, as in other mammals. We further anticipate that orangutans will have a higher proportion of type I fibres throughout the triceps surae group because orangutans are predominantly arboreal, with their locomotion characterised by slow, cautious predominantly orthograde (upright-trunked) behaviours (Thorpe & Crompton, 2005, 2006). In contrast, the locomotor behaviour of chimpanzees is dominated by terrestrial quadrupedalism, and involves more powerful, swift plantarflexions (Goodall, 1990; Hunt, 1992a,b;), which is likely to result in a higher proportion of type II fibres in the gastrocnemius. Further investigation at the microscopic level (i.e. muscle fibre typing) may reveal in more detail how these animals meet the differing functional demands in their daily repertoire. The aim of the present study, therefore, was to determine the proportion and distribution patterns of type I and type II fibre types in the triceps surae group at five levels along the proximo-distal axis in chimpanzees and orangutans, using immunohistochemistry. Our goal was to contribute further to our understanding of how the musculoskeletal system of apes has adapted in the face of changing functional demands.
Materials and methods
Specimens and sample preparation
The distributions of type I and type II muscle fibres were studied in two chimpanzees (Pan troglodytes; Blumenback, 1799) and one orangutan (Pongo abelii; Lesson, 1827; see Table 1 for subject information), obtained after death in European zoos (the male chimpanzee was obtained from Zoologischer Garten Halle, Germany; the female chimpanzee from Belfast Zoological Gardens, Northern Ireland; and the orangutan from Tierpark Hagenbeck, Hamburg, Germany). All animals had no known musculoskeletal problems, and were considered healthy, active individuals prior to death. Animals were eviscerated during post mortem examination and frozen for transport to Friedrich-Schiller-University Jena, Germany. The cadavers were then skinned and fixed in 4% formalin via immersion in a natural position. Once fixed, the triceps surae muscle group from the right leg was dissected out for further analysis.
Table 1.
Subject information and gross muscle architecture
| Subject | Sex | Age (years) | Mass (kg) | Cause of death | Muscle | Muscle mass (g)(% of total body mass)* | Muscle length (cm)(% of body mass1/3)* |
|---|---|---|---|---|---|---|---|
| Chimpanzee | Female | 23 | 56 | ? | S | 158.7 (0.3) | 21.5 (56.2) |
| GL and GM† | 155.7 (0.3) | 22.9 (59.9) | |||||
| Chimpanzee | Male | 34 | 62 | Pulmonary embolism | S | 190.3 (0.3) | 24.5 (61.9) |
| GL | 120.3 (0.2) | 28.2 (71.3) | |||||
| GM | 96.7 (0.2) | 21.8 (55.1) | |||||
| Orangutan | Female | 12 | 42 | Drowning | S | 63.8 (0.2) | 16.9 (48.6) |
| GL | 40.4 (0.1) | 16.3 (46.9) | |||||
| GM | 77.2 (0.2) | 19.0 (54.7) |
Following Alexander et al. (1981).
Separate data for the gastrocnemius lateralis and gastrocnemius medialis muscles were not available for the female chimpanzee.
GL, gastrocnemius lateralis; GM, gastrocnemius medialis; S, soleus.
Muscles were removed from their points of origin to point of insertion, external tendons and fascia were removed prior to the muscles being weighed and belly lengths measured (information provided in Table 1). The muscles were then frozen (−18 °C) and divided into muscle blocks along the proximo-distal axis to obtain five equally distributed cross-sections along the length of the muscle belly (Fig. 1). The number of blocks within each cross-section varied depending on the overall width of the muscle (up to two blocks), as they could be no larger than 3 × 3 cm for the histological processing. The gastrocnemius medialis and lateralis from both chimpanzees and the orangutan, and the orangutan soleus were simply divided into five overall cross-sections (e.g. Fig. 1A), whereas the chimpanzee soleus muscles were wider than 3 cm and therefore additionally divided proximo-distally into a medial and a lateral block, resulting in 10 muscle blocks in total (e.g. Fig. 1B). Once the immunohistochemical labelling was performed on sections from the middle of each block, the overall muscle cross-section was re-assembled to visualise the distribution of muscle fibres across the complete cross-section, and the five overall cross-sections were put together to analyse fibre proportion along the muscle's proximo-distal axis.
Fig. 1.
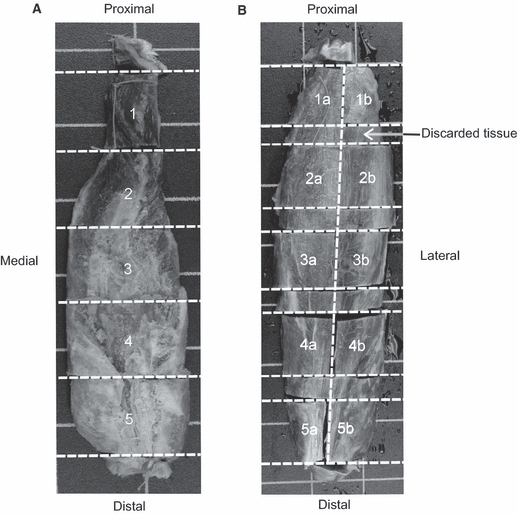
Preparation of the muscle blocks for sampling the histological cross-sections from the five proximo-distal levels. Example of the (A) orangutan soleus and (B) female chimpanzee soleus (divided into two blocks at each level). Note that the investigated cross-sections were from the centre of each muscle block.
Immunohistochemistry
The muscle blocks were washed in distilled water and dehydrated with a graded series of ethanol (30%, 50%, 70% and 96%) and propanol over a period of 4 days, before being embedded in paraffin. Histological serial cross-sections were prepared (HM360 microtome, 10 μm; Microm, Germany), and several sections were sampled from the centre of each tissue block for immunohistochemical labelling.
Commercially available mouse monoclonal antibodies, raised against rabbit skeletal muscle, were used to identify fast-twitch (type II) and slow-twitch (type I) fibres (for detailed protocol, see Schmidt & Schilling, 2007). To cross-check fibre identification, enzyme histochemistry following Ziegan's protocol (Ziegan, 1979; modified after von Mering & Fischer, 1999) was additionally performed on a fresh sample from the male chimpanzee (Fig. 2). From this, we established that, firstly, type I fibres correlated with slow fibres, and type II fibres correlated with the fast fibres. Furthermore, among the type II fibres, type IIa fibres were present in the chimpanzee, in addition to other subtypes (Fig. 2). However, to identify the subtypes of type II fibres, fresh material frozen immediately after death is necessary, which is generally not possible with primate species or larger muscles, and therefore immunohistochemistry on fixed material was the only viable option, allowing us to identify type I and type II fibres only.
Fig. 2.
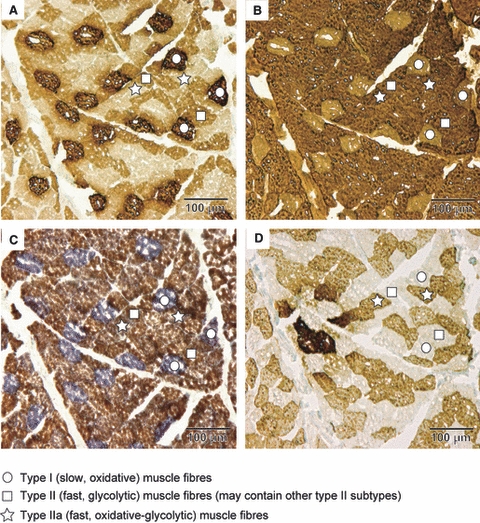
Results of immuno- and enzyme-histochemical fibre identification in the example of the male chimpanzee. (A) Anti-slow immune reaction. (B) Anti-fast immune reaction. Note the complementary staining result. (C) Ziegan's enzyme reaction. (D) Anti-fast IIa immune reaction.
Initial testing on the fixed material for all three individuals found that the primary antibody to fast myosin (MHC II, Clone MY-32; Sigma-Aldrich, Germany) produced both the greatest staining intensity and ease of distinction between the two fibre types, and was therefore used for all samples. In summary, the immunoreactivity of the muscle sample was first reinstated using trypsin (0.1%) in phosphate-buffered saline (PBS, 0.01 m, pH 7.4), peroxidise activity was then blocked using 3% H2O2 in methanol before treating the cross-sections with normal goat serum (1.5% in PBS). The primary antibody was added and stained using a Mouse ExtrAvidin® Peroxidase staining kit (Sigma-Aldrich), consisting of ExtrAvidin® Peroxidase and Biotinylated Purified Goat Antibody to Mouse IgG. To visualise the reaction, muscle samples were covered with a diaminobenzidine–H2O2 substrate. This stained the fibres containing the type II MHC (type II fibres) brown. Counterstaining was carried out using methylene blue (to contrast type I fibres), and slides were mounted with Euparal (Chroma, Germany) and cover-slipped.
Quantification of fibre type distribution
To determine the distribution of type II (fast) and type I (slow) fibre types throughout the triceps surae muscle group, one of the serial histological sections collected from each tissue block was photographed using a digital camera mounted to a Zeiss® Axiolab microscope and analySIS® software (Soft Imaging System GmbH, Münster, Germany). Each section was approximately 3 × 3 cm or smaller, thus to identify and count individual muscle fibres, multiple images were taken of each section in a systematic, grid-like pattern using the motor-driven object table of the microscope. There was no overlap between adjacent photos.
To assess the percentage of fibre types within the muscles, the numbers of type I and type II fibres were counted in evenly spaced images based on a grid system and marked with the aid of ImageJ® to prevent double-counting. Each image contained no more than 350 fibres, with the number of photos counted per muscle cross-section ranging from 30 to 155 depending on overall size. The proportion of each fibre type was then calculated as a percentage of all the fibres in a single image. In addition, the mean proportion of each fibre type was calculated for each cross-section. In the case of the chimpanzee soleus muscles, the numbers of fibres from both the medial and lateral blocks were added together to obtain a mean proportion for the entire cross-section of the muscle at each proximo-distal level.
Statistical analysis
Mean percentages ± SE of type I and type II fibres for each cross-section and for whole muscles were calculated using spss v.15 (SPSS, USA). To ascertain whether there were statistically significant differences between cross-sections within a muscle and at a given proximo-distal level between subjects, general linear models (GLMs) with binomial error distribution were conducted using the statistical package R v. 2.10.1 (http://www.R-project.org). All data conformed to the assumptions of normality. In cases where a significant difference was established, Tukey's post hoc testing was applied for all pair-wise comparisons to establish which cross-sections differed significantly along the proximo-distal axis within a muscle, and in cases of variance heterogeneity (established by a Levene's test), a sandwich estimator of the covariance matrix was additionally applied (Hothorn et al. 2008). Significance was taken at the P < 0.05 level throughout.
Results
The percentages of type I and type II fibres for all subjects and their means are presented in Table 2. The two chimpanzees were found to differ in the mean proportion of type I fibres across the majority of comparable muscle cross-sections: the male chimpanzee had a statistically significantly greater mean proportion of type I fibres in the soleus (52% compared with 41%; GLM: F9,1850 = 70.29, P < 0.001) and gastrocnemius lateralis (18% compared with 13%; GLM: F9,1016 = 59.9, P < 0.001) compared with the female chimpanzee. In contrast, there was a similar proportion of type I fibres in the gastrocnemius medialis of both chimpanzees, although there was again a significant overall difference between respective cross-sections (GLM: F9,979 = 40.4, P < 0.001). Within each chimpanzee, the two heads of the gastrocnemius muscle did not differ significantly in their proportion of type I fibres across equivalent cross-sections (e.g. gastrocnemius lateralis section one and gastrocnemius medialis section one in the female; Table 2). Results for the female chimpanzee only differed significantly between the lateral and medial heads across the second most proximal cross-section, with the gastrocnemius medialis having more type I fibres (Tukey: P < 0.01). Results for the male chimpanzee differed significantly only across the second and fourth cross-sections, with the gastrocnemius lateralis having more type I fibres (Tukey: P < 0.001).
Table 2.
Percentages of type I and type II fibres from all muscles of the triceps surae group (values are mean percentages ± SE)
| Soleus | Gastrocnemius lateralis | Gastrocnemius medialis | ||||
|---|---|---|---|---|---|---|
| Muscle cross-section | Type I fibres | Type II fibres | Type I fibres | Type II fibres | Type I fibres | Type II fibres |
| Orangutan | ||||||
| 1 (proximal) | 87 ± 1.5a,b* | 13 ± 1.5 | 31 ± 1.7a,b,c* | 69 ± 1.7 | 47 ± 1.5a,b,c* | 49 ± 1.6 |
| 2 | 84 ± 1.7 | 16 ± 1.7 | 37 ± 1.0a | 61 ± 1.4 | 51 ± 1.1a,d,e,f | 47 ± 1.1 |
| 3 | 79 ± 1.7 | 18 ± 0.8 | 36 ± 1.4b,d | 62 ± 1.6 | 49 ± 1.2d,g,h | 50 ± 1.2 |
| 4 | 68 ± 1.2a,c | 30 ± 1.0 | 37 ± 1.0c,e | 63 ± 1.0 | 41 ± 0.9b,e,g | 57 ± 1.0 |
| 5 (distal) | 64 ± 1.2b,c | 33 ± 1.0 | 32 ± 1.8d,e | 63 ± 2.6 | 34 ± 2.5c,f,h | 56 ± 3.4 |
| Overall muscle | 72 ± 0.8 | 26 ± 0.6 | 35 ± 0.6 | 63 ± 0.7 | 47 ± 0.6 | 51 ± 0.6 |
| Female chimpanzee | ||||||
| 1 (proximal) | 41 ± 0.9h,i | 58 ± 1.0 | 12 ± 0.5h,i,j | 79 ± 2.2 | 15 ± 1.2h,i | 85 ± 1.2 |
| 2 | 39 ± 0.8j,k,l | 57 ± 1.0 | 15 ± 0.5h,k,l | 80 ± 1.6 | 17 ± 0.5h,j,k,l | 82 ± 0.9 |
| 3 | 46 ± 0.9h,j,m,n | 52 ± 0.9 | 15 ± 0.5i,m,n | 83 ± 1.2 | 14 ± 0.5i,j,m | 78 ± 2.0 |
| 4 | 41 ± 0.9k,m,n | 56 ± 1.0 | 12 ± 0.4j,k,m | 88 ± 0.4 | 11 ± 0.6k,m,n | 84 ± 1.9 |
| 5 (distal) | 36 ± 1.0i,l,m,n | 61 ± 1.2 | 11 ± 0.7l,n | 84 ± 2.4 | 14 ± 1.1l,n | 86 ± 1.1 |
| Overall muscle | 41 ± 0.4 | 57 ± 0.5 | 13 ± 0.2 | 82 ± 0.8 | 14 ± 0.3 | 83 ± 0.8 |
| Male chimpanzee | ||||||
| 1 (proximal) | 50 ± 1.1o,p | 47 ± 1.1 | 16 ± 0.6o,p | 82 ± 1.3 | 17 ± 0.6o,p | 80 ± 1.5 |
| 2 | 50 ± 1.1q,r | 45 ± 1.0 | 21 ± 0.5o,q,r,s | 78 ± 0.9 | 17 ± 0.4q,r | 81 ± 1.0 |
| 3 | 49 ± 1.4s | 45 ± 1.4 | 18 ± 0.5q,t | 82 ± 0.8 | 16 ± 0.5s,t | 84 ± 0.7 |
| 4 | 55 ± 1.3o,q | 43 ± 1.3 | 17 ± 0.7r,u | 79 ± 1.7 | 14 ± 0.5o,q,s,u | 83 ± 1.6 |
| 5 (distal) | 55 ± 1.3p,r,s | 40 ± 1.1 | 11 ± 2.0p,s,t,u | 89 ± 2.0 | 12 ± 0.7p,r,t,u | 86 ± 2.1 |
| Overall muscle | 52 ± 0.6 | 44 ± 0.6 | 18 ± 0.3 | 81 ± 0.6 | 16 ± 0.2 | 82 ± 0.6 |
Paired letters in subscript refer to a significant difference (P < 0.05) in the percentage of type I fibres between the muscle cross-sections using Tukey's post hoc test, for example orangutan soleus section 1 is significantly different from section 4 (as indicated by lettera).
Nevertheless, taken as a whole, the mean proportions of type I and type II fibres in the two chimpanzees were clearly more similar to each other than either were to those of the orangutan for all muscles. The orangutan had a much higher mean percentage of type I fibres across all muscle sections compared with both chimpanzees, with an overall mean of 72% type I fibres in the soleus compared with 41% and 52% in the female and male chimpanzees, respectively. The overall mean proportion of type I fibres in the two heads of the gastrocnemius muscle varied slightly in the orangutan, with the medial head having a significantly higher percentage than the lateral head (47% and 35%, respectively; GLM: F9,670 = 59.48, P < 0.001). When comparing equivalent cross-sections there was a significant difference between the proximal four sections, with gastrocnemius medialis having a higher proportion of type I fibres (Tukey: P < 0.01), but there was no significant difference between the most distal cross-sections (Tukey: P = 0.569).
The proportion of fibre types differed significantly along the proximo-distal axis of the muscle for all muscles in both chimpanzees (female chimpanzee: soleus GLM: F4,906 = 24.13, P < 0.001; gastrocnemius medialis: F4,452 = 51.02, P < 0.001; gastrocnemius lateralis GLM: F4,525 = 40.59, P < 0.001; male chimpanzee: soleus GLM: F4,944 = 10.8, P < 0.001; gastrocnemius medialis GLM: F4,527 = 13.22, P < 0.001; gastrocnemius lateralis GLM: F4,491 = 59.61, P < 0.001). Tukey's pairwise relationships (shown as paired subscript letters in Table 2) show that almost all cross-sections within each muscle contributed to the overall significant difference (taken at the P < 0.05 level). Overall, proximo-distal variation was also apparent in all orangutan muscles, with there being a significant difference between the majority of cross-sections (soleus GLM: F4,428 = 79.05, P < 0.001; gastrocnemius lateralis GLM: F4279 = 10.99, P < 0.001; gastrocnemius medialis GLM: F4391 = 61.2, P < 0.001; see Table 2 for Tukey's pairwise relationships across the cross-sections within each individual muscle).
However, the pattern of variation within all muscles of the triceps surae along the proximo-distal axis differed between the chimpanzees and the orangutan (Table 2; Fig. S1). The male chimpanzee showed a slight increase in type I fibre proportion from 50% to 55% towards the distal end of the soleus muscle, but this was the opposite gradient to that observed in the orangutan where the number of type I fibres decreased significantly from 87% proximally to 64% distally. The female chimpanzee had a slight peak in proportion of type I fibres in the middle of the soleus muscle. The pattern of fibre proportion within gastrocnemius lateralis was similar in the chimpanzees in that the number of type I fibres slightly decreased towards the distal end of the muscle. The same was true for the male chimpanzee's gastrocnemius medialis. In the orangutan gastrocnemius lateralis there was a slight peak in type I fibres in the middle of the muscle belly, this was also the case in the orangutan and female chimpanzee's gastrocnemius medialis muscles.
Overall there was little variation along the superficial-deep and medio-lateral axes in all muscles (Fig. 1A), although some variation was apparent in the medio-lateral axis of the chimpanzee soleus muscles. In the female chimpanzee soleus there was a decrease in the proportion of type I fibres from ∼50% on the medial side and in the centre of the muscle to ∼20% along the lateral edge (Fig. S1D). The proportion of type I fibres in the male chimpanzee soleus muscle decreased from ∼70% on the medial side and in the centre of the muscle to ∼30% in the lateral quarter (Fig. S1G).
Discussion
Sample selection
The origins and insertions of all muscles in this study agreed with previous studies (Swindler & Wood, 1973; Thorpe et al. 1999; Payne et al. 2006; J.P. Myatt, R.H. Crompton and S.K.S. Thorpe, unpublished data), and the masses of muscles sampled in this study and their percentage of total body mass are similar to the range previously observed (Thorpe et al. 1999; Payne et al. 2006; J.P. Myatt, unpublished data), showing that this was a normal, healthy sample. Housing conditions of captive animals may influence fibre type populations compared with their wild counterparts due to factors such as support type and activity level. However, all of the individuals in this study were considered healthy, active individuals immediately prior to death, and their captive conditions allowed for varied support use, mimicking that observed in the wild. Furthermore, Williams et al. (1997) found that the fibre type composition in wild cheetah compared with less active captive cheetah did not differ significantly; therefore, we consider the overall proportion of fibres observed in this study to be representative for chimpanzees and orangutans, although we acknowledge that a larger sample size would be beneficial and hope that the number of individuals sampled of both sexes and different ages can be increased over time. Due to the limitations of the small sample size, the following interpretations are speculative in nature, with the collection of further data required to test our results.
Intraspecific comparison between chimpanzees
There was a significant difference in the absolute proportions of type I fibres between the cross-sections of the triceps surae muscles of the two chimpanzees (mean difference: soleus 11%; gastrocnemius lateralis 5%; gastrocnemius medialis 2%), and this could be related to a range of factors, including genetic variability (e.g. Simoneau & Bouchard, 1995), sex (e.g. Deschenes, 2004), age (e.g. Deschenes, 2004), activity level (e.g. Monster et al. 1978) and body size (Kram & Taylor, 1990; Seow & Ford, 1991). In this instance the observed difference in fibre proportion between the two chimpanzees is unlikely to be related to age as both chimpanzees were fully-grown adults. As the chimpanzees were different sexes we could expect that the difference in body mass may have had some effect (the male chimpanzee was 11% heavier than the female), as with increasing body mass there is usually an increase in the proportion of type I fibres to cope with the increasing forces necessary to support body weight against gravity (see Kram & Taylor, 1990; Seow & Ford, 1991). Unidentified genetic factors are also thought to account for up to 25% of the variability in fibre type distribution between individuals, with differences in behaviour and the level of muscular contractile activity accounting for 30% (Simoneau & Bouchard, 1995). Wild and captive male chimpanzees often carry out patrols of their territories, lasting a number of hours and covering distances as great as 35 km (Goodall, 1990; Watts & Mitani, 2001; S.K.S. Thorpe, personal observation). This may explain some of the greater type I proportion observed in the male's triceps surae muscle, as they would need more stamina. However, captive chimpanzees cover much smaller distances than their wild counterparts, more similar to females (S.K.S. Thorpe, personal observation), supporting the idea that most of the variation between the two chimpanzees could be explained by genetic variation. The greatest mean difference between the two chimpanzees was 11%; therefore, theoretically, all of the variation could be accounted for by genetic variation (Simoneau & Bouchard, 1995); however, other factors should also be considered.
Variations along the different axes within the muscles of the triceps surae were similar between the chimpanzees. A slight increase in the proportion of type I fibres distally was apparent in the soleus muscle, although this was not as clear as the medial to lateral variation, whereby the proportion of type I fibres decreased towards the medial side. This is in contrast to the homogeneity of the predominantly type I population usually observed in the soleus muscle of mammals (e.g. Ariano et al. 1973; Edgerton et al. 1975). It is possible that this lateral increase in type I fibres may help to stabilise the foot and prevent it rotating outwards onto the lateral side, as the highest pressure is present under the lateral mid-foot during mid-stance when chimpanzees walk quadrupedally (Wunderlich & Ford, 2000; Vereecke, 2006).
Interspecific comparison between chimpanzee, orangutan and other mammals
Although the two chimpanzees differed in their fibre type proportions, these differences were small in comparison to those between the chimpanzees together and the orangutan. The chimpanzees and orangutan were similar in that their soleus muscles had more type I fibres than either head of the gastrocnemius muscle, as is apparent in most mammals (e.g. Ariano et al. 1973; Edgerton et al. 1975). The greater proportion of type I fibres in the soleus is generally evident in all synergistic muscle groups whereby the deeper muscle consists predominantly of type I fibres and may reflect optimisation of the lever arm for different tasks (Kernell, 1998; Dickx et al. 2010). The orangutan, however, had a much larger proportion of type I fibres across all muscles. This agrees with our predictions as orangutans move much more slowly than chimpanzees, which requires slow, sustained contractions, as has also been observed in other, but rather slower, arboreal animals such as the slow loris (Sickles & Pinkstaff, 1981b) and sloth (Bárány et al. 1967).
Whilst it was anticipated that the chimpanzees would generally have a greater proportion of type II fibres compared with the orangutan, the proportion of type II fibres in their soleus muscles (female: 41%; male: 52%) was high compared with that in most mammals studied, for example human: 20–30% type II fibres (Edstrom & Nystrom, 1969; Gollnick, 1974; Edgerton, 1975; Dahmane et al. 2005); long-tailed macaque: ∼10% type II fibres (Acosta & Roy, 1987); cat (0%); rat (16%); guinea pig (0%); bush baby (13%) and slow loris (28%; Ariano et al. 1973). An increase in the proportion of type II fibres in a postural muscle can result from an extreme lack of use (see Loughna et al. 1990; Jaenicke et al. 1991; Scott et al. 2001), but this is unlikely to be the case here as the chimpanzees were healthy, active individuals immediately prior to death. The higher proportions of type II fibres in the chimpanzee soleus muscle seem to suggest that this muscle plays a role in functions other than maintenance of posture, and may reflect a reduced need for type I fibres. This is possibly related to their use of column-like hindlimb postures when quadrupedal, similar to that used during human bipedal walking, thus reducing the need for an increased proportion of type I fibres to counteract knee and ankle flexion (Edstrom & Nystrom, 1969; Gollnick, 1974; Edgerton, 1975; Dahmane et al. 2005). Alternatively, type II fibres are able to produce force more rapidly than type I fibres, and may also have greater peak power output (see Walmsley et al. 1978; Bottinelli et al. 1996; Widrick et al. 1996). At a macroscopic level, force production can be increased by having shorter muscle fascicles and a larger PCSA, but this results in restricted joint mobility and velocity of shortening (Thorpe et al. 1999; Payne et al. 2006). An increase in type II fibres may thus be an important mechanism by which chimpanzees facilitate increased power and acceleration when terrestrial, despite their gross morphological adaptations (long muscle fascicles and smaller PCSAs) to the greater joint mobility required when moving in an arboreal habitat (Thorpe et al. 1999; Payne et al. 2006). Hindlimb power and acceleration will be important for chimpanzees during conflict and social interactions involving both sexes (Goodall, 1990), and during high-speed terrestrial travel.
In contrast to the chimpanzees, where both the medial and lateral gastrocnemius had a similar proportion of type I fibres, there was a greater difference in the proportion of type I fibres between the two heads of the gastrocnemius muscle in the orangutan. In the orangutan, the gastrocnemius lateralis had a greater proportion of type II fibres. This possibly indicates that this muscle is more likely to be recruited at faster locomotor speeds, for example for mobilisation or dynamic stabilisation (Schilling, 2009) during vertical climbing where the ankle joint is more strongly inverted (Isler, 2005; DeSilva, 2009; J.P. Myatt, personal observation), although more data are required to confirm this.
The orangutan also showed a clear proximal to distal gradient in the soleus muscle, with the proportion of type I fibres decreasing distally. This places more type II fibres at the distal end of the muscle belly, in close proximity to the Achilles tendon, including its internal portion (approximately 18.34% of the total muscle length in the orangutan sampled in the present study). At the muscle–tendon junction type II fibres have a greater surface area dedicated to force transmission than type I fibres (Trotter & Baca, 1987). Therefore, positioning the type II fibres closer to the tendon of insertion may enable more effective and immediate force transmission for dynamic stabilisation to compensate for sudden, unexpected movements in their constantly shifting environment. This, along with the variable gearing mechanism of pennate muscles (Azizi et al. 2008) and the placement of different fibre types throughout the muscle belly, may reflect the ability of a muscle to adapt depending on the mechanical demands of a particular behaviour, although further study and in vivo analysis would be necessary to investigate the behaviour of this muscle–tendon unit in more detail.
The smaller length of the Achilles tendon in chimpanzees and orangutans, compared with humans and cursorial mammals, implies that they rely less on energy return from this tendon during their locomotion. Rather, they benefit in an arboreal habitat by having more muscle that is better able to cope with irregularities and flexibility in habitat structure, by controlling the level of contractile activity and being able to tune muscle contractions more precisely through variable placement of the muscle fibre types, rather than being limited to passive extension like tendon (Roberts, 2002). Chimpanzees and orangutans further benefit in an arboreal habitat by being able to produce moderate forces over a greater range of joint mobility; an adaptation that is reflected in the macro-architecture of their muscles (Payne et al. 2006; Thorpe & Crompton, 2006). However, by possessing longer muscle fibres and smaller PCSAs, chimpanzees and orangutans are limited in their ability to produce high forces. Unlike orangutans, chimpanzees undertake quadrupedal running in short bursts, requiring high accelerations and forces, and long-distance patrols, behaviours which may benefit from an Achilles tendon to some extent. Adaptations to their arboreal habitat, however, possibly outweigh the advantages of an Achilles tendon. Chimpanzees, therefore, appear to have a greater proportion of type II fibres throughout their triceps surae muscle relative to orangutans, to enable them to perform those behaviours requiring speed and power. The locomotor repertoire of orangutans, on the other hand, does not often include behaviours requiring more power or speed, and their need to maintain controlled contractions in their arboreal habitat increases the need for type I over type II fibres. Therefore, orangutans may be limited to slow locomotion by their muscle physiology, although the risky, fragile nature of their arboreal habitat also imposes its own restrictions.
Concluding remarks
This study is a first step to providing insight into how the different great apes are adapted to their habitats and lifestyles at the micro-architecture level, although we acknowledge the limitations of the study with regards to sample size and the ability to discern between fast and slow fibre types only. Further studies, increasing both the number of individuals and species studied, are crucial; gibbons, in particular, would be of interest as they combine a long Achilles tendon with an arboreal locomotor repertoire. Overall, this study has shown that not only do orangutans and chimpanzees have very different muscle fibre profiles that reflect their locomotor repertoires, but it also shows how adaptations in micro-architecture provide an additional parameter by which the output of a muscle can be modulated, and the fine tuning of control is possible. Only by combining data from all aspects of muscle architecture can we truly appreciate the subtle links between form and function and increase our knowledge of great ape locomotor repertoires.
Acknowledgments
Animals were kindly provided through collaboration with Dr A. Kitchener (National Museums Scotland, UK), PD Dr T. Kaiser (Universität Hamburg, Germany) and the Tierpark Hagenbeck (Hamburg, Germany), as well as J. Heuer (Zoologischer Garten Halle, Germany). We wish to thank I. Weiβ for all her technical assistance and training in the preparation of cross-sections and the immunohistochemical procedure, and B. Hesse for her help and training in both the preparation and analysis of samples. Additional thanks go to M. Krüger for technical support. Thanks to J. Neufuβ, J. Schmidt, S. Myatt, K. Miller and A. Ben-Aribia for their assistance with the analysis, and P. Cassey for his input into the statistical analysis. We also thank three anonymous reviewers for their comments on this manuscript. This study was funded by The Royal Society, UK (to S.K.S.T. and N.S.), and the Biotechnology and Biological Sciences Research Council (BBSRC), UK (to J.P.M. and S.K.S.T.).
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Fig. S1 Cross-sections for the five proximo-distal levels (top to bottom) for each muscle showing the percentage of type I fibres in each image counted.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Acosta L, Roy RR. Fiber-type composition of selected hindlimb muscles of a primate (Cynomolgus monkey) Anat Rec. 1987;218:136–141. doi: 10.1002/ar.1092180207. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Energy-saving mechanisms in walking and running. J Exp Biol. 1991;160:55–69. doi: 10.1242/jeb.160.1.55. [DOI] [PubMed] [Google Scholar]
- Alexander RM. A model of bipedal locomotion on compliant legs. Phil Trans Roy Soc B. 1992;338:189–198. doi: 10.1098/rstb.1992.0138. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Tendon elasticity and muscle function. Comp Biochem Physiol. 2002;133:1001–1011. doi: 10.1016/s1095-6433(02)00143-5. [DOI] [PubMed] [Google Scholar]
- Alexander RM, Vernon A. The dimensions of the knee and ankle muscles and the forces they exert. J Hum Mov Stud. 1975;1:115–123. [Google Scholar]
- Alexander RM, Jayes AA, Maloiy GMO, et al. Allometry of the leg muscles of mammals. J Zool Lond. 1981;194:539–552. [Google Scholar]
- Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of 5 mammals. J Histochem Cytochem. 1973;21:51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Saubert CW, IV, Seeherman HJ, et al. Distribution of fiber types in locomotory muscles of dogs. Am J Anat. 1982;163:87–98. doi: 10.1002/aja.1001630107. [DOI] [PubMed] [Google Scholar]
- Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. PNAS. 2008;105:1745–1750. doi: 10.1073/pnas.0709212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M, Conover TE, Schliselfeld LH, et al. Relation of properties of isolated myosin to those of intact muscles of the cat and sloth. Eur J Biochem. 1967;2:156–164. doi: 10.1111/j.1432-1033.1967.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, et al. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol Lond. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Zajac FE, et al. Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Carlson KJ. Muscle architecture of the common chimpanzee (Pan troglodytes): perspectives for investigating chimpanzee behavior. Primates. 2006;47:218–229. doi: 10.1007/s10329-005-0166-4. [DOI] [PubMed] [Google Scholar]
- Channon AJ, Gunther MM, Crompton RH, et al. Mechanical constraints on the functional morphology of the gibbon hind limb. J Anat. 2009;215:383–400. doi: 10.1111/j.1469-7580.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon AJ, Crompton RH, Gunther MM, et al. Muscle moment arms of the gibbon hind limb: implications for hylobatid locomotion. J Anat. 2010;216:446–462. doi: 10.1111/j.1469-7580.2009.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52:129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Dahmane R, Djordjevič S, Šimunič B, et al. Spatial fiber type distribution in normal human muscle: histochemical and tensiomyographical evaluation. J Biomech. 2005;38:2451–2459. doi: 10.1016/j.jbiomech.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Demirel HA, Powers SK, Naito H, et al. Exercise-induced alterations in skeletal muscle myosin heavy chain phenotype: dose-response relationship. J Appl Physiol. 1999;86:1002–1008. doi: 10.1152/jappl.1999.86.3.1002. [DOI] [PubMed] [Google Scholar]
- Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- DeSilva JM. Functional morphology of the ankle and the likelihood of climbing in early hominins. PNAS. 2009;106:6567–6572. doi: 10.1073/pnas.0900270106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickx N, Cagnie B, Achten E, et al. Differentiation between deep and superficial fibers of the lumbar multifidus by magnetic resonance imaging. Eur Spine J. 2010;19:122–128. doi: 10.1007/s00586-009-1171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem J. 1975;7:259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- Edstrom L, Nystrom B. Histochemical types and sizes of fibres in normal human muscles. A biopsy study. Acta Neurol Scand. 1969;45:257–269. doi: 10.1111/j.1600-0404.1969.tb01238.x. [DOI] [PubMed] [Google Scholar]
- Eng CM, Smallwood LH, Rainiero MP, et al. Scaling of muscle architecture and fiber types in the rat hindlimb. J Exp Biol. 2008;211:2336–2345. doi: 10.1242/jeb.017640. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol. 2001;204:3201–3208. doi: 10.1242/jeb.204.18.3201. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Sjödin B, Karlsson J, et al. Human soleus muscle: a comparison of fiber composition and enzyme activities with other leg muscles. Pflug Arch Eur J Phy. 1974;348:247–255. doi: 10.1007/BF00587415. [DOI] [PubMed] [Google Scholar]
- Goodall J. Through a Window: Thirty Years with the Chimpanzees of Gombe. Great Britain: George Weidenfeld and Nicolson; 1990. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Hunt KD. Positional behavior of Pan troglodytes in the Mahale Mountains and Gombe Stream national parks, Tanzania. Am J Phys Anthropol. 1992a;87:83–107. doi: 10.1002/ajpa.1330870108. [DOI] [PubMed] [Google Scholar]
- Hunt KD. Social rank and body size as determinants of positional behavior in Pan troglodytes. Primates. 1992b;33:347–357. [Google Scholar]
- Isler K. 3D-kinematics of vertical climbing in hominoids. Am J Phys Anthropol. 2005;126:66–81. doi: 10.1002/ajpa.10419. [DOI] [PubMed] [Google Scholar]
- Jaenicke T, Martindale J, Loughna PT, et al. Mechanical signals involved in the expression of the slow type genes and the determination of muscle fiber-phenotype in the mammalian soleus. J Muscle Res Cell Motil. 1991;12 [Google Scholar]
- Kernell D. Muscle regionalization. Can J Appl Physiol. 1998;23:1–22. doi: 10.1139/h98-001. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y. Comparative analysis of muscle architecture in primate arm and forearm. Anat Histol Embryol. 2009;39:93–106. doi: 10.1111/j.1439-0264.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- Kimura T. Composition of psoas major muscle fibers compared among humans, orangutans, and monkeys. Z Morph Anthropol. 2002;83:305–314. [PubMed] [Google Scholar]
- Kram R, Taylor CR. Energetics of running – a new perspective. Nature. 1990;346:265–267. doi: 10.1038/346265a0. [DOI] [PubMed] [Google Scholar]
- Langdon JH. Variations in cruropedal musculature. Int J Primatol. 1990;11:575–606. [Google Scholar]
- Lieber RL. Skeletal Muscle Structure, Function and Plasticity, the Physiological Basis of Rehabilitation. USA: Lippincott Williams and Wilkins; 2002. [Google Scholar]
- Loughna PT, Izumo S, Goldspink G, et al. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development. 1990;109:217–223. doi: 10.1242/dev.109.1.217. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. Tensile properties of the in vivo human gastrocnemius tendon. J Biomech. 2002;35:1639–1646. doi: 10.1016/s0021-9290(02)00240-3. [DOI] [PubMed] [Google Scholar]
- von Mering F, Fischer MS. Fibre type regionalization of forelimb muscles in two mammalian species, Galea musteloides (Rodentia, Caviidae) and Tupaia belangeri (Scandentia, Tupaiidae), with comments on postnatal myogenesis. Zoomorphology. 1999;119:117–126. [Google Scholar]
- Michilsens F, Vereecke EE, D'Aout K, et al. Functional anatomy of the gibbon forelimb: adaptations to a brachiating lifestyle. J Anat. 2009;215:335–354. doi: 10.1111/j.1469-7580.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster AW, Chan HC, O'Connor D. Activity patterns of human skeletal muscles: relation to muscle fiber type composition. Science. 1978;200:314–317. doi: 10.1126/science.635587. [DOI] [PubMed] [Google Scholar]
- Moritz S, Fischer MS, Schilling N. Three-dimensional fibre-type distribution in the paravertebral muscles of the domestic ferret (Mustela putorius f. furo) with relation to functional demands during locomotion. Zoology. 2007;110:197–211. doi: 10.1016/j.zool.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Ohira Y, Yoshinaga T, Nonaka I, et al. Histochemical responses of human soleus muscle fibers to long-term bedrest with or without countermeasures. Jpn J Physiol. 2000;50:41–47. doi: 10.2170/jjphysiol.50.41. [DOI] [PubMed] [Google Scholar]
- Oishi M, Ogihara N, Endo H, et al. Muscle architecture of the upper limb in the orangutan. Primates. 2008;49:204–209. doi: 10.1007/s10329-008-0082-5. [DOI] [PubMed] [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans I. Muscle architecture. J Anat. 2006;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Punkt K. Fibre Types in Skeletal Muscles: Advances in Embryology and Cell Biology 162. New York: Springer-Verlag; 2002. [DOI] [PubMed] [Google Scholar]
- Roberts TJ. The integrated function of muscles and tendons during locomotion. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1087–1099. doi: 10.1016/s1095-6433(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Rome LC, Funke RP, Alexander RM, et al. Why animals have different muscle fibre types. Nature. 1988;335:824–827. doi: 10.1038/335824a0. [DOI] [PubMed] [Google Scholar]
- Schilling N. Metabolic profile of the perivertebral muscles in small therian mammals: implications for the evolution of the mammalian trunk musculature. Zoology. 2009;112:279–304. doi: 10.1016/j.zool.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Schilling N. Fiber type distribution in the shoulder muscles of the tree shrew, the cotton top tamarind, and the squirrel monkey related to shoulder movements and forelimb loading. J Hum Evol. 2007;52:401–419. doi: 10.1016/j.jhevol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther. 2001;81:1810–1816. [PubMed] [Google Scholar]
- Seow CY, Ford LE. Shortening velocity and power output of skinned muscle-fibers from mammals having a 25 000-fold range of body-mass. J Gen Physiol. 1991;97:541–560. doi: 10.1085/jgp.97.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickles DW, Pinkstaff CA. Comparative histochemical study of prosimian primate hindlimb muscles. I. Muscle fiber types. Am J Anat. 1981a;160:175–186. doi: 10.1002/aja.1001600204. [DOI] [PubMed] [Google Scholar]
- Sickles DW, Pinkstaff CA. Comparative histochemical study of prosimian primate hindlimb muscles. II. Populations of fiber types. Am J Anat. 1981b;160:187–194. doi: 10.1002/aja.1001600205. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Bouchard C. Genetic determinism of fiber type proportion in human skeletal muscle. FASEB J. 1995;9:1091–1095. doi: 10.1096/fasebj.9.11.7649409. [DOI] [PubMed] [Google Scholar]
- Smith JL, Edgerton VR, Betts B, et al. EMG of slow and fast ankle extensors of cat during posture, locomotion, and jumping. J Neurophysiol. 1977;40:503–513. doi: 10.1152/jn.1977.40.3.503. [DOI] [PubMed] [Google Scholar]
- Spector SA, Gardiner PF, Zernike RF, et al. Muscle-architecture and force-velocity characteristics of cat soleus and medial gastrocnemius - implications for motor control. J Neurophysiol. 1980;44:951–960. doi: 10.1152/jn.1980.44.5.951. [DOI] [PubMed] [Google Scholar]
- Swindler DR, Wood CD. An Atlas of Primate Gross Anatomy. USA: University of Washington Press; 1973. [Google Scholar]
- Thorpe SKS, Crompton RH. Locomotor ecology of wild orangutans (Pongo abelii) in the Gunung Leuser ecosystem, Sumatra, Indonesia: a multivariate analysis using log-linear modelling. Am J Phys Anthropol. 2005;127:58–78. doi: 10.1002/ajpa.20151. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH. Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea. Am J Phys Anthropol. 2006;131:384–401. doi: 10.1002/ajpa.20422. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Gunther MM, et al. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (Pan troglodytes) Am J Phys Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Chamberlain A. Bipedal behaviour in captive chimpanzees (Pan troglodytes) In: Harcourt CS, Sherwood BR, editors. New Perspectives in Primate Evolution and Behaviour. Great Britain: Westbury Academic & Scientific Publishing; 2002. pp. 231–248. [Google Scholar]
- Trotter JA, Baca JM. A stereological comparison of the muscle-tendon junctions of fast and slow fibers in the chicken. Anat Rec. 1987;218:256–266. doi: 10.1002/ar.1092180306. [DOI] [PubMed] [Google Scholar]
- Vereecke EE. Antwerp: University of Antwerp; 2006. The functional morphology and bipedal locomotion of Hylobates lar and its implications for the evolution of bipedalism in hominins. PhD Thesis. [Google Scholar]
- Vereecke EE, D'Août K, Payne R, et al. Functional analysis of the foot and ankle myology of gibbons and bonobos. J Anat. 2005;206:453–476. doi: 10.1111/j.1469-7580.2005.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978;41:1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Watts DP, Mitani JC. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour. 2001;138:299–327. [Google Scholar]
- Widrick JJ, Trappe SW, Costill DL, et al. Force-velocity and force-power properties of single muscle fibers from elite master runners and sedentary men. Am J Physiol Cell Physiol. 1996;271:C676–C683. doi: 10.1152/ajpcell.1996.271.2.C676. [DOI] [PubMed] [Google Scholar]
- Williams TM, Dobson GP, Mathieu-Costello O, et al. Skeletal muscle histology and biochemistry of an elite sprinter, the African cheetah. J Comp Physiol B. 1997;167:527–535. doi: 10.1007/s003600050105. [DOI] [PubMed] [Google Scholar]
- Wunderlich RE, Ford KR. EMED Scientific Meeting, August 2000. Munich, Germany: 2000. Plantar pressure distribution during bipedal and quadrupedal walking in the chimpanzee (Pan troglodytes) [Google Scholar]
- Ziegan J. Kombinationen enzymhistochemischer Methoden zur Fasertypendifferenzierung und Beurteilung der Skeletmuskulatur. Acta Histochem. 1979;65:34–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


