Abstract
Patients with metastatic prostate cancer who undergo androgen ablation therapy invariably relapse and develop incurable castration-resistant disease. Activation of the pro-survival Akt pathway accompanies androgen ablation. We discovered that androgen receptor (AR) induces expression of the tumor suppressor inositol polyphosphate 4-phosphatase type II (INPP4B) but not PTEN in prostate cancer cells. Optimal induction of INPP4B by AR required expression of the transcriptional co-activator NCoR. INPP4B dephosphorylates phosphatidylinositol-3, 4-bisphosphate, which leads to reduced phosphorylation and activity of Akt. In support of a key role for INPP4B in Akt control, INPP4B depletion activated Akt and increased cellular proliferation. The clinical significance of INPP4B in androgen-dependent prostate cancers was determined in normal or primary tumor prostate tissues derived from radical prostatectomy specimens. In primary tumors, expression of both INPP4B and PTEN was significantly reduced compared to normal tissue. Further, decreased expression of INPP4B reduced the time to biochemical recurrence. Thus, androgen ablation can activate the Akt pathway via INPP4B downregulation, thereby mitigating the antitumor effects of androgen ablation. Our findings reinforce the concept that patients undergoing androgen ablation may benefit from Akt targeting therapies.
Keywords: Prostate cancer, androgen receptor, INPP4B, PTEN
Introduction
Androgen ablation therapy through suppression of testicular androgen production or treatment with androgen receptor (AR) antagonist remains the cornerstone of systemic prostate cancer treatment. Although initially successful at controlling advanced tumors, the disease inevitably progresses to a more aggressive state termed hormone-refractory, castration resistant, or androgen independent prostate cancer. However, castration resistant tumors retain AR and select AR-regulated gene expression in the absence or in low levels of circulating androgens, demonstrating that AR signaling continues to play a significant role in patients with castration resistant disease (1–3).
In the normal mature prostate AR is functionally dichotomous: supporting proliferative epithelial renewal while maintaining terminal differentiation of secretory epithelium. Prostate specific deletion of the AR leads to dedifferentiation and increased proliferation of luminal epithelial cells (4). In addition, androgens regulate the expression of growth suppressing genes such as Nkx3.1 and AS3 (5–8). Hence androgens regulate growth of prostate epithelial cells through the regulation of a select subset of AR target genes. Although AR plays a pivotal role in maintaining cellular quiescence and terminal differentiation of normal prostate epithelium, during the development and progression of prostate cancer there is a gradual shift in AR function to predominantly proliferative. Expression of differentiation markers such as prostate specific antigen (PSA) demonstrates that the AR retains some differentiating function in prostate cancer. The functional shift in AR activity is potentially mediated through altered expression of multiple AR co-regulatory proteins and the activation of extracellular signaling pathways (9). Coregulators of the AR, such as the p160 family of coactivators potentiate AR function and demonstrate increased expression in prostate cancer that correlates with poor patient outcome (10–12). Promoter specific modulation of AR function by coregulators, may account for the selective reactivation of AR signaling pathways that favor growth in advanced prostate cancers (9).
Androgen ablation therapies routinely utilized in the treatment of advanced prostate cancers and androgen-independent tumors are associated with increased Akt signaling (13, 14). Furthermore, androgen starvation of prostate cancer cells leads to increased PI3K/Akt activity, which supports survival and androgen independent growth that can be suppressed by dihydrotestosterone (DHT) (15, 16). Androgens therefore control proliferation of prostate epithelial cells in part through down regulation of Akt signaling. Activated Akt signaling stimulates cellular proliferation, cell survival, cell cycle progression, growth, migration and angiogenesis (17). As an example, pro-apoptotic Forkhead transcription factor class-O family (FOXO) members are phosphorylated by Akt, which targets them for degradation (18). Deregulation of Akt signaling is associated with numerous human cancers including prostate cancer. Expression of activated Akt is elevated in prostate cancer compared to normal tissue and is associated with reduced time to biochemical recurrence (19).
Akt activity is dependent on the availability of phosphatidylinositol-3,4,5-trisphosphate (PI(3,4,5)P3) and phosphatidylinositol-3,4-bisphosphate (PI(3,4)P2). The signaling lipids PI(3,4,5)P3 and PI(3,4)P2 are generated through PI3K (Phosphoinositide 3-kinase) activity and are degraded by PTEN (Phosphatase and Tensin homolog deleted on chromosome 10) and INPP4B (Inositol polyphosphate 4-phosphatase type II) respectively (20). The PTEN substrate PI(3,4,5)P3 contributes predominantly to Thr308 phosphorylation and membrane associated activation of Akt, whereas PI(3,4)P2, INPP4Bs substrate, contributes mostly to Ser473 phosphorylation and cytoplasmic activation of Akt (21). PTEN is a dual specificity phosphatase that dephosphorylates PI(3,4,5)P3 as well as serine, threonine, and tyrosine residues in vitro, and focal adhesion kinase in vivo. PTEN loss of function or expression is frequently observed in human cancers and loss of PTEN in mice results in a number of different tumor types (22–24). Homozygous gene deletions, loss of heterozygosity (LOH) and inactivating mutations of PTEN in prostate cancers show that PTEN activity is lost in a large percentage of prostate cancers and likely contributes to prostate cancer progression (25, 26).
INPP4B is a class II phosphatase that preferentially hydrolyzes the 4 position of PI(3,4)P2. Silenced INPP4B expression in malignant proerythroblast was associated with increased activated Akt levels that could be alleviated by re-expression of INPP4B (27). In a nonbiased RNAi-based genetic screen, loss of INPP4B was shown to facilitate anchorage independent growth of human mammary epithelial cells (28). Significantly, INPP4B was recently suggested to be a tumor suppressor gene in breast and ovarian cancers that suppresses PI3K/AKT signaling. Reduced INPP4B mRNA levels were identified in BRCA1 and basal-like breast tumors (29). Decreased levels of INPP4B protein in breast and ovarian cancer correlated with decreased survival as determined by tissue microarray expression analysis (29).
Given that activated Akt is elevated in human prostate cancers and is associated with castration resistance, we investigated the regulation of INPP4B and its possible implication in prostate cancer. Significantly, we found that AR directly regulates INPP4B but not PTEN expression in prostate cancer cells. We show that INPP4B regulates Akt activation and cellular proliferation in prostate cancer cells. Using prostate cancer tissue microarrays we observed decreased expression of both PTEN and INPP4B in prostate cancer compared to benign tissue. Our data indicates that decreased expression of INPP4B has similar predictive value to PTEN for prostate cancer recurrence and is therefore potentially equally as important as PTEN in the etiology of prostate cancer.
Materials and Methods
Cell Culture
LNCaP and VCaP prostate cancer cells were purchased from ATCC and were maintained in RPMI 1640 or DMEM media respectively and supplemented with 10% FBS according to ATCC guidelines. All media were purchased from Invitrogen (Carlsbad, CA), FBS and charcoal stripped serum (css) were purchased from Sigma-Aldrich (St. Louis, MO). R1881 was purchased from Perkin Elmer (Waltham, MA), bicalutamide was purchased from LKT Laboratories (St. Paul, MN), cycloheximide was purchased from Sigma-Aldrich and epidermal growth factor (EGF) was purchased from Becton, Dickinson and Company (Franklin Lakes, NJ).
Constructs
Full length human INPP4B was obtained from Open Biosystems (Huntsville, AL). FLAG-INPP4B was generated by PCR amplification (Forward: aattaattagcggccgcgaaattaaagaggaaggggc, Reverse: aattaatgcggccgcttaggtgtcagcttttccataagtc) of the INPP4B CDS and insertion into the NotI site of p3×FLAG-CMV-10 (Sigma-Aldrich).
Transfection
LNCaP cells were transfected with siRNA using Lonza electroporation buffer R as recommended by the manufacturer (Lonza, Walkerswille, MD). Briefly, 2×106 cells were electroporated with 800 pmoles of the indicated siRNA. Cells were seeded onto poly-D-lysine coated plates, treated as described per experiment and harvested for RNA and protein analysis. NCoR down regulation was performed using siRNA previously described (30). INPP4B down regulation was performed using silencer siRNAs and non-coding silencer siRNA was used as a control (Ambion, Austin, TX). For overexpresssion of INPP4B, LNCaP cells were seeded at 2.5×105 cells per well in 6 well plates. Cells were transfected with 3 μg of FLAG-INPP4B or empty vector per well using Lipofectamine as described by the manufacturer (Invitrogen).
Western Blot Analysis
Protein was extracted with buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1mM EDTA, 1% Triton-X 100), including protease and phosphatase inhibitors (Roche and Calbiochem respectively). For each sample 50 μg of protein was resolved on 7.5% or 4–15% SDS-PAGE and transferred to nitrocellulose membranes. Immunoblotting was performed as previously described (31) using antibodies against INPP4B (1:1000), AR (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA), total Akt (1:1000), phospho-Akt Thr308 (1:1000), phospho-Akt Ser473 (1:1000), FOXO3a (1:1000), phospho-FOXO3a S253 (1:1000) (Cell Signaling, Danvers, MA), M2 FLAG epitope (1:1000) (Sigma-Aldrich), β-actin (1:5000) (Sigma-Aldrich), and β-Tubulin (1:2000) (Millipore, Billerica, MA). Luminescent signals were captured on a Gel Logic 2000 imaging system with Kodak Molecular Imaging software (Kodak, Rochester, NY).
Proliferation assay
Proliferation assays were performed using a Roche DP RCTA Xcelligence machine as described by the manufacturer (Roche, Germany). Background impedance was determined by incubating E-Plates with 100 μl of RPMI 1640 with 10% FBS at room temperature for 30 min. LNCaP cells were electroporated with 800 pmol control noncoding or INPP4B specific siRNAs and 2×104 cells were seeded per well. Cells were incubated at room temperature for 30 min prior to placement into the Real Time Cell Analyzer (RTCA). Cells were grown for 50 hours and impedance measured every 15 min. Impedance is represented by Cell Index (CI) and was calculated as follows: CI = (Zi−Z0)/15Ω. Where Zi is the impedance at an individual time point and Z0 is the background impedance. Average CI was calculated from a minimum of three wells per time point and per experiment. Raw CI values were normalized to a time point following cell adherence, but prior to proliferation. Normalized cell index (NCIti) was calculated as the cell index CIti at a given time point divided by the cell index CInml_time at the normalized time point (nml_time)(NCIti = CIti/CInml_time).
CHIP assays
ChIP assays were performed exactly as previously described (31). Briefly, LNCaP cells were grown in medium supplemented with 10% css for 36 hours, crosslinked, sonicated and immunoprecipitated with either 5 μg of AR antibody or 5 μg of rabbit IgG. Crosslinking was reversed overnight and immunoprecipitated DNA examined by real time quantitative PCR using the Roche Universal Probe library. The primers and probe sets used to detect AR recruitment were: PSA Enhancer (31), INPP4B ARE1 (Forward: aggtgagctacaagcaaggaa, Reverse: tctgaataactcatgatattgggaaa, Probe 46), INPP4B ARE2 (Forward: attggtggctcaaaatccaa, Reverse: gcaagagaaagaagatacaaaacca, Probe 24), and Negative region (Forward: atgcgctctagctaatatcaacc, Reverse: cctataagcctcctcagagtagaaga, Probe 59).
Real-Time PCR Analysis
RNA was prepared from cell lines using Trizol reagent as described by the manufacturer (Invitrogen). First strand cDNA was synthesized using the Superscript III kit (Invitrogen). The PSA primer and probe set was previously described (11). The Roche Universal Probe library and primers were used to amplify the following genes; INPP4B (Forward: gaaagcttccactcgtggtg, Reverse: tgtttcgctggtttcaagg, Probe 63), ERG (Forward: ggttaatgcatgctagaaacaca, Reverse: agatggttgagcagctttcg, Probe 64), PTEN (Forward: ggggaagtaaggaccagagac, Reverse: tccagatgattctttaacaggtagc, Probe 48), PMEPA1 (Forward: ctgcacggtccttcatcag, Reverse ttgcctgacactgtgctctc, Probe 76), and the reference gene 18S (Forward: gcaattattccccatgaacg, Reverse: gggacttaatcaacgcaagc, Probe 48). Real-time PCR amplification was performed on a Roche 480 LightCycler (all PCRs were carried out with annealing temp of 58).
Immunohistochemical analysis of human tissue microarrays
Tissue microarrays used in this study were described previously (32). Samples were procured from radical prostatectomies of 640 patients who received no adjuvant therapy. Immunohistochemical analysis for INPP4B was performed using INPP4B goat polyclonal antibody (Santa Cruz) exactly as previously described (29). Samples were scanned using a Bliss automated slide scanner to generate high resolution digital images. Staining was evaluated in normal luminal epithelial cytoplasm in normal samples or epithelial tumor cells of prostate cancer samples. Staining index was calculated as a product of average staining intensity (0 to 3) and average extent of staining (0 to 3) yielding a staining index of 0–9 as described previously. PTEN, and Ki67 staining and quantitation have been reported previously (33, 34).
Statistical analysis
Corresponding Independent samples T-tests were used after testing the equal variances assumption for INPP4B protein levels in in vitro experiments. Spearman correlation coefficients were used to evaluate relationships between INPP4B and clinical-pathological parameters. Comparisons of levels of INPP4B and PTEN between normal and tumor tissues were done using Wilcoxon Signed Ranks test. Mann-Whitney test was used to compare INPP4B and PTEN levels among Gleason Grade groups. Boxplots were used for illustration of these results. Kaplan-Meier recurrence-free survival curves for different levels of INPP4B, INPP4B/Ki67, PTEN and PTEN/Ki67 combinations were plotted. Minimum p-value method was used to divide the patient population into low and high expressing recurrence-free groups. Cox proportional hazard regression modeling of biochemical recurrence was used to compare groups and to develop multivariate survival models. For quantitative PCR analysis, statistical significance was determined by Student’s t-test.
Results
INPP4B is a primary AR target gene
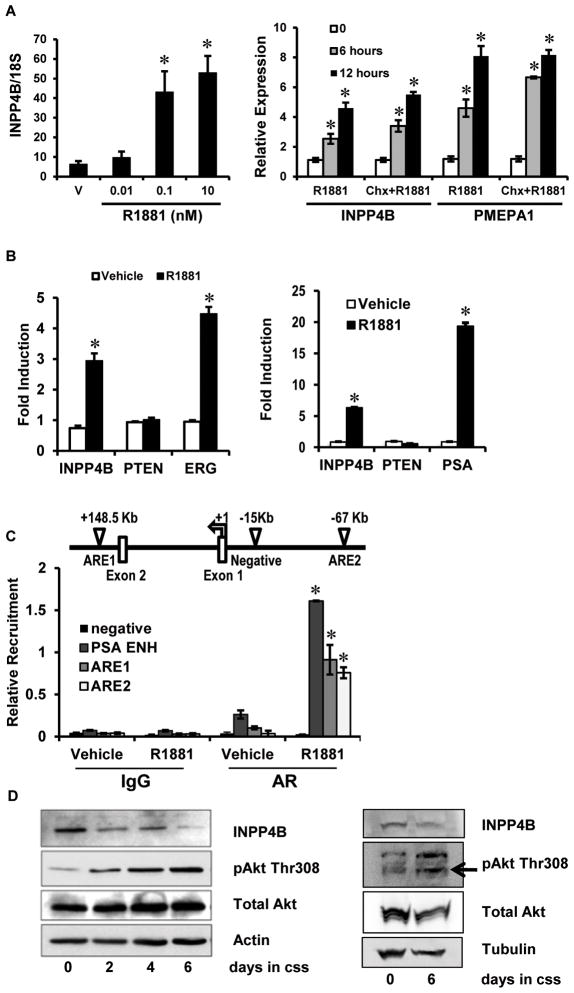
In the absence of androgens, Akt and phospho-Akt protein levels are increased in LNCaP cells, and this is reversed by treatment with DHT (16). LNCaP cells lack functional PTEN due to a frameshift mutation (35, 36), thus we sought to determine if INPP4B expression was responsive to androgens and potentially mediated androgen regulation of Akt signaling. To determine if INPP4B was an androgen responsive gene, LNCaP cells were cultured in the absence of androgens and subsequently treated with the synthetic androgen R1881 and INPP4B expression evaluated by quantitative RT-PCR. Significantly, INPP4B demonstrated both dose and time dependent regulation by R1881 in LNCaP prostate cancer cells (Figure 1A). Pretreatment with cycloheximide to inhibit de novo protein synthesis did not reduce R1881 induction of a primary AR target gene PMEPA1 (37) or INPP4B (Figure 1A), indicating that INPP4B is regulated by androgens at the level of transcription. INPP4B mRNA expression was induced in both LNCaP and VCaP AR expressing prostate cancer cells. VCaP express functional PTEN and although LNCaP cells lack functional PTEN, they retain mRNA expression (38, 39). No induction of PTEN transcription was observed in LNCaP or VCaP cells (Figure 1B). PSA and the TMPRSS2-ERG fusion gene were evaluated in LNCaP and VCaP cells respectively as known direct AR target genes and controls for hormone induction. Examination of a data set reported by Wang et. al. indicated the presence of two AR binding regions in the INPP4B locus in LNCaP cells (Figure 1C) (40). Direct recruitment of AR to both AR binding regions in the INPP4B locus was evaluated by ChIP analysis (Figure 1C). As expected AR was recruited to the PSA enhancer region (41). Androgen stimulation of LNCaP cells significantly enhanced AR recruitment to both binding regions of the INPP4B locus, further confirming INPP4B as a direct AR target gene. No recruitment was observed to a negative control region 15 kilobases upstream of the INPP4B transcription initiation start site (Figure 1C). In agreement with our INPP4B expression analysis, culturing LNCaP cells in the absence of androgens led to decreased INPP4B expression and elevation of activated Akt (Figure 1D). In addition, androgen starvation of VCaP cells, which are PTEN positive, led to decreased expression of INPP4B and elevated levels of activated Akt (Figure 1D). Thus, INPP4B contributes to AR driven suppression of Akt activation.
Figure 1. INPP4B is an AR target gene.
A. LNCaP cells grown in 10% css medium for 36 hours were treated with vehicle or the indicated concentrations of R1881. Cells were harvested 48 hours post-treatment, RNA was extracted and INPP4B and 18S expression analyzed by quantitative RT-PCR (Left graph). LNCaP cells were grown in 10% css medium for 48 hours, pretreated with 10 μM cycloheximide or vehicle for an hour, 0 hour time point cells were harvested and cycloheximide and vehicle treated cells were then treated with 1 nM R1881 for 6 or 12 hours. RNA was extracted and analyzed for INPP4B, PMEPA1, and 18S expression by quantitative RT-PCR (Right graph). B. LNCaP cells were grown 48 hours in 10% css medium. Cells were then treated with 1 nM R1881 or vehicle (ethanol (vehicle)) for 24 hours. Expression of INPP4B, PTEN, PSA and 18S were analyzed by quantitative RT-PCR (Left graph). VCaP cells cultured 48 hours in 10% css and then treated with 1 nM R1881 for 24 hours. Expression of INPP4B, PTEN, TMPRSS2-ERG (ERG) and 18S were analyzed by quantitative RT-PCR (Right graph). C. Schematic diagram of the INPP4B locus demonstrating the location of AREs and the negative control region relative to the transcription initiation start site. LNCaP cells were grown in 10% css medium for 36 hours and treated with either vehicle (ethanol) or 1 nM R1881 for 12 hours. AR recruitment to the INPP4B ARE1, INPP4B ARE2, negative control and PSA enhancer was determined by ChIP analysis and quantitative PCR. D. LNCaP (Left panel) and VCaP (Right panel) cells were plated in 10% FBS media; the next day (Day 0) medium was replaced with 10% css, and cells were harvested at the indicated time points. Protein was extracted and expression of INPP4B, phospho-Akt (Thr308), total Akt, and β-actin or tubulin was analyzed by Western blot. Star indicates statistically significant difference from vehicle treated control with p<0.05.
Depletion of INPP4B activates Akt and stimulates proliferation
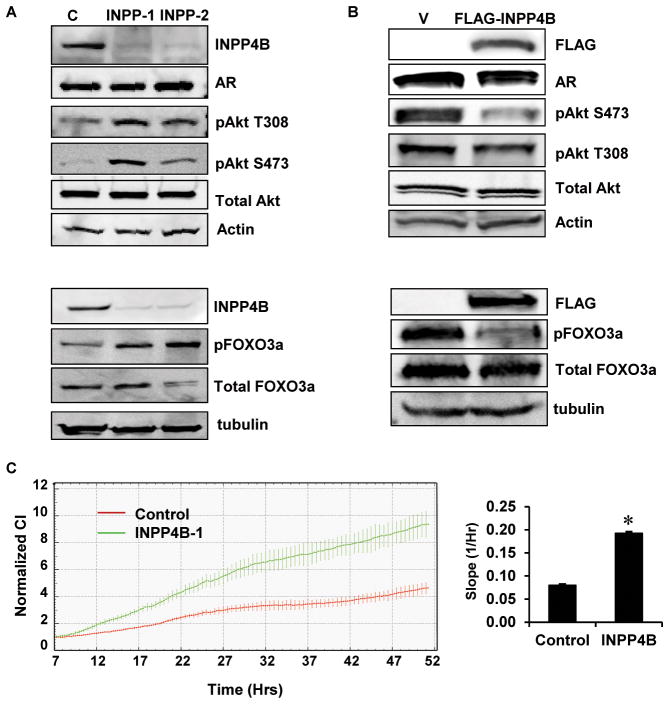
Gewinner et al previously reported that INPP4B depletion in breast cancer cells increased proliferation (29). Since LNCaP cells lack functional PTEN we were interested to know if depleting INPP4B could further activate PI3K/Akt signaling and cellular proliferation. LNCaP cells treated with two independent siRNAs specifically targeting INPP4B showed significantly reduced INPP4B levels after 48 hours, without appearing to affect AR or total Akt steady state levels (Figure 2A). In agreement with Figure 1D, depletion of INPP4B in LNCaP cells increased the levels of activated Akt (Figure 2A). Further, depletion of INPP4B increased phosphorylation of FOXO3a (Ser253), a direct Akt substrate (Figure 2A). Correspondingly, overexpression of FLAG-INPP4B in LNCaP cells cultured in 10% css decreased the levels of activated Akt without significantly altering AR and total Akt (Figure 2B). In order to demonstrate INPP4B regulation of Akt downstream targets, we overexpressed INPP4B in LNCaP cells and measured phosphorylation of FOXO3a following a 30 minute stimulation with EGF prior to protein extraction. Exogenous expression of INPP4B clearly impeded phosphorylation of FOXO3a in LNCaP cells stimulated with EGF (Figure 2B). Depletion of INPP4B in LNCaP cells significantly increased the rate of proliferation of LNCaP cells as measured by cellular index (Figure 2C). Cellular index is a measure of electrical impedance, which is proportional to the number of adherent cells on the electrode grid integrated into the bottom of the plate. The average slope of the impedance curve was calculated between 9 and 50 hours posttransfection to allow for depletion of INPP4B protein. INPP4B depletion routinely decreased the doubling time of LNCaP cells by 25–30% (Data not shown).
Figure 2. INPP4B regulates proliferation and Akt phosphorylation in prostate cancer cells.
A. Two million LNCaP cells were electroporated with either noncoding control (C) or two independent INPP4B specific siRNAs (INPP-1 or INPP-2) (Ambion). One million cells were plated per 10 cm dish for each siRNA. Cells were grown for 48 hours in medium supplemented with 10% FBS. Cellular protein extracts were prepared and analyzed by Western blot for INPP4B, AR, phospho-Akt (Thr308 and Ser473), total Akt and β-actin (Top panels). LNCaP cells were electroporated as described above and cultured for 48 hours post-transfection. Cellular protein extracts were prepared from cells and analyzed by Western blot for INPP4B, phospho-FOXO3a (Ser253), total FOXO3a and β-tubulin (Bottom panels) B. LNCaP cells were transfected with 3×FLAG-INPP4B or empty vector and cells grown in medium supplemented with 10% css. Protein extracts were prepared 48 hours post-transfection and analyzed by Western blot for INPP4B, AR, phospho-Akt (Thr308 and Ser473), total Akt and β-actin (Top panels). LNCaP cells were transfected as described above, grown for 48 hours and prior to protein extraction cells were treated with 100 ng/ml EGF for 30 minutes. Protein was extracted and analyzed by Western blot for INPP4B, phospho-FOXO3a (Ser253), total FOXO3a and β-tubulin (Bottom panels). C. LNCaP cells were electroporated as in A and seeded at 2×104 cells per well of an E-Plate 16 and cellular impedance as a measure of proliferation was monitored continuously for 50 hours (Left graph). Quantification of the proliferation slopes (Right graph).
INPP4B and PTEN are reduced in prostate cancer and are associated with reduced time to biochemical recurrence
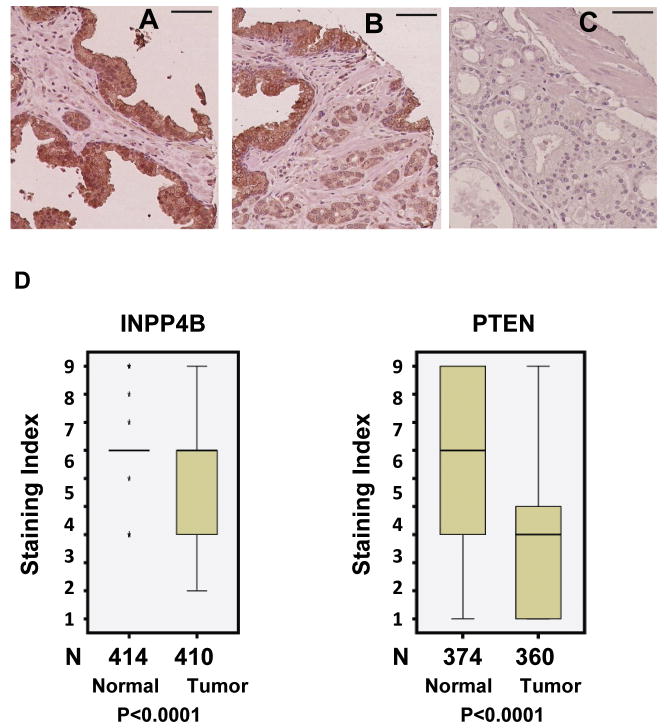
To determine whether INPP4B is expressed in normal human prostate tissue and if its expression was lost during the development and progression of prostate cancer we screened a prostate tissue microarray. We observed specific staining for INPP4B in luminal epithelial cells in normal prostate specimens and in cancer cells (Figure 3A–C). Examination of clinical prostate specimens showed a significant decrease in INPP4B expression in human prostate cancers compared to normal tissue (p<0.0001) (Figure 3D). We observed quite consistent staining for INPP4B in normal tissue with all but 5 samples showing expression with a staining index of 6, while half of the tumor samples scored less than 6 (Figure 3D). In the previously reported tissue microarray analysis of breast and ovarian cancer, INPP4B also demonstrated epithelial compartment expression (27). Consistent with previous reports we found that PTEN expression is decreased in prostate cancer specimens compared to normal tissue (Figure 3D) (p<0.0001).
Figure 3. Expression of INPP4B is reduced in prostate cancer.
Examples of immunohistochemical staining of prostate tissue microarrays with anti-INPP4B antibody. A. Normal prostate with strong staining of the epithelium. B. Infiltrating cancer glands with moderate staining adjacent to normal epithelium (left and top). C. Prostate cancer with no staining. Scale bar: 150 microns. D. Tissue microarrays constructed from radical prostatectomy patient tissues were evaluated for INPP4B and PTEN expression in normal and tumor tissue by IHC. Wilcoxon Signed-Ranks test was used to compare INPP4B (Left graph) and PTEN (Right graph) expression between normal and tumor tissues. N denotes the number of patients per group and 3 cores were analyzed per patient. * Denotes outliers.
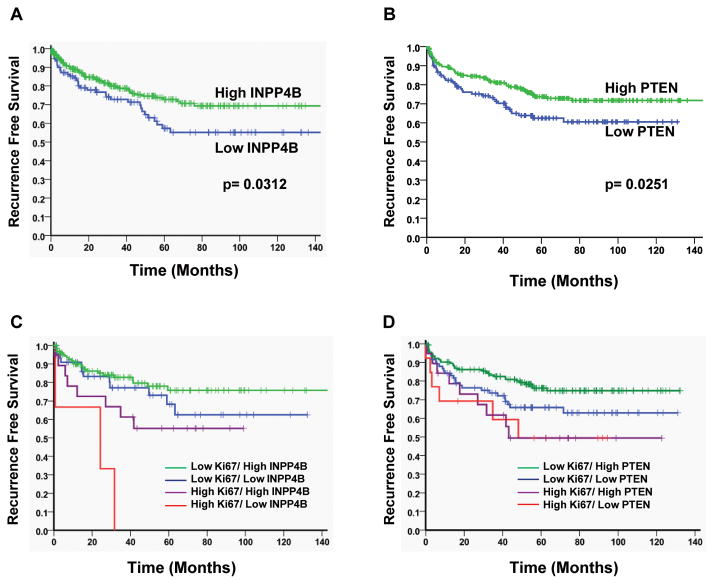
Since we observed a decrease in INPP4B and PTEN in prostate cancer, we sought to further identify correlations of these two proteins that regulate the Akt pathway with clinical parameters. Significantly, patients with lower INPP4B expression (≤ 5) recurred earlier compared to patients with higher INPP4B expression (≥6) (p=0.0312) (Fig 4A). Patients, who lost PTEN expression showed a similar decrease in recurrence free survival. (p=0.0263) (Figure 4B).
Figure 4. Loss of INPP4B and PTEN correlates with reduced recurrence free survival.
A. Recurrence free survival graph for prostate cancer tissue stained for INPP4B expression. Tissues from prostate cancer patients were stained for INPP4B and scored for expression levels (High expression ≥6, Low expression ≤5). Patients with low INPP4B expression showed significantly shorter time to biochemical recurrence compared to patients with high INPP4B expression (p=0.0312). B. Recurrence free survival graph for prostate cancer tissue stained for PTEN expression. Tissues from prostate cancer patients were stained for PTEN and scored for expression levels (High expression ≥1, Low expression = 0). Patients with loss of PTEN expression showed significantly shorter time to biochemical recurrence compared to patients that retained PTEN expression (p=0.00251). C. Prostate cancer tissues were analyzed for correlation of INPP4B expression and expression of the proliferation marker Ki67 with recurrence free survival. Loss of INPP4B significantly increased biochemical recurrence in highly proliferative prostate cancers (p=0.0368). D. Prostate cancer tissues were analyzed for correlation of PTEN expression and expression of the proliferation marker Ki67 with biochemical recurrence. Loss of PTEN has no correlation with aggressively growing prostate cancers but tends to separate slower-growing cancer patients into a higher risk group (p=0.0567).
Since prostate cancer is a heterogeneous disease ranging from an indolent to aggressively proliferating malignancy, INPP4B and PTEN expression were correlated with the recurrence rate of patients with high and low expression levels of the proliferation marker Ki67, which was determined previously in this same array. Our data suggest that in rapidly proliferating tumors loss of INPP4B coincides with accelerated recurrence (p=0.0312) (Figure 4C). Loss of INPP4B in more slowly proliferating cancers did not significantly alter the time to biochemical recurrence. Interestingly, loss of PTEN in combination with Ki67 showed no correlation with aggressively growing prostate cancers but tended to separate patients with slower-growing cancers into a higher risk group (p=0.0567) (Figure 4D).
INPP4B is regulated by NCoR
Expression of AR target genes is regulated by numerous coactivators and corepressors. We and others have shown dynamic changes in coregulator expression between normal and tumor tissue in patients (10, 11, 42–44). Investigating the role of the corepressor NCoR on the AR transcriptome in LNCaP cells (manuscript in preparation), we found that INPP4B was the most down regulated gene following NCoR depletion. Since it has previously been shown that NCoR modulates agonist bound AR activity (45, 46), we examined whether NCoR depletion affects INPP4B expression. To deplete NCoR in LNCaP cells we used NCoR specific siRNA (Figure 5A). Note that while NCoR protein levels decreased, the levels of AR did not significantly change (Figure 5A). As expected and in accordance with previous reports, PSA induction increased following NCoR depletion (Figure 5B). Surprisingly, INPP4B expression requires NCoR for optimal expression both with and without androgen (Figure 5C). While AR does not lose the ability to induce INPP4B, basal INPP4B transcription is compromised. Similarly, while bicalutamide does not lose its ability to repress transcription of INPP4B in full serum, overall expression is decreased (Figure 5D).
Figure 5. Nuclear Corepressor (NCoR) is required for basal and androgen induced expression of INPP4B.

A. LNCaP cells were transfected with either control noncoding (C) or NCoR specific siRNA. Cells were plated in full serum medium, harvested 24 hours later and expression of NCoR, AR, and β-actin were analyzed by Western blot. B. LNCaP cells were transfected as in A and plated in css medium and treated with either vehicle (ethanol (V)) or 1 nM R1881. Cells were harvested 24 hours post-treatment and relative PSA expression was determined by quantitative RT-PCR. C. Cells were transfected as in A, plated in css medium and treated with either vehicle (ethanol (V)) or 1 nM R1881 for 24 hours. RNA was extracted and analyzed for INPP4B and 18S expression by quantitative RT-PCR. D. LNCaP cells transfected in parallel with A were plated in full serum and treated with either vehicle (ethanol (V)) or 10 μM bicalutamide (bic) for 24 hours. RNA was extracted and analyzed for INPP4B and 18S expression by quantitative RT-PCR. Star indicates statistically significant difference in expression in NCoR siRNA treated compared to the noncoding control treated cells with p<0.05.
Discussion
Elevated PI3K/Akt signaling is routinely associated with androgen ablation therapies and particularly with castration resistant recurrent prostate cancers (13, 14). Here, we present evidence that INPP4B is an androgen-regulated gene in human prostate cancer cells that suppresses Akt activation. INPP4B expression was induced by androgens in both a time and dose dependent manner in LNCaP cells. This is distinct from the well characterized tumor suppressor gene (TSG), PTEN, which did not demonstrate androgen-regulated expression. Whereas PTEN is a global TSG, INPP4B is an androgen regulated TSG in prostate epithelium that enables the AR to control proliferation through modulating Akt activity. Luminal epithelial specific deletion of the AR in the mature prostate facilitates increased proliferation and loss of differentiation in the mouse (4). Therefore, INPP4B in association with genes such as Nkx3.1 forms a subset of androgen regulated genes that control growth and maintain differentiation. Significantly, deregulation or loss of INPP4B in primary prostate cancers may lead to androgen driven proliferation and loss of differentiation.
In agreement with previous studies in breast cancer, knock down of INPP4B in LNCaP cells enhanced proliferation. The observed increase in proliferation was associated with increased levels of activated Akt. However, unlike the study by Gewinner et al (29), enhanced phosphorylation of Akt did not require added stimulation of cells with insulin or activation of other growth receptor pathways. This difference may be accounted for by the different cell types and/or reflect tissue specificity. Significantly we observed INPP4B regulation of Akt activation in prostate cancer cell lines in both the presence and absence of functional PTEN. This further strengthens the association between androgen withdrawal, INPP4B depletion and activation of Akt in prostate cancer. The proapoptotic transcription factor FOXO3a is phosphorylated by Akt, which leads to its cytoplasmic retention and degradation, thus impeding apoptosis (18). We found that INPP4B status was implicated in the extent of Akt dependent phosphorylation of FOXO3a, confirming its regulatory function in Akt signaling.
Extracellular growth receptor pathways, including EGFR, IGF-1R and HER2/Neu have been implicated in prostate cancer and the development of castration resistant disease (47–49). Significantly, activation of these pathways induces PI3K/Akt signaling. Furthermore, growth receptor pathways and intracellular kinase signaling pathways modulate AR function, though predominantly through modulation of AR coregulators (50–54). Hence, AR regulation of INPP4B suggests that INPP4B may be an important mediator in the cross talk between extracellular growth signals and AR regulated growth pathways. Significantly, PTEN has also been implicated in regulating AR turnover and activity (55, 56) and as such, tight regulation and coordination of AR and PI3K signaling appears to be crucial for the correct regulation of prostate epithelial proliferation and maintenance of cellular differentiation.
Importantly, immunohistochemical analysis of human prostate specimens showed luminal epithelial specific staining of INPP4B, the site of prostate cancer initiation. In primary prostate cancers INPP4B expression was significantly decreased. The occurrence of INPP4B mutations and the presence of splice variants and their correlation with clinical outcomes were not evaluated in the present study. Mutations of PI3K are rare in prostate cancer, with elevated PI3K signaling in prostate cancer previously more commonly associated with inactivating mutations of PTEN (26). A recent publication by Taylor et al identified alterations in the PI3K pathway in 42% of primary and 100% of metastatic prostate cancers (57). In the study by Taylor et al INPP4B was decreased at the RNA level by outlier analysis in 8% of clinically localized disease, which was twice the rate of PTEN (4%) (57). Interestingly, INPP4B was decreased in 47% of metastatic samples (compared to 42% for PTEN). In combination with our findings these data suggest an important role for INPP4B in prostate cancer. Our work confirms the RNA studies at the protein level in clinically localized disease using a much larger sample size. Mutational inactivation and differential isoform activities of INPP4B may further contribute to the etiology of prostate cancer and explain the subgroup that has elevated mRNA levels of INPP4B. Androgen regulation of INPP4B suggests that its expression would be decreased or lost following androgen ablation therapies. Prostatic tissue used in the current tissue microarray was obtained from patients that had not received hormone ablation therapies prior to surgery. Hence, in future studies it will be important to correlate INPP4B status with therapy response.
Our results suggest that decreased expression of either INPP4B or PTEN correlates with poor outcome for prostate cancer patients undergoing radical prostatectomy. In the present study loss of PTEN expression correlated with reduced time to biochemical recurrence, however only a decrease in INPP4B expression was sufficient for a correlation with an increased recurrence rate. This is significant since our data indicates that castration therapies targeting androgen signaling likely lead to further down regulation of INPP4B and subsequent up regulation of PI3K/Akt signaling and the progression of prostate cancer. Loss of expression and inactivating mutations of PTEN are more frequently associated with late stage and metastatic prostate cancers (26). Recent data suggest that both PTEN and INPP4B likely play significant roles in prostate cancer metastasis (57). Hence, loss of INPP4B potentially plays a significant role in the development of prostate cancer and the establishment of androgen independence and metastases. Our data indicates that INPP4B is an important TSG in prostatic epithelium and may serve an equally important role to PTEN in regulating cellular proliferation.
An interesting observation from this study was that knock down of the corepressor NCoR in LNCaP cells suppressed basal expression and prevented full androgen induction of INPP4B. Steroid receptor coactivators potentiate the AR and in addition to cell signaling pathways regulate AR function in a promoter specific manner (9). Elevated expression of coactivators and over-activation of growth receptor signaling pathways are likely involved in the functional switch of the AR to pro-proliferative. It has previously been shown that NCoR suppresses agonist dependent AR transcriptional activity (58). Significantly, we found that NCoR expression is reduced in prostate cancer, which coincided with deceased expression of INPP4B (paper in preparation). Furthermore, microarray analysis of LNCaP cells following NCoR depletion identified INPP4B as the most down regulated transcript in NCoR depleted cells (paper in preparation). Therefore as in LNCaP cells, NCoR likely regulates the expression of INPP4B in the human prostate either in a direct or indirect manner. Loss of NCoR and the associated loss of INPP4B further highlight the significant role of AR coregulators in normal prostate biology and prostate cancer etiology.
Inactivation of INPP4B in primary prostate cancers or preneoplastic lesions in association with other epigenetic and genetic alterations potentially facilitates the switch from androgen-regulated differentiation to proliferation. Loss of INPP4B may provide a marker for prostate cancer patients that would benefit from combined androgen ablation therapy and PI3K/Akt inhibitors.
Acknowledgments
We would like to acknowledge the expert technical assistance of William E Bingman 3rd.
Grant support
Department of Defense grant BC097064 (I.U.A), NIH grant 1R21CA129265-01A1 (I.U.A), NIH CA58504 SPORE in Prostate Cancer (L.J.S, A.F, R.L, L.E.P, G.A, M.M.I, N.L.W) and NIH DK65252 (N.L.W).
Abbreviations
- AR
androgen receptor
- INNP4B
inositol polyphosphate phosphatase type II
- PTEN
phosphatase and tensin homolog
- NCoR
nuclear receptor corepressor
References
- 1.Balk SP. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60:132–8. doi: 10.1016/s0090-4295(02)01593-5. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 2.Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, Hittmair A. Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 1995;55:3068–72. [PubMed] [Google Scholar]
- 3.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu CT, Altuwaijri S, Ricke WA, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104:12679–84. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieberich CJ, Fujita K, He WW, Jay G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem. 1996;271:31779–82. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- 6.Geck P, Maffini MV, Szelei J, Sonnenschein C, Soto AM. Androgen-induced proliferative quiescence in prostate cancer cells: the role of AS3 as its mediator. Proc Natl Acad Sci U S A. 2000;97:10185–90. doi: 10.1073/pnas.97.18.10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geck P, Szelei J, Jimenez J, Lin TM, Sonnenschein C, Soto AM. Expression of novel genes linked to the androgen-induced, proliferative shutoff in prostate cancer cells. J Steroid Biochem Mol Biol. 1997;63:211–8. doi: 10.1016/s0960-0760(97)00122-2. [DOI] [PubMed] [Google Scholar]
- 8.Magee JA, Abdulkadir SA, Milbrandt J. Haploinsufficiency at the Nkx3.1 locus. A paradigm for stochastic, dosage-sensitive gene regulation during tumor initiation. Cancer Cell. 2003;3:273–83. doi: 10.1016/s1535-6108(03)00047-3. [DOI] [PubMed] [Google Scholar]
- 9.Tindall D, Mohler J. Androgen action in prostate cancer. New York: Springer; 2009. [Google Scholar]
- 10.Agoulnik IU, Vaid A, Bingman WE, 3rd, et al. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005;65:7959–67. doi: 10.1158/0008-5472.CAN-04-3541. [DOI] [PubMed] [Google Scholar]
- 11.Agoulnik IU, Vaid A, Nakka M, et al. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66:10594–602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- 12.Agoulnik IU, Weigel NL. Androgen receptor coactivators and prostate cancer. Adv Exp Med Biol. 2008;617:245–55. doi: 10.1007/978-0-387-69080-3_23. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto H, Altuwaijri S, Cai Y, Messing EM, Chang C. Inhibition of the Akt, cyclooxygenase-2, and matrix metalloproteinase-9 pathways in combination with androgen deprivation therapy: potential therapeutic approaches for prostate cancer. Mol Carcinog. 2005;44:1–10. doi: 10.1002/mc.20121. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 15.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 16.Rokhlin OW, Taghiyev AF, Guseva NV, Glover RA, Syrbu SI, Cohen MB. TRAIL-DISC formation is androgen-dependent in the human prostatic carcinoma cell line LNCaP. Cancer Biol Ther. 2002;1:631–7. doi: 10.4161/cbt.311. [DOI] [PubMed] [Google Scholar]
- 17.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 19.Ayala G, Thompson T, Yang G, et al. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin Cancer Res. 2004;10:6572–8. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- 20.Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer. 10:342–52. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- 21.Ma K, Cheung SM, Marshall AJ, Duronio V. PI(3,4,5)P3 and PI(3,4)P2 levels correlate with PKB/akt phosphorylation at Thr308 and Ser473, respectively; PI(3,4)P2 levels determine PKB activity. Cell Signal. 2008;20:684–94. doi: 10.1016/j.cellsig.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto H, Hamada K, Saunders M, et al. Physiological functions of Pten in mouse tissues. Cell Struct Funct. 2003;28:11–21. doi: 10.1247/csf.28.11. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez F, Sellers WR. The PTEN tumor suppressor protein: an antagonist of phosphoinositide 3-kinase signaling. Biochim Biophys Acta. 2000;1470:M21–35. doi: 10.1016/s0304-419x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 25.Facher EA, Law JC. PTEN and prostate cancer. J Med Genet. 1998;35:790. doi: 10.1136/jmg.35.9.790-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 27.Barnache S, Le Scolan E, Kosmider O, Denis N, Moreau-Gachelin F. Phosphatidylinositol 4-phosphatase type II is an erythropoietin-responsive gene. Oncogene. 2006;25:1420–3. doi: 10.1038/sj.onc.1209187. [DOI] [PubMed] [Google Scholar]
- 28.Westbrook TF, Martin ES, Schlabach MR, et al. A genetic screen for candidate tumor suppressors identifies REST. Cell. 2005;121:837–48. doi: 10.1016/j.cell.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Gewinner C, Wang ZC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–25. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon HG, Chan DW, Huang ZQ, et al. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22:1336–46. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agoulnik IU, Bingman WE, 3rd, Nakka M, et al. Target gene-specific regulation of androgen receptor activity by p42/p44 mitogen-activated protein kinase. Mol Endocrinol. 2008;22:2420–32. doi: 10.1210/me.2007-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayala G, Wang D, Wulf G, et al. The prolyl isomerase Pin1 is a novel prognostic marker in human prostate cancer. Cancer Res. 2003;63:6244–51. [PubMed] [Google Scholar]
- 33.Li R, Wheeler T, Dai H, Frolov A, Thompson T, Ayala G. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004;28:928–34. doi: 10.1097/00000478-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Ayala G, De Marzo A, et al. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res. 2002;8:3419–26. [PubMed] [Google Scholar]
- 35.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 36.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 37.Masuda K, Werner T, Maheshwari S, et al. Androgen receptor binding sites identified by a GREF_GATA model. J Mol Biol. 2005;353:763–71. doi: 10.1016/j.jmb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Hermans KG, van Alewijk DC, Veltman JA, van Weerden W, van Kessel AG, Trapman J. Loss of a small region around the PTEN locus is a major chromosome 10 alteration in prostate cancer xenografts and cell lines. Genes Chromosomes Cancer. 2004;39:171–84. doi: 10.1002/gcc.10311. [DOI] [PubMed] [Google Scholar]
- 39.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–3. [PubMed] [Google Scholar]
- 40.Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell. 2005;19:631–42. doi: 10.1016/j.molcel.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Zhou HJ, Yan J, Luo W, et al. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005;65:7976–83. doi: 10.1158/0008-5472.CAN-04-4076. [DOI] [PubMed] [Google Scholar]
- 43.Debes JD, Sebo TJ, Lohse CM, Murphy LM, Haugen DA, Tindall DJ. p300 in prostate cancer proliferation and progression. Cancer Res. 2003;63:7638–40. [PubMed] [Google Scholar]
- 44.Majumder S, Liu Y, Ford OH, 3rd, Mohler JL, Whang YE. Involvement of arginine methyltransferase CARM1 in androgen receptor function and prostate cancer cell viability. Prostate. 2006;66:1292–301. doi: 10.1002/pros.20438. [DOI] [PubMed] [Google Scholar]
- 45.Cheng S, Brzostek S, Lee SR, Hollenberg AN, Balk SP. Inhibition of the dihydrotestosterone-activated androgen receptor by nuclear receptor corepressor. Mol Endocrinol. 2002;16:1492–501. doi: 10.1210/mend.16.7.0870. [DOI] [PubMed] [Google Scholar]
- 46.Hodgson MC, Astapova I, Cheng S, et al. The androgen receptor recruits nuclear receptor CoRepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J Biol Chem. 2005;280:6511–9. doi: 10.1074/jbc.M408972200. [DOI] [PubMed] [Google Scholar]
- 47.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 48.Culig Z, Hobisch A, Cronauer MV, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor and epidermal growth factor. Eur Urol. 1995;27 (Suppl 2):45–7. doi: 10.1159/000475232. [DOI] [PubMed] [Google Scholar]
- 49.Signoretti S, Montironi R, Manola J, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–25. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 50.Gregory CW, Fei X, Ponguta LA, et al. Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem. 2004;279:7119–30. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- 51.Lin HK, Hu YC, Yang L, et al. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J Biol Chem. 2003;278:50902–7. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- 52.Manin M, Baron S, Goossens K, et al. Androgen receptor expression is regulated by the phosphoinositide 3-kinase/Akt pathway in normal and tumoral epithelial cells. Biochem J. 2002;366:729–36. doi: 10.1042/BJ20020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–27. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 54.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin HK, Hu YC, Lee DK, Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol Endocrinol. 2004;18:2409–23. doi: 10.1210/me.2004-0117. [DOI] [PubMed] [Google Scholar]
- 56.Nan B, Snabboon T, Unni E, Yuan XJ, Whang YE, Marcelli M. The PTEN tumor suppressor is a negative modulator of androgen receptor transcriptional activity. J Mol Endocrinol. 2003;31:169–83. doi: 10.1677/jme.0.0310169. [DOI] [PubMed] [Google Scholar]
- 57.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon HG, Wong J. The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor. Mol Endocrinol. 2006;20:1048–60. doi: 10.1210/me.2005-0324. [DOI] [PubMed] [Google Scholar]