Abstract
Akt (also known as PKB) signaling orchestrates many aspects of biological functions and, importantly, its deregulation is linked to cancer development. Akt activity is well-known regulated through its phosphorylation at T308 and S473 by PDK1 and mTORC2, respectively. Although in the last decade the research has been primarily focused on Akt phosphorylation and its role in Akt activation and functions, other posttranslational modifications on Akt have never been reported. Until very recently, a novel posttranslational modification on Akt termed ubiquitination was identified and shown to play an important role in Akt activation. The cancer-associated Akt mutant recently identified in a subset of human cancers displays enhanced Akt ubiquitination, in turn contributing to Akt hyperactivation, suggesting a potential role of Akt ubiquitination in cancers. Thus, this novel posttranslational modification on Akt reveals an exciting avenue that has advanced our current understandings of how Akt signaling activation is regulated.
Keywords: Akt, kinase, ubiquitination, phosphorylation, TRAF6, E3 ligase, PDK1, mTOC2, NF-κB, tumorigenesis
Introduction
Growth factors are important nutrients for cell growth and survival. The way cells respond to such extracellular clues is to initiate serial signaling cascades involving several protein kinases for their growth and survival. One critical player that helps coordinate these events is serine (S)/threonine (T) protein kinase Akt. Since discovered in 1991, 1–3 Akt has thereafter become one of the major research areas addressing the wide ranges of the biological systems. Thus, the great advances on the understanding of Akt functions and its regulation have been made during the last decade. Because of the important roles of Akt in cell signaling and cancers, it’s not a surprise that the Akt research will continue to grow in the next decade.
Akt signaling regulates many aspects of biological functions including cell proliferation, survival, metabolism, cell migration, and metastasis. Importantly, the deregulated Akt pathway is associated with a variety of human cancers, and several mouse models with activated Akt pathway support the role of Akt in cancer development. Given the important role of the Akt signal in cancers, small molecule inhibitors targeting Akt have been developed and already tested in the clinical trial.4, 5 However, Akt signal is also important for normal cell functions, so targeting Akt is expected to raise serious side effects. Therefore, the comprehensive understanding of how Akt signal is regulated is of importance and of relevance in cancers and will help to design better therapeutic strategies targeting human cancers with limited side effects.
Posttranslational modifications, such as phosphorylation, ubiquitination, sumoylation, and methylation, often regulate protein activity and stability. Although it is known that Akt activation is regulated through the phosphorylation of Akt, it remains unclear whether other types of posttranslational modifications occur on Akt and may regulate Akt activity and functions. Our recent study reveals that in addition to phosphorylation, Akt also undergoes a novel type of the posttranslational modification named ubiquitination,6 which appears to play an important role in Akt signaling activation. In this review, we will summarize recent advances in the regulation of Akt activity with a particular emphasis on the Akt ubiquitination and its potential implications in cancers.
Ubiquitin pathways in protein degradation and activation
Ubiquitination plays a critical role in numerous biological functions including cell cycle control, cell growth, apoptosis, DNA damage repair, immune functions, as well as neuron degeneration.7–9 Protein ubiquitination is originally thought to target proteins for 26S proteasome-dependent degradation.10 Very recently, protein ubiquitination is also found to play non-proteolytic functions including protein trafficking, DNA damage repair, activation of signal transduction pathways, such as activation of IKK (IκB kinase)/NF-κB (nuclear transcription factor kappaB) pathway in the immune function (Fig. 1).7, 11, 12
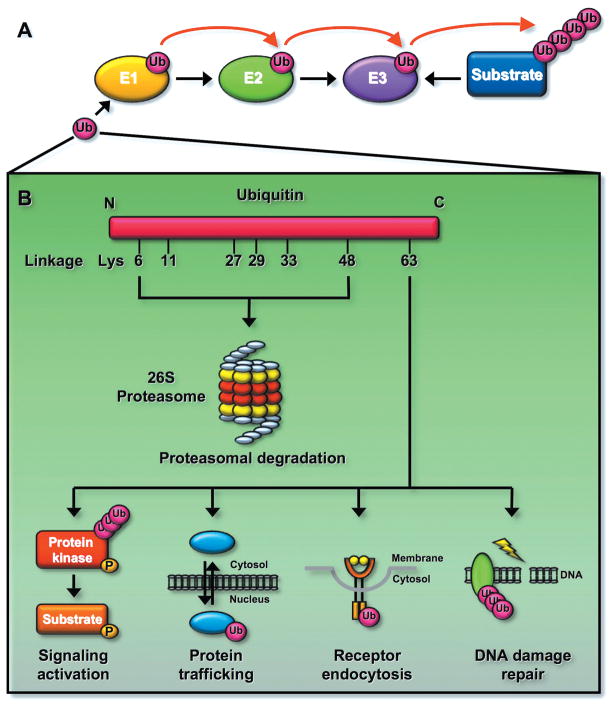
Figure 1.
Ubiquitination can regulate protein degradation or activation. (A) Ubiquitination reaction involves three enzymes. Ubiquitin is activated by the E1 and is transferred to the E2. The E3 recognizes its protein substrates and brings them to the E2, resulting in protein ubiquitination. (B) Ubiquitination regulates the fate of proteins. Ubiquitin consists of seven lysine (K) residues. The K48-linked ubiquitination is recognized by the 26S proteasome and results in protein degradation. In contrast, the K63-linked ubiquitination does not induce protein degradation since it is not recognized by the proteasome, instead it regulates signaling activation involved in distinct biological functions including DNA damage response, receptor endocytosis, and protein trafficking. Although the function for other types of ubiquitnation is less clear, it is proposed that these types of ubiquitination may also regulate protein degradation.
Ubiquitin consists of 76-amino acid polypeptides that are highly conserved through the evolution. Ubiquitination is a covalent reaction that attaches the ubiquitin(s) to one or more lysine residues of proteins. It is triggered by an enzymatic cascade involving three distinct classes of enzymes termed E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme), and E3 (ubiquitin ligase) (Fig. 1A). There are seven lysine (K) residues (K6, K11, K27, K29, K33, K48, and K63) within the ubiquitin, and ubiquitination chains involving those sites have been reported (Fig. 1B). Protein ubiquitination involving K48-linked ubiquitination is recognized by the 26S proteasome and is therefore targeted for protein degradation, whereas K63-linked ubiquitination plays non-proteolytic functions (Fig. 1B).7, 10 Although the role of other types of ubiquitination is less clear, recent studies suggest that they may be also involved in triggering protein degradation (Fig. 1B). 13
In essence, the E2 determines which type of ubiquitin chains is formed, while the E3 provides the substrate specificity. In human genome, 2 E1s, roughly 50 E2s, and 600 E3s have been identified.7 The K48-linked ubiquitination can be triggered by most E2s except UBC13. The UBC13 E2 is a major E2 known to trigger K63-linked ubiquitination with assistance of its cofactor UEV1A. The E3s are categorized to 2 major types, one containing a homologous to the HECT (E6-AP carboxyl terminus) domain and the other containing RING (a really interesting new gene) or RING-like domain (e.g. U-Box and PHD domain).7 Most of E3s recognized target proteins for K48-linked ubiquitination, although there are also very few E3s, such as TRAF6 (tumor necrosis factor receptor-associated factor 6), HectH9, c-IAP1/2 (cellular inhibitor of apoptosis protein 1/2), and RNF8 (ring finger protein 8) (see below), 7, 14–16 targeting proteins for K63-linked ubiquitination.
The ubiquitination is a reversible process in which the polyubiquitin chain on proteins can be removed by the deubiquitinating enzymes (DUBs). There are about 90 DUBs in human genome that can be divided into five subfamilies: ubiquitin-specific proteases (USP), ubiquitin carboxyl-terminal hydrolases (UCH), ovarian tumor-like proteases (OTU), JAMM/MPN metalloproteases, and Machado–Jakob-disease proteases (MJD).7, 9, 17 The DUBs are known to play important roles in controlling key signal transduction pathways involved in oncogenic and tumor suppressor pathways such as the NF-κB pathway and PTEN (tumor suppressor phosphatase and tensin homolog)/PI3K (phosphatidylinositol-3-OH kinase)/Akt pathway, suggesting their roles in cancer development and tumor suppression. 7, 18 One of the characteristics for the DUBs is that the ubiquitin binding domain (UBD) is found in most DUBs, so the DUBs can bind to the ubiquitinated proteins and induce protein deubiquitination. The HAUSP (also known as USP7), a DUB that is known to inhibit the nuclear translocation of the tumor suppressor PTEN by deubiquitinating PTEN, is amplified in human prostate cancer and may play an important role in cancer development.19, 20 However, other DUBs, such as A20 and CYLD (cylindromatosis tumor suppressor), negatively regulate the NF-κB pathway.7, 21 In support of the role of CYLD and A20 in NF-κB regulation, recent genetic evidence and the mutation analysis show that knockout of CYLD in mice or mutations on CYLD and A20 genes in patients causes the hyperactivation of NF-κB resulting in tumor susceptibility or tumor formation.22–25
The K63-linked ubiquitination provides a molecular platform for protein/protein interaction important for signaling activation, DNA damage repair, protein trafficking, and receptor endocytosis (Fig. 1). In the case of the DNA damage repair, RNF8 E3 ligase is recruited to the DNA damage sites upon γ-irradiation and triggers K63-linked ubiquitination of histone H2A and H2AX.16, 26 The polyubiquitination of H2A or H2AX is recognized by RAP80/Abrax/BRCA1 complex important for the DNA damage repair.9, 27 RAP80 contains two ubiquitin-binding motifs (UIM), which bind preferentially to K63-linked ubiquitin chains and is required to recruit BRCA1 and Abrax to the DNA damage sites.9, 27 With regard to the endocytosis, the K63-linked ubiquitination of the receptor regulates the receptor internalization to the early and late endosome.28, 29 For instance, prolactin receptor (PRLr) ubiquitination upon the stimulation with its ligand prolactin facilitates the interaction of PRLr with the AP2 complex, leading to the internalization of PRLr to the late endosome.29
The role of Akt in cell cycle regulation and tumorigenesis
The PI3K/Akt pathway plays a central role in various biological functions including cell survival, cell proliferation, cell metabolism, and protein translation.30–34 The PI3K contains the p85 regulatory domain and p110 catalytic domain. The p85 regulatory domain possesses two src-homology 2 (SH2) domains and a src-homology 3 (SH3) domain.24,25 PI3K phosphorylates the inositol ring of PI(4,5)P2 at the D-3 position to form PI(3,4,5)P3, which is required to activate Akt kinase in the plasma membrane. The recruitment of Akt from the cytosol to the plasma membrane requires its binding to PI(3,4,5)P3 phospholipid in the membrane through its pleckstrin homology (PH) domain within the N-terminal of Akt.31, 32 Akt is then phosphorylated at T308 within its catalytic domain by PDK1 (phosphoinositol-dependent kinase 1) and at S473 within its C-terminal regulatory domain by mTORC2 (mammalian target of rapamycin complex 2), resulting in full activation of Akt kinase.32, 35 The PI3K/Akt pathway is negatively regulated by PTEN, a lipid phosphatase dephosphorylating PI(3,4,5)P3 at the D3 position of the inositol ring.18, 36, 37
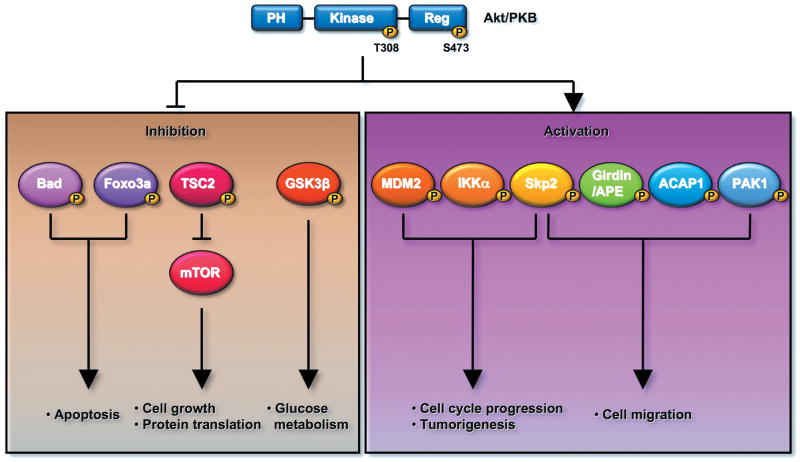
The PI3K/Akt pathway is activated by numerous growth factors and cytokines through their cognate receptors.31, 32 It provides the survival signal in diverse cell types, and activation of PI3K/Akt signal can rescue cells from apoptosis in response to growth factor deprivation.31, 32, 38 It has become clear that Akt can phosphorylate and inhibit proapoptotic proteins like Bad and Foxo3a to prevent cell apoptosis (Fig. 2).31, 32 Akt can also phosphorylate and activate numerous oncogenic proteins involved in cell cycle progression and tumorigenesis, such as MDM2 (murine double minute), IKKα, and Skp2 (S-phase kinase-associated protein 2) E3 ligase.39–45 Akt regulates cell growth and protein translation by phosphorylating and inactivating TSC2 (tuberous sclerosis 2), resulting in activation of the mTOR pathway.46, 47 Akt also regulates glucose metabolism through phosphorylating and inactivating GSK3β (glycogen synthase kinase 3β).48 The PI3K/Akt pathway also has an important role in cell migration. Several Akt substrates, such as Girdin/APE, ACAP1 (ArfGAP with coiled-coil, ankyrin repeat and PH domains 1), PAK1 (p21 protein-activated kinase 1), and Skp2, are known to be phosphorylated by Akt and play an important role in cell migration (Fig. 2).42, 49–51 It remains to be determined whether phosphorylation of these proteins is indeed required for Akt-mediated cell motility.
Figure 2.
Akt regulates numerous biological functions by phosphorylating distinct protein substrates. For instance, Akt protects cells from apoptosis by phosphorylating and inactivating proapoptotic proteins, such as Bad and Foxo3a. Akt regulates cell growth and protein translation by phosphorylating and inactivating TSC2, resulting in activation of the mTOR pathway. Akt regulates cell cycle progression and tumorigenesis by phosphorylating and activating oncogenic proteins, such as Skp2, Mdm2, and IKKα. Akt can orchestrate glucose metabolism by regulating the activity of GSK3β. Akt may also regulate cell migration by inducing the phosphorylation and activation of Skp2, Girdin/APE, ACAP1, and PAK1.
The role of PI3K/Akt signaling in cancer development has been well documented. For instance, overexpression of insulin-like growth factor–binding protein-5 helps accelerate progression to androgen independence in the prostate tumor model through activation of the PI3K/Akt pathway.52 Aberrant Akt activation is observed in various human cancers, and importantly, Akt1, Akt2 and Akt3 isoforms are found to be overexpressed in human cancers.53–58 Recent studies show that Akt1 mutations were observed in a subset of human cancers and associated with Akt hyperactivation.59–65 The role of Akt in cancer development has been supported by numerous animal tumor models. For example, Pten+/− mice with aberrant Akt activation develop multiple tumors, which can be inhibited by Akt1 deficiency.66, 67 In addition, the prostate-specific expression of constitutively active Akt1 in mice leads to prostate intraepithelial neoplasia (PIN).68, 69 Accordingly, these results highlight the critical role of the PI3K/Akt pathway in cancer development.
Akt activity is regulated by phosphorylation of Akt
Engagement of Growth factor receptor by growth factors leads to Akt phosphorylation and activation. As aforementioned, the full activation of Akt requires phosphorylation of Akt at T308 and S473 by PDK1 and mTORC2, respectively.35, 70–72 T308 phopshoryaltion of Akt directly controls Akt activity. Although S473 phopshorylation does not directly control Akt activity, it may facilitate T308 phosphorylation. In contrast, dephosphorylation of Akt leads to the termination of Akt actvation. PP2A (protein phosphatase 2A) negatively regulates Akt activity by inducing Akt T308 dephosphorylation, whereas PHLPP (PH domain leucine-rich repeat protein phosphatase) suppresses Akt activity by dephosphorylating Akt at S473 (Fig. 3).73–77 The interaction of PHLPP with Akt is regulated by FKBP51 (FK506 binding protein 51), which serves as a scaffold for Akt and PHLPP and negatively regulates Akt phosphorylaiton at S473 (Fig. 3).78 Accordingly, Akt activity is controlled by the phosphorylation and dephosophorylation cycles within Akt molecules.
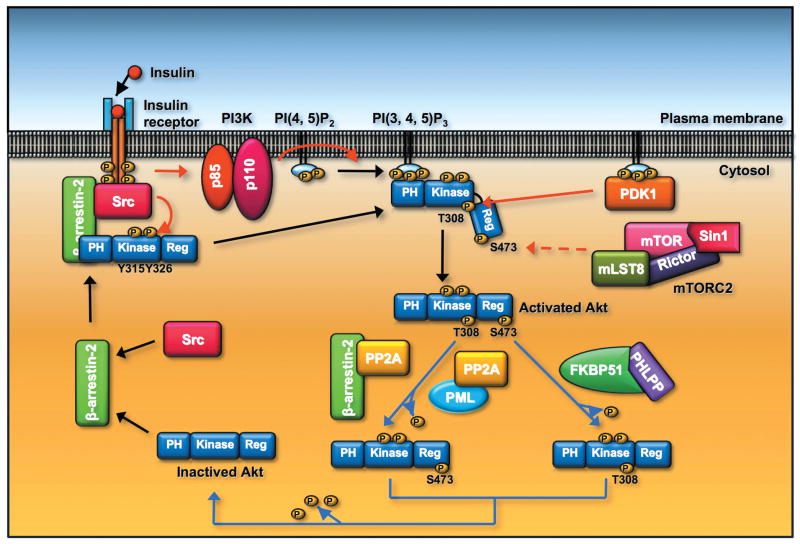
Figure 3.
The activity of Akt is regulated by phosphorylation and dephosphorylation of Akt. β-arrestin-2 recruits Src and Akt to the activated insulin receptor, and phosphoryaltion of Akt at Y315 and Y326 by Src is a prerequisite for Akt T308 and S473 phosphorylaiton induced by PDK1 and mTOC2, respectively, leading to the full activation of Akt. Akt T308 dephosphorylation is triggered by PP2A phosphatase. PML or β-arrestin-2 recruits PP2A and facilitates Akt T308 dephosphorylation. Dephosphorylation of Akt at S473 is induced by PHLPP phosphates. The adaptor protein FKBP51 recruits PHLPP to elicit Akt dephosphorylation at S473.
Akt phosphorylation and activation is also regulated by PML (promyelocytic leukemia protein) tumor suppressor. PML forms a complex with PP2A and Akt and facilitates PP2A-mediated Akt dephosphorylation at T308 (Fig. 3).79 As a consequence, PML cooperates with PTEN tumor suppressor to restrict prostate cancer development in the animal model.79 Additionally, PP2A also interacts with β-arrestin-2, on which PP2A-mediated Akt dephosphorylation in response to dopamine is dependent in neurons (Fig. 3).80 This result supports the notion that β-arrestin-2 is a signaling molecule that functions as a scaffold to regulate signal transduction pathways such as Akt signaling.
In contrast to its negative role in Akt activation in neurons, β-arrestin-2 is recently shown to be required for phosphorylation and activation of Akt in response to insulin in muscles.81 Mice deficient for β-arrestin-2 have defects in Akt phosphorylation and develop type 2 diabetes, whereas β-arrestin-2 overexpression in mice promotes Akt activation and ameliorates the diabetes phenotype. 81 Accumulating evidence reveals that tyrosyl phopshorylation of Akt at Y315 and Y326 by Src is a prerequisite for T308 and S473 phosphorylation and activation of Akt.81–85 Pei and colleagues further showed that β-arrestin-2 is required for Akt tyrosyl phopshorylaiton by recruiting Akt and Src to the activated insulin receptors (Fig. 3).81 Thus, β-arrestin-2 is once again a critical scaffold that regulates Akt activation and insulin sensitivity by forming a signaling Akt complex for Akt activation.
Accordingly, these results suggest that β-arrestin-2 has distinct functions in Akt signaling activation dependent on various stimuli and tissue types. Future experiments will be required to understand what is a molecular switch that determines the distinct effects of β-arrestin-2 on Akt activation.
Akt undergoes a novel type posttranslational modification critical for Akt phosphorylation and activation
Akt phosphorylation plays a critical role in Akt activation and its oncogenic activity. The research on Akt study in the past decade has been centered on the role of Akt phosphorylation and how this phosphorylation is regulated. Akt was recently found to also undergo ubiquitination when cells were treated with growth factors or cytokines known to induce Akt activation.6, 86 Akt ubiquitination is correlated well with Akt T308 phosphorylation and activation. Interestingly, this ubiquitination acts through the K63-linked ubiquitination, but not the K48-linked ubiquitination. Akt ubiqiutination does not affect Akt stability, but it is important for Akt signaling activation.6, 86
Subsequent experiments identify TRAF6 as an E3 ligase for Akt. Traf6−/− primary cells have defects in Akt ubiquitination and phosphorylation in response to growth factors such as insulin-like growth factor-1 (IGF-1) and serum treatment.6, 86. Reconstitution of TRAF6, but not TRAF6 E3 ligase dead mutant, restores Akt ubiquitination and subsequent Akt phosphorylation, suggesting that TRAF6 E3 ligase is required for Akt phosphorylation and activation.6, 86 The further mutation analysis reveals that Akt ubiquitination occurs at K8 and K14 within the PH domain of Akt, and mutations on these sites (from K to R) abrogates Akt phosphorylation and activation,77 highlighting the critical role of Akt ubiquitination in Akt signaling activation. Thus, Akt ubiquitination represents a novel posttranslational modification that plays a key role in Akt activation.
Akt consists of three isoforms: Akt1, Ak2 and Akt3. Extensive studies suggest that although these isoforms share some similar functions, they also play distinct functions in normal cell physiology and cancer pathogenesis. Akt1 null mice are viable, but display the reduction in body weight and thymus apoptosis.87 Akt2 null mice develop severe type 2 diabetes, whereas Akt3 null mice exhibit impaired brain development.87 Akt1 promotes cell migration in mouse embryonic fibrablasts (MEFs), but inhibits breast cancer cell migration.88, 89 In contrast, Akt2 negatively regulates cell migration in MEFs, but positively regulates breast cancer cell migration.88, 89 The exact mechanisms responsible for these distinct functions played by Akt isoforms remain still elusive. Although Akt1 and Akt2 appear to regulate diverse biological functions, the operating machinery responsible for their activation may act through the same mechanism. Supporting evidence came from the observation that both of Akt1 and Akt2 interacted with TRAF6 with a similar efficiency, and that the levels of the ubiquitination between Akt1 and Akt2 induced by TRAF6 are comparable.6, 86 Thus, TRAF6 likely regulates the phosphorylation and activation of Akt1 and Akt2 in a similar fashion.
Although the interaction between Akt and TRAF6 was shown, the detailed interaction region between Akt and TRAF6 has not been mapped yet. Sequence alignment reveals that Akt consists of the TRAF6 consensus binding motif (Pro-X-Glu-X-X-(Ar/Ac) within the N-terminal PH domain (Fig. 5),90, 91 which is evolutionary conserved from Drosophila to human (data not shown). Importantly, it is also conserved among all three Akt isoforms (Fig. 5). These observations imply that TRAF6 may bind to Akt and regulate Akt signaling activation in the lower organisms. The TRAF6 consensus binding motif found in several TRAF6 interacting proteins plays an important role in TRAF6’s interaction and functions.91 Future experiments will be warranted to decipher the interaction regions between Akt and TRAF6, and to determine whether mutation on the TRAF6 consensus site within Akt disrupts the interaction between Akt and TRAF6, leading to abrogation of Akt ubiquitination and activation. These experiments will be warranted to provide a novel molecular insight into how TRAF6 regulates Akt signaling.
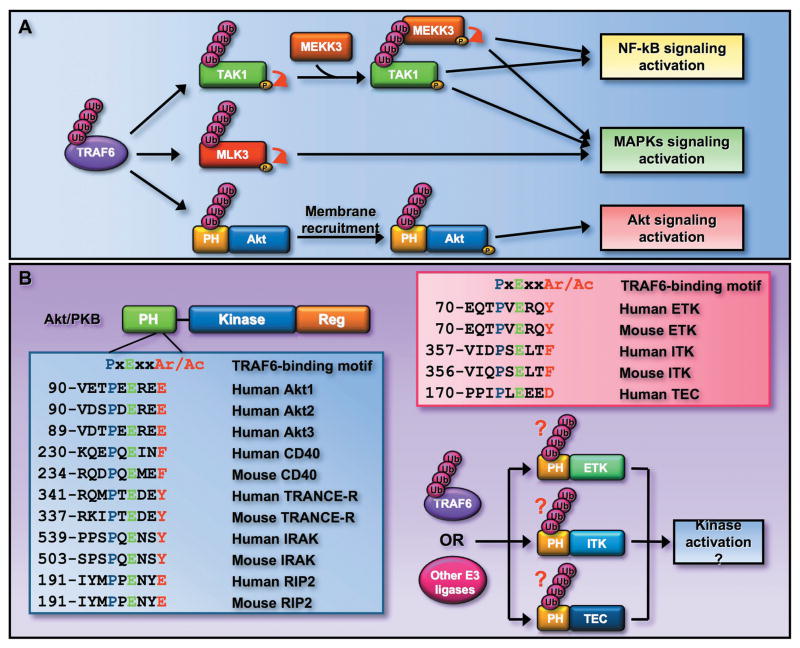
Figure 5.
K63-linked ubiquitination may serve as a general mechanism for kinase activation. (A) TRAF6 induces the activation of TAK1 and MLK3 by triggering their K63-linked ubiquitination. TRAF6 also induces K63 ubiquitination of Akt within its PH domain and regulates Akt activation. (B) TRAF6 consensus binding motif is conserved among all three Akt isoforms and is found in several other TRAF6 interacting proteins. The PH domain is also found in several other kinases, such as ETK, ITK, and TEC, which are important downstream effectors for growth factor receptor and TCR signaling. Interestingly, these kinases also contain the TRAF6 consensus binding motif, suggesting that TRAF6 or other E3 ligases may induce the K63-linked ubiquitinaiton of these kinases and in turn regulates their activation.
Ubiquitination of Akt regulate Akt membrane recruitment
Akt normally resides in the cytosol and translocalizes to the plasma membrane in response to various growth factor stimuli. Although the Akt PH domain is clearly important for PI(3,4,5)P3 lipid binding required for membrane localization, it is also proposed to play an important role in protein/protein interaction required for membrane recruitment. Several Akt interaction partners known to regulate Akt activation, such as JIP1 (JNK-interacting protein 1) and TCL1 (T cell leukemia-1), bind to the PH domain of Akt,30, 92, 93 raising the possibility that the PH domain of the Akt may not only serve as an important region for PI(3,4,5)P3 binding, but also provides the platform for recruiting the critical adaptors important for Akt membrane localization and phosphorylation. This notion was supported by the evidence that overexpression of the PH domain of Akt inhibited endogenous Akt phosphorylation, whereas the Akt PH domain mutant retaining the PI(3,4,5)P3 binding did not.94
Although the PH domain of Akt is clearly known to mediate PI(3,4,5)P3 lipid binding required for Akt membrane localization, it was not clear in the first place how the Akt is recruited to the plasma membrane, where it can bind to the PI(3,4,5)P3 lipid and is subsequently activated by PDK1 and mTORC2. The current model proposed by the Tsichlis group suggests that before Akt can be recruited to the plasma membrane to interact with PI(3,4,5)P3 phospholipid, the inactive Akt may need to interact with the critical adaptors by using its PH domain in the cytosol, which then facilitate the Akt membrane recruitment.95
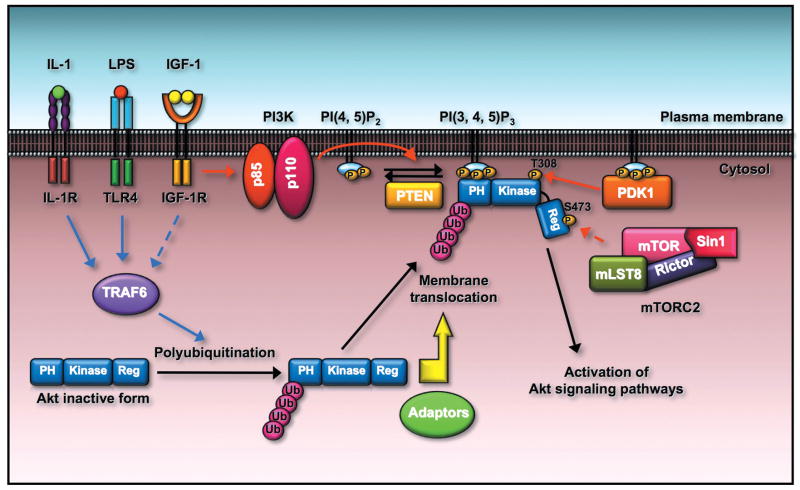
This model was indeed supported by our recent report demonstrating that Akt undergoes K63-linked ubiquitination at K8 and K14 within its PH domain by TRAF6, which is critical for Akt membrane recruitment and phosphorylation.6, 86 Notably, TRAF6 overexpression promotes Akt ubiquitination, membrane recruitment, and phosphorylation, whereas TRAF6 deficiency inhibits these processes.6, 86 These results underscore the critical role of TRAF6-mediated Akt ubiquitinaiton in Akt membrane localization and activation, supporting the notion that the K63-linked ubiquitination plays an important role in protein trafficking and signaling activation.28, 29
However, TRAF6 does not appear to affect Akt activation once Akt is already on the plasma membrane, since its overexpression could promote neither T308 nor S473 phosphorylation of the myristoylated Akt mutant, which displays the constitutive Akt membrane localization (our unpublished observations). TRAF6-mediated Akt ubiquitination does not affect the ability of Akt to interact with PI(3,4,5)P3,6 suggesting that Akt ubiquitination is dispensable for the binding of Akt to PI(3,4,5)P3. This is in line with the observation that mutation of the critical ubiquitination site of Akt on the K8 residue, which is not located in the PI(3,4,5)P3 lipid binding pocket, does not affect the ability of Akt to interact with PI(3,4,5)P3.6 Our data suggest that both events (Akt ubiquitination and PI(3,4,5)P3 lipid binding) are required for Akt membrane recruitment and phosphorylation, and Akt ubiquitination is likely an event that precedes the PI(3,4,5)P3 lipid binding (Fig. 4).
Figure 4.
Akt membrane localization and activation is regulated by ubiquitination of Akt. TRAF6 E3 ligase is activated by engagement of IGF-1, IL-1, and LPS to their cognate receptors. The activated TRAF6 then interacts with Akt and triggers K63-linked ubiquitination of Akt, which facilitates Akt membrane recruitment and subsequent phosphorylation by PDK1 and mTORC2 through an unknown mechanism. It is possible that the ubiquitinated Akt may recruit the essential adaptors to facilitate Akt membrane localization.
How does the Akt ubiquitination regulate Akt membrane localization? As the K63-linked ubiquitination plays an important role in protein/protein interaction, the K63-linked ubiquitination of Akt on its PH domain may serve as a molecular platform to recruit the essential adaptors for Akt, in turn facilitating Akt membrane recruitment and activation (Fig. 4). The validation of this hypothetical model will require a thorough analysis towards identifying such factors using the systematic approach, which may help understand how ubiquitination of Akt regulates Akt membrane localization and activation. Interestingly, PAK1, a protein kinase involved in cell migration and tumorigenesis,96 was recently shown to interact with Akt and help recruit Akt to the plasma membrane.97 How exactly PAK1 regulates Akt membrane localization and activation remains unclear. It will be interesting in the future to examine whether Akt ubiquitination orchestrates the interaction between Akt and PAK1, therefore promoting Akt membrane recruitment.
Hyperubiquitination contributes to hyperactivation of the Akt cancer-associated mutant
Recent studies show that a mutation within the Akt PH domain (E17K) was identified in a subset of human cancers, such as colon and breast cancer.53–57 The Akt E17K cancer-associated mutant displays a constitutive Akt membrane localization and T308 phosphorylation presumably due to its higher PI(3,4,5)P3 lipid binding, leading to aberrant oncogenic potential.60 Interestingly, Akt E17K mutant was also found to display the enhanced Akt ubiquitination, and abrogating this ubiquitination resulted in a marked reduction in Akt membrane recruitment and phosphorylation.6, 86 Accordingly, these results suggest that Akt E17K gains two important properties (enhanced Akt ubiquitination and PI(3,4,5)P3 lipid binding) that contribute to constitutive Akt membrane recruitment and phosphorylation. Our results therefore suggest that targeting Akt ubiquitination may offer promising strategies for the treatment of human cancers.
In addition to the E17K mutant, another Akt mutant (Akt E49K) was recently indetifed in bladder cancer patients.59 Similar to the Akt E17K mutant, the Akt E49K mutant also displays hyperphosphorylation and activation of Akt compared to wild-type Akt,59 although the molecular mechanism responsible for its hyperactivation is unclear. Since the mutation located at the PH domain of Akt gains an additional lysine residue, it will be interesting to determine in the future whether the hyperubiquitination is also observed in Akt E49K mutant and contributes to hyper-oncogenic potential of the Akt E49K mutant.
Can K63-linked ubiquitination serve as a general mechanism for kinase activation?
The activation of protein kinase is normally regulated through protein phosphorylation. The K63-linked ubiquitination has recently emerged to also play an important role in the regulation of protein kinase activation. For instance, TAK1 (transforming growth factor-β activating kinase-1) was previously known to be activated by TGF-β (transforming growth factor β) through an unknown mechanism. Recent studies suggest that TAK1 undergoes K63-linked ubiquitination upon stimulation with TGF-β. Importantly, K63-linked ubiquitination of TAK1 is induced by TRAF6, which is prerequisite for TAK1 autoposphorylation and subsequent activation.98, 99 Another study further show that the ubiquitination of TAK1 serves as a docking site to recruit mitogen-activated MEKK3 (protein kinase kinase kinase 3) to activate the MAP kinase pathway (Fig. 5A).100
Another example is MLK3 (mixed linage kinase 3). MLK3 is a family of mitogen-activated protein kinase kinase kinase (MAP3K) responsible for activation of multiple mitogen-activated protein kinase (MAPK) pathways in response to stress, growth factors, and the pro-inflammatory cytokines, such as tumor necrosis factor (TNF). The K63-linked ubiquitination of MLK3 triggered by TRAF6 is critical for activation of MLK3 (Fig. 5A).101 These results indicated that K63-linked ubiquitination of kinases appears to be important for kinase activation. In further support of this notion, Akt kinase also undergoes K63-linked ubiquitination triggered by TRAF6, which is required for Akt activation. The K63-linked ubiquitination of Akt does not directly activate Akt kinase activity, instead is required for Akt membrane recruitment and subsequent phosphorylation and activation (Fig. 5A).
Accordingly, these results raise the interesting and important question whether or not K63-linked ubiquitination represents a general mechanism for kinase activation? Akt ubiquitination occurs within the Akt PH domain, which is also found in other protein kinases known to be involved in growth factor and T-cell receptor (TCR) signaling, such as ETK (endothelial/epithelial tyrosine kinase), ITK (IL-2 inducible T-cell kinase), TEC (Tec protein tyrosine kinase).102, 103 Interestingly, these kinases also contain the TRAF6 consensus binding motif as Akt does (Fig. 5B), raising the possibility that TRAF6 may induce the ubiquitination of these kinases. Thus, it is of significance to examine whether these PH domain-containing kinases also undergo K63-linked ubiquitination by TRAF6 or other E3 ligases, which may play an important role in kinase activation. Answering this question may advance our understanding of how growth factor and TCR signaling elicits the kinase cascades to regulate cell survival and proliferation.
TRAF6 E3 ligase regulates multiple signal transduction pathways
TLR4 (Toll-like receptor 4) and IL-1R (interleukin-1 receptor) signaling pathways play important roles in NF-κB activation and the innate immune response.7, 11, 21 The key protein that engages the TLR4/IL-1R signaling to NF-κB activation is TRAF6. TRAF6 is a RING domain containing E3 ligase that is recruited to the receptor upon lipopolysaccharide (LPS) or IL-1 stimulation where it can be activated by IRAK4 (IL-1 receptor-associated kinase 4)/IRAK1/IRAK2 complex by inducing its oligomeraization and K63-linked ubiquitination.7, 11, 21 The K63-linked ubiquitination of TRAF6 was proposed to serve as a platform to recruit the kinase complex containing the TAB2/TAB3/TAK1,7, 21 although this model was recently challenged.104, 105 The association of TAK1 with TAB2/TAB3 triggers its autophosphorylation, therefore activating TAK1. TAK1 then phosphorylates IKKβ to activate the IKK complex consisting of IKKα, IKKβ and the regulatory protein NEMO (NF-κB essential modulator, also known as IKKγ). Active IKK complex then phosphorylates IκB and results in K48-linked ubiquitination and degradation of IκB. As such, the p65, a subunit of NF-κB is released from IκB, translocates to the nucleus, where it can turn on several target genes important for the proinflammatory response.7, 11, 21
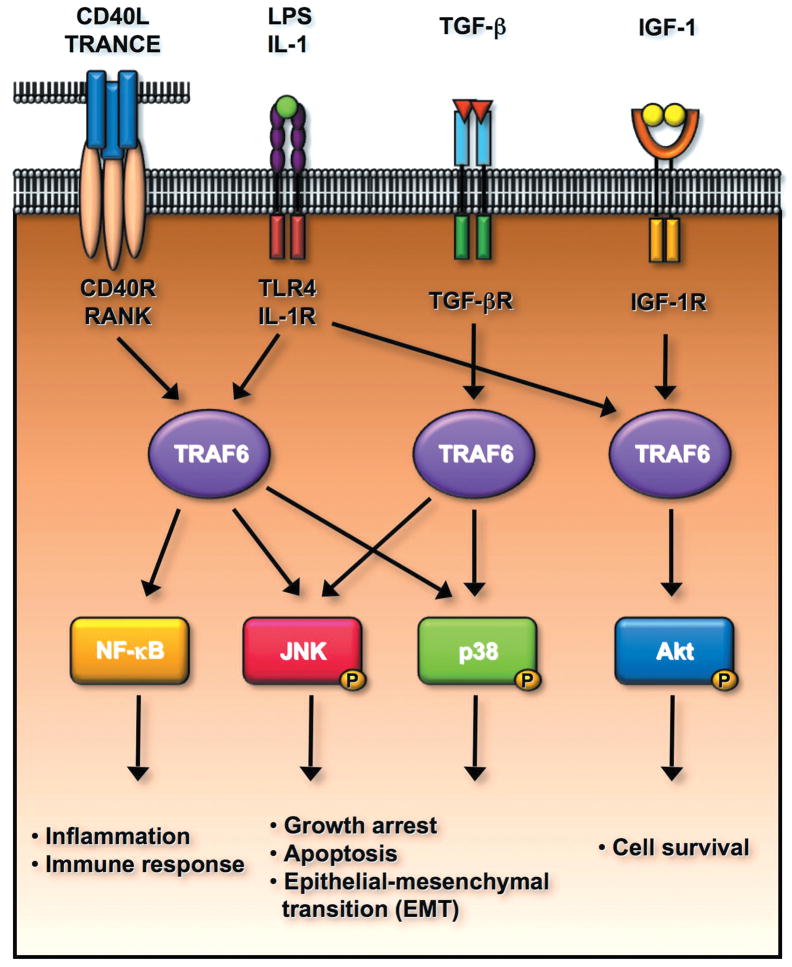
TRAF6 appears to be essential for prenatal and postnatal survival. When bred with Traf6 heterozygote mice, only 11% of Traf6−/− mice were born and some Traf6−/− mice did not survive up to three weeks.106 Interestingly, some Traf6−/− mice, which could survive more than 3-week old displayed an osteopetrotic phenotypes.106 Examining the Traf6−/− cells revealed that TRAF6 is required for LPS, IL-1, CD40, and RANKL-induced NF-κB activation (Fig. 6).106 Recent study revealed that TRAF6 is also engaged in TGF-β-induced p38 phosphorylation and activation, but not Smad2/3 phosphorylation.98, 99, 107 TGF-β promotes the association of TRAF6 with TGF-β receptor I and induces TRAF6 ubiquitination and activation, in turn eliciting K63-linked ubiquitination of TAK1 and subsequent p38 and JNK phosphorylation.98, 99, 107 TRAF6 was further shown to be required for TGF-β-induced cell apoptosis and epithelial-mesenchymal transition (EMT) (Fig. 6).98, 99, 107 Thus, TRAF6 may play a dual role in TGF-β-mediated tumor suppression and metastasis.
Figure 6.
TRAF6 E3 ligase regulates multiple signal transduction pathways involved in the plethora of biological functions including the innate immune response, cell survival, apoptosis, and epithelial-mesenchymal transition (EMT). TRAF6 is a critical mediator for NF-κB activation upon the engagement of CD40L, TRANCE, LPS and IL-1 to their receptors, in turn regulating inflammation and innate immune response. TRAF6 is activated by TGF-β/TGF-β receptor and is required for TGF-β-mediated p38 and JNK activation. The activated p38 and JNK positively regulate cell apoptosis and EMT. Interestingly, TRAF6 is also activated upon the binding of IGF-1 to IGF-1 receptor and regulates cell survival by inducing Akt activation.
In addition to NF-κB and p38 activation upon cytokine stimulation, TRAF6 is also required for Akt signaling activation and cell survival in response to growth factor receptor signaling by inducing the K63-linked ubiquitination of Akt (Fig. 6).6, 86 Interestingly, TRAF6 ubiquitination and activation is also induced by IGF-1 receptor (IGF-1R) signaling and is correlated with Akt ubiquitination.6, 86 TRAF6 was shown to physically interact with IGF-1R and this interaction was disrupted by IGF-1 treatment.6, 86 Further study will be required to understand the mechanism by which IGF-1/IGF-1R induces TRAF6 ubiquitination and activation.
Proinflammatory cytokine IL-1 and endotoxin LPS were known to induce Akt phosphorylation and are important for cellular survival,108–110 although the underlining mechanism by which these molecules activate Akt remains poorly understood. Our results provide the first clue as to how they activate Akt. IL-1 and LPS act through TRAF6 to trigger Akt K63-linked ubiquitination, phosphorylation, and activation (Fig. 6).6, 86 Accordingly, these results suggest that TRAF6 is engaged in multiple signal transduction pathways important for cell survival, apoptosis, and inflammation.
Although the deregulated NF-κB is clearly associated with human cancer development,111, 112 it is still unclear whether TRAF6 is involved in cancer development. The hint that TRAF6 may be involved in tumorigenesis came from a recent report demonstrating that p62, known to interact with TRAF6 and required for TRAF6 ubiquitination and activation,113 is induced by oncogenic Ras and required for Ras-induced NF-κB activation, cell transformation, and lung cancer formation.114 The first evidence proving the role of TRAF6 in tumorigenesis came from our recent report demonstrating that TRAF6 knockdown in prostate cancer cells impair IGF-1-mediated Akt phosphorylation and reduce tumorigenic potential of prostate cancer cells by using the xenograft tumor models.6 This expands the role of TRAF6 not only in TLR signaling and innate immune response, but also in growth factor receptor signaling and oncogenic pathways, suggesting that TRAF6 may be a previously unrecognized oncogene that plays an important role during the cancer progression. Our results therefore suggest that targeting TRAF6 may offer promising strategies for the treatment of human cancers. It will be important to determine in the future whether TRAF6, like its regulator p62, also participates in cellular transformation and tumorigenesis in oncogenic Ras activation or other oncogenic events such as PTEN inactivation.
Perspective
Growth-factor receptors undergo ubiquitination upon ligand engagement, which is critical for receptor internalization to the early endosome. Our findings demonstrating that Akt also undergoes ubiquitination upon growth factor stimulation, which is critical for Akt membrane recruitment and subsequent activation, provides the direct evidence that ubiquitination of the signaling components downstream of these receptors is also critical for protein trafficking and signaling activation. This discovery opens up a new and exciting avenue for the Akt kinase field, suggesting that Akt ubiquitination appears to be as equally important as the phosphorylation does on Akt. We envision that the research efforts studying the regulation of Akt ubiquitination and its role in cancer development represent novel and important areas for further understanding of growth factor receptor signaling, and it is anticipated that more and more exciting findings on this area will be made in the next decade.
Several outstanding questions remain mysterious and warrant for future investigations. How is TRAF6 activated by growth factors? Are there additional E3 ligases responsible for Akt ubiquitination and activation? Does the K63-linked ubiquitination serve as a general mechanism for the activation of kinases downstream of growth factor signaling? Addressing these important questions will lead to comprehensive understanding of the complicate modes of TRAF6 and Akt activation and shed new light into the role of ubiquitination in the regulation of growth factor-mediated signal transduction pathways.
Acknowledgments
We thank the members of the Lin’s lab for their critical reading and comments on our manuscript. We apologize to many investigators whose important works were not cited here due to space limitations. This work is supported by M.D. Anderson Research Trust Scholar Fund to H.K. Lin, a National Science Council fund (NSC-97-2911-I-182-004-2) from Taiwan to C.Y. Wu, and a China Scholarship Council fund to J. Wu.
References
- 1.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–7. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 2.Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–81. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 3.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991;88:4171–5. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 5.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG, Lin HK. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–8. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–7. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 8.Giasson BI, Lee VM. Are ubiquitination pathways central to Parkinson’s disease? Cell. 2003;114:1–8. doi: 10.1016/s0092-8674(03)00509-9. [DOI] [PubMed] [Google Scholar]
- 9.Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–44. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- 10.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–86. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–52. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 13.Adhikari A, Chen ZJ. Diversity of polyubiquitin chains. Dev Cell. 2009;16:485–6. doi: 10.1016/j.devcel.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Adhikary S, Marinoni F, Hock A, Hulleman E, Popov N, Beier R, Bernard S, Quarto M, Capra M, Goettig S, Kogel U, Scheffner M, Helin K, Eilers M. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell. 2005;123:409–21. doi: 10.1016/j.cell.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–14. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun SC. Deubiquitylation and regulation of the immune response. Nat Rev Immunol. 2008;8:501–11. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–14. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–56. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. NEDD4–1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–39. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–65. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano A, Bhagat G, Chadburn A, Dalla-Favera R, Pasqualucci L. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–21. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato M, Sanada M, Kato I, Sato Y, Takita J, Takeuchi K, Niwa A, Chen Y, Nakazaki K, Nomoto J, Asakura Y, Muto S, Tamura A, Iio M, Akatsuka Y, Hayashi Y, Mori H, Igarashi T, Kurokawa M, Chiba S, Mori S, Ishikawa Y, Okamoto K, Tobinai K, Nakagama H, Nakahata T, Yoshino T, Kobayashi Y, Ogawa S. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459:712–6. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- 25.Novak U, Rinaldi A, Kwee I, Nandula SV, Rancoita PM, Compagno M, Cerri M, Rossi D, Murty VV, Zucca E, Gaidano G, Dalla-Favera R, Pasqualucci L, Bhagat G, Bertoni F. The NF-{kappa}B negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood. 2009;113:4918–21. doi: 10.1182/blood-2008-08-174110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Elledge SJ. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc Natl Acad Sci U S A. 2007;104:20759–63. doi: 10.1073/pnas.0710061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehat B, Andersson S, Girnita L, Larsson O. Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis. Cancer Res. 2008;68:5669–77. doi: 10.1158/0008-5472.CAN-07-6364. [DOI] [PubMed] [Google Scholar]
- 29.Varghese B, Barriere H, Carbone CJ, Banerjee A, Swaminathan G, Plotnikov A, Xu P, Peng J, Goffin V, Lukacs GL, Fuchs SY. Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol Cell Biol. 2008;28:5275–87. doi: 10.1128/MCB.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 31.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 32.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–8. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1998;95:15587–91. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–90. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 38.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–6. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 39.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 40.Ecker K, Hengst L. Skp2: caught in the Akt. Nat Cell Biol. 2009;11:377–9. doi: 10.1038/ncb0409-377. [DOI] [PubMed] [Google Scholar]
- 41.Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer S, Tempst P, Pandolfi PP. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–32. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. Embo J. 2002;21:4037–48. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–8. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–6. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell. 2005;9:663–73. doi: 10.1016/j.devcel.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Zhou GL, Zhuo Y, King CC, Fryer BH, Bokoch GM, Field J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol Cell Biol. 2003;23:8058–69. doi: 10.1128/MCB.23.22.8058-8069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyake H, Nelson C, Rennie PS, Gleave ME. Overexpression of insulin-like growth factor binding protein-5 helps accelerate progression to androgen-independence in the human prostate LNCaP tumor model through activation of phosphatidylinositol 3′-kinase pathway. Endocrinology. 2000;141:2257–65. doi: 10.1210/endo.141.6.7520. [DOI] [PubMed] [Google Scholar]
- 53.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Benedetti Panici P, Mancuso S, Neri G, Testa JR. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–5. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 54.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992;89:9267–71. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–41. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–7. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L, Robertson GP. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 58.Nakatani K, Thompson DA, Barthel A, Sakaue H, Liu W, Weigel RJ, Roth RA. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J Biol Chem. 1999;274:21528–32. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 59.Askham JM, Platt F, Chambers PA, Snowden H, Taylor CF, Knowles MA. AKT1 mutations in bladder cancer: identification of a novel oncogenic mutation that can cooperate with E17K. Oncogene. 2009 doi: 10.1038/onc.2009.315. [DOI] [PubMed] [Google Scholar]
- 60.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, Thomas JE. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 61.Kim MS, Jeong EG, Yoo NJ, Lee SH. Mutational analysis of oncogenic AKT E17K mutation in common solid cancers and acute leukaemias. Br J Cancer. 2008;98:1533–5. doi: 10.1038/sj.bjc.6604212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malanga D, Scrima M, De Marco C, Fabiani F, De Rosa N, De Gisi S, Malara N, Savino R, Rocco G, Chiappetta G, Franco R, Tirino V, Pirozzi G, Viglietto G. Activating E17K mutation in the gene encoding the protein kinase AKT1 in a subset of squamous cell carcinoma of the lung. Cell Cycle. 2008;7:665–9. doi: 10.4161/cc.7.5.5485. [DOI] [PubMed] [Google Scholar]
- 63.Mohamedali A, Lea NC, Feakins RM, Raj K, Mufti GJ, Kocher HM. AKT1 (E17K) mutation in pancreatic cancer. Technol Cancer Res Treat. 2008;7:407–8. doi: 10.1177/153303460800700509. [DOI] [PubMed] [Google Scholar]
- 64.Shoji K, Oda K, Nakagawa S, Hosokawa S, Nagae G, Uehara Y, Sone K, Miyamoto Y, Hiraike H, Hiraike-Wada O, Nei T, Kawana K, Kuramoto H, Aburatani H, Yano T, Taketani Y. The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br J Cancer. 2009;101:145–8. doi: 10.1038/sj.bjc.6605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zilberman DE, Cohen Y, Amariglio N, Fridman E, Ramon J, Rechavi G. AKT1 E17 K pleckstrin homology domain mutation in urothelial carcinoma. Cancer Genet Cytogenet. 2009;191:34–7. doi: 10.1016/j.cancergencyto.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Chen ML, Xu PZ, Peng XD, Chen WS, Guzman G, Yang X, Di Cristofano A, Pandolfi PP, Hay N. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20:1569–74. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–4. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 68.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 69.Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR, Loda M, Sellers WR. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A. 2003;100:7841–6. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bayascas JR. Dissecting the role of the 3-phosphoinositide-dependent protein kinase-1 (PDK1) signalling pathways. Cell Cycle. 2008;7:2978–82. doi: 10.4161/cc.7.19.6810. [DOI] [PubMed] [Google Scholar]
- 71.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–70. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 72.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 73.Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24:7746–55. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- 74.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–91. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 75.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 76.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Mendoza MC, Blenis J. PHLPPing it off: phosphatases get in the Akt. Mol Cell. 2007;25:798–800. doi: 10.1016/j.molcel.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 78.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–66. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–7. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Luan B, Zhao J, Wu H, Duan B, Shu G, Wang X, Li D, Jia W, Kang J, Pei G. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature. 2009;457:1146–9. doi: 10.1038/nature07617. [DOI] [PubMed] [Google Scholar]
- 82.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276:31858–62. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 83.Datta K, Bellacosa A, Chan TO, Tsichlis PN. Akt is a direct target of the phosphatidylinositol 3-kinase. Activation by growth factors, v-src and v-Ha-ras, in Sf9 and mammalian cells. J Biol Chem. 1996;271:30835–9. doi: 10.1074/jbc.271.48.30835. [DOI] [PubMed] [Google Scholar]
- 84.Jiang T, Qiu Y. Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J Biol Chem. 2003;278:15789–93. doi: 10.1074/jbc.M212525200. [DOI] [PubMed] [Google Scholar]
- 85.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–9. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 86.Restuccia DF, Hemmings BA. Cell signaling. Blocking Akt-ivity. Science. 2009;325:1083–4. doi: 10.1126/science.1179972. [DOI] [PubMed] [Google Scholar]
- 87.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–5. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 88.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–34. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McKenna LB, Zhou GL, Field J. Isoform-specific functions of Akt in cell motility. Cell Mol Life Sci. 2007;64:2723–5. doi: 10.1007/s00018-007-7247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darnay BG, Besse A, Poblenz AT, Lamothe B, Jacoby JJ. TRAFs in RANK signaling. Adv Exp Med Biol. 2007;597:152–9. doi: 10.1007/978-0-387-70630-6_12. [DOI] [PubMed] [Google Scholar]
- 91.Ye H, Arron JR, Lamothe B, Cirilli M, Kobayashi T, Shevde NK, Segal D, Dzivenu OK, Vologodskaia M, Yim M, Du K, Singh S, Pike JW, Darnay BG, Choi Y, Wu H. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–7. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 92.Kim AH, Sasaki T, Chao MV. JNK-interacting protein 1 promotes Akt1 activation. J Biol Chem. 2003;278:29830–6. doi: 10.1074/jbc.M305349200. [DOI] [PubMed] [Google Scholar]
- 93.Noguchi M, Ropars V, Roumestand C, Suizu F. Proto-oncogene TCL1: more than just a coactivator for Akt. Faseb J. 2007;21:2273–84. doi: 10.1096/fj.06-7684com. [DOI] [PubMed] [Google Scholar]
- 94.Varnai P, Bondeva T, Tamas P, Toth B, Buday L, Hunyady L, Balla T. Selective cellular effects of overexpressed pleckstrin-homology domains that recognize PtdIns(3,4,5)P3 suggest their interaction with protein binding partners. J Cell Sci. 2005;118:4879–88. doi: 10.1242/jcs.02606. [DOI] [PubMed] [Google Scholar]
- 95.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–25. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 96.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–71. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 97.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–64. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 98.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landstrom M. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 99.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31:918–24. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, Akira S, Hayashi H, Su B, Inoue J. Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci Signal. 2009;2:ra66. doi: 10.1126/scisignal.2000387. [DOI] [PubMed] [Google Scholar]
- 101.Korchnak AC, Zhan Y, Aguilar MT, Chadee DN. Cytokine-induced activation of mixed lineage kinase 3 requires TRAF2 and TRAF6. Cell Signal. 2009;21:1620–5. doi: 10.1016/j.cellsig.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 103.Qiu Y, Kung HJ. Signaling network of the Btk family kinases. Oncogene. 2000;19:5651–61. doi: 10.1038/sj.onc.1203958. [DOI] [PubMed] [Google Scholar]
- 104.Walsh MC, Kim GK, Maurizio PL, Molnar EE, Choi Y. TRAF6 autoubiquitination-independent activation of the NFkappaB and MAPK pathways in response to IL-1 and RANKL. PLoS One. 2008;3:e4064. doi: 10.1371/journal.pone.0004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xia ZP, Sun L, Chen X, Pineda G, Jiang X, Adhikari A, Zeng W, Chen ZJ. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 2009;461:114–9. doi: 10.1038/nature08247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–24. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166–76. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 108.Neumann D, Lienenklaus S, Rosati O, Martin MU. IL-1beta-induced phosphorylation of PKB/Akt depends on the presence of IRAK-1. Eur J Immunol. 2002;32:3689–98. doi: 10.1002/1521-4141(200212)32:12<3689::AID-IMMU3689>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 109.Vivarelli MS, McDonald D, Miller M, Cusson N, Kelliher M, Geha RS. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med. 2004;200:399–404. doi: 10.1084/jem.20040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wong F, Hull C, Zhande R, Law J, Karsan A. Lipopolysaccharide initiates a TRAF6-mediated endothelial survival signal. Blood. 2004;103:4520–6. doi: 10.1182/blood-2003-06-2118. [DOI] [PubMed] [Google Scholar]
- 111.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dey A, Verma CS, Lane DP. Updates on p53: modulation of p53 degradation as a therapeutic approach. Br J Cancer. 2008;98:4–8. doi: 10.1038/sj.bjc.6604098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moscat J, Diaz-Meco MT, Wooten MW. Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 114.Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–54. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]