Abstract
A nitrogen oxides (NOx; x=1, 2) optical sensor with an extremely low detection limit in the range of fractions of ppbV (0.3 ppbV for 20s sample injection) is presented. Phenylenediamine derivatives are utilized as molecular probes in solid state on a nanoporous membrane to produce a miniaturized and low cost sensing platform for use as a wearable personal monitor.
Keywords: Nitrogen oxides, nanoporous membrane, optical detection, biomedical sensor, environmental sensor
In spite of significant advances in sensor development, a portable device that can reliably detect personal exposure of chemicals in a real environment still faces many challenges.1 These challenges include extremely low yet potentially toxic levels of chemicals in many practical scenarios, variable environmental conditions and the need of low-cost and highly portable detection methods. To address these challenges, we present here a nitrogen oxides (NOx; (x=1, 2)) sensor based on new solid-state molecular probes, alumina nanoparticles, and a low-cost and high performance webcam-sensing platform. NOx are studied here because they are among the most toxic pollutants from traffic and industrial processes, which affect a large population.1,2 Detection of NOx has been a leading priority for environmental monitoring agencies, industrial hygienists and epidemiologists.3–6 This is also important for pulmonologists because NO in exhaled breath has been identified as an asthma biomarker.
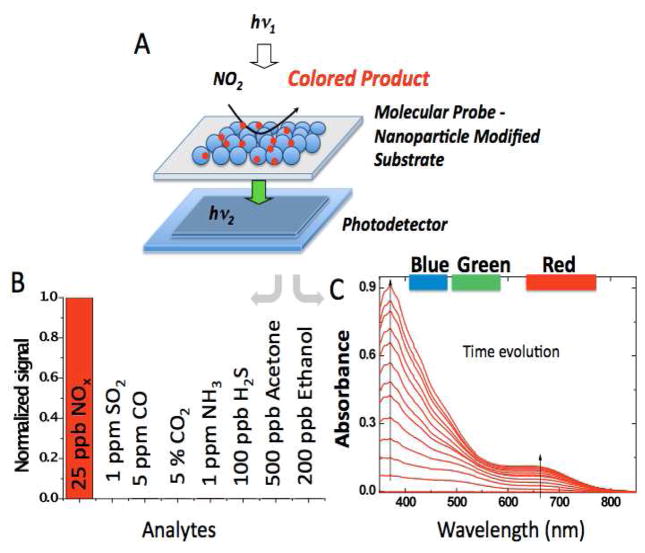
The molecular probes are phenylenediamine derivatives, o-phenylenediamine and diethyl-p-phenylenediamine, and are immobilized on 50-nm alumina nanoparticles (Fig. 1A) supported on a microporous cellulose membrane. The nano-composite sensing material provides a large effective surface area, which is one of the key factors for the high sensitivity. Exposure of the phenylenediamine derivative-modified nanoparticles to NO2 produces distinctive color changes and allows for unambiguous detection of the analyte. The sensing mechanism is immune to most common compounds present in polluted ambient air, except ozone, which can also trigger a reaction but can be scrubbed with a simple ozone filter. Fig. 1B shows the response of one of the molecular probes, o-phenylenediamine, to 25 ppb NO2 compared to the response of interferents at concentrations typically found in polluted environments. In addition, selectivity coefficients of both molecular probes (obtained at much larger concentrations of interferents and calculated as the NO2 sensitivity/interferent sensitivity) are higher than 104 (Supp. info., Section 1, Selectivity of the molecular probes). Fig. 1C shows visible spectrophotometric changes of the molecular probe, o-phenylenediamine, upon reaction with NO2. Increases of absorbance in the range of 350 to 550 nm are clearly observed, which forms the basis of the sensing mechanism7 (see below). Similar features of increasing absorbance upon exposure of NO2 are found for diethyl-p-phenylenediamine. The reaction of the highly selective probes is fast, and the response of the sensor is mass transport controlled even at high flow rates of 500 mL.min−1 (Supp. info., Section 2, Mass transport study). This renders a NO2 capture efficiency of ~ 90%, and an apparent reaction efficiency of ~95 % (Supp. info, Section 9, Apparent reaction efficiency).
Figure 1.
A) Schematic representation of the sensing platform. B) Normalized response of o-phenylenediamine upon exposure to NO2 and various environmental gases. A similar response pattern is found for diethyl-p-phenylenediamine. C) UV-Visible spectrum of o-phenylenediamine upon reaction with NO2 (part-per-million (ppm) level) taken at increasing reaction times.
Unlike existing detection methods for NO2, which involve the catalytic conversion of NO2 into NO followed by chemiluminescence detection,8 the new sensing platform directly detects NO2 without the need of additional supplies of reagents, low pressure sensing environments, and expensive photodetectors. Furthermore, by adding a small oxidizing tubing (based on NaMnO4), NO can be converted into NO2, enabling detection of NO, and total NOx (NO2 + NO) (see Supp. info, Section 3. Oxidizing tubing for conversion of NO into NO2).
Another key enabling feature of the present sensing platform is the sensor configuration, including the detector and signal processing (Fig 2A). A basic version of the sensor consists of two elements, one for sensing and one for reference correction. The optical signal is taken with an optical imaging device based on a complementary semiconductor metal oxide (CMOS) imager. The image is acquired and controlled with a program developed in our lab. The program has the capability of extracting Red, Green, and Blue intensity values (R(t), G(t), and B(t), respectively) from the image with a reasonably fast acquisition rate (~5Hz). The logarithmic ratio between the sensing and the reference elements for each color component is evaluated as an output signal. The overall approach affords not only increased sensitivity but also selectivity. 1) An extremely low detection limit in the range of fractions of ppbV (~ 0.3 ppbV, see Fig. 2B) is achieved due to the low noise level of the system (~ 2 × 10−5 absorbance units for a 20-s period (sample injection period)) (Supp. info., Section 10, NOx field-testing); 2) Excellent selectivity against common interferents, such as carbon dioxide, is obtained because the molecular probes are insensitive to pH changes and have high oxidation potential, and the capability of reading individually RGB components provides a distinct color pattern for the analyte. In addition, the sensing platform is also provided with a nafion tubing, which makes the sensor immune to humidity changes (Supp. info., Section 5, Humidity effect conditioning).
Figure 2.
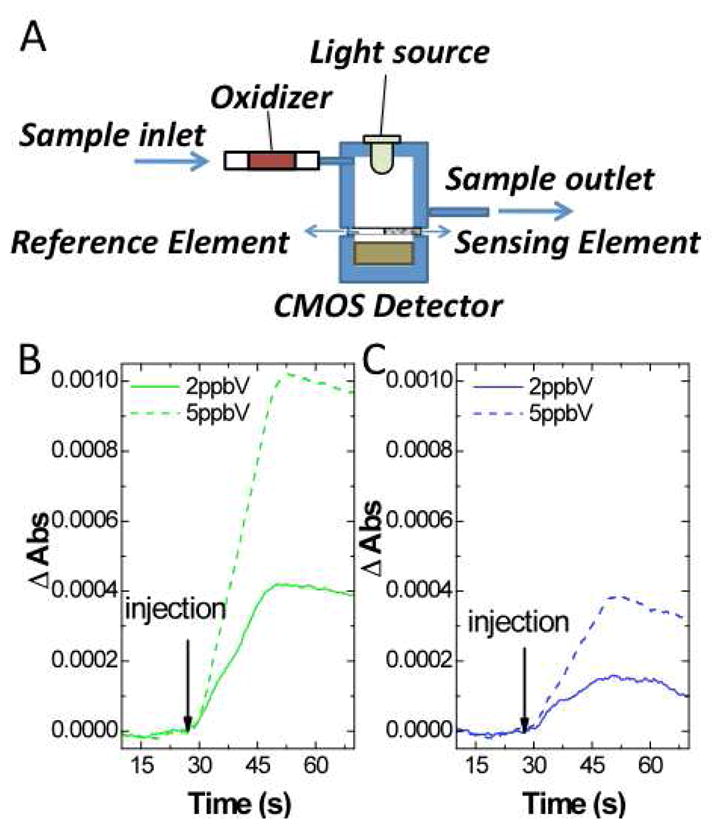
(A) Configuration of the sensing platform. (B) Green- and (C) Blue- component signal corresponding to the injections of 2 and 5 ppbV of NO2. The sensor probe is diethyl-p-phenylenediamine and the signal is the −log(sensing area light intensity/reference area light intensity). Flow rate = ~450 mL/min.
The new solid-state molecular probes provide the needed sensitivity, selectivity and response time for many real-world applications. The device hardware is rather simple and compact, adding the benefit of low cost and high portability. One of the key elements of the sensing platform is the use of the new molecular probes without the need of additional binders or co-reagents. The molecular probes are casted on alumina nanoparticles immobilized on a semi-transparent support (Supp. info. Section 4, Preparation of the sensing element). The support provides: (1) an excellent host of high surface concentration for the molecular probes, enabling a high dynamic detection range and lifetime (Supp. info., Section 7, Sensor stability (under NO2 sensing)), (2) a good medium for quick diffusion and transport of NO2, (3) a medium that promotes the formation of colored products, and (4) a highly stable support to store the sensor for several months (4 months tested so far) (Supp. info., Section 6, Sensor stability (under storage)).
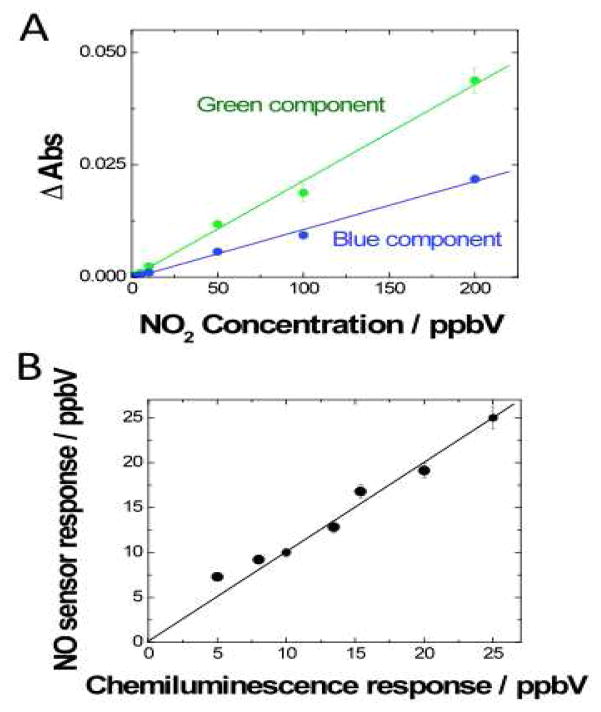
We have applied this new sensing platform to detect NO2 and NO. Fig. 3A shows as example a calibration plot obtained for detection of NO2 from few ppbV to 200 ppbV using diethyl-p-phenylenediamine molecular probe. Since the absorption wavelengths of the reaction products of both molecular probes mostly range from 350 to 550 nm (e.g. Fig. 1C), we found the blue and the green components of the sensor output signal to be the most sensitive color components. This observation is consistent with the corresponding optical absorption spectra of the probes. In addition, the response curves are linear over a wide dynamic range, which enable the use of the sensor for quantitative detection and analysis of NO2 and NO for environmental monitoring applications.
Figure 3.
Analytical performance of the sensing platform. (A) Calibration plots for NO2 using: Green and Blue absorbance changes vs. concentration of NO2. Signal changes were recorded upon 20 s injections of NO2, using diethyl-p-phenylenediamine as molecular probe. (B) Evaluation of the sensor accuracy for real sample analysis. The sensing platform includes an oxidizing filter made of NaMnO4 to convert NO into NO2 and thus the sensor detects NO. The molecular probe is o-phenylenediamine. Comparison of NO concentration determined by the present sensor vs. NO concentration determined by the gold standard method (chemiluminescence). (A&B) Flow rate = ~ 450 mL/min.
We have characterized the reaction products. In the case of o-phenylenediamine, the extinction coefficient of the NO2 reaction product has been estimated to be ~2,000 M−1cm−1 in solid phase. The product is believed to be a phenazine derivative according to the study using liquid chromatography/mass spectrometry (LC/MS), and Fourier transform spectroscopy (FTIR) (see Supp. info., Section 8. Reaction product characterization) and previously published studies.9–11 We note that even though the extinction coefficient of the o-phenylenediamine product is modest, a high sensitivity and low detection limit are achieved because of good reference correction, efficient sample delivery and conditioning, and low noise optical detection.12
We have calibrated and compared our sensor with a standard reference chemiluminescence method using both artificial and real breath and environmental samples. Levels of NO determined with the present sensing platform and the reference method are in excellent agreement as shown in Fig. 3B. We have also carried out preliminary field test and demonstrated that the sensor is capable of monitoring levels of NOx in high and low traffic areas (see Supp. info., Section 10. NOx field-testing).
In summary, a highly selective, sensitive and low-cost sensing platform is developed based on new molecular probe-functionalized nanoparticles coated on a membrane and a high-performance CMOS imaging-sensing platform. High selectivity is achieved due to intrinsic chemical properties of the probes with NO2 and distinct RGB color patterns of the reaction products. High sensitivity is possible because of the large surface concentration given by alumina nanoparticles and the fast reaction kinetics. Using an inexpensive webcam we have achieved a detection limit of fractions of ppbV and demonstrated selective detection of NO2. Furthermore, the combination of the sensing platform with an appropriate oxidant preconditioning oxidizing tubing has also allowed accurate detection of NO, enabling the detection of total NOx levels.
Supplementary Material
Acknowledgments
The authors thank funding from NSF under grant ECCS#0925496 and NIH/NIEHS under Grant U01 ES016064-02 as well as Max Porter, Ben Davis and John Neff from the Maricopa County Air Quality Department.
Footnotes
BRIEFS. A highly selective, sensitive, low-cost, wearable sensing platform is developed based on new solid-state molecular probes for NO2 and an integrated sample collection and colorimetric approach.
SUPPORTING INFORMATION PARAGRAPH. Selectivity factors, sensor preparation, mass transport study, sensor stability, reaction product characterization, apparent reaction efficiency, NOx field testing. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.United States Environmental Protection Agency. Technical Bulletin. 1998. EPA-456/F-98-005. [Google Scholar]
- 2.Han X, Naeher LP. Environment International. 2006;32:106–120. doi: 10.1016/j.envint.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 3.United States Environmental Protection Agency. Technical Bulletin. 1999. EPA 456/F-99-006R. [Google Scholar]
- 4.Ashmore MR, Dimitroulopoulou C. Atmospheric Environment. 2009;43:128–141. [Google Scholar]
- 5.Geupel A, Schönauer D, Röder-Roith U, Kubinski DJ, Mulla S, Ballinger TH, Chen HY, Visser JH, Moos R. Sensors and Actuators B. 2010;145:756–761. [Google Scholar]
- 6.Ohira SH, Dasgupta PK, Schug KA. Anal Chem. 2009;81:4183–4191. doi: 10.1021/ac801756z. [DOI] [PubMed] [Google Scholar]
- 7.Hobbs AP. Anal Chem. 1962;34(5):91R. [Google Scholar]
- 8.Dunlea EJ, et al. J Atmos Chem Phys. 2007;7:2691–2704. [Google Scholar]
- 9.He D, Wu Y, Xu BQ. European Polymer Journal. 2007;43:3703–3709. [Google Scholar]
- 10.Niu S, Zhang S, Ma L, Jiao K. Bull Korean Chem Soc. 2004;25:829–832. [Google Scholar]
- 11.Chai L, Zhang L, Wang H, Yu W, Sang P. Materials Letter. 2010;64:1193–1196. [Google Scholar]
- 12.Tao NJ, Forzani ES, Iglesias RA. US Provisional Patent Application: Integrated optoelectrochemical sensor for nitrogen oxides in gaseous samples. 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.