Abstract
Objective
To describe a case of childhood-onset progressive multiple sclerosis with dementia and brain biopsy evidence of extensive cortical demyelination.
Design
Case report
Patient
A 26-year-old gentleman with a history of behavioral changes starting at the age of 13 years followed by progressive dementia.
Interventions
Neurological examination, MRI, CSF studies, neuropsychological testing, and brain biopsy.
Results
MRI showed numerous T2W hyperintensities throughout the central nervous system not associated with contrast enhancement. Brain biopsy showed cortical and subcortical demyelination. All three types of cortical demyelinating lesions were
observed
leukococortical, intracortical, and subpial. Lesions were associated with profound microglial activation. The patient continued to progress despite attempts to treat with multiple sclerosis disease-modifying therapies.
Conclusions
Multiple sclerosis should be considered in the diagnosis of progressive dementia in children and young adults. Cortical demyelination may contribute to cognitive decline in patients with dementia due to multiple sclerosis.
Cognitive impairment in multiple sclerosis (MS) has been reported in 40-65% of patients, with Double Inversion Recovery (DIR) MRI studies demonstrating significant correlation between cortical lesion burden and cognitive decline.1 However, dominant cognitive impairment due to MS is uncommon in childhood-onset MS.2 Furthermore, a primary progressive course in pediatric MS is rare.3, 4 We present a case of progressive dementia in childhood-onset MS associated with extensive cortical demyelination (CDM) on brain biopsy.
Report of a case
A 26-year-old gentleman first developed behavioral symptoms at age 13 characterized by inattention and personality change and was diagnosed with attention deficit disorder. His academic performance progressively declined with grades dropping from Bs in middle school, to Cs/Ds in high school. By age 17, he developed subtle gait, and sphincter difficulties, although his progressive cognitive symptoms predominated. He graduated high school and attended community college with difficulty. Over the subsequent years he became increasingly apathetic with reduced motivation. At the age of 25 years he had his first brain MRI which revealed global brain atrophy and numerous T2W hyperintense and T1W hypointense periventricular, subcortical, juxtacortical, brainstem, and spinal cord lesions (Figure A-C). The lesions did not enhance with contrast. Diagnostic work-up included CBC, comprehensive metabolic panel, CRP, thyroid function tests, ANA, vitamin B12 level, vitamin D level, HIV, lipid profile, homocystine, and Lyme serology that were all normal. CSF studies showed a WBC count of 16, RBC count of 0, glucose 61 mg/dl, protein 68.9 mg/dl, 20 oligoclonal bands, IgG index of 1.07 and IgG synthesis rate of 28.1 mg/dl. CSF VDRL, Lyme PCR, and cytology were negative. Neuropsychological testing demonstrated evidence of severe impairment in multiple modalities including intellectual function, language, executive function, sensorimotor function, visual-spacial abilities, processing speed, attention/concentration, and memory. His performance was in the dementia range. He had relative preservation of academic performance (reading, comprehension, and math). He also met criteria for major depression and generalized anxiety. He was diagnosed with MS. Despite the absence of relapses, he was treated with several courses of steroids, glatiramer acetate, monthly IVIg, and natalizumab without response. Neurological examination at age 26 revealed a score of 20/36 on the Kokmen Mini-Mental Status exam. Other pertinent positives included saccadic smooth pursuits, a left extensor plantar response, and moderate difficulty on tandem gait. Given the prominent cortical atrophy and dominant cognitive presentation, a diagnostic right frontal brain biopsy was performed to exclude a superimposed primary neurodegenerative disorder.
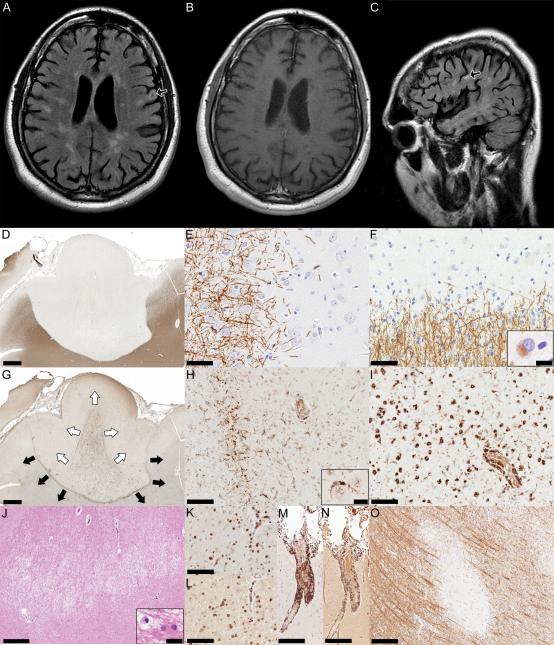
Figure.
(A and C) Axial and sagittal FLAIR MRI showing typical periventricular lesions of high signal. Some lesions appear to involve the cortical gray matter (arrows). (B) Axial T1W MRI with gadolinium showing absence of enhancing lesions. (D) Subpial lesion showing loss of myelin that extends in the subcortical white matter (PLP, scale bar = 1.6mm). (E) Cortical edge of the subpial lesion showing lack of active demyelination (PLP, scale bar = 50μm). (F) White matter border of the subpial lesion is mostly inactive, but rare macrophages containing myelin degradation products within their cytoplasm (inset) are still present (PLP, scale bar = 50μm, inset scale bar = 10μm). (G) Intersecting subpial and leukocortical lesions; the subpial lesion bordered by a rim of microglia appears to expand towards subcortical white matter (black arrows), while a leukocortical lesion revealed by profound macrophage activation appears to extend towards the pial surface (white arrows) (KiM1P, scale bar = 1.6mm). (H) The subpial lesion is bordered by a rim of microglia and some microglia are seen in close apposition to neurons (inset) (KiM1P, scale bar = 100μm, inset scale bar = 20μm). (I) Macrophages are the predominant cells in the leukocortical lesion (KiM1P, scale bar = 100μm). (J) The white matter located in the intersection zone of the subpial and leukocortical plaques shows a destructive area; inset shows a reactive astrocyte located in the proximity of a macrophage (H&E, scale bar = 500μm, inset scale bar = 20μm). (K) Parenchymal and perivascular CD3+ lymphocytes (CD3, scale bar = 100μm), and (L) Parenchymal and perivascular CD8+ lymphocytes (CD8, scale bar = 100μm) are components of the inflammatory infiltrates located in the demyelinated white matter of the subpial and/or leukocortical lesion. (M) Perivascular meningeal CD3+ lymphocytes (CD3, scale bar = 200μm), and (N) Perivascular meningeal CD8+ lymphocytes (CD8, scale bar = 200μm) are components of the meningeal inflammatory infiltrates. (O) Intracortical lesion (PLP, scale bar = 200μm).
Neuropathological analysis included routine staining and immunocytochemistry according to previously published protocols5 using the following markers: hematoxylin and eosin, luxol fast blue and proteolipid protein (LFB and PLP for myelin), glial fibrillary acidic protein (GFAP for astrocytes), neurofilament (NF for axons), KiM1P for macrophages, and CD3 and CD8 for T cells. All 3 cortical MS plaque types were observed and included subpial (figure, D-I), intracortical (figure O) and leukocortical (not shown) lesions. Subpial and leukocortical lesions were sharply demarcated (figure D) and surrounded by a rim of microglia (figure, G and H). As previously reported for chronic MS,6, 7 active demyelination was absent in the cortical gray matter (figure E), however myelin-laden macrophages were rarely present in the underlying white matter (figure F, inset, and I). Profound microglial activation was present within cortical lesions, with occasional microglia in close apposition to neurons (figure H, inset). The white versus gray matter component of the lesions was more destructive (figure J), and contained parenchymal and perivascular CD3+ (figure K) and CD8+ (figure L) lymphocytic infiltrates. Reactive astrocytosis was present (figure J, inset). Marked diffuse and perivascular meningeal inflammation composed of CD3+ (figure M) and CD8+ (figure N) lymphocytes were present, but not topographically associated with areas of CDM. Interestingly, whereas the myelin stain showed a subpial lesion extending into the subcortical white matter (figure D), the macrophage/microglial stain indicated the intersection of both a subpial and leukocortical lesion which appeared to advance in opposite directions (figure G).
Comment
This is the first study to describe pathological evidence of CDM in the setting of childhood onset progressive MS presenting with dominant cognitive impairment. The lack of a clear history of relapses suggests a primary progressive disease course (PPMS). PPMS is rare in children and only 4% of childhood-onset MS cases develop a secondary progressive course during childhood.3, 4 Cognitive impairment as the predominant presentation of MS is also uncommon and has been previously described in a cohort of 23 adults with relapsing-remitting or progressive MS; 14 of which had progressive dementia.2 Although our patient carried an established diagnosis of MS prior to brain biopsy, the atypical presentation, coupled with pronounced cortical atrophy, raised concerns for an alternative or superimposed explanation for the progressive dementia. Nevertheless, among young adults presenting with progressive dementia, MS has been reported to be the sole cause in 11%.8 Amato et al conducted 6-monthly serial cognitive testing on a cohort of patients with relapsing-remitting MS aged 10.9-20.6 years compared to healthy controls. Cognitive impairment was found in 57% of patients <15 years of age and in 70% of patients ≥15 years old.9 75% had deterioration in cognitive function with time. This did not correlate with disease duration, use of disease modifying therapy, or EDSS. An earlier study by MacAllister et al reported cognitive impairment among 35% of 37 children with clinically definite MS.10 Interestingly, similar to our case, one of the included cases had ADHD; however, cognitive testing was otherwise normal. Our case underscores the importance of considering MS in the differential diagnosis of pediatric patients presenting with progressive cognitive decline. This case also demonstrates the challenges in the clinical diagnosis of childhood behavioral and cognitive abnormalities.
Pathological and imaging studies indicate CDM is extensive in patients with progressive MS.11 Subpial cortical lesions have a predilection for anatomical regions involved with cognitive functions, including cingulate, temporal, insular and cerebellar cortex.11 However, the precise contribution of CDM to clinical signs is difficult to determine, particularly in the setting of extensive white matter lesion burden, as was observed in our patient. There are however several case reports of cognitive dominant MS associated with widespread subpial CDM in the complete absence of white matter lesions, suggesting CDM may be an important pathological substrate for cognitive decline in some MS patients.6 Previous pathological studies describing extensive CDM in MS were based on chronic autopsy archival material. Whereas analysis of MS brain biopsies offers the advantage of evaluating tissue pathology from earlier disease phases, there are several inherent limitations, including selection and sampling bias. Although none of the sampled cortical regions in our patient's biopsy were normal, the extent of CDM and relative burden of cortical disease cannot be reliably determined on biopsy alone. Furthermore, the absence of cortical demyelination on biopsy does not exclude the presence of CDM in another cortical region. Nevertheless, the pathological evidence demonstrating all three cortical plaque types in our case, as well as the presence of severe cortical atrophy and numerous cortical/juxtacortical lesions on MRI, suggest CDM may have contributed to the predominant cognitive clinical manifestations observed in this case.
Supplementary Material
Acknowledgments
We wish to thank Patricia Ziemer, for her expert technical assistance. This study was supported by grants RO1-NS049577-01-A2 from the National Institutes of Health (Dr. Lucchinetti) and NMSS RG 3185-B-3 from the National Multiple Sclerosis Society (Dr. Lucchinetti).
Disclosures:
Dr. Lucchinetti receives royalties from the publication of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010); and receives research support from the NIH (NS49577-R01 [PI]), and the National MS Society (RG 3185-B-3 [PI]).
Dr. Parisi serves on scientific advisory boards for the US Government Defense Health Board and the Subcommittee for Laboratory Services and Pathology; serves as a Section Editor for Neurology; receives royalties from the publication of Principles & Practice of Neuropathology, 2nd ed. (Oxford University Press, 2003); and receives research support from the NIH (NS32352-13 [co-investigator]).
References
- 1.Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66:1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- 2.Staff NP, Lucchinetti CF, Keegan BM. Multiple sclerosis with predominant, severe cognitive impairment. Arch Neurol. 2009;66:1139–1143. doi: 10.1001/archneurol.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gusev E, Boiko A, Bikova O, et al. The natural history of early onset multiple sclerosis: comparison of data from Moscow and Vancouver. Clin Neurol Neurosurg. 2002;104:203–207. doi: 10.1016/s0303-8467(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 4.Mikaeloff Y, Caridade G, Assi S, Suissa S, Tardieu M. Prognostic Factors for Early Severity in a Childhood Multiple Sclerosis Cohort. PEDIATRICS. 2006;118:1133–1139. doi: 10.1542/peds.2006-0655. [DOI] [PubMed] [Google Scholar]
- 5.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Bø L, Vedeler CA, Nyland HI, Trapp BD, Mørk SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 7.Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 8.Kelley BJ, Boeve BF, Josephs KA. Young-onset dementia: demographic and etiologic characteristics of 235 patients. Arch Neurol. 2008;65:1502–1508. doi: 10.1001/archneur.65.11.1502. [DOI] [PubMed] [Google Scholar]
- 9.Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features in childhood and juvenile MS: two-year follow-up. Neurology. 2010;75:1134–1140. doi: 10.1212/WNL.0b013e3181f4d821. [DOI] [PubMed] [Google Scholar]
- 10.MacAllister WS, Belman AL, Milazzo M, et al. Cognitive functioning in children and adolescents with multiple sclerosis. Neurology. 2005;64:1422–1425. doi: 10.1212/01.WNL.0000158474.24191.BC. [DOI] [PubMed] [Google Scholar]
- 11.Kutzelnigg A, Lassmann H. Cortical demyelination in multiple sclerosis: a substrate for cognitive deficits? J Neurol Sci. 2006;245:123–126. doi: 10.1016/j.jns.2005.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.