Abstract
Angiogenesis is a key event in cancer progression and therefore a promising target in cancer treatment. Galectin-1, a β-galactoside binding lectin, is up-regulated in the endothelium of tumors of different origin and has been shown to be the target for anginex, a powerful anti-angiogenic peptide with anti-tumor activity. Here we show that when bound to anginex, galectin-1 binds various glycoproteins with hundred- to thousand-fold higher affinity. Anginex also interacts with galectin-2, -7, -8N, and -9N but not with galectin-3, -4, or -9C.
Keywords: Cancer Therapy, Carbohydrate, Fluorescence, Glycoprotein, Lectin, Peptide Interactions, Angiogenesis, Fluorescence Polarization, Galactose, Galectin
Introduction
Anginex is a 33-mer cytokine-like artificial β-peptide (the sequence is given in supplemental Fig. S1) that inhibits endothelial cell growth by specifically blocking adhesion and migration of activated endothelial cells leading to angiogenesis in vitro and in vivo (1, 2). It inhibits microvessel formation but not the larger, pre-existing vessels, pointing toward tumor specificity (1). The profound anti-tumor effect is found both on tumor growth and on established tumors in human xenograft mouse models (2).
A two-hybrid screen using anginex as bait revealed the small soluble lectin galectin-1 as a potential target, and the requirement of galectin-1 for the anti-angiogenic effect of anginex was demonstrated using galectin-1-null mutant mice (3). This and other studies on galectin-1 (4–6) suggested a rate-limiting role of galectin-1 in tumor angiogenesis and indicated that anginex interferes with the pro-angiogenic effect of galectin-1.
Galectin-1 consists of an ∼135-amino acid canonical galectin carbohydrate recognition domain (CRD),2 which exists as a mixture of monomers and dimers at physiological concentrations (7, 8). Similar to other galectins, its main known biochemical function is to bind β-galactoside-containing glycoproteins and cross-link them, which may result in various cellular signals and consequent effects in immunity, inflammation, and cancer (5, 9). Therefore we analyzed the effect of anginex on the carbohydrate binding activity of galectin-1, with the hypothesis that it would act as an inhibitor. Surprisingly, we instead found that anginex may strongly enhance binding of galectin-1 to certain glycoconjugates.
EXPERIMENTAL PROCEDURES
Galectins, Glycoproteins, and Chemicals
Plasmids encoding human galectin-1 (gift from Professor Richard Cummings, Department of Biochemistry, Emory School of Medicine, Atlanta, GA) and human galectin-1 C3S (gift from Dr. Jun Hirabayashi, Research Center for Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan) were expressed in Escherichia coli, and galectins were purified by affinity chromatography on lactosyl-Sepharose and characterized as described before (8). The other galectins were produced and purified in an analogous way as described (10, 11). The oxidation-resistant mutant, galectin-1 C3S (galectin-1), was used in most experiments to avoid having to include β-mercaptoethanol, which may perturb interacting glycoprotein ligands, and was shown to have the same specificity and affinity as wild type galectin-1 for a wide range of saccharides, probes, and glycoproteins (8, 12). Anginex and an inactive control peptide (supplemental Fig. S1) (1, 13) and fluorescein-tagged saccharide probes (supplemental Fig. S2) (8, 14) were as described before. Asialofetuin (ASF), fetuin, haptoglobin, and bovine serum albumin (BSA) were from Sigma. All dilutions were prepared in 10% PBS buffer (12 mm NaCl, 7 mm Na/K-phosphate, pH 7.2) because anginex stock solutions precipitate in high salt buffers; there was no evidence for any difference in affinity and specificity of galectin-1 for small saccharides and probes between PBS and 10% PBS buffer. High concentration of galectin-1 did not cause aggregation of ASF in 10% PBS buffer as it does in PBS, but the affinities were only marginally lower in 10% PBS buffer (Kd ∼5 μm as compared with ∼3 μm for ASF and 50 μm as compared with 40 μm for fetuin).
Fluorescence Anisotropy
Affinities of probes and inhibitors for galectin-1 were measured with fluorescence anisotropy (FA) and calculated as described (8). Details are given in the figure legends. All graphs were generated using Prism 4 (GraphPad Software Inc.).
RESULTS AND DISCUSSION
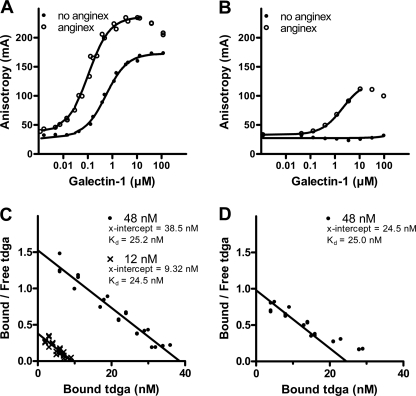
The interaction between anginex and galectin-1 was investigated using an FA assay (8, 15). In this assay, the interactions of galectin-1 with fluorescein-tagged saccharide probes were studied, as well as the perturbing effects of potential inhibitors/modifiers. With a fixed concentration of a newly designed high affinity tdga-probe (8) (supplemental Fig. S2A) and increasing concentrations of galectin-1, the anisotropy rose from a value of ∼30 mA (A0 = anisotropy of the free probe) to ∼170 mA (Amax = anisotropy of the galectin-probe complex) following a curve of shape consistent with a 1:1 interaction and position along x axis consistent with a Kd ∼0.4 μm. With the same probe and galectin concentrations in the presence of 8 μm anginex (Fig. 1A), the curve shifted to the left, indicative of higher binding affinity (Kd ∼0.02 μm), and Amax became higher (∼235 mA), indicative of more restricted mobility of the fluorescein tag in the galectin-probe complex. With the LNT-probe (supplemental Fig. S2B), there was no rise in the anisotropy with increasing galectin-1 concentrations, due in part to lower affinity (Kd ∼100 μm) but also higher segmental mobility of the fluorescein tag even in the galectin-probe complex, probably because the galectin binds the terminal disaccharide (as indicated in supplemental Fig. S2B and discussed in detail for other galectins (10, 14)). In the presence of anginex, however, the LNT-probe also gave a clear rise in anisotropy (to ∼120 mA) and a curve consistent with a Kd ∼5 μm (Fig. 1B). With both probes, in the presence of anginex, the anisotropy values started to drop when the galectin concentration started to exceed the anginex concentration, most likely due to competition by anginex-free galectin for the probe.
FIGURE 1.
Effect of anginex on the interaction of galectin-1 with fluorescein-labeled saccharide probes. A and B, a range of different concentrations of galectin-1 was added to a microtiter plate together with a fixed concentration (0.1 μm) of tdga-probe (A) or LNT-probe (B) in 8 μm anginex or 10% PBS buffer, and fluorescence anisotropy was measured using a PolarStar plate reader. Binding curves were generated by plotting anisotropy against increasing concentrations of galectin-1. C and D, fixed low concentrations of galectin-1 (12 or 48 nm) were mixed with a range of concentrations of tdga-probe in the presence of 8 μm (C) or 4 μm (D) anginex. Fractions of bound probe were calculated ((A − A0)/(Amax − A0)), and from this, fractions of free probe (total − bound) were calculated. Finally, bound tdga-probe was plotted against bound/free tdga-probe to construct Scatchard plots. In D, linear regression was made with a fixed Kd = 25 nm, as found for 8 μm anginex. Graphs were made using Prism 4 (GraphPad Software Inc.). The structures of the probes are shown in supplemental Fig. S2.
For a more detailed assessment of the affinity of the tdga-probe for the galectin-1-anginex complex, fixed low concentrations of galectin-1 (12 and 48 nm, not causing significant probe binding by themselves) were mixed with 8 μm anginex and analyzed with a range of different concentrations of the probe. From the anisotropy data, the concentrations of free and bound probe were calculated and used to construct Scatchard plots. These showed that the tdga-probe interacted with the galectin-1-anginex complex with Kd ∼25 nm (Fig. 1C), which is ∼16-fold higher affinity than for free galectin-1. The estimated concentration of these high affinity sites (from the x-intercept) was ∼39 nm with 48 nm galectin-1 and 9.3 nm with 12 nm galectin-1; that is, about 80% of the available galectin would be in complex with anginex. With 4 μm anginex, the Scatchard plot was consistent with ∼25 nm high affinity sites in 48 nm galectin-1 (∼50% of the added galectin), and in addition, lower affinity sites were detected at the highest probe concentrations, probably due to free galectin-1. These data are consistent with the affinity of anginex for immobilized galectin-1 in surface plasmon resonance (3).
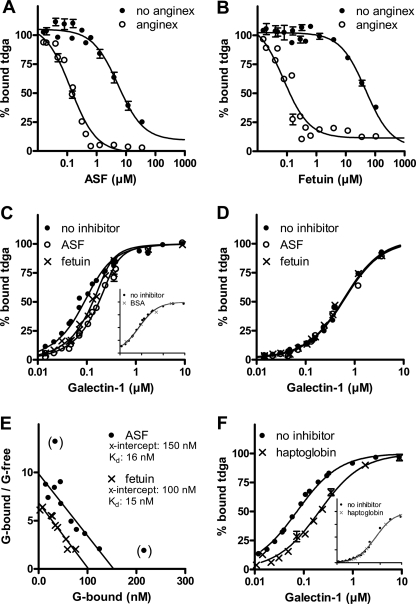
To analyze the effect of anginex on non-labeled ligands, we added these as inhibitors of the galectin-1-probe interaction as described previously (8). In the first type of assay, a range of concentrations of the glycoproteins ASF and fetuin was added to fixed low concentrations of galectin-1 (0.25 μm) and tdga-probe (0.1 μm) in the presence or absence of 8 μm anginex, anisotropy was measured, and the percentages of bound probe and Kd values were calculated. In the absence of anginex, this produced Kd values (assuming one binding site) of ∼5 μm for ASF and ∼50 μm for fetuin (Fig. 2, A and B), in agreement with the previous results (Table 2 and supplemental Table S3 in Ref. 8). In the presence of 8 μm anginex, there was a dramatic shift of the inhibition curves to the left, indicative of much lower Kd values, ∼10 nm for ASF and 20 nm for fetuin, an increase in inhibitory potency of 500 and 2500 times, respectively.
FIGURE 2.
Effect of anginex on galectin-1 interaction with glycoproteins ASF, fetuin, and haptoglobin. A and B, increasing concentrations of ASF (A) or fetuin (B) were mixed with 0.1 μm tdga-probe and 0.25 μm galectin-1 in the presence or absence of 8 μm anginex, and anisotropy was measured. The percentage of bound probe was calculated as 100 × (A − A0)/(Ano inhibitor − A0) and plotted against inhibitor concentration. The inhibition curve goes down to 0% bound probe for ASF because after the anginex-galectin-1 complex has been saturated, the ASF concentration (low μm) is also enough to inhibit remaining free galectin-1 (A). However, this is not the case for fetuin, where a residual of about 10–20% bound probe is observed even at higher fetuin concentrations, which may be due to the fact that fetuin is a poor inhibitor for free galectin-1. C and D, increasing concentrations of galectin-1 were added to a fixed concentration of tdga-probe (0.1 μm) and 0.1 μm ASF or fetuin in the presence of 8 μm anginex (C) or without anginex (D). The percentage of bound tdga-probe was calculated and plotted against increasing concentration of galectin-1. BSA (C, inset) was run as a non-binding control. E, the percentage of bound probe was used to estimate the concentration of free galectin-1 in the presence of glycoprotein using the values in its absence as a standard curve, and then the concentration of glycoprotein-bound galectin-1 was calculated (total − free − probe-bound), and Scatchard plots were constructed as described previously (Fig. 6 in Ref. 8). Data points in parenthesis were not included in the linear regression calculation. F, same as in C, but with 0.1 μm haptoglobin as inhibitor and without anginex (as in D) as inset.
To determine how many of the nine potential galectin-1 binding sites on ASF (16) become occupied, we used a second type of assay, which previously identified one high affinity galectin-1 binding site (Kd ∼3 μm) using the tdga-probe and another eight lower affinity sites using another low affinity probe (8). In this assay type, a range of galectin concentrations was mixed with fixed concentrations of tdga-probe (0.1 μm), glycoprotein (0 or 0.1 μm), and anginex (0 or 8 μm), the percentage of bound probe was calculated from the fluorescence anisotropy (Fig. 2C), and the concentrations of free and glycoprotein-bound galectin-1 were calculated to construct Scatchard plots (Fig. 2E). Inhibition by the glycoprotein is seen as a shift of the binding curve to the right (Fig. 2C). Without anginex, there was no inhibition (Fig. 2D) because the glycoprotein concentration used here (0.1 μm) was much lower as compared with that used previously (20–40 μm) (8). However, in 8 μm anginex, there was a clear shift of the curves for both glycoproteins (Fig. 2C). BSA, which does not bind galectin-1, did not give a curve shift even in the presence of anginex (Fig. 2C, inset), which validates the assay. The Scatchard plots indicated about 1.5 binding sites per molecule for ASF and about 1 for fetuin, similar to that found with the tdga-probe in the absence of anginex (8), but with several hundred- to thousand-fold higher affinities (Kd ∼15 nm). We also analyzed the interaction of galectin-1 with haptoglobin, a naturally occurring human serum glycoprotein (Fig. 2F). A clear shift of the binding curve was found in the presence of anginex but not in its absence (inset), suggesting enhanced affinity of galectin-1 also for this glycoprotein in the presence of anginex. In contrast, the inhibitory potencies of small saccharides, Galβ1-4Glcβ-O-Me and Galβ1-4GlcNAcβ-O-Me, known to bind galectin-1 (8), were not enhanced in the presence of anginex (not shown).
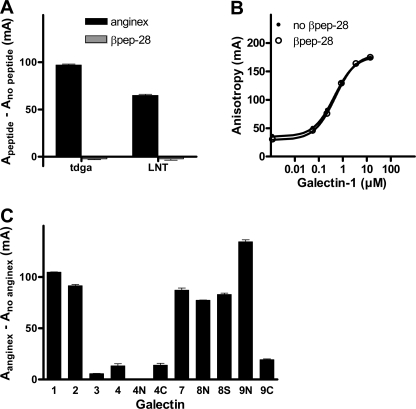
Anginex was selected based on its anti-angiogenic activity from a panel of ∼30 different designed peptides with small variations in sequence (1). From this panel of peptides, βpep-28 (supplemental Fig. S1) has been shown to have a similar structure as anginex (13) but to be biologically inactive, and hence, it has been used as a negative control (1, 2). In the FA assay, this peptide also had no effect either when it was analyzed with galectin-1 and the two probes, at concentrations where the addition of anginex would result in the maximal increase in fluorescence anisotropy (Fig. 3A), or when screened with a range of galectin-1 concentrations (Fig. 3B), as done with anginex in Fig. 1A. This shows that the interaction of anginex with galectin-1 in the FA assay is specific and correlates with its biological activity. The concentrations (low μm) of anginex required for the effects in the FA assay described above are also in the same range as those required for its biological effects (1).
FIGURE 3.
Effect of a control peptide, βpep-28, and the effect of anginex on other galectins. A, 0.1 μm tdga-probe or LNT-probe was mixed with 0.1 or 3 μm galectin-1, respectively, in the presence of 8 μm anginex, 8 μm βpep-28, or no peptide, and anisotropy was measured. Free probes (A0) were run in parallel. Increased anisotropy (Apeptide − Ano peptide) (y axis) was calculated with Apeptide = A − A0 in anginex or βpep-28 and Ano peptide = A − A0 in the absence of peptide. The amino acid sequences of anginex and βpep-28 are shown in supplemental Fig. S1. B, a range of different concentrations of galectin-1 was analyzed with a fixed concentration (0.1 μm) of tdga-probe in the same way as in Fig. 1A but in the presence or absence of 8 μm βpep-28 instead of anginex. C, different combinations of probe (0.1 μm) and galectin (tdga-probe: 0.5 μm galectin-1, 10 μm galectin-2, 3 μm galectin-4N, 2 μm galectin-9C; A-tetra-saccharide-probe (14): 1 μm galectin-3, 0.5 μm galectin-4, 0.5 μm galectin-4C; LNnT-probe (14): 2 μm galectin-7; LNnT-probe (23): 0.4 μm galectin-8N, 0.4 μm galectin-8S, and 1 μm galectin-9N) were analyzed in the presence or absence of 16 μm anginex. The different probe-galectin combinations and concentrations were chosen to ensure binding in the absence of anginex. 16 μm anginex was used instead of 8 μm to exceed the highest galectin concentration (10 μm for galectin-2). Free probes were measured in parallel, and increased anisotropy (Aanginex − Ano anginex) (y axis) was calculated with Aanginex = A − A0 in anginex and Ano anginex = A − A0 without anginex. Error bars in A and C indicate S.E.
We finally assessed the effect of anginex on other members of the galectin family. Anginex was added to different galectins, at fixed concentrations with different probes selected based on our extensive use of the FA assay, to test galectin inhibitors (11). Galectin-2 and -7 and the N-terminal CRDs of galectin-8 and -9, but not galectin-3 and -4 and the C-terminal CRD of galectin-9, showed an increased anisotropy, indicating interaction with anginex (Fig. 3C).
In summary, we have found that the synthetic anti-angiogenic peptide anginex dramatically enhances the affinity of galectin-1 for certain ligands, including biologically relevant glycoproteins. In fact, this apparently monovalent interaction has among the highest affinities (Kd low nm) ever reported for a lectin. To elucidate the precise mechanism of this and its consequences in cellular systems will require further study.
Because anginex tends to form dimers and larger aggregates (3, 13), the mechanism for its effect may entail increased cross-linking of galectin-1. However, more specific conformational modification of a single CRD is also possible.
One possible general explanation for the biological effects of anginex may be due to shifts of galectin-1 receptor binding equilibria. Many of the proposed regulatory effects of galectin-1 appear to involve reversible binding to receptors with affinities in the low μm range (17–19), perhaps with reversible formation and dissolution of cross-linked lattices (4, 9, 20, 21). If affinity for certain ligands were increased hundred- to thousand-fold, as seen here, such equilibria would be dramatically altered.
The anginex effect on galectin-1 may also mimic a normal function; it is hard to believe that an artificial peptide can show such dramatic effects without speculating that there is a natural counterpart in vivo. This also becomes an intriguing thought because galectin-1 in some cases showed biological effects at nm concentrations (e.g. Fig. 3 in Ref. 6) or surprisingly little was needed to rescue the null phenotype in mice in vivo (22) despite the fact that, as mentioned above, most known ligands have affinities in the μm range. There are many text book cases where strong binding activity of one protein is induced by another protein or smaller molecule, but it has not been found before for the carbohydrate binding activity of galectins, or perhaps not even lectins in general (except for cations). The fluorescence anisotropy assay used here provides a simple screening method to find natural modifiers of galectin ligand binding and also to select designed peptides with similar effects, for example, in the search for new improved versions of anginex as anti-cancer agents.
Acknowledgments
We thank Professor Kevin Mayo, Department of Biochemistry, University of Minnesota, Minneapolis, MN for providing the peptides anginex and βpep-28 and Barbro Kahl-Knutson for excellent technical help and valuable comments.
This work was supported by grants from the Lund University Research School in Pharmaceutical Sciences, the Swedish Research Council Grants 2009-5656 (to H. L.) and 2009-5326 (to U. J. N.), and Dutch Cancer Society Grants UM2008-4101 and VU2009-4358 (to V. L. T. and A. W. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- CRD
- carbohydrate recognition domain
- FA
- fluorescence anisotropy
- tdga
- thiodigalactoside amide
- LNT
- lacto-N-tetraose (Galβ1-3GlcNAcβ1-3Galβ1-4Glc)
- ASF
- asialofetuin
- LNnT
- lacto-N-neotetraose (Galβ1-4GlcNAcβ1-3Galβ1-4Glc).
REFERENCES
- 1. Griffioen A. W., van der Schaft D. W., Barendsz-Janson A. F., Cox A., Struijker Boudier H. A., Hillen H. F., Mayo K. H. (2001) Biochem. J. 354, 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Schaft D. W., Dings R. P., de Lussanet Q. G., van Eijk L. I., Nap A. W., Beets-Tan R. G., Bouma-Ter Steege J. C., Wagstaff J., Mayo K. H., Griffioen A. W. (2002) FASEB J. 16, 1991–1993 [DOI] [PubMed] [Google Scholar]
- 3. Thijssen V. L., Postel R., Brandwijk R. J., Dings R. P., Nesmelova I., Satijn S., Verhofstad N., Nakabeppu Y., Baum L. G., Bakkers J., Mayo K. H., Poirier F., Griffioen A. W. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15975–15980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thijssen V. L., Poirier F., Baum L. G., Griffioen A. W. (2007) Blood 110, 2819–2827 [DOI] [PubMed] [Google Scholar]
- 5. Le Mercier M., Fortin S., Mathieu V., Kiss R., Lefranc F. (2010) Brain Pathol. 20, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thijssen V. L., Barkan B., Shoji H., Aries I. M., Mathieu V., Deltour L., Hackeng T. M., Kiss R., Kloog Y., Poirier F., Griffioen A. W. (2010) Cancer Res. 70, 6216–6224 [DOI] [PubMed] [Google Scholar]
- 7. Leffler H., Carlsson S., Hedlund M., Qian Y., Poirier F. (2004) Glycoconj. J. 19, 433–440 [DOI] [PubMed] [Google Scholar]
- 8. Salomonsson E., Larumbe A., Tejler J., Tullberg E., Rydberg H., Sundin A., Khabut A., Frejd T., Lobsanov Y. D., Rini J. M., Nilsson U. J., Leffler H. (2010) Biochemistry 49, 9518–9532 [DOI] [PubMed] [Google Scholar]
- 9. Rabinovich G. A., Toscano M. A. (2009) Nat. Rev. Immunol. 9, 338–352 [DOI] [PubMed] [Google Scholar]
- 10. Salomonsson E., Carlsson M. C., Osla V., Hendus-Altenburger R., Kahl-Knutson B., Oberg C. T., Sundin A., Nilsson R., Nordberg-Karlsson E., Nilsson U. J., Karlsson A., Rini J. M., Leffler H. (2010) J. Biol. Chem. 285, 35079–35091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cumpstey I., Salomonsson E., Sundin A., Leffler H., Nilsson U. J. (2008) Chemistry 14, 4233–4245 [DOI] [PubMed] [Google Scholar]
- 12. Nishi N., Abe A., Iwaki J., Yoshida H., Itoh A., Shoji H., Kamitori S., Hirabayashi J., Nakamura T. (2008) Glycobiology 18, 1065–1073 [DOI] [PubMed] [Google Scholar]
- 13. Arroyo M. M., Mayo K. H. (2007) Biochim. Biophys. Acta 1774, 645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlsson S., Oberg C. T., Carlsson M. C., Sundin A., Nilsson U. J., Smith D., Cummings R. D., Almkvist J., Karlsson A., Leffler H. (2007) Glycobiology 17, 663–676 [DOI] [PubMed] [Google Scholar]
- 15. Sörme P., Kahl-Knutsson B., Huflejt M., Nilsson U. J., Leffler H. (2004) Anal. Biochem. 334, 36–47 [DOI] [PubMed] [Google Scholar]
- 16. Dam T. K., Gabius H. J., André S., Kaltner H., Lensch M., Brewer C. F. (2005) Biochemistry 44, 12564–12571 [DOI] [PubMed] [Google Scholar]
- 17. Cederfur C., Salomonsson E., Nilsson J., Halim A., Oberg C. T., Larson G., Nilsson U. J., Leffler H. (2008) Glycobiology 18, 384–394 [DOI] [PubMed] [Google Scholar]
- 18. Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W. E., Yagi F., Kasai K. (2002) Biochim. Biophys. Acta 1572, 232–254 [DOI] [PubMed] [Google Scholar]
- 19. Leppänen A., Stowell S., Blixt O., Cummings R. D. (2005) J. Biol. Chem. 280, 5549–5562 [DOI] [PubMed] [Google Scholar]
- 20. Brewer C. F., Miceli M. C., Baum L. G. (2002) Curr. Opin. Struct. Biol. 12, 616–623 [DOI] [PubMed] [Google Scholar]
- 21. Garner O. B., Baum L. G. (2008) Biochem. Soc. Trans. 36, 1472–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blois S. M., Ilarregui J. M., Tometten M., Garcia M., Orsal A. S., Cordo-Russo R., Toscano M. A., Bianco G. A., Kobelt P., Handjiski B., Tirado I., Markert U. R., Klapp B. F., Poirier F., Szekeres-Bartho J., Rabinovich G. A., Arck P. C. (2007) Nat. Med. 13, 1450–1457 [DOI] [PubMed] [Google Scholar]
- 23. Oberg C. T., Carlsson S., Fillion E., Leffler H., Nilsson U. J. (2003) Bioconjug. Chem. 14, 1289–1297 [DOI] [PubMed] [Google Scholar]