Abstract
Atherosclerosis is an inflammatory disease characterized by the accumulation of macrophages in the arterial intima. The activated macrophages secreted more pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, which promote the development of the disease. Apolipoprotein A-I (apoA-I), the major component of high density lipoprotein, is involved in reverse cholesterol transport of lipid metabolism. Recently, it has been found that apoA-I suppresses inflammation via repression of inflammatory cytokine expression; the mechanisms of the apoA-I-suppressive action, however, are not yet well characterized. In this study, we have for the first time found that apoA-I suppresses the expression of some inflammatory cytokines induced by lipopolysaccharide via a specific post-transcriptional regulation process, namely mRNA destabilization, in macrophages. Our further studies have also shown that AU-rich elements in the 3′-untranslated region of TNF-α mRNA are responsive to the apoA-I-mediated mRNA destabilization. The apoA-I-induced inflammatory cytokine mRNA destabilization was associated with increased expression of mRNA-destabilizing protein tristetraprolin through a JAK2/STAT3 signaling pathway-dependent manner. When blocking interaction of apoA-I with ATP-binding membrane cassette transporter A1 (ABCA1), a major receptor for apoA-I in macrophages, it would almost totally abolish the effect of apoA-I on tristetraprolin expression. These results present not only a novel mechanism for the apoA-I-mediated inflammation suppression in macrophages but also provide new insights for developing strategies for modulating vascular inflammation and atherosclerosis.
Keywords: Apolipoprotein Genes, Atherosclerosis, Cholesterol Metabolism, Inflammation, STAT Transcription Factor, ATP-binding Cassette Transporter A1, Apolipoprotein A-I, Inflammation, Reverse Cholesterol Transport
Introduction
It is well known that inflammation plays a key role in the development of atherosclerosis (1). Inflammatory cells, mainly macrophages and T-lymphocytes, produce a wide range of inflammatory cytokines in atherosclerotic lesions, which are critically important in the progress of the disease (2). Human population studies have shown that plasma levels of high density lipoproteins (HDL) are inversely associated with risk for cardiovascular disease (3, 4). One of the most investigated mechanisms of HDL to prevent atherosclerosis is the reverse cholesterol transport process, in which accumulated cholesterol is transported by HDL from the vessel wall to the liver for excretion (5). Recently, HDL has been described to have various anti-inflammatory properties (6), which may provide new insight into the prevention of atherosclerosis (7, 8). The exact mechanisms for reducing inflammation by HDL, which usually presents as multifunctional lipoprotein complexes, however, are not fully understood.
Apolipoprotein A-I (apoA-I), the major component of the HDL that promotes cellular cholesterol efflux mainly via a cell membrane protein called ATP-binding membrane cassette transporter A1 (ABCA1) (9), is widely considered as the major underlying factor with anti-inflammatory function of HDL (10). It has been reported that apoA-I, but not other components of the HDL, inhibited expression of the integrin CD11b on the monocyte surface (11). In addition, apoA-I can inhibit the production of inflammatory cytokines by blocking the contact-mediated activation of monocytes by T-lymphocytes (12). Moreover, apoA-I knock-out mice exhibit increased inflammatory activity as enhanced macrophage infiltration and inflammatory cytokine productions in many tissues (13). The molecular pathways underlying inhibition of inflammatory response by apoA-I, however, remain unclear. At present, it is clear that one of the anti-inflammation mechanisms of apoA-I is due to its effect on exporting lipids from cells, which modifies plasma membrane lipid rafts and impairs LPS signaling (14, 15). However, homeostatic traffic of cholesterol from the plasma membrane to extracellular acceptors, such as apoA-I in particular, can itself regulate cellular signaling by determining the selective localization of signaling proteins to plasma membrane microdomains (14, 16). We have previously reported that apoA-I or its mimetic peptides dramatically increased the activation of Rho GTPase CDC42, as well as its downstream kinases via an ABCA1-dependent manner (17, 18). Recently, two candidate signal transducer and activator of transcription 3 (STAT3)-docking sites in ABCA1 have been found to contribute to the function of apoA-I in regulation of macrophage inflammation (19). These reports suggest that the anti-inflammatory effect of apoA-I is not limited to its lipid transport function but is also related to its direct role in stimulating the intercellular signaling pathways.
In this study, we have found that apoA-I strongly inhibits some LPS-induced inflammatory cytokine production in macrophages mainly due to the alteration of the rate of mRNA decay. Furthermore, the regulation of cytokine mRNA destabilization by apoA-I depends on the up-regulation of tristetraprolin (TTP),2 which has been described to destabilize mRNAs of several inflammatory cytokines containing class II AU-rich elements (AREs) in the 3′-untranslated regions (UTR) (20). The apoA-I-mediated increasing expression of TTP is inhibited by treatment with STAT3 siRNA and AG-490, a JAK2 inhibitor, suggesting a JAK2/STAT3-dependent pathway for up-regulation of TTP induced by apoA-I. Although HDL acts through various receptors to decrease monocyte activation (21, 22), our studies have shown that ABCA1 is the major contributing receptor for apoA-I in increasing the expression of TTP.
EXPERIMENTAL PROCEDURES
Cells
Human THP-1 cells were cultured as described previously (9, 23). After 3–4 days, cells were treated with phorbol 12-myristate 13-acetate (160 nmol/liter) for 12 h, and then the medium was replaced by serum-free medium containing oxLDL (50 μg/ml) for 48 h to become fully differentiated macrophage foam cells before their use in experiments. Human monocytes from healthy control subjects were isolated by using the OptiPrep procedure (Axis Shield, Norway). The blood was collected and mixed with OptiPrepTM in a ratio of 8:1, and the mixture covered with 0.5 ml of TBS was centrifuged at 1500 × g for 30 min at room temperature. There were three fractions after separation, and the upper fraction contained the monocytes (at the top of plasma, >90% monocyte purity). Monocytes were allowed to adhere to the flask in RPMI 1640 medium and differentiated into macrophages by culturing them for 7 days in the presence of macrophage colony-stimulating factor as described previously (24). Cells were loaded with oxLDL (50 μg/ml) overnight and stimulated with LPS (10 ng/ml) and/or apoA-I (10 μg/ml) unless otherwise indicated.
Antibodies and Reagents
STAT1, phospho-STAT1, STAT6, phospho-STAT6, TTP, HuR, TNF-α, and COX-2 antibodies and histone H1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for phosphorylation and total STAT3 and phosphor-JAK2 were purchased from Cell Signaling Technology. ABCA1 antibody was purchased from Abcam. The JAK2-specific inhibitor AG490, phosphatidylcholine, and cholesterol were purchased from Sigma. HDL was isolated from the plasma of healthy human donors by sequential ultracentrifugation within the density ranges ρ = 1.063–1.250 g/ml (25). Recombinant human apoA-I and apoB were obtained from Protein Specialists (Prospec, Israel).
Cytokine ELISA
Cells were plated in 6-well plates and treated as described above. Culture supernatants were collected and stored at −20 °C until analysis. The concentrations of IL-1β, IL-6, IFNγ, and MCP-1 in supernatants were measured by enzyme-linked immunosorbent assay (ELISA) (DuoSet ELISA Development System, R&D Systems, Abingdon, UK) following the manufacturer's instructions. The cytokine standards were used to generate standard curves. Quantitative determinations in three different experiments were performed.
RNA Isolation and Real Time PCR Analysis
Total RNA from cells was extracted by using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Relative quantitative real time PCR (RT-PCR), using SYBR Green detection chemistry, was performed on the Mx3000 Multiplex quantitative PCR system (Stratagene, La Jolla, CA). Melt curve analyses of all real time PCR products were performed and shown to produce a single DNA duplex. Quantitative measurements were determined using the ΔΔCt method, and expression of β-actin was used as the internal control.
Nuclear Run-on Assay
Nuclear run-on reactions were performed as described previously (26). Briefly, nuclei were prepared using nuclei isolation kits (Axis Shield, Norway) and were resuspended in 100 μl of glycerol buffer (40% glycerol, 50 mm Tris-HCl, pH 8.8, 5 mm MgCl2, 0.1 mm EDTA). Reaction buffer containing 5 mm Tris-HCl, 300 mm KCl, 5 mm MgCl2, 5 mm DTT, and 1 mm each of ATP, CTP, and GTP, 100 units/μl RNase inhibitor (Sigma), and 250 μCi of 32P-labeled UTP (R&D Systems, Minneapolis, MN) were added to the nuclei for 30 min at room temperature. RNA was isolated using TRIzol reagent and ammonium acetate precipitation. Equal amounts of labeled RNA were hybridized to target DNA immobilized on nylon membranes. After overnight hybridization at 65 °C, membranes were washed twice with 2× saline sodium citrate (SSC) containing 1% SDS at 65 °C and once in 0.1× SSC at room temperature. Filters were exposed to x-ray film, and spots were quantitated by densitometry. Results were normalized to the β-actin.
Construction of a TNF-α Promoter and 3′-UTR Chloramphenicol Acetyltransferase Reporter Vector
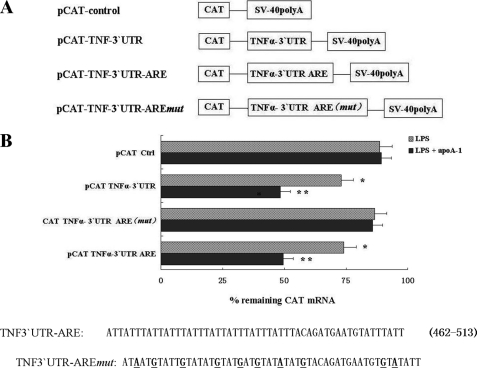
The TNF-α promoter reporter was constructed using the chloramphenicol acetyltransferase (CAT) reporter pCAT3 (Promega) as described previously with modifications (27). Briefly, a TNF-α promoter fragment (−993 to +110) was amplified from THP-1 genomic DNA by PCR, and two restriction enzyme sites (KpnI at the 5′ end and BglII at the 3′ end) were generated by incorporating them into their respective primers. The sense primer was GGGGTACCCCCATGTGAGATATGGCCACAT; the antisense primer was GAAGATCTTCACCGTCGAACAGTCCCCTA. The promoter fragment was cloned into the KpnI and BglII restriction sites located upstream of the CAT gene in the pCAT3-basic plasmid (Promega) to create the pCAT-TNF-α-promoter reporter gene construct. The CAT and TNF-α 3′-UTR chimeric constructs were generated as described before (28, 29). Briefly, pCAT-TNF-α 3′-UTR and pCAT-TNF-α 3′-UTR ARE were generated by cloning a DNA fragment corresponding to TNF-α 3′-UTR and 3′-UTR ARE (nt 441–520 of TNF 3′-UTR) that were subcloned into pCAT3 vector (Promega) at the XbaI site immediately downstream of CAT coding region. Another construct that contains similar TNF-α 3′-UTR ARE sequences with the mutation in clustered AU pentamers between nt 461 and 95 was generated by PCR-based mutagenesis (Fig. 2B). The nucleotide sequences for cloned fragments were determined at Biology Engineering Corp. (Shanghai, China).
FIGURE 2.
TNFα 3′-UTR AREs confer mRNA instability to apoA-I on CAT mRNA. A, schematic representation of the TNF-α/CAT chimeric constructs detailed under “Experimental Procedures.” B, THP-1 macrophages transiently transfected with TNF-α/CAT chimeric vectors were stimulated by LPS for 3 h with or without pretreatment of apoA-I and then treated with act D. Cultures were harvested immediately (0 time point) or at 2 h after treatment with act D. Total cellular RNA was prepared, and levels of CAT mRNA were assayed by RT-PCR. Remaining CAT mRNA at 2 h (% of 0 time point) after act D treatment from pCAT-TNFα 3′-UTR transfected cells was compared with that of pCAT-Ctrl transfected cells. *, p < 0.05 compared with pCAT-Ctrl group; **, p < 0.01 compared with pCAT-Ctrl group. All of the data represent the means ± S.E. from three separate experiments with triplicate samples. Nucleotide sequence of the ARE between nt 462 and 513 within the 3′ UTR of the human TNF-α gene is shown. Substitution mutations in TNFα 3′-UTR AREmut (disruption of reiterated cluster of ARE pentamers) are underlined.
Transfection and CAT Assay
THP-1 macrophages were transfected as described previously with modifications (30, 31). Briefly, 2 × 107 THP-1 cells, grown in RPMI 1640 medium supplemented with 10% FCS, were transiently transfected with 3 μg of TNF-α/CAT chimeric plasmid and 0.5 μg of a pCMV-β-galactosidase vector (Promega) as internal control using Effectene transfection reagent (Qiagen, Germany) at a DNA/Effectene ratio of 1:10 according to the manufacturer's instructions. During transfection, cell viability was confirmed by trypan blue staining. Following transfection, the cells were placed into the 24-well plate (2 × 105 cells/well) for 4 h, treated with phorbol 12-myristate 13-acetate (160 nmol/liter) for 12 h, and then incubated with oxLDL (50 μg/ml) in medium containing 1% FCS for 24 h. CAT activity was measured by using a CAT ELISA kit (Roche Diagnostics) according to the manufacturer's instruction. β-Galactosidase activity was measured using β-galactosidase assay kit (Beyotime Institute of Biotechnology, Shanghai, China). The relative CAT activity was determined as a ratio of CAT/β-gal in three independent experiments.
Transfection of siRNA
The siRNA against STAT3, TTP, and an irrelevant nonfunctional 21-nucleotide siRNA duplex, which was used as a control, was purchased from Biology Engineering Corp., Shanghai, China. The respective sequences are as follows: STAT3 sense, 5′-AACUUCAGACCCGUCAACAAA-3′, and antisense, 5′-AAAGUCAGGUUGCUGGUCAAA-3′; TTP sense, 5′-UCGCCACCCCAAGUACAAAtt-3′, and antisense, 5′-CUCUGCCACAAGUUCUACCtt-3′. ABCA1 siRNA was purchased from Santa Cruz Biotechnology (sc-41136), which consisted of three target-specific 20–25-nt siRNAs designed to knock down ABCA1 gene expression. Scrambled siRNA sense, 5′-UGUGGAUGACUGAGUACCUGA-3′, and antisense, 5′-UCAGGUACUCAGUCAUCCACA-3′, were used. THP-1 macrophage-derived foam cells (2 × 106 cells/well) were incubated in 6-well plates, washed with serum-free DMEM, and then transfected with siRNA using Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen). 48 h after transfection, real time RT-PCR was performed.
mRNA Decay Assay
LPS-stimulated THP-1 macrophage-derived foam cells and human peripheral macrophages were treated with apoA-I for 3 h and then exposed to actinomycin D (act D) to inhibit transcription, and total RNA was harvested after 0, 30, 60, and 120 min. Inflammatory cytokines mRNA levels at each time point were quantified using RT-PCR and were normalized against the β-actin. Remnant inflammatory cytokine mRNAs in the percentage of the amount at time point 0 of act D treatment were depicted.
RNA Electrophoretic Mobility Shifts Assays (EMSA)
Cytoplasmic extracts were prepared from treated cell cultures as described previously (32). 30 μg of protein were incubated with 2 nm biotin-labeled RNA probes in RNA-EMSA buffer containing 10 mm HEPES, 50 mm KCl, 5% glycerol, and 1 mm DTT for 30 min at room temperature. For competition experiments, the cytoplasmic extract was incubated with 100-fold molar excess of unlabeled RNA for 10 min before incubation with the labeled RNA. Samples were subjected to electrophoresis on an 8% native acrylamide gel in 0.5× TBE buffer (45 mm Tris-HCl, 45 mm borate, and 2.5 mm EDTA) and transferred to positive-charge nylon membrane. The signals of RNA-EMSA reaction were detected by LightShift chemiluminescent kit (Pierce). Membranes were exposed to x-ray film for 5 min. RNA-protein complex contents were calculated by densitometry using Labworks analysis software.
Western Blot Analysis
Western blot analyses were performed as described before (23). Proteins (20 μg of extracts) were loaded on 8% SDS-polyacrylamide electrophoresis gel and electrophoresed for 2 h at 100 V in buffer containing 25 mm Tris base, 250 mm glycine, and 0.1% SDS. After electrophoresis, the proteins were electrically transferred to the Immobilon-P transfer membrane in buffer containing 25 mm Tris, 192 mm glycine, 20% methanol, and 0.005% SDS. After transfer, the membrane was blocked in TBST (20 mm Tris base, pH 7.6, 150 mm NaCl, 0.1% Tween 20) containing 5% skimmed milk for 4 h at room temperature. The membrane was then incubated with antibodies against STAT1 or pSTAT1, STAT3 or pSTAT3, STAT6 or pSTAT6, TTP, HuR, TNF-α, COX-2, or ABCA1 in the blocking solution at 4 °C overnight. Thereafter, the membrane was washed three times with TBST for 30 min, incubated with secondary antibody in the blocking solution for 50 min at room temperature, and washed three times with TBST for 30 min. The proteins were visualized using a chemiluminescence method (ECL Plus Western blotting Detection System; Amersham Biosciences).
Statistical Analysis
Data are expressed as means ± S.D. Results were analyzed by one-way analysis of variance and Student's t test, using SPSS 13.0 software. Statistical significance was obtained when p values were less than 0.05.
RESULTS
ApoA-I Suppresses Some Inflammatory Cytokine Expression by Increasing the mRNA Decay
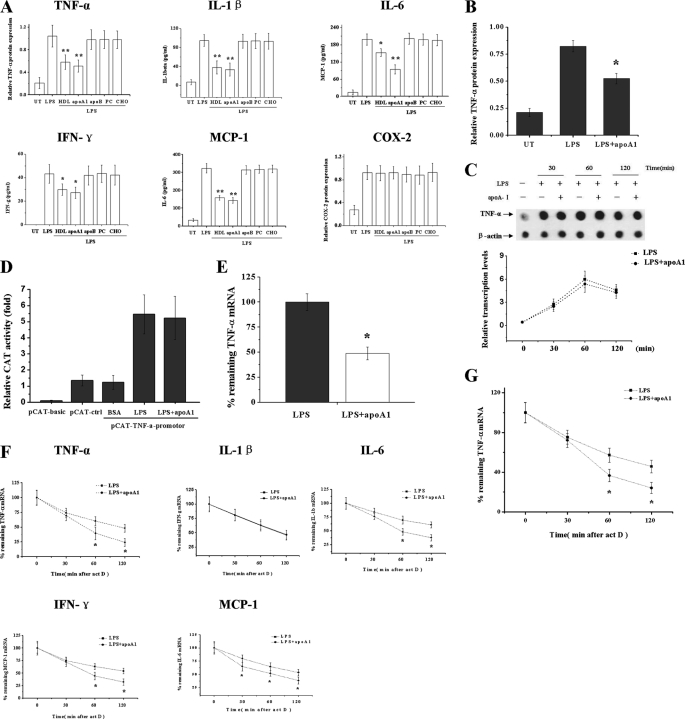
THP-1 macrophage-derived foam cells were stimulated with LPS (10 ng/ml) in the presence or absence of pretreatment of native HDL (30 μg/ml) and HDL constituents, including apoA-I (10 μg/ml), apoB (10 μg/ml), phosphatidylcholine (25 μg/ml), or cholesterol (50 ng/ml) for 6 h. Protein was extracted and subjected to ELISA or Western blot analysis. As shown in Fig. 1A, native HDL and apoA-I significantly suppressed the LPS-induced up-regulation of TNF-α, IL-1β, IL-6, IFNγ, and MCP-1, but it had no obvious effects on COX-2 expression. Other components of the HDL, such as apoB, phosphatidylcholine, and cholesterol, had no obvious effects on the LPS-induced up-regulation of these inflammatory cytokines. In human primary macrophages, apoA-I had inhibited the LPS-induced up-regulation of TNF-α as well (Fig. 1B).
FIGURE 1.
ApoA-I suppresses LPS-induced inflammatory cytokine expression in macrophages through increasing the mRNA decay. A, THP-1 macrophages were treated with LPS for 6 h with or without pretreatment of native HDL, apoA-I, apoB, phosphatidylcholine, and cholesterol for 3 h, and TNF-α and COX-2 levels were determined by Western blots and compared with β-actin. The concentrations of IL-1β, IL-6, IFN-γ, and MCP-1 were measured by ELISA. *, p < 0.05 versus LPS group. **, p < 0.01 versus LPS group. UT, untreated. B, human primary macrophages were treated with LPS for 6 h with or without pretreatment of apoA-I for 3 h, and TNF-α levels were determined by Western blots. *, p < 0.05 versus LPS group. C, THP-1 macrophages were treated with LPS for 30, 60, and 120 min with or without pretreatment of apoA-I. Nuclei were isolated from cells, and run-on reactions were performed. Nuclear RNAs were isolated and hybridized to membranes containing cDNA of TNF-α and β-actin (as a control). D, CAT reporter vector of basic, control, and TNF-α promoter were cotransfected with pCMV vector into THP-1 cells. The CAT and β-galactosidase activity were assayed by ELISA, and the relative CAT activity was determined as a ratio of CAT/β-gal. E, TNF-α mRNA levels were measured by RT-PCR and normalized to the levels of β-actin, *, p < 0.05 versus LPS group. F, THP-1 macrophages were treated with LPS alone or with apoA-I for 3 h followed by addition of act D (5 μg/ml) to stop transcription. After the indicated time points, cytokines mRNA were quantified using RT-PCR and normalized to β-actin expression. Remnant cytokine mRNA in percentage of the amount at the time point 0 of act D treatment was depicted, *, p < 0.05 versus LPS group. G, human primary macrophages were treated with LPS alone or with pretreatment of apoA-I for 3 h, and TNF-α mRNA decay rate was determined by the method mentioned above. All of the data represent the mean ± S.E. from three separate experiments with triplicate samples.
To investigate whether the apoA-I-mediated inhibition of inflammatory cytokines resulted from differences in gene transcription, we analyzed the rate of de novo transcription of TNF-α in nuclear run-on experiments at the indicated time points (30, 60, and 120 min). As shown in Fig. 1C, LPS-induced transcription of TNF-α seemed to be largely unaltered in the presence of apoA-I. We then transfected THP-1 cells with a TNF-α promoter fragment fused upstream to a CAT reporter gene and found that the transcriptional activity of TNF-α promoter was not significantly influenced by apoA-I (Fig. 1D), further suggesting that the inhibition effect of apoA-I on TNF-α expression may be via a post-transcriptional manner.
To test this hypothesis, we first examined the amounts of TNF-α mRNA in LPS-stimulated THP-1 macrophages with or without apoA-I. The RT-PCR results in Fig. 1E showed that apoA-I treatment caused significant reduction of TNF-α mRNA in LPS-stimulated macrophages, suggesting a necessary role of mRNA decay in apoA-I-mediated decrease of TNF-α expression. Therefore, we investigated the effect of apoA-I on the stability of TNF-α as well as other inflammatory cytokine mRNAs induced by LPS. THP-1 macrophages were treated with LPS (10 ng/ml) in the presence or absence of apoA-I (10 μg/ml) for 3 h followed by treatment of act D (5 μg/ml) to stop the transcription. The results of RT-PCR indicated that apoA-I induces a marked increase in TNF-α, IL-6, IL-1β, and MCP-1 mRNA degradation without affecting that of IFNγ (Fig. 1F), suggesting that apoA-I suppresses inflammatory cytokine expression, at least partially, through promoting the mRNA destabilization. In human primary macrophages, apoA-I had also significantly increased the TNF-α mRNA decay (Fig. 1G).
ApoA-I-mediated TNFα mRNA Destabilization Depends upon Its 3′-UTR Sequence
To determine whether apoA-I-induced TNF-α mRNA decay is related to TNF-α 3′-UTR AREs, we investigated the levels of CAT mRNA in the THP-1 macrophages, which transiently transfected the CAT reporter cDNA fused with a series of 3′-UTR sequences of the TNF-α (Fig. 2A). The cells were stimulated with LPS alone or with apoA-I for 3 h, followed by treatment with act D to prevent further transcription. Total RNA was prepared and used to determine the levels of CAT mRNA by RT-PCR. As shown in Fig. 2B, CAT mRNA transcribed from pCAT control (pCAT-Ctrl) was highly stable over the period of the experiment, whereas the intrinsic half-life of CAT mRNA containing the TNF-α 3′-UTRs (pCAT TNFα 3′-UTRs) was significantly reduced after LPS treatment. ApoA-I treatment further destabilized CAT mRNA containing the TNF-α 3′-UTR (pCAT TNFα 3′-UTR) and TNF-α 3′-UTR ARE (pCAT TNFα 3′-UTR ARE), although it did not significantly alter the stability of CAT mRNA containing the TNF-α 3′-UTR ARE mut (pCAT TNFα-3′-UTR AREmut) by the apoA-I stimulation. The half-life of CAT mRNA in construct containing the control nucleotides (pCAT Ctrl) was insensitive to apoA1 treatment, as shown in Fig. 2B.
ApoA-I-stimulated TTP Expression Inhibits TNF-α Production in LPS-induced Macrophages
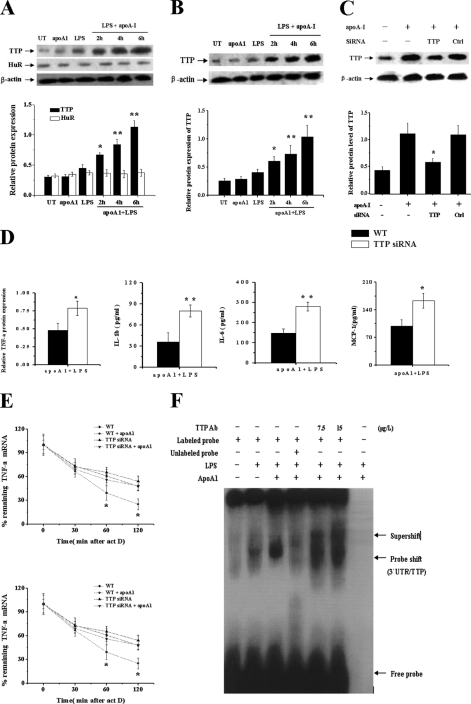
Both TTP and another ARE-binding protein HuR, a member of the elav-like family of RNA-binding proteins, are known to control intrinsic inflammatory gene mRNA decay (33). Therefore, we investigated whether TTP and HuR play roles in apoA-I-mediated inflammatory regulation. As shown in Fig. 3A, apoA-I treatment substantially increased the expression of TTP, but it had no effect on HuR expression in LPS-stimulated THP-1 macrophage-derived foam cells, suggesting that TTP may be involved in the anti-inflammatory functions of apoA-I. Similar results were also obtained in primary macrophages, and apoA-I increased the expression of TTP (Fig. 3B).
FIGURE 3.
ApoA-I-induced TTP expression limits expression of some inflammatory cytokines in LPS-stimulated macrophages. A, THP-1 macrophage-derived foam cells; B, human primary macrophages were treated with LPS or apoA-I for 4 h or with LPS + apoA-I for different times (2, 4, and 6 h). Total protein extracts from cells were subjected to Western blot analyses for TTP and HuR. *, p < 0.05 versus LPS group. **, p < 0.01 versus LPS group. C, THP-1 macrophages were transfected with control (WT) or TTP siRNA for 48 h, and protein samples were immunoblotted with TTP and β-actin antibodies. *, p < 0.05 versus WT cells treated by apoA1. D, THP-1 macrophages transfected with control (WT) or TTP siRNA were treated by LPS alone or with apoA-I for 6 h, and the levels of cytokines were measured. *, p < 0.05 versus WT group; **, p < 0.01 versus WT group. E, THP-1 macrophages transfected with control (WT) or TTP siRNA were treated by LPS alone or with pretreatment of apoA-I for 3 h followed by addition of act D (5 μg/ml) to stop transcription. After the indicated time points, TNF-α and IL-6 mRNA were quantified using RT-PCR. Values were normalized against β-actin. Remnant TNF-α mRNA in percentage of the amount at the time point 0 of act D treatment was depicted. *, p < 0.05 compared with other groups. F, protein extracts (30 μg) of variously treated THP-1 cells were analyzed for in vitro binding of TTP to biotin-labeled RNA probe (3′-UTR AREs of TNF-α, 80 bp, 2 nm) using RNA EMSA. 1st lane, free probe; 2nd lane, LPS; 3rd lane, LPS + apoA-I; 4th lane, LPS + apoA-I + 200× 3′-UTR ARE cold probe; 5th lane, LPS + apoA-I + TTP Ab (7.5 μg/liter); 6th lane, LPS + apoA-I + TTP antibody (15 μg/liter); 7th lane, negative control with protein and TTP antibody. All of the data represent the mean ± S.E. from three separate experiments with triplicate samples.
We further investigated the effect of TTP siRNA on the down-regulation of inflammatory cytokines induced by apoA-I. Treatment with siRNA for TTP significantly down-regulated apoA-I-induced TTP protein expression in LPS-treated THP-1 cells, whereas treatment with control siRNA had no effect (Fig. 3C). We then measured the amount of TNF-α, IL-1β, IL-6, and MCP-1 in LPS-stimulated TTP siRNA knockdown cells that were pretreated with apoA-I for 3 h. After treatment with LPS for 6 h, the expression of TNF-α, IL-1β, IL-6, and MCP-1 in control WT cells was 0.48 (relative value compared with β-actin), 36.2, 148.4, and 98.5 pg/ml respectively, whereas in TTP siRNA knockdown cells, 0.78 (relative value compared with β-actin), 79.9, 279.8, and 163.1 pg/ml, respectively, were observed (Fig. 3D). To investigate whether the incomplete apoA-I-mediated inhibition of cytokine production in TTP siRNA cells resulted from differences in mRNA decay, we analyzed the amounts of TNF-α and IL-6 mRNA in LPS-stimulated TTP siRNA cells with or without apoA-I pretreatment. In LPS-stimulated control WT cells, apoA-I caused a reduction of TNF-α and IL-6 mRNA to 53.1 and 60.3% of the amount present in cells treated with LPS alone for 2 h, whereas in TTP siRNA knockdown cells, apoA-I caused a reduction to 88.9 and 90.3% of the level in cells treated with LPS alone (Fig. 3E).
To directly address the apoA-I effect on TTP binding to TNF-α 3′-UTR-ARE, we performed the RNA-protein complex formation in EMSA experiments. Proteins extracted from variously treated THP-1 cells were incubated with a synthesized biotin-labeled probe consisting of bases 441–520 of TNF 3′-UTR containing AREs. The free probe migrated to the bottom (Fig. 3F, 1st lane), representing an oligomeric form. In the presence of protein extracted from LPS-stimulated THP-1 cells, the probe was shifted upward (Fig. 3F, 2nd lane), indicating the interaction between probe and a protein present in the extract. The extent of this shift was significantly increased when protein extracted from apoA-I-treated cells was added (Fig. 3F, 3rd lane). Addition of a 100-fold excess of unlabeled probe to the RNA/protein mixture prevented the probe shift, indicating competition from excess nonlabeled RNA (Fig. 3F, 4th lane). The RNA-protein bands were further retarded (supershifted) after the protein extracts were preincubated with different levels of monoclonal TTP antibody (Fig. 3F, 5th and 6th lanes), indicating that TTP forms a complex with the sequence of TNF-α 3′-UTR containing AREs.
ApoA-I-induced TTP Expression Is Mediated by JAK2/STAT3 Pathway
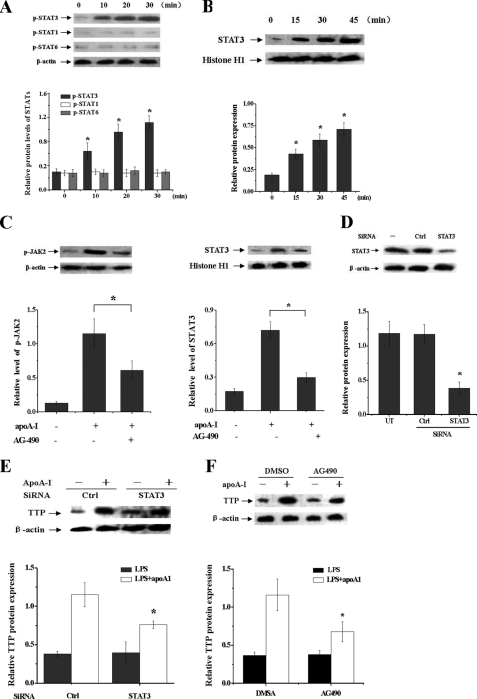
TTP expression has been reported to be controlled by activated STAT1, STAT3, and STAT6 (28, 34, 35). In mouse macrophages, the JAK2/STAT3 pathway has been demonstrated to be activated by apoA-I (19). We treated THP-1 macrophage-derived foam cells with apoA-I, and phosphorylation of STAT3 but not STAT1 or STAT6 was detected 10 min after addition of apoA-I, and it was further enhanced up to 30 min (Fig. 4A). Because phosphorylated signal transducer and activator of transcriptions dimerize and translocate to the nucleus (36), we investigated the nuclear translocation of STAT3 in apoA-I-stimulated THP-1 macrophage-derived foam cells. The level of STAT3 in the nucleus increased in a time-dependent manner after addition of apoA-I into the culture (Fig. 4B). Then we tested the action of AG-490, a JAK2 inhibitor, on STAT3 activation by measuring its effect on nuclear translocation of STAT3 in apoA-I-stimulated cells. AG-490 (30 μm) significantly inhibited the phosphorylation of JAK2 as well as the nuclear translocation of STAT3 (Fig. 4C), implying that the activation of STAT3 signaling induced by apoA-I was mediated by JAK2.
FIGURE 4.
STAT3 activation is involved in the apoA-I-mediated increase of TTP expression in LPS-treated macrophages. A, THP-1 macrophages were treated with apoA-I for different times as indicated. Proteins were extracted, and the phosphorylated STAT1, STAT3, and STAT6 levels were measured by immunoblot analyses with antibodies specific for phosphorylated signal transducer and activator of transcriptions. Values were normalized against β-actin. *, p < 0.05 versus 0 min. B, THP-1 macrophages were treated with apoA-I for different times as indicated. The nuclear proteins extracted from cells were subjected to immunoblot analyses with antibodies against STAT3 and histone H1. *, p < 0.05 versus 0 min. C, THP-1 macrophages were pretreated with apoA-I for 30 min. Thereafter, the medium was replaced with fresh medium containing the AG-490 (30 μm). Then cells were incubated for another 30 min, and total or nuclear proteins were subjected to immunoblot analyses with antibody against p-JAK2 and STAT3. *, p < 0.05. D, THP-1 macrophages were transfected with control (WT) or STAT3 siRNA for 48 h, and protein samples were immunoblotted with STAT3 and β-actin antibodies. *, p < 0.05 versus control group. E, THP-1 macrophages transfected with control or STAT3 siRNA were incubated with LPS for 4 h with or without pretreatment of apoA-I. Protein samples were immunoblotted with TTP and β-actin antibodies. *, p < 0.05 versus control group. F, THP-1 macrophages were pretreated with AG-490 or DMSO for 1 h, and cells were then incubated with LPS for another 4 h with or without pretreatment of apoA-I. Protein samples were immunoblotted with TTP and β-actin antibodies. *, p < 0.05 compared with DMSO group. All of the results are the mean ± S.D. of quadruplicate values from three separate experiments.
We further used siRNA transfections to determine whether apoA-I is exerting its TTP induction effects through activated STAT3. THP-1 macrophage transfected with STAT3 siRNA has reduced STAT3 protein levels by 74% compared with cells transfected with a scrambled siRNA (Fig. 4D). The ability of apoA-I to induce TTP expression in LPS-stimulated macrophages was significantly impaired by siRNA of STAT3 (Fig. 4E) or AG-490 (Fig. 4F), suggesting that apoA-I-induced TTP expression is mediated by the JAK2/STAT3 pathway.
ApoA-I Increased TNF-α mRNA Decay in an ABCA1-dependent Manner
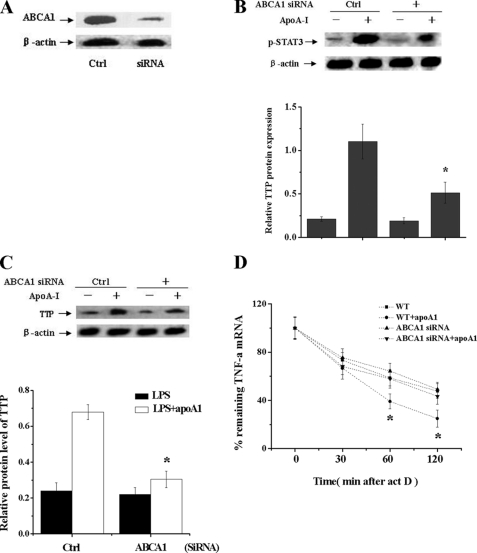
To test the role of ABCA1 on the effect of apoA-I on TNF-α mRNA decay in THP-1 cells, we first incubated THP-1 macrophage-derived foam cells with 100 nm ABCA1 siRNA, and the expression of ABCA1 was almost completely inhibited by siRNA (Fig. 5A). The ability of apoA-I to stimulate phosphorylation of STAT3 in THP-1 macrophage-derived foam cells was also significantly impaired in ABCA1 siRNA-treated cells (Fig. 5B). We then confirmed a role of ABCA1 in the apoA-I-mediated increase of TTP expression by measuring the effects of apoA-I on TTP expression in ABCA1 knockdown cells. The effect of apoA-I on TTP expression in ABCA1 siRNA cells was significantly abolished compared with control siRNA-untreated cells (Fig. 5C), indicating an ABCA1-dependent manner for the induction effects of apoA-I to TTP. We further examined the role of ABCA1 in the apoA-I-mediated TNF-α mRNA decay, and the rate of apoA1-induced TNFα mRNA decay in ABCA1 siRNA cells was markedly decreased compared with control siRNA-untreated cells (Fig. 5D).
FIGURE 5.
ABCA1 is involved in the apoA1-mediated increase of TTP expression in LPS-treated macrophages. A, THP-1 macrophages were transfected with control (WT) or ABCA1 siRNA for 48 h, and protein samples were immunoblotted with ABCA1 antibody. Ctrl, control. B, THP-1 macrophages transfected with control (WT) or ABCA1 siRNA were treated with apoA-I for 20 min. p-STAT3 expression was analyzed by Western blot. *, p < 0.05 versus control group. C, THP-1 macrophages transfected with control (WT) or ABCA1 siRNA were treated with LPS alone or with pretreatment of apoA-I for 4 h. TTP expression was analyzed by Western blot. *, p < 0.05 versus control group. D, THP-1 macrophage-derived foam cells transfected with control (WT) or ABCA1 siRNA (ABCA1−/−) were treated by LPS alone or with apoA-I for 3 h followed by addition of act D (5 μg/ml) to stop transcription. After the indicated time points, TNF-α mRNA was quantified using RT-PCR. Values were normalized against β-actin. Remnant TNF-α mRNA in percentage of the amount at the time point 0 of act D treatment was depicted. *, p < 0.05 compared with other groups. All of the data represent the mean ± S.E. from three separate experiments with triplicate samples.
DISCUSSION
The production of pro-inflammatory mediators in mononuclear phagocytes is a pivotal event in vascular inflammation and atherosclerosis (1, 37). Lipid-free apoA-I promotes cellular cholesterol efflux through a membrane microsolubilization process with consequent attenuation of Toll-like receptor signaling, which is thought to exert its anti-inflammatory effects (16, 38). Here, we reveal a novel intracellular molecular mechanism that is involved in the anti-inflammatory activity of apoA-I. Our studies provide evidence that the suppressive function of apoA-I in inflammation is mediated, at least partially, by antagonism of LPS-induced inflammatory genes in mRNA stability. It has been reported that dysregulation of mRNA stability is associated with some pathologies such as chronic inflammation and cardiovascular diseases (39, 40). LPS, a classic stimulator of Toll-like receptor 4 (TLR4)-mediated inflammation, has also been reported to promote stabilization of pro-inflammatory mRNAs such as TNF-α and IL-6 (41, 42). Regulation of mRNA decay by RNA-destabilizing factors therefore illuminates a promising approach for the treatment of inflammatory-related diseases. Using act D to inhibit transcription in this study, we first found that apoA-I significantly promoted some inflammatory gene mRNA decay, which might result either from the direct effect of apoA-I on inflammatory gene mRNA stability or from the ability of apoA-I to interfere with stabilization in response to LPS. Therefore, we utilized a chimeric construct, including a general mRNA decay element, which is from inflammatory gene mRNA such as TNF-α, to identify these alternative mechanisms. ApoA-I treatment destabilized mRNA containing the TNF-α mRNA decay element, although it did not alter the stability of control mRNA, suggesting a direct effect of apoA-I on TNF-α mRNA stability.
Although the mechanisms involved in mRNA decay are complicated, the AREs located in the 3′-UTR of labile genes is well reported to be a key element to regulate mRNA stability (43). In this study, we have shown for the first time that apoA-I-mediated inhibition of TNF-α mRNA expression depends upon TNF-α 3′-UTR sequences. ARE is a consensus sequence present in the 3′-UTR region of TNF-α mRNAs, as well as other inflammatory gene mRNAs, including IL-6, IL-1, interferons, etc. (44–46). ARE-mediated mRNA decay is regulated by several RNA-binding proteins collectively called ARE-binding proteins (ARE-BPs) (47, 48), and these proteins can be divided into two groups as follows: destabilizing and stabilizing ARE-BPs (47, 49). TTP, a zinc finger protein, is one of the well known destabilizing ARE-BPs for its mRNA-destabilizing activity (20). HuR, a ubiquitous member of the Hu family, is recognized as one of several proteins shown to stabilize ARE mRNAs (50). Whether apoA-I modulates the expression or function of these ARE-BPs, however, remains to be elucidated. Here, we have found that apoA-I treatment increases the expression of TTP, although it has no effect on HuR expression, which results in the enhanced degradation of some inflammatory gene mRNAs. In our studies, THP-1 macrophage with TTP knockdown showed compensation for decreased inflammatory cytokine expression as well as increased inflammatory gene mRNA decay to apoA-I, suggesting apoA-I inhibited some inflammatory cytokines in a TTP-dependent manner.
The present data have found that apoA-I increases the expression of TTP via activating STAT3, which is consistent with previous findings that TTP was up-regulated by the STAT3 pathway in LPS-treated mouse macrophages (32). In this respect, it is worthy to note that apoA-I seems to facilitate the up-regulation of TTP in TLR4-triggered macrophages, although it has no obvious effect on normal expression of TTP, suggesting that a functional interaction exists between TLR4 and STAT3. Nuclear factor κB (NF-κB) is induced by LPS through activation of TLR4. Several studies have already reported the functional cross-talk between NF-κB and STAT3 in various cell types (51, 52). It has been reported that activated STAT3 can form a complex with NF-κB p65 under inflammatory conditions followed by the interaction with sequences at some gene promoter for the NF-κB response element that is essential for the synergistic induction of the genes by cytokines (51, 53). Therefore, the activated STAT3 induced by apoA1 may be playing an essential role in supporting the transactivation of the TLR4/NF-κB pathway, although the exact mechanisms remain to be further studied.
Interleukin-6 (IL-6) has also been demonstrated to activate STAT3 and then stimulate TTP expression (32); however, IL-6 has not been shown to be able to inhibit TNF-α, which is consistent with its pro-inflammatory properties (32, 54). As it has been shown that activated p38 MAPK phosphorylates TTP protein (55) and then the phosphorylated TTP loses its activity (56), and considering that IL-6 can also activate the p38 MAPK pathway (32, 57), it would be easy to conclude that inhibition of p38 MAPK activity is necessary for promoting TTP-mediated function (58, 59). Accordingly, apoA-I has been found to be able to inhibit stress (60) and LPS-induced activation of p38 MAPK signaling by modulating the lipid raft through ABCA1-mediated cholesterol efflux (15, 61). Recently, Karwatsky et al. (62) have found that ABCA1-mediated cholesterol efflux to apoA-I initiated the activation of calcineurin, which has been found to promote down-regulation of p38 MAPK activity by enhancing MAPK phosphatase-1 expression (63). These results may suggest the hypothesis that there may be two ways for apoA-I regulatory roles in forming cross-talk to modulate the inflammatory response as follows: one is that apoA-I increases TTP expression via a STAT3-dependent manner in LPS-treated macrophages, and another one is that apoA-I decreases the activity of p38 MAPK via its cholesterol export activity in LPS-treated macrophages, thus releasing TTP from the p38 MAPK-mediated inhibition.
ABCA1, a member of the ABCA subfamily, is highly expressed in cholesterol-loaded macrophages, and it plays a central role in mediating the transport of lipids across cellular membranes to lipid-poor apoA-I (15). Recent experiments have revealed ABCA1 can function as an anti-inflammatory receptor to mediate the suppression of inflammatory cytokines by apoA-I (19). In our studies, we found that blocking ABCA1 expression significantly abolished the effect of apoA-I on TTP expression, suggesting that ABCA1 can directly mediate the effect of apoA-I on inflammatory gene mRNA decay. These results are also consistent with the observations of Murphy et al. (11), who proposed that the apoA-1/ABCA1 interaction is likely a major pathway mediating the anti-inflammatory effect of HDL.
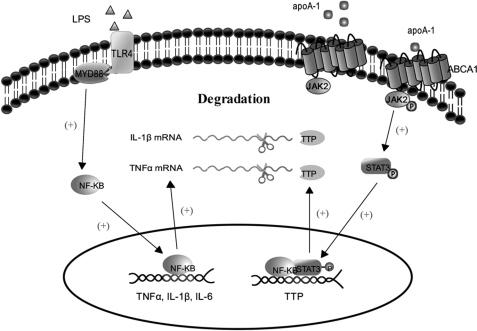
In conclusion, the present studies have detailed the mechanisms by which apoA-I modulates inflammatory cytokine expression and have provided novel evidence that apoA-I-mediated inflammation suppression in macrophages relates to the promotion of mRNA decay. Although there is no direct clinical data available to verify that the apoA-I suppressed the inflammatory responses in vivo by up-regulating TTP, it has been found that TTP is highly induced in human plaque macrophages, even if the exact mechanism of induction is currently unknown (64). This observation shows that up-regulation of TTP by apoA1 will have an impact on the further study of it as a potential mechanism that modulates inflammatory related disease in vivo. Moreover, we have also revealed a crucial role for the ABCA1/JAK2/STAT3/TTP pathway in apoA-I-mediated anti-inflammation function (Fig. 6). Although the exact cross-talk between cholesterol transport and the STAT3/TTP signaling pathway mediated by apoA-I/ABCA1 interaction remains to be further elucidated, it might be speculated that the anti-inflammatory ability of apoA-I is related to its direct effect on stimulating the intercellular signaling pathways.
FIGURE 6.
Schematic representation of the effects of apoA-I on TNF-α mRNA stabilization in LPS-stimulated macrophages. The results of the present studies revealed the following scheme for the possible mechanisms: apoA-I down-regulates the expression of TNF-α via a post-transcriptional regulation manner in macrophages; when macrophages are treated with apoA-I, the JAK2/STAT3 signaling pathway is activated, and the STAT3 dimers bind to the regulated elements of TTP in the nucleus. Then the expression of TTP is up-regulated, which in turn promotes the degradation of inflammatory cytokine mRNA through its 3′-UTR AREs. Plus sign indicates activation; scissors indicate degradation.
This work was supported by National Natural Sciences Foundation of China Grants 30470720 and 81070220, Post-doctoral Sciences Foundation of China Grant 2005037157, Heng Yang Joint Funds of Hunan Provincial Natural Sciences Foundation of China Grant 10JJ9019, Hunan Post-graduate Innovative Project Grant CX2010B379, Hunan Provincial Natural Sciences Foundation of China Grant 06jj5058, and Science and Technology Department Funds of Heng Yang of Hunan Province Grant 2010kj17.
- TTP
- tristetraprolin
- ARE
- AU-rich element
- ARE-BP
- ARE-binding protein
- act D
- actinomycin D
- nt
- nucleotide.
REFERENCES
- 1. Hansson G. K. (2005) N. Engl. J. Med. 352, 1685–1695 [DOI] [PubMed] [Google Scholar]
- 2. Luo N., Liu J., Chung B. H., Yang Q., Klein R. L., Garvey W. T., Fu Y. (2010) Diabetes 59, 791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asztalos B. F., Collins D., Cupples L. A., Demissie S., Horvath K. V., Bloomfield H. E., Robins S. J., Schaefer E. J. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 2185–2191 [DOI] [PubMed] [Google Scholar]
- 4. Barter P., Gotto A. M., LaRosa J. C., Maroni J., Szarek M., Grundy S. M., Kastelein J. J., Bittner V., Fruchart J. C. (2007) N. Engl. J. Med. 357, 1301–1310 [DOI] [PubMed] [Google Scholar]
- 5. Rader D. J., Alexander E. T., Weibel G. L., Billheimer J., Rothblat G. H. (2009) J. Lipid Res. 50, S189–S194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel S., Drew B. G., Nakhla S., Duffy S. J., Murphy A. J., Barter P. J., Rye K. A., Chin-Dusting J., Hoang A., Sviridov D., Celermajer D. S., Kingwell B. A. (2009) J. Am. Coll. Cardiol. 53, 962–971 [DOI] [PubMed] [Google Scholar]
- 7. Rye K. A., Barter P. J. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1890–1891 [DOI] [PubMed] [Google Scholar]
- 8. Barter P. J., Puranik R., Rye K. A. (2007) Curr. Cardiol. Rep. 9, 493–498 [DOI] [PubMed] [Google Scholar]
- 9. Tang C. K., Tang G. H., Yi G. H., Wang Z., Liu L. S., Wan S., Yuan Z. H., He X. S., Yang J. H., Ruan C. G., Yang Y. Z. (2004) Acta Biochim. Biophys. Sin. 36, 218–226 [DOI] [PubMed] [Google Scholar]
- 10. Kim K. D., Lim H. Y., Lee H. G., Yoon D. Y., Choe Y. K., Choi I., Paik S. G., Kim Y. S., Yang Y., Lim J. S. (2005) Biochem. Biophys. Res. Commun. 338, 1126–1136 [DOI] [PubMed] [Google Scholar]
- 11. Murphy A. J., Woollard K. J., Hoang A., Mukhamedova N., Stirzaker R. A., McCormick S. P., Remaley A. T., Sviridov D., Chin-Dusting J. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 2071–2077 [DOI] [PubMed] [Google Scholar]
- 12. Hyka N., Dayer J. M., Modoux C., Kohno T., Edwards C. K., 3rd, Roux-Lombard P., Burger D. (2001) Blood 97, 2381–2389 [DOI] [PubMed] [Google Scholar]
- 13. Zabalawi M., Bharadwaj M., Horton H., Cline M., Willingham M., Thomas M. J., Sorci-Thomas M. G. (2007) J. Lipid Res. 48, 52–65 [DOI] [PubMed] [Google Scholar]
- 14. Smoak K. A., Aloor J. J., Madenspacher J., Merrick B. A., Collins J. B., Zhu X., Cavigiolio G., Oda M. N., Parks J. S., Fessler M. B. (2010) Cell Metab. 11, 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yin K., Liao D. F., Tang C. K. (2010) Mol. Med. 16, 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tellier E., Canault M., Poggi M., Bonardo B., Nicolay A., Alessi M. C., Nalbone G., Peiretti F. (2008) J. Cell. Physiol. 214, 687–693 [DOI] [PubMed] [Google Scholar]
- 17. Liu X. H., Xiao J., Mo Z. C., Yin K., Jiang J., Cui L. B., Tan C. Z., Tang Y. L., Liao D. F., Tang C. K. (2010) J. Cardiovasc. Pharmacol. 56, 309–319 [DOI] [PubMed] [Google Scholar]
- 18. Hu Y. W., Ma X., Li X. X., Liu X. H., Xiao J., Mo Z. C., Xiang J., Liao D. F., Tang C. K. (2009) Atherosclerosis 204, e35–43 [DOI] [PubMed] [Google Scholar]
- 19. Tang C., Liu Y., Kessler P. S., Vaughan A. M., Oram J. F. (2009) J. Biol. Chem. 284, 32336–32343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson P., Phillips K., Stoecklin G., Kedersha N. (2004) J. Leukocyte Biol. 76, 42–47 [DOI] [PubMed] [Google Scholar]
- 21. Tölle M., Pawlak A., Schuchardt M., Kawamura A., Tietge U. J., Lorkowski S., Keul P., Assmann G., Chun J., Levkau B., van der Giet M., Nofer J. R. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1542–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bocharov A. V., Baranova I. N., Vishnyakova T. G., Remaley A. T., Csako G., Thomas F., Patterson A. P., Eggerman T. L. (2004) J. Biol. Chem. 279, 36072–36082 [DOI] [PubMed] [Google Scholar]
- 23. Hao X. R., Cao D. L., Hu Y. W., Li X. X., Liu X. H., Xiao J., Liao D. F., Xiang J., Tang C. K. (2009) Atherosclerosis 203, 417–428 [DOI] [PubMed] [Google Scholar]
- 24. Drouineaud V., Sagot P., Garrido C., Logette E., Deckert V., Gambert P., Jimenez C., Staels B., Lagrost L., Masson D. (2007) Mol. Hum. Reprod. 13, 373–379 [DOI] [PubMed] [Google Scholar]
- 25. Ramsamy T. A., Boucher J., Brown R. J., Yao Z., Sparks D. L. (2003) J. Lipid Res. 44, 733–741 [DOI] [PubMed] [Google Scholar]
- 26. Ma P., Cui X., Wang S., Zhang J., Nishanian E. V., Wang W., Wesley R. A., Danner R. L. (2004) J. Leukocyte Biol. 76, 278–287 [DOI] [PubMed] [Google Scholar]
- 27. Skoog T., van't Hooft F. M., Kallin B., Jovinge S., Boquist S., Nilsson J., Eriksson P., Hamsten A. (1999) Hum. Mol. Genet. 8, 1443–1449 [DOI] [PubMed] [Google Scholar]
- 28. Rajasingh J., Bord E., Luedemann C., Asai J., Hamada H., Thorne T., Qin G., Goukassian D., Zhu Y., Losordo D. W., Kishore R. (2006) FASEB J. 20, 2112–2114 [DOI] [PubMed] [Google Scholar]
- 29. Jacob C. O., Tashman N. B. (1993) Nucleic Acids Res. 21, 2761–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larigauderie G., Furman C., Jaye M., Lasselin C., Copin C., Fruchart J. C., Castro G., Rouis M. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 504–510 [DOI] [PubMed] [Google Scholar]
- 31. Serio K. J., Hodulik C. R., Bigby T. D. (2000) Am. J. Respir. Cell Mol. Biol. 23, 234–240 [DOI] [PubMed] [Google Scholar]
- 32. Schaljo B., Kratochvill F., Gratz N., Sadzak I., Sauer I., Hammer M., Vogl C., Strobl B., Müller M., Blackshear P. J., Poli V., Lang R., Murray P. J., Kovarik P. (2009) J. Immunol. 183, 1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krishnamurthy P., Rajasingh J., Lambers E., Qin G., Losordo D. W., Kishore R. (2009) Circ. Res. 104, e9–e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sauer I., Schaljo B., Vogl C., Gattermeier I., Kolbe T., Müller M., Blackshear P. J., Kovarik P. (2006) Blood 107, 4790–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suzuki K., Nakajima H., Ikeda K., Maezawa Y., Suto A., Takatori H., Saito Y., Iwamoto I. (2003) J. Exp. Med. 198, 1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ivashkiv L. B., Hu X. (2004) Arthritis Res. Ther. 6, 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kleemann R., Zadelaar S., Kooistra T. (2008) Cardiovasc. Res. 79, 360–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yvan-Charvet L., Welch C., Pagler T. A., Ranalletta M., Lamkanfi M., Han S., Ishibashi M., Li R., Wang N., Tall A. R. (2008) Circulation 118, 1837–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheneval D., Kastelic T., Fuerst P., Parker C. N. (2010) J. Biomol. Screen. 15, 609–622 [DOI] [PubMed] [Google Scholar]
- 40. Eberhardt W., Doller A., Akool el-S., Pfeilschifter J. (2007) Pharmacol. Ther. 114, 56–73 [DOI] [PubMed] [Google Scholar]
- 41. Neininger A., Kontoyiannis D., Kotlyarov A., Winzen R., Eckert R., Volk H. D., Holtmann H., Kollias G., Gaestel M. (2002) J. Biol. Chem. 277, 3065–3068 [DOI] [PubMed] [Google Scholar]
- 42. Brook M., Sully G., Clark A. R., Saklatvala J. (2000) FEBS Lett. 483, 57–61 [DOI] [PubMed] [Google Scholar]
- 43. Lykke-Andersen J., Wagner E. (2005) Genes Dev. 19, 351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guhaniyogi J., Brewer G. (2001) Gene 265, 11–23 [DOI] [PubMed] [Google Scholar]
- 45. Khabar K. S. (2007) J. Leukocyte Biol. 81, 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 1670–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chou C. F., Mulky A., Maitra S., Lin W. J., Gherzi R., Kappes J., Chen C. Y. (2006) Mol. Cell. Biol. 26, 3695–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maitra S., Chou C. F., Luber C. A., Lee K. Y., Mann M., Chen C. Y. (2008) RNA 14, 950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bevilacqua A., Ceriani M. C., Capaccioli S., Nicolin A. (2003) J. Cell. Physiol. 195, 356–372 [DOI] [PubMed] [Google Scholar]
- 50. Fan X. C., Steitz J. A. (1998) EMBO J. 17, 3448–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hagihara K., Nishikawa T., Sugamata Y., Song J., Isobe T., Taga T., Yoshizaki K. (2005) Genes Cells 10, 1051–1063 [DOI] [PubMed] [Google Scholar]
- 52. Han S. S., Yun H., Son D. J., Tompkins V. S., Peng L., Chung S. T., Kim J. S., Park E. S., Janz S. (2010) Mol. Cancer 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoshida Y., Kumar A., Koyama Y., Peng H., Arman A., Boch J. A., Auron P. E. (2004) J. Biol. Chem. 279, 1768–1776 [DOI] [PubMed] [Google Scholar]
- 54. Geisterfer M., Richards C., Baumann M., Fey G., Gywnne D., Gauldie J. (1993) Cytokine 5, 1–7 [DOI] [PubMed] [Google Scholar]
- 55. Rigby W. F., Roy K., Collins J., Rigby S., Connolly J. E., Bloch D. B., Brooks S. A. (2005) J. Immunol. 174, 7883–7893 [DOI] [PubMed] [Google Scholar]
- 56. Mahtani K. R., Brook M., Dean J. L., Sully G., Saklatvala J., Clark A. R. (2001) Mol. Cell. Biol. 21, 6461–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meng F., Yamagiwa Y., Taffetani S., Han J., Patel T. (2005) Am. J. Physiol. Cell Physiol. 289, C971–C981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ronkina N., Kotlyarov A., Dittrich-Breiholz O., Kracht M., Hitti E., Milarski K., Askew R., Marusic S., Lin L. L., Gaestel M., Telliez J. B. (2007) Mol. Cell. Biol. 27, 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. King E. M., Kaur M., Gong W., Rider C. F., Holden N. S., Newton R. (2009) J. Pharmacol. Exp. Ther. 330, 575–585 [DOI] [PubMed] [Google Scholar]
- 60. Van Linthout S., Spillmann F., Riad A., Trimpert C., Lievens J., Meloni M., Escher F., Filenberg E., Demir O., Li J., Shakibaei M., Schimke I., Staudt A., Felix S. B., Schultheiss H. P., De Geest B., Tschöpe C. (2008) Circulation 117, 1563–1573 [DOI] [PubMed] [Google Scholar]
- 61. Zhu X., Lee J. Y., Timmins J. M., Brown J. M., Boudyguina E., Mulya A., Gebre A. K., Willingham M. C., Hiltbold E. M., Mishra N., Maeda N., Parks J. S. (2008) J. Biol. Chem. 283, 22930–22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Karwatsky J., Ma L., Dong F., Zha X. (2010) J. Lipid Res. 51, 1144–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lim H. W., New L., Han J., Molkentin J. D. (2001) J. Biol. Chem. 276, 15913–15919 [DOI] [PubMed] [Google Scholar]
- 64. Patino W. D., Kang J. G., Matoba S., Mian O. Y., Gochuico B. R., Hwang P. M. (2006) Circ. Res. 98, 1282–1289 [DOI] [PubMed] [Google Scholar]