Abstract
The activities of signaling pathways are critical for fungi to survive antifungal attack and to maintain cell integrity. However, little is known about how fungi respond to antifungals, particularly if these interact with multiple cellular targets. The antifungal protein AFP is a very potent inhibitor of chitin synthesis and membrane integrity in filamentous fungi and has so far not been reported to interfere with the viability of yeast strains. With the hypothesis that the susceptibility of fungi toward AFP is not merely dependent on the presence of an AFP-specific target at the cell surface but relies also on the cell's capacity to counteract AFP, we used a genetic approach to decipher defense strategies of the naturally AFP-resistant strain Saccharomyces cerevisiae. The screening of selected strains from the yeast genomic deletion collection for AFP-sensitive phenotypes revealed that a concerted action of calcium signaling, TOR signaling, cAMP-protein kinase A signaling, and cell wall integrity signaling is likely to safeguard S. cerevisiae against AFP. Our studies uncovered that the yeast cell wall gets fortified with chitin to defend against AFP and that this response is largely dependent on calcium/Crz1p signaling. Most importantly, we observed that stimulation of chitin synthesis is characteristic for AFP-resistant fungi but not for AFP-sensitive fungi, suggesting that this response is a successful strategy to protect against AFP. We finally propose the adoption of the damage-response framework of microbial pathogenesis for the interactions of antimicrobial proteins and microorganisms in order to comprehensively understand the outcome of an antifungal attack.
Keywords: Aspergillus, Calcium, Cell Wall, Signal Transduction, Yeast, Antifungal, Antimicrobial, Defense
Introduction
The emergence and spread of pathogenic microorganisms that are resistant to virtually all available antimicrobials represents a serious challenge for medicine and agriculture and has stepped up efforts to develop new antimicrobials. The use of “smarter” antibiotics, also called “dirty drugs” affecting multiple cellular targets is one discussed strategy to prevent the development of resistance mechanisms (1, 2). Of special interest is the exploitation of antimicrobial peptides (AMPs),2 which are natural products of pro- and eukaryotic organisms and function as defense molecules to combat nutrient competitors, colonizers or invaders (3, 4). The currently known and studied AMPs have been catalogued in the antimicrobial peptide data base (5, 6), which at present contains more than 1,600 members. Although AMPs are different in sequence and secondary structure, they share common attributes. Most of them are derived from precursors, are small in size (<100 amino acids with frequent use of glycine, alanine, lysine, and cysteine), display cationic and amphipathic properties, and resist proteolytic degradation due to stabilizing disulfide bonds (2, 4, 5). Their positive net charge attracts them electrostatically to negatively charged cell surfaces of microorganisms, where hydrophobic and/or receptor-based interactions allow them to bind, traverse, or permeabilize biological membranes (4, 7, 8). Thereby provoked membrane alterations cause dysfunctions, such as loss of ion homeostasis and/or cell wall biopolymer synthesis, which in turn represents a potentially lethal stress situation. Additional intracellular interactions of translocated AMPs with molecules displaying negative net charges (e.g. proteins, nucleic acids, and oligosaccharides) can eventually lead to cell death (7).
One very promising AMP for employment in antifungal treatments is the antifungal protein AFP produced by the filamentous fungus Aspergillus giganteus. AFP is a 5.8-kDa small, cysteine-rich, amphipathic protein with a positive net charge and is secreted by A. giganteus especially under non-favorable growth conditions (9, 10). The protein is active against filamentous fungi, including serious human and plant pathogens, but inactive against bacteria, yeast, plants, or mammalian cells and successfully protects plants from colonization or invasion of filamentous fungi (11, 12). AFP heavily localizes to the cell wall and plasma membrane of sensitive filamentous fungi, where it provokes membrane stretching and permeabilization (13–15). It comprises a chitin-binding domain and inhibits chitin synthesis in sensitive filamentous fungi (13). In collapsed and dead cells, AFP can also be found intracellularly (14, 15), where it might bind via its oligonucleotide/oligosaccharide-binding fold to anionic molecules, such as nucleic acids (16). A shorter version of AFP exhibiting the chitin-binding domain but lacking the hydrophobic domain (sAFP) does not disturb the cell wall/cell membrane integrity of AFP-sensitive filamentous fungi, although it is able to bind to chitin and nucleic acids under in vitro conditions (13). This loss of bioactivity implies that the primary inhibitory effect of AFP is exerted at the cell surface, where a potential target(s) of AFP resides. Most importantly, not all filamentous fungi are equally susceptible toward AFP; some are highly sensitive (minimal inhibitory concentration, 0.1–10 μg/ml; Aspergillus niger, Fusarium oxysporum) or moderately sensitive (100 < minimal inhibitory concentration < 400 μg/ml; A. giganteus), and others are resistant (Penicillium chrysogenum).
Microorganisms confronted with multifunctional AMPs, such as AFP, have to resist these protein activities to ensure cell survival. Some microorganisms may be inherently resistant because their cell surfaces lack electrostatic affinity or receptors for AMPs. Others comprising these targets can potentially counteract the attack of AMPs (e.g. by shielding their surface via remodeling of their cell wall/cell membranes, by extracellular trapping of AMPs at specific cell surface areas, by expressing specific proteases, or by modification of intracellular targets) (2, 7). Prerequisite for these adaptive survival responses are the activities of signaling cascades, which sense and transduce stress signals to activate and coordinate the defense reaction in a timely fashion (17).
Using A. niger as model system, we could recently show that one defense mechanism to counteract AFP inhibitory effects is induction of the cell wall integrity (CWI) pathway, a highly conserved signaling cascade that secures cell surface protection in yeast and filamentous fungi (17–20) and results in A. niger in increased expression of the agsA gene, encoding an α-1,3-glucan synthase (13). However, when induction of the CWI pathway is meant to protect A. niger, why then is the fungus killed by AFP? One compelling explanation is that up-regulation of the CWI pathway might not be the most adequate response to counteract AFP. To pursue that hypothesis, we studied in this work the counteractive potential of the AFP-resistant yeast Saccharomyces cerevisiae. We choose S. cerevisiae as a model system because the architecture of its cell wall shares many similarities with the cell wall of filamentous fungi (21) and because a genome-wide deletion mutant collection is available. We speculated that mutations affecting processes that normally guarantee cell wall and plasma membrane integrity could render an AFP-resistant organism, such as S. cerevisiae, AFP-sensitive. Thus, by means of screening mutants of an AFP-resistant fungus for AFP-sensitive phenotypes, it may be possible to spot and analyze defense mechanisms that are essential to survive an AFP attack.
EXPERIMENTAL PROCEDURES
Strains, Growth Conditions, and Molecular Techniques
All strains used in this study are given in Table 1 and supplemental Table S1. S. cerevisiae strains are from the EUROSCARF collection (Frankfurt, Germany) containing gene deletions in the MATa background strain BY4741 (22). Yeast strains were cultivated in YPD medium (2% peptone, 1% yeast extract, 2% dextrose, pH 6.5), whereas A. niger and P. chrysogenum were grown in YPD medium at pH 4.5. Alternatively, A. niger strains were cultivated in minimal medium (23) containing 1% glucose as a carbon source (if not otherwise stated) or in complete medium, consisting of minimal medium supplemented with 1% yeast extract and 0.5% casamino acids. 10 mm uridine was added when required. Transformation of A. niger, selection procedures, genomic DNA extraction, and diagnostic PCR were performed using recently described protocols (24). Standard PCR, general cloning procedures in E. coli, and Southern and Northern analyses were done according to Ref. 25.
TABLE 1.
Grouping of deletion mutants according to their susceptibility against AFP
* Each 103 cells were cultivated in YPD medium containing 400 μg/ml AFP for 28 h. Growth is expressed as a percentage compared with the control (cells not treated with AFP). The growth of the wild-type strain BY4741 is 100% in both the absence and presence of AFP.
Preparation of AFP
A. giganteus was cultivated in complete medium at 28 °C for 4 days, after which the culture was cultivated for an additional 20 h at 37 °C to foster expression and secretion of AFP. The protein was isolated and purified from the culture broth according to the protocol described in Ref. 14.
Sensitivity Tests toward AFP and Calcofluor White
Sensitivity of yeast and filamentous fungal strains against AFP was determined using a protocol according to Ref. 14. In brief, 103 cells or spores were used to inoculate 150 μl of YPD medium in the absence or presence of different AFP amounts (0.1–400 μg/ml). Cultivations were carried out in technical triplicates in microtiter plate format (28 °C, 28 h, 120 rpm) and repeated at least twice. Growth was assessed by measuring the optical density at 600 nm. Calcofluor white (CFW) sensitivity was determined using the protocol according to Ref. 26 by spotting serial dilutions of exponentially growing cultures on YPD agar containing 50 μg/ml CFW. Growth was assessed after 48 h of incubation at 28 °C.
SYTOX Green Uptake Assay
The assay was carried out in microtiter plate format using a slightly modified method described recently (14). 105 yeast cells were cultivated at 28 °C in 150 μl of YPD medium for 12–16 h until they reached the midlogarithmic growth phase. 1 μm SYTOX Green and AFP (up to final concentrations of 400 μg/ml) were added. Fluorescence values were measured over time using a CytoFluor 2350 fluorescence measurement system (excitation, 480 nm; emission, 530 nm) and corrected by subtracting values from AFP-untreated samples. All measurements were carried out in triplicates.
Cell Wall Polymer Quantification
106 yeast cells or fungal spores were inoculated per ml of YPD medium and incubated in the presence (150 μg/ml) or absence of AFP. For some experiments, 50 nm BAPTA or 10 nm FK506 were added as well. After cultivations for 28 h at 28 °C, biomass was harvested, and the amount of chitin and β-1,3-glucan was determined. Cell wall chitin was isolated according to Ref. 27, measured according to Refs. 28 and 29, and calculated per dry biomass. β-1,3-Glucan levels were determined according to Ref. 30 and calculated per dry biomass. All isolations and measurements were done from at least two independent experiments.
Microscopy
Cells or conidia (3 × 105) of S. cerevisiae or A. niger strains were inoculated in 3 ml of liquid medium (YPD or minimal medium supplemented with 0.003% yeast extract) and cultivated on coverslips in the presence or absence of 400 μg/ml AFP for 28 h at 28 °C. For chitin visualizations, cells adherent to the coverslips were incubated for 10 min in 10 μg/ml CFW and rinsed thereafter with water before being subjected to microscopy. Samples were observed with an Axioplan 2 microscope (Zeiss) equipped with a DKC-5000 digital camera (Sony) using differential interference contrast or DAPI settings. Images were captured with a ×100 objective and processed using Adobe Photoshop 6.0 (Adobe Systems Inc.).
Construction of chs1Δcrz1Δ Deletion Strain of S. cerevisiae
The CRZ1 gene was deleted in the chs1Δ knock-out strain Y02020 (EUROSCARF) by replacing it with the selection marker URA3 of Kluyveromyces lactis. For this purpose, the URA3 cassette from plasmid pGEMT-URA3 (kindly provided by Udo Schmidt, Berlin University of Technology)3 was amplified by PCR, which also introduced CRZ1 homologous flanks necessary for homologous recombination (for primers, see supplemental Table S2). S. cerevisiae transformants were selected by uracil prototrophy, and CRZ1 deletion was verified using diagnostic PCR.
Construction of an A. niger csmB Deletion Strain
A fusion PCR approach was used to construct a csmB deletion cassette. In brief, ∼1 kb of the promoter and terminator regions of csmB (An02g02340) was amplified from genomic DNA of A. niger N402 using primer pairs given in supplemental Table S2. As a selection marker, we used the A. fumigatus pyrG gene, which was amplified from GFP-AfpyrG cassette (31) using primers SMP1 and GFP2. The three amplified fragments were purified and subjected to the fusion PCR using primers PP1 and GSP4. The PCR product obtained was used to transform the pyrG− strain MA70.15, which allows targeted integration at high frequencies (32). Uracil-prototroph transformants were selected, purified, and subjected to Southern analyses according to Ref. 24 to confirm gene deletions.
Construction of an A. niger chsD Overexpression Strain and Chitin Assay
The open reading frame of the chsD gene (An09g02290) was PCR-amplified from genomic DNA of the A. niger wild-type strain N402 and cloned downstream of the doxycycline-responsive promoter TetO7::Pmin in plasmid pVG2.2 (67). Plasmid pVG2.2 contains three cassettes: (i) PgpdA::rtTA2S-M2::TcgrA ensures constitutive expression of the doxycycline-responsive transcription factor rtTA2S-M2; (ii) TetO7::Pmin::TtrpC allows rtTA2S-M2-dependent expression of a gene of interest when cloned downstream of Pmin; and (iii) pyrG* targets the complete plasmid to the pyrG locus of A. niger, after which uracil-prototrophy is restored (34). pVG2.2-chsD was transformed into the AFP-sensitive A. niger strain MA169.4 (35), and uracil-prototroph transformants were selected and purified. Southern analyses according to Ref. 24 confirmed integration of PgpdA::rtTA2S-M2::TcgrA-TetO7::Pmin::chsD::TtrpC at the pyrG locus in clone JP3-K4. 106 conidia/ml JP3-K4 and VG5.1 (control strain containing plasmid pVG2.2 at the pyrG locus) were used to inoculate liquid complete medium containing 5 μg/ml doxycycline (Dox) to induce expression of chsD. As a control, no Dox was added. After 16 h of cultivation, 10 μg/ml AFP was added to the cultures, and after an additional incubation for 3 h in the presence or absence of AFP, chitin levels were determined as described above. The assay was repeated twice.
RESULTS
We selected from the S. cerevisiae deletion strain collection 100 strains, all being deleted in a single non-essential gene (Table 1). This set of strains included mutants affected in cell wall and plasma membrane assembly and mutants disturbed in signaling processes, such as CWI signaling, calcium signaling, high osmolarity glycerol signaling, protein kinase A (PKA) signaling, and TOR signaling, known to be important to fortify and preserve the yeast cell wall (17, 21, 36). Because for several yeast systems it has been reported that an elevated cell wall chitin content very often correlates with hypersensitivity to the chitin antagonist CFW (36–38), we also wished to determine whether there is a general relationship between chitin levels in S. cerevisiae and susceptibility to AFP. Hence, the selected strain collection also contained mutants displaying increased or decreased chitin levels compared with the wild-type S. cerevisiae.
AFP Has the Capacity to Cause Damage to S. cerevisiae
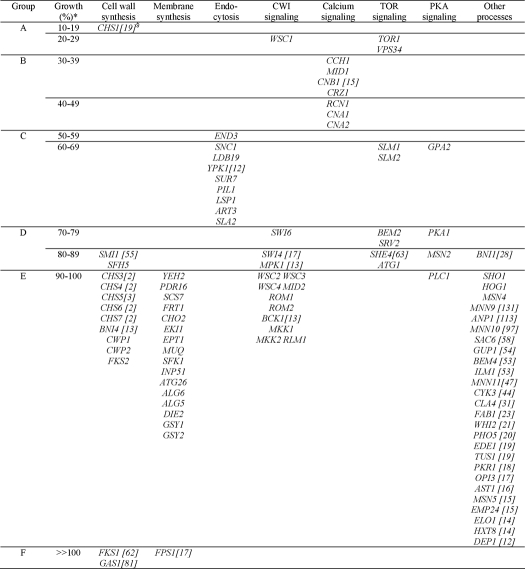
All mutant strains were grown in YPD medium in the presence of 400 μg/ml AFP, and cell growth was compared with cultivations in the absence of AFP. Under such high AFP concentrations, growth of the S. cerevisiae WT strain BY4741 is unaffected (Fig. 1) (14). In contrast, growth of 35 of 100 deletion strains became compromised by AFP (Fig. 1 and Table 1), demonstrating that S. cerevisiae can become moderately sensitive toward AFP. The inhibitory effect of AFP ranged from strong inhibition (residual growth of 10–29%, group A) to weak inhibition (residual growth of 70–89%, group D). For the majority of the strains, growth remained unaffected by AFP (59 strains, group E). Surprisingly, we also observed improved growth (group F).
FIGURE 1.
AFP susceptibilities of the S. cerevisiae wild-type strain BY4741 and selected deletion mutants when cultivated in YPD medium in the presence of 400 μg/ml AFP. Growth is expressed as percentages compared with the negative control, which consisted of the strains cultivated in the absence of AFP. Error bars, S.D. for triplicate experiments.
From the growth data obtained, three main conclusions were drawn. First, S. cerevisiae mutants with higher chitin content do not simply become AFP-sensitive (strains with up to 10 times higher WT chitin levels were tested) (Table 1), suggesting that the overall chitin content is not per se important for the susceptibility against AFP. Second, from seven genes tested, known to orchestrate and catalyze chitin synthesis in S. cerevisiae (CHS1, CHS3, CHS4, CHS5, CHS6, CHS7, and BNI4; note that CHS2 is an essential gene (21)), only deletion of CHS1 rendered S. cerevisiae AFP-sensitive, making Chs1p the prime chitin synthase responsible for the resistance of S. cerevisiae against AFP. Third, and most interestingly, deletions of almost all genes constituting the classical CWI signaling pathway (17) (i.e. WSC2, WSC3, WSC4, MID2, ROM1, ROM2, BCK1, MKK1, MKK2, MPK1, RLM1, SWI4, and SWI6) did not render or only marginally rendered S. cerevisiae sensitive toward AFP, suggesting that CWI signaling is of only minor importance to counteract any detrimental effects provoked by AFP. The only exception was the wsc1Δ strain, which, like chs1Δ, fell into the sensitive group A.
S. cerevisiae Becomes Vulnerable to AFP during Cell Separation
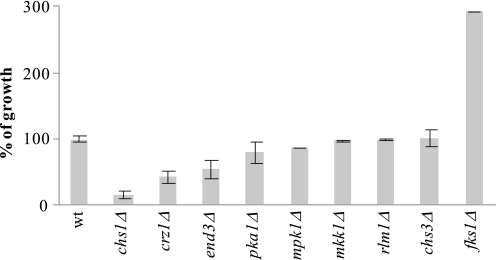
Four mutants constituted the group of the most susceptible strains: chs1Δ, wsc1Δ, vps34Δ, and tor1Δ (Table 1). Chs1p acts as a septum repair chitin synthase to replenish chitin lost through chitinase activity during mother-daughter cell separation (39). Wsc1p is, like Wsc2p, Wsc3p, and Mid2p, a cell wall- and cell membrane-spanning sensor that signals cell wall stress to the CWI pathway (21, 40). Some features of Wsc1p, however, distinguish it from the other sensors (e.g. only Wsc1p localizes to sites of polarized growth, cycles in a cell cycle-dependent manner between the cytoplasm and the plasma membrane, and becomes rapidly internalized by endocytosis after completion of cell separation) (41). The gene VPS34 encodes a phosphatidylinositol 3-kinase required for phosphatidylinositol metabolism, endocytic uptake, and vacuole partitioning between mother and daughter cells during cell division (42, 43). Moreover, its activity is also functionally linked to the Tor1p protein in S. cerevisiae and higher eukaryotes (44, 45). Tor1p is a membrane-localized phosphatidylinositol 3-kinase-related kinase and, as a subunit of the TORC1 complex, implicated in phosphatidylinositol metabolism and myriads of other processes, including cell cycle regulation and endocytosis (46).
Common to all four proteins is a function during cell separation, suggesting that S. cerevisiae might become vulnerable to AFP in a cell cycle-dependent manner. We thus questioned whether AFP affects cell separation in these mutants and, as shown for all sensitive filamentous fungi so far (13, 14), is able to permeabilize their plasma membranes. As shown in Fig. 2A, the budding pattern of the WT strain was not compromised by AFP (i.e. cells separated well after budding). In contrast, chs1Δ, wsc1Δ, tor1Δ, and vps34Δ cells remained attached to each other and formed aggregates in the presence of AFP, suggesting that AFP interrupts cell division. In addition, the plasma membranes of all four deletion strains became readily permeabilized by AFP within 10 min after AFP addition, which was not the case for the WT strain (Fig. 2B). These results suggested that S. cerevisiae can be attacked by AFP, especially when its plasma membrane is exposed and not protected sufficiently in a timely manner (e.g. by the formation of new cell wall chitin).
FIGURE 2.
S. cerevisiae cell morphology and membrane integrity in the absence and presence of AFP. Cells were treated as described under “Experimental Procedures.” A, differential interference contrast micrographs of WT BY4741 and mutant strains. Bars, 10 μm. B, AFP-mediated membrane-permeabilizing effect on mutant and wild-type strains of S. cerevisiae using the SYTOX Green uptake assay. This dye can only enter plasma membranes when compromised, and previous work has shown that the degree of AFP activity can be correlated with the extent of fluorescence (13, 14).
S. cerevisiae Counteracts AFP with Increased Chitin Synthesis
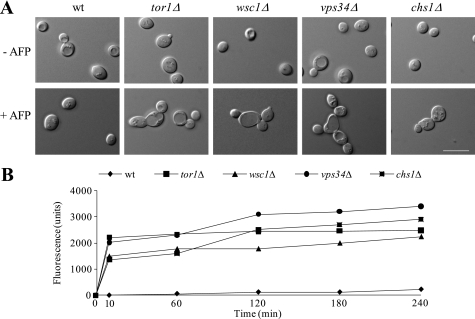
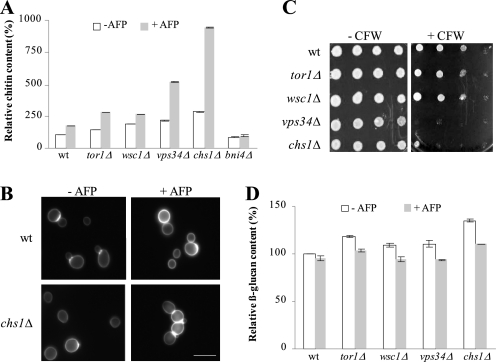
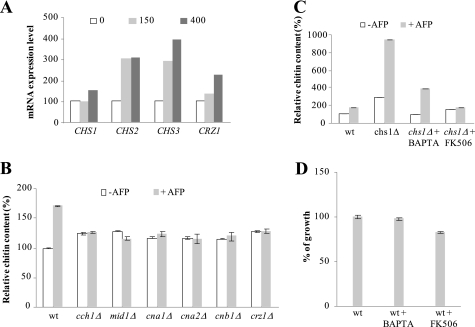
Having the moderately sensitive strains chs1Δ, wsc1Δ, tor1Δ, and vps34Δ in hand, we sought to identify mechanisms for how these strains respond to AFP and which defense strategies are used to counteract AFP-inhibitory effects. For this purpose, we cultivated the WT and mutant strains in the presence and absence of AFP and quantified chitin and β-1,3-glucan levels of their cell walls. All five strains responded with increased chitin synthesis to AFP, whereby the strongest response was observed in chs1Δ and vps34Δ cells (Fig. 3, A and B). These data indicate that one counteracting mechanism of S. cerevisiae against AFP is fortification of the chitin layer, a defense strategy also used by S. cerevisiae to circumvent CFW-inhibitory effects (38). Notably, AFP obviously affected more cellular processes than CFW, because only chs1Δ and vps34Δ cells (and not wsc1Δ and tor1Δ) are vulnerable to CFW (Fig. 3C). β-1,3-glucan contents were slightly but significantly reduced in all five strains (Fig. 3D), suggesting that the stress-induced increase in chitin levels is paralleled by down-regulated glucan synthesis.
FIGURE 3.
Cell wall remodeling of S. cerevisiae in response to AFP. Amount of chitin (A) and β-1,3-glucan (D) in mutants of S. cerevisiae in the absence and presence of 150 μg/ml AFP. The data are given relative to the chitin/glucan amount determined in the WT strain BY4741 in the absence of AFP (set as 100%). Error bars, S.D. for quadruple experiments. B, microscopic images of WT and chs1Δ strains stained with CFW. Pictures were taken using fixed exposure time (100 ms). The increase in CFW fluorescence intensity in the presence of AFP (400 μg/ml) reflects enhanced chitin levels at cell walls. Bar, 10 μm. C, plate sensitivity assay using 50 μg/ml CFW. Equivalent numbers of cells were serially diluted, and aliquots were spotted on YPD medium containing or lacking CFW. Plates were photographed after 3 days of incubation at 28 °C.
To assess whether the increase in cell wall chitin content in S. cerevisiae WT and mutant strains was due to the activities of Chs2p or Chs3p, we measured chitin levels in a bni4Δ strain stressed with AFP. Bni4p is a scaffold protein that specifically tethers Chs3p to the bud neck, thus regulating chitin synthesis at the chitin ring (47). If Chs3p is the AFP-responsive chitin synthase, increased chitin synthesis would be abolished in a bni4Δ background; however, if Chs2p is the counteractive chitin synthase, increased chitin levels would still be observable in response to AFP treatment. As shown in Fig. 3A, the chitin response toward AFP was completely lost in bni4Δ cells, strongly suggesting that AFP-stimulated chitin synthesis in S. cerevisiae WT and mutant strains is largely dependent on Chs3p.
The AFP-induced Chitin Response Is Transcriptionally Regulated via Crz1p Signaling
Next, we investigated how S. cerevisiae enforced chitin synthesis in response to AFP. Northern experiments revealed that the S. cerevisiae WT strain responded with increased transcription of all three chitin synthase genes, CHS1, CHS2, and CHS3, whereby expression of CHS3 was stimulated most (Fig. 4A). Because our mutant screen revealed that the CWI pathway was not the main pathway that rescued S. cerevisiae from AFP but that deletions in the calcineurin/Crz1p pathway rendered S. cerevisiae more susceptible to AFP (Table 1), we suspected that the activity of the latter might be responsible for these transcriptional up-regulations. Supportive of this assumption was the observation that CRZ1 expression levels also increased when the WT was treated with AFP (Fig. 4A). The increase in chitin content was in fact absent in strains deleted for components of the calcineurin/Crz1p pathway, CCH1, MID1, CNA1, CNA2, CNB1, and CRZ1 (Fig. 4B; note that calmodulin gene CMD1 is an essential gene). Furthermore, when the chs1Δ strain was co-treated with AFP and the calcium chelator BAPTA, the increase in chitin content was reduced. The chitin response was almost fully abolished when chs1Δ was treated with AFP in the presence of the calcineurin inhibitor FK506 (Fig. 4C), strongly suggesting that an intact calcineurin/Crz1p signaling pathway mediates increased chitin synthesis in response to AFP and that the activity of this pathway is sufficient to protect S. cerevisiae against AFP. In agreement, we observed a mild sensitization of the WT strain when co-treated with BAPTA or FK506 (Fig. 4D).
FIGURE 4.
Calcium-dependent cell wall remodeling in S. cerevisiae. A, BY4741 cells harvested from a logarithmically grown culture were treated with different amounts of AFP (0, 150, and 400 μg/ml) for 1 h, after which total RNA was isolated and analyzed. Transcript levels were quantified by densitometry using the 18 S rRNA signal for calibration. All values are expressed relative to the respective untreated control. Data from a representative experiment are shown. B and C, amount of chitin in S. cerevisiae strains in the absence or presence of 150 μg/ml AFP. The data are given relative to the chitin amount determined in the WT strain BY4741 in the absence of AFP (set as 100%). Error bars, S.D. for triplicate experiments. D, AFP sensitivity assay of BY4741 (using 150 μg/ml AFP) in the presence of 50 nm BAPTA or 10 nm FK506. These concentrations of BAPTA and FK506 were chosen because they are the maximum concentrations that do not inhibit growth of BY4741 (data not shown). The data are given relative to the growth of the WT strain BY4741 in the absence of AFP (set as 100%). Error bars, S.D. for quadruple experiments.
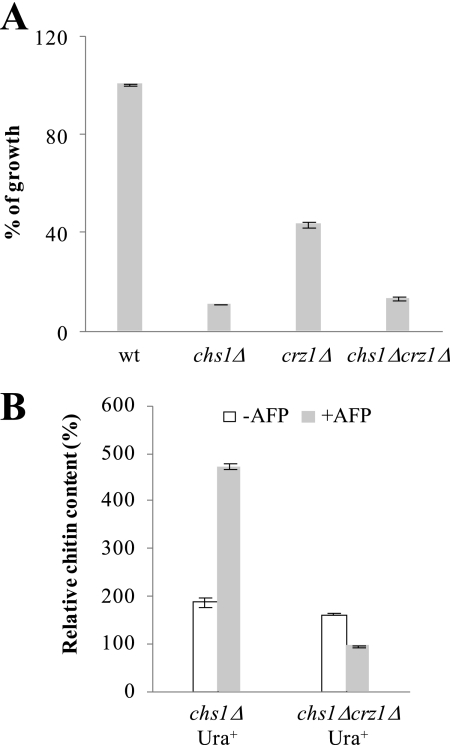
To finally prove that increased chitin synthesis is largely controlled by Crz1p, we deleted the CRZ1 gene in the chs1Δ background and determined the growth-inhibitory effect of AFP and chitin levels in the double mutant in the presence or absence of AFP. As depicted in Fig. 5A, the growth-inhibitory effect of AFP against chs1Δcrz1Δ is stronger when compared with the crz1Δ strain and comparable with that of the chs1Δ strain. Importantly, the increase in chitin amounts in response to AFP became completely lost in the chs1Δcrz1Δ strain (Fig. 5B), demonstrating that Crz1p is the main regulator responsible for the Chs3p-mediated chitin response.
FIGURE 5.
Crz1p-dependent chitin response in S. cerevisiae. A, growth of BY4741 and derived mutants in the presence of 400 μg/ml AFP. Growth is expressed as percentages compared with the negative control, which consisted of the strains cultivated in the absence of AFP. Error bars, S.D. for triplicate experiments. B, amount of chitin in the chs1Δcrz1Δ double mutant (strain JPSc1.4) when cultivated in the absence or presence of 150 μg/ml AFP. As a control, a chs1Δ strain was used in which the URA3 selection marker, used to delete CRZ1 (see “Experimental Procedures”), was integrated heterologously into the genome of the chs1Δ strain (strain JPSc1.6). We used this strain as a reference to avoid artifacts due to nonmatching auxotrophies between mutant and reference strain (66). The data are given relative to the chitin amount determined in the WT strain BY4741 in the absence of AFP (set as 100%). Mean values of a duplicate experiment are given.
Increased Chitin Synthesis Is a Consistent Response of Moderately Sensitive and Resistant Fungi
Previously, we have shown that chitin synthase activities are inhibited by AFP in sensitive fungi, such as A. niger, Aspergillus oryzae, and F. oxysporum (13). Because the present work revealed that resistant and moderately sensitive S. cerevisiae strains responded with increased chitin synthesis to AFP, we speculated that elevated chitin synthesis may be a general cellular strategy of moderately sensitive and resistant fungi to defend themselves against AFP. To prove or refute this assumption, we determined chitin levels in response to AFP in the AFP-resistant strain P. chrysogenum and in an A. niger mutant strain, in which we have deleted the chitin synthase csmB (An02g02340), which is a predicted class V chitin synthase). We have decided to delete this chitin synthase for several reasons. Class V chitin synthases are specific for filamentous fungi (48), csmB expression levels are strongly affected during cell wall stress (19), and deletion of the class V chitin synthase chsV in F. oxysporum made the strain hyperresistant toward AFP (13). Indeed, for all yeast and fungal strains tested, we found a correlation between susceptibility and chitin synthesis (Table 2), i.e. reduced sensitivity is paralleled by increased chitin levels in response to AFP treatment in S. cerevisiae, F. oxsporum chsV mutant, A. niger ΔcsmB mutant, and P. chrysogenum. A higher sensitivity toward AFP, however, is observed in strains with down-regulated chitin synthesis, e.g. in wild-type strains of A. niger, A. oryzae, and F. oxysporum.
TABLE 2.
Relative chitin response in selected fungal strains in response to the addition of AFP
| Strain | Relevant genotype | Changea | MICb | Reference/Source |
|---|---|---|---|---|
| -fold | μg/ml | |||
| A. niger 15/1801 | WT | 0.28 | 1 | Ref. 13 |
| A. niger JP1 | ΔcsmB | 1.33 | 10 | This work |
| S. cerevisiae BY4741 | WT | 1.69 | >400 | This work |
| S. cerevisiae Y02020 | chs1Δ | 7.19 | >400 | This work |
| S. cerevisiae Y05149 | vps34Δ | 4.37 | >400 | This work |
| F. oxysporum 4287 | WT | 0.62 | 1 | Ref. 13 |
| F. oxysporum ChsV | ΔchsV | 1.57 | >400 | Ref. 13 |
| A. oryzae A1560 | WT | 0.60 | 1 | Ref. 13 |
| P. chrysogenum ATCC 10002 | WT | 4.6 | >400 | This work |
a Chitin amounts were determined after the addition of AFP as described under “Experimental Procedures” and related to the respective untreated controls (set as 1). S.D. is less than 15%.
b Minimal inhibitory concentration.
Stimulation of Cell Wall Salvage Pathways Lowers AFP Sensitivity of A. niger
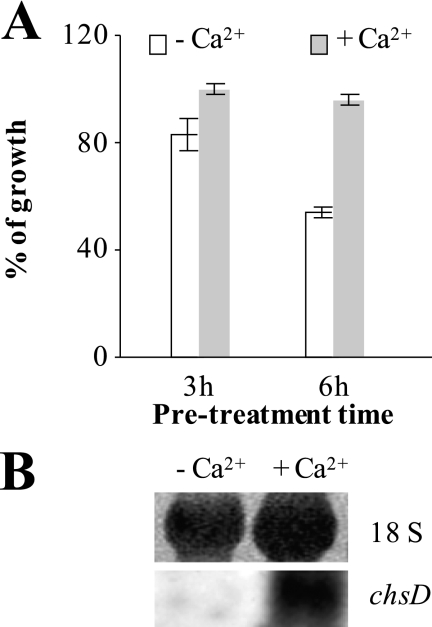
Recently, it was shown that pretreatment of Candida albicans with activators of the calcium signaling pathway activated chitin synthesis and reduced the susceptibility of C. albicans toward the cell wall stressor caspofungin (49). We thus questioned whether the probability of survival also increases for a WT A. niger strain when pretreated with calcium. We thus allowed spores of A. niger to germinate and added CaCl2 for defined incubation times, after which we removed it by washing the cells with fresh medium. After this procedure, growth of A. niger was assessed in the absence or presence of 10 μg/ml AFP. In addition, transcript levels of the chsD gene encoding the predicted S. cerevisiae Chs3p ortholog of A. niger (An09g02290) (50) were followed. As shown in Fig. 6A, AFP-induced growth inhibition was less severe when germlings were primed with CaCl2, especially when a preincubation time of 6 h was applied. The fact that improved survival rates were paralleled by markedly increased transcript levels of chsD (Fig. 6B) suggested that a stimulated calcium signaling machinery can also confer a higher protection against AFP in a filamentous fungus.
FIGURE 6.
Effects of stimulated calcium signaling on AFP susceptibility of A. niger. 107 spores/ml of the WT strain N402 were allowed to germinate in YPD medium for 5 h, after which 100 mm of CaCl2 were added (as a negative control, H2O was added). After 3 or 6 h of further cultivation, germlings were washed twice with YPD and thereafter incubated in YPD supplemented without or with 10 μg/ml AFP. A, growth was assessed by measuring A600 after 24 h and is expressed as percentages compared with the growth of N402 in the absence of AFP (set as 100%). Error bars, S.D. for triplicate experiments. B, Northern analysis of chsD gene expression after 6 h of calcium priming. 5 μg of total RNA from calcium treated and non-treated samples were hybridized with a chsD probe. Methylene blue-stained 18 S RNA confirmed equal loading.
We finally wished to test the idea whether an artificially stimulated chitin synthesis can also improve survival rates of a WT A. niger strain. To examine this, we constructed an A. niger strain (JP3-K4), in which the chsD gene was put under control of the inducible Dox-dependent promoter. The addition of 5 μg/ml Dox provoked about 31.2 ± 3.1% more chitin in JP3-K4. Because the minimal inhibitory concentration of AFP against JP3-K4 increased as well (from 1 μg/ml in the absence of Dox to 3 μg/ml in the presence of Dox), we concluded that reinforced chitin synthesis indeed has the potential to protect A. niger against AFP attack.
DISCUSSION
With the hypothesis that the susceptibility of fungi toward AFP is also dependent on the cell's capacity to counteract AFP inhibitory effects, we sought to screen the yeast genomic deletion collection for AFP-sensitive mutants. The isolation of AFP-susceptible strains and their analysis in the present study strongly support the view that S. cerevisiae protects itself against AFP via stimulation of chitin synthesis, which is mainly accomplished on the transcriptional level via the calcium/calcineurin/Crz1p signaling pathway. A very likely candidate protein for the chitin rescue response is Chs3p because the chitin response was lost in a bni4Δ background. Most importantly, reinforcement of chitin synthesis is a response not merely specific for S. cerevisiae; it is a distinctive feature of other AFP-moderate sensitive and AFP-resistant fungi, supporting the conclusion that an elevation of chitin levels provides the necessary response to survive an AFP attack. Such a defense strategy seems not to be realized in AFP-sensitive fungi. Here, the classical CWI pathway becomes activated, the output of which is an increase in glucan but not chitin synthesis (13). This response, however, obviously fails to counteract AFP.
The Damage-Response Framework of AMP
Microbial Interactions
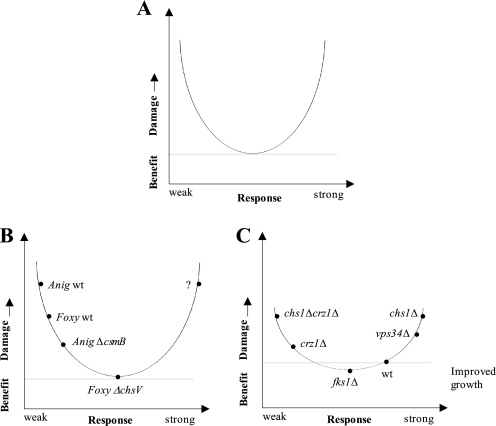
The microbial defense strategy is thus an important parameter that determines the susceptibility of fungi against AFP, a conclusion that most likely can be extrapolated to the interactions of microorganisms with other AMPs. If the most adequate response has been initiated, the microorganism can resist an AMP attack; if a too weak or an inappropriate response has been chosen, the microorganism becomes damaged or killed. Importantly, many signaling mechanisms, such as calcium signaling meant to rescue from cell stress, have the capacity to damage the microorganism itself (51, 52). Thus, a microbial response has to be envisioned as a tightrope walk between survival and death. To comprehensively understand the inhibitory effect of AFP and in general of AMPs, we therefore propose that not only the defense strategy has to be taken into account but also potentially self-induced damage by the microorganism. We therefore recommend the adoption of the concept of the “damage-response framework of microbial pathogenesis” put forward by Pirofksi and Casadevall in 1999 (53–55) for the analysis of interactions between AMPs and microorganisms. When translating the tenets of this conceptual approach to microbial-AMP interactions, one can postulate that the outcome of an AMP attack is dependent on (i) the innate susceptibility of the microorganism, (ii) the damage potential of the AMP defined by its concentration and target-(non)specific molecular interactions, and (iii) the microbial response, which can be appropriate, too weak, or too strong and thus detrimental to the host. Consequently, the damage-response framework plots the microbial damage as a function of the microbial response (Fig. 7A) and predicts that the cytotoxic capacity of a given AMP is relative and depends not only on the genetic background and AMP concentration but also on other variables, such as microbial survival strategies.
FIGURE 7.
Cytotoxic activities of AMPs and the microbial response. A, the damage-response framework of microbial pathogenesis is reflected by a parabolic curve (53–55). When translating this concept to the AMP-microorganism interaction, the y axis denotes microbial damage, which is defined as a perturbation of cell homeostasis. The microbial damage is a function of the microbial response, which, in terms of quality and quantity, can be considered as weak, appropriate, or (too) strong. Both a too weak and a too excessive response will damage or kill the microorganism. B, the csmB and the chsV mutation are protective for A. niger and F. oxysporum, respectively, whereby the latter makes F. oxysporum insensitive against AFP. With a question mark we denote the possible scenario in which a signaling pathway meant to protect the microorganisms causes self-damage when deregulated (e.g. increased cytoplasmic calcium concentrations might induce apoptosis). C, the curve in C is more flattened compared with B to indicate that S. cerevisiae is in general less susceptible to AFP than filamentous fungi. Plotted are different S. cerevisiae deletion mutants, which we observed to enhance or diminish AFP-induced damage based on their chitin response. Most importantly, the damage-response framework also opens the possibilities to include the paradoxical effect of improved growth in the presence of AFP, which we have observed for three S. cerevisiae mutants (Table 1, group F).
Fungal Interactions
A common feature of all AFP-sensitive and moderately sensitive fungi analyzed so far is that their plasma membranes become permeabilized within minutes (13–15) (Fig. 2), suggesting that the plasma membrane represents the Achilles heel of yeast and filamentous fungi when exposed to AFP. The damage-response framework of AFP-fungal interactions opens up many possible explanations for why some fungi survive the plasma membrane attack and some do not survive or only partially survive (Fig. 7, B and C). For example, a fungus can harbor a hypothetical AFP target but is resistant because of being fully competent in mitigating AFP (e.g. via increased chitin synthesis in S. cerevisiae and in P. chrysogenum) or because the target might be shielded because the cell wall is remodeled (e.g. as supposed for the ΔchsV mutant of F. oxysporum (56) and as also possible in the A. niger ΔcsmB strain). Alternatively, a fungus may contain a modified AFP target or less of it but is nevertheless susceptible because it is incapable of counteracting AFP (e.g. the chs1Δcrz1Δ mutant of S. cerevisiae). One imaginable example for a too strong response would be an excessive calcium response, a hypothetical scenario that remains to be scrutinized. However, for the AFP-related protein PAF, it has been shown that the PAF-sensitive fungus Neurospora crassa reacts with strongly increased intracellular calcium levels to PAF exposure (57). It is thus conceivable that such a response might be causally linked to the induced programmed cell death phenotype observed in A. nidulans after treatment with PAF (58).
S. cerevisiae Interactions
Of 100 yeast deletion strains selected, the growth of 35 became compromised by AFP. These mutants were affected in different cellular processes (mainly chitin synthesis and endocytosis) and also in different signaling pathways (calcium signaling, TOR signaling, cAMP-PKA signaling, and CWI signaling; Table 1). On one hand, this suggests that interference with chitin synthesis and endocytosis might render S. cerevisiae vulnerable to AFP, and on the other hand, it suggests that the concerted activities of these signaling pathways might be important to resist AFP.
Why are chitin synthesis and endocytosis important for the resistance of S. cerevisiae against AFP? The chs1Δ mutant showed the highest susceptibilities toward AFP, suggesting that S. cerevisiae can be attacked by AFP, especially during mother-daughter cell separation. This process is indeed very critical for cell integrity maintenance because the controlled activity of chitin synthesis (Chs1p) and chitin degradation (Cts1p) is necessary to separate both cells (39). If the chitin layer is not closed in a timely manner, the plasma membrane might not be shielded sufficiently and could potentially be permeabilized by AFP. This scenario could also explain why the wsc1Δ strain is very sensitive toward AFP, because the main function of the mechanosensor Wsc1p (but not Mid2p) is to sense cell wall stress during cell separation (40). If such a stress signal is not generated, S. cerevisiae might not respond to the AFP attack sufficiently or in a timely manner. Interestingly, Chs1p and Chs3p follow the same exocytotic and endocytotic route using chitosomes as transport vesicles (21, 59). They become mobilized to the plasma membrane in a cell cycle-dependent manner to synthesize chitin at the bud neck, after which they become endocytosed into chitosomes. Hence, endocytosis is essential to maintain the chitosome pool (21, 59). Our growth-inhibitory screen uncovered nine endocytotic mutants (group C, Table 1), two of which, end3Δ and sla2Δ, have been shown to contain reduced Chs3p levels in purified chitosomes (59, 60), and also vps34Δ, whose function is important for endocytic sorting (61). It is thus tempting to speculate that the blockage of endocytosis might interfere with the chitosomic reservoir of Chs1p and Chs3p, which in turn cannot be mobilized during AFP attack.
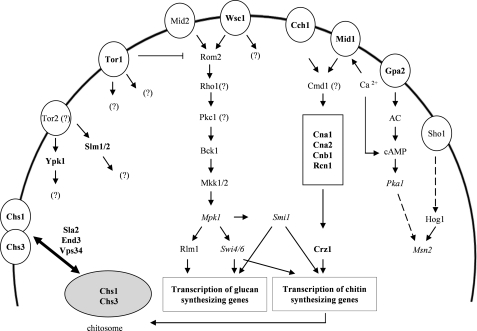
Notably, not all S. cerevisiae signaling mutants were similarly impaired in growth, indicating that the respective pathways contribute to a varying extent to AFP counteraction (Fig. 8). Based on the growth inhibition assays, we conclude that the CWI pathway is only of minor importance to protect S. cerevisiae against AFP (residual growth of 80–100%; the only exception is Wsc1p, with 25%). We caution, however, that Rho1p and Pkc1p could still be involved in the survival responses because both proteins escaped from our analysis (rho1Δ and pkc1Δ strains are not viable). However, if an AFP stress signal, supposedly recognized by Wsc1p, is transmitted to both proteins, the signal is probably not channeled into the MAPK cascade of the CWI pathway. A clear growth inhibition (residual growth 60–89%) was observed when strains deleted for components of the cAMP-PKA signaling pathway (GPA2, PKA1, and MSN2) were subjected to AFP. cAMP-PKA signaling is in fact a nutrient-sensing pathway that controls the cell cycle and transmits a glucose signal to various effectors, one of which can be the stress transcription factor Msn2p (62, 63). Interestingly, cAMP-PKA signaling is intertwined with both calcium and TOR signaling via Msn2p under nutrient starvation conditions (63, 64) and with Wsc1p under thermal stress conditions (65).
FIGURE 8.
Proteins and signaling pathways of S. cerevisiae involved in AFP counteraction. Proteins that are of major importance for the defense against AFP are indicated in boldface type, and proteins that contribute to a lesser extent are given in italics. Proteins that were not included in the screening assay (e.g. because of essential cell functions) are indicated with a question mark. The interconnection of proteins into signaling networks is based on Refs. 17, 21, and 36).
Calcium signaling and TOR signaling seemed to be the most crucial pathways for the rescue of S. cerevisiae from AFP attack (residual growth of 20–50%). Our data showed that stimulation of the chitin defense response is virtually completely dependent on the calcium signaling machinery, emphasizing the importance of calcium signaling for survival. Remarkably, all deletion strains of this signaling cascade (cch1Δ, mid1Δ, cna1Δ, cna2Δ, cnb1Δ, rcn1Δ, and crz1Δ) showed similar susceptibilities toward AFP, suggesting that once a calcium signal had entered the pathway (hypothetically as calcium influx via the plasma membrane channels Cch1p and/or Mid1p), the signal was directly channeled to its effector Crz1p.
The tor1Δ mutant was among the most susceptible strains, probably due to the fact that Tor1p occupies a central position in the regulatory network that balances growth, proliferation, and survival of S. cerevisiae (46, 65). 229 genetic and 175 physical interactions are recorded for Tor1p in the Saccharomyces Genome Data base, demonstrating its broad scope of cellular and regulatory functions. Among the AFP-susceptible strains identified in this study were also mutants deleted in Tor1p effector proteins, such as Sla2p, She4p, Vps34p, Ypk1p (endocytosis (44, 66)), Mpk1p (CWI signaling (17)), and Pka1p (cAMP-PKA signaling (62)), hinting at the possibility that Tor1p might contribute to the cellular defense against AFP via multiple pathways.
Conclusions
The genetic approach followed in this work allowed the identification of a valuable collection of AFP-sensitive S. cerevisiae deletion strains, whose initial analysis revealed that a concerted action of calcium signaling, TOR signaling, cAMP-PKA signaling, and CWI signaling is likely to safeguard S. cerevisiae against AFP. Biochemical analyses of selected strains uncovered that the cell wall of S. cerevisiae is fortified with chitin in the presence of AFP and that this response is largely dependent on transcriptional stimulation via the calcium/calcineurin/Crz1p pathway. A comparative analysis of different fungi showed a correlation between chitin response and AFP susceptibility, suggesting that an increase in cell wall chitin is the best strategy for yeast and filamentous fungi to survive AFP. Further analyses will decipher how other fungal survival strategies contribute to the defense against AFP and to what extent the respective signaling pathways are interconnected. We finally propose the adoption of the damage-response framework of microbial pathogenesis for the interactions of AMPs and microorganisms in order to comprehensively understand microbial survival strategies.
This work was supported by Federal Ministry of Economics and Technology (AiF) Grant 13305N) and by a grant from the German Academic Exchange Service (to J. P. O.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
U. Schmidt, unpublished.
- AMP
- antimicrobial peptide
- CWI
- cell wall integrity
- CFW
- calcofluor white
- Dox
- doxycycline
- PKA
- protein kinase A
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
REFERENCES
- 1. Frantz S. (2005) Nature 437, 942–943 [DOI] [PubMed] [Google Scholar]
- 2. Peschel A., Sahl H. G. (2006) Nat. Rev. Microbiol. 4, 529–536 [DOI] [PubMed] [Google Scholar]
- 3. Epand R. M., Vogel H. J. (1999) Biochim. Biophys. Acta 1462, 11–28 [DOI] [PubMed] [Google Scholar]
- 4. Zasloff M. (2002) Nature 415, 389–395 [DOI] [PubMed] [Google Scholar]
- 5. Wang G., Li X., Wang Z. (2009) Nucleic Acids Res. 37, D933–D937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z., Wang G. (2004) Nucleic Acids Res. 32, D590–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeaman M. R., Yount N. Y. (2003) Pharmacol. Rev. 55, 27–55 [DOI] [PubMed] [Google Scholar]
- 8. Aerts A. M., François I. E., Cammue B. P., Thevissen K. (2008) Cell Mol. Life Sci. 65, 2069–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer V., Wedde M., Stahl U. (2002) Mol. Genet. Genomics 266, 747–757 [DOI] [PubMed] [Google Scholar]
- 10. Campos-Olivas R., Bruix M., Santoro J., Lacadena J., Martinez del Pozo A., Gavilanes J. G., Rico M. (1995) Biochemistry 34, 3009–3021 [DOI] [PubMed] [Google Scholar]
- 11. Barakat H., Spielvogel A., Hassan M., El-Desouky A., El-Mansy H., Rath F., Meyer V., Stahl U. (2010) Appl. Microbiol. Biotechnol. 87, 617–624 [DOI] [PubMed] [Google Scholar]
- 12. Meyer V. (2008) Appl. Microbiol. Biotechnol. 78, 17–28 [DOI] [PubMed] [Google Scholar]
- 13. Hagen S., Marx F., Ram A. F., Meyer V. (2007) Appl. Environ. Microbiol. 73, 2128–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theis T., Wedde M., Meyer V., Stahl U. (2003) Antimicrob. Agents Chemother. 47, 588–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Theis T., Marx F., Salvenmoser W., Stahl U., Meyer V. (2005) Res. Microbiol. 156, 47–56 [DOI] [PubMed] [Google Scholar]
- 16. Martinez Del Pozo A., Lacadena V., Mancheno J. M., Olmo N., Onaderra M., Gavilanes J. G. (2002) J. Biol. Chem. 277, 46179–46183 [DOI] [PubMed] [Google Scholar]
- 17. Levin D. E. (2005) Microbiol. Mol. Biol. Rev. 69, 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damveld R. A., Arentshorst M., Franken A., vanKuyk P. A., Klis F. M., van den Hondel C. A., Ram A. F. (2005) Mol. Microbiol. 58, 305–319 [DOI] [PubMed] [Google Scholar]
- 19. Meyer V., Damveld R. A., Arentshorst M., Stahl U., van den Hondel C. A., Ram A. F. (2007) J. Biol. Chem. 282, 32935–32948 [DOI] [PubMed] [Google Scholar]
- 20. Fujioka T., Mizutani O., Furukawa K., Sato N., Yoshimi A., Yamagata Y., Nakajima T., Abe K. (2007) Eukaryot. Cell 6, 1497–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lesage G., Bussey H. (2006) Microbiol. Mol. Biol. Rev. 70, 317–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., Hieter P., Boeke J. D. (1998) Yeast 14, 115–132 [DOI] [PubMed] [Google Scholar]
- 23. Bennett J. W., Lasure L. (1991) More Gene Manipulations in Fungi, Academic Press, Inc., San Diego [Google Scholar]
- 24. Meyer V., Ram A. F., Punt P. J. (2010) in Manual of Industrial Microbiology and Biotechnology (Demain A. L., Davis J. eds) pp. 318–329, 3rd Ed., John Wiley & Sons, Inc., New York [Google Scholar]
- 25. Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory Press, New York [Google Scholar]
- 26. Ram A. F., Klis F. M. (2006) Nat. Protoc. 1, 2253–2256 [DOI] [PubMed] [Google Scholar]
- 27. Ram A. F., Arentshorst M., Damveld R. A., vanKuyk P. A., Klis F. M., van den Hondel C. A. (2004) Microbiology 150, 3315–3326 [DOI] [PubMed] [Google Scholar]
- 28. Tracey M. V. (1955) in Modern Methods of Plant Analysis, Vol. 2 (Peach P., Tracey M. V. eds) pp. 264–274, Springer Verlag, Berlin [Google Scholar]
- 29. Popolo L., Gilardelli D., Bonfante P., Vai M. (1997) J. Bacteriol. 179, 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fortwendel J. R., Juvvadi P. R., Pinchai N., Perfect B. Z., Alspaugh J. A., Perfect J. R., Steinbach W. J. (2009) Antimicrob. Agents Chemother. 53, 476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang L., Ukil L., Osmani A., Nahm F., Davies J., De Souza C. P., Dou X., Perez-Balaguer A., Osmani S. A. (2004) Eukaryot. Cell 3, 1359–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyer V., Arentshorst M., El-Ghezal A., Drews A. C., Kooistra R., van den Hondel C. A., Ram A. F. (2007) J. Biotechnol. 128, 770–775 [DOI] [PubMed] [Google Scholar]
- 33. Chopra R., Sharma V. M., Ganesan K. (1999) Appl. Environ. Microbiol. 65, 2267–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Gorcom R. F., van den Hondel C. A. (1988) Nucleic Acids Res. 16, 9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carvalho N. D., Arentshorst M., Jin Kwon M., Meyer V., Ram A. F. (2010) Appl. Microbiol. Biotechnol. 87, 1463–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lesage G., Shapiro J., Specht C. A., Sdicu A. M., Ménard P., Hussein S., Tong A. H., Boone C., Bussey H. (2005) BMC Genet. 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Plaine A., Walker L., Da Costa G., Mora-Montes H. M., McKinnon A., Gow N. A., Gaillardin C., Munro C. A., Richard M. L. (2008) Fungal Genet. Biol. 45, 1404–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roncero C., Valdivieso M. H., Ribas J. C., Durán A. (1988) J. Bacteriol. 170, 1945–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cabib E., Silverman S. J., Shaw J. A. (1992) J. Gen. Microbiol. 138, 97–102 [DOI] [PubMed] [Google Scholar]
- 40. Rodicio R., Heinisch J. J. (2010) Yeast 27, 531–540 [DOI] [PubMed] [Google Scholar]
- 41. Grossmann G., Malinsky J., Stahlschmidt W., Loibl M., Weig-Meckl I., Frommer W. B., Opekarová M., Tanner W. (2008) J. Cell Biol. 183, 1075–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slessareva J. E., Routt S. M., Temple B., Bankaitis V. A., Dohlman H. G. (2006) Cell 126, 191–203 [DOI] [PubMed] [Google Scholar]
- 43. Herman P. K., Emr S. D. (1990) Mol. Cell. Biol. 10, 6742–6754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zurita-Martinez S. A., Puria R., Pan X., Boeke J. D., Cardenas M. E. (2007) Genetics 176, 2139–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang Y. Y., Juhász G., Goraksha-Hicks P., Arsham A. M., Mallin D. R., Muller L. K., Neufeld T. P. (2009) Biochem. Soc. Trans. 37, 232–236 [DOI] [PubMed] [Google Scholar]
- 46. Inoki K., Ouyang H., Li Y., Guan K. L. (2005) Microbiol. Mol. Biol. Rev. 69, 79–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanz M., Castrejón F., Durán A., Roncero C. (2004) Microbiology 150, 3229–3241 [DOI] [PubMed] [Google Scholar]
- 48. Roncero C. (2002) Curr. Genet. 41, 367–378 [DOI] [PubMed] [Google Scholar]
- 49. Walker L. A., Munro C. A., de Bruijn I., Lenardon M. D., McKinnon A., Gow N. A. (2008) PLoS Pathog. 4, e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pel H. J., de Winde J. H., Archer D. B., Dyer P. S., Hofmann G., Schaap P. J., Turner G., de Vries R. P., Albang R., Albermann K., Andersen M. R., Bendtsen J. D., Benen J. A., van den Berg M., Breestraat S., Caddick M. X., Contreras R., Cornell M., Coutinho P. M., Danchin E. G., Debets A. J., Dekker P., van Dijck P. W., van Dijk A., Dijkhuizen L., Driessen A. J., d'Enfert C., Geysens S., Goosen C., Groot G. S., de Groot P. W., Guillemette T., Henrissat B., Herweijer M., van den Hombergh J. P., van den Hondel C. A., van der Heijden R. T., van der Kaaij R. M., Klis F. M., Kools H. J., Kubicek C. P., van Kuyk P. A., Lauber J., Lu X., van der Maarel M. J., Meulenberg R., Menke H., Mortimer M. A., Nielsen J., Oliver S. G., Olsthoorn M., Pal K., van Peij N. N., Ram A. F., Rinas U., Roubos J. A., Sagt C. M., Schmoll M., Sun J., Ussery D., Varga J., Vervecken W., van de Vondervoort P. J., Wedler H., Wösten H. A., Zeng A. P., van Ooyen A. J., Visser J., Stam H. (2007) Nat. Biotechnol. 25, 221–231 [DOI] [PubMed] [Google Scholar]
- 51. Zhang N. N., Dudgeon D. D., Paliwal S., Levchenko A., Grote E., Cunningham K. W. (2006) Mol. Biol. Cell 17, 3409–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berridge M. J., Lipp P., Bootman M. D. (2000) Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 53. Casadevall A., Pirofski L. A. (1999) Infect. Immun. 67, 3703–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Casadevall A., Pirofski L. A. (2000) Infect. Immun. 68, 6511–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Casadevall A., Pirofski L. A. (2003) Nat. Rev. Microbiol. 1, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Madrid M. P., Di Pietro A., Roncero M. I. (2003) Mol. Microbiol. 47, 257–266 [DOI] [PubMed] [Google Scholar]
- 57. Binder U., Chu M., Read N. D., Marx F. (2010) Eukaryot. Cell 9, 1374–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leiter E., Szappanos H., Oberparleiter C., Kaiserer L., Csernoch L., Pusztahelyi T., Emri T., Pócsi I., Salvenmoser W., Marx F. (2005) Antimicrob. Agents Chemother. 49, 2445–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ziman M., Chuang J. S., Schekman R. W. (1996) Mol. Biol. Cell 7, 1909–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ziman M., Chuang J. S., Tsung M., Hamamoto S., Schekman R. (1998) Mol. Biol. Cell 9, 1565–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Strahl T., Thorner J. (2007) Biochim. Biophys. Acta 1771, 353–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tamaki H. (2007) J. Biosci. Bioeng. 104, 245–250 [DOI] [PubMed] [Google Scholar]
- 63. Ohdate T., Izawa S., Kita K., Inoue Y. (2010) Genes Cells 15, 59–75 [DOI] [PubMed] [Google Scholar]
- 64. Beck T., Hall M. N. (1999) Nature 402, 689–692 [DOI] [PubMed] [Google Scholar]
- 65. Fuchs B. B., Mylonakis E. (2009) Eukaryot. Cell 8, 1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aronova S., Wedaman K., Anderson S., Yates J., 3rd, Powers T. (2007) Mol. Biol. Cell 18, 2779–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meyer V., Wanka F., van Gent J., Arentshorst M., van den Hondel C. A., Ram A. F. (2011) Appl. Environ. Microbiol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]