Abstract
A pivotal step in the mitochondrial pathway of apoptosis is activation of Bak and Bax, although the molecular mechanism remains controversial. To examine whether mitochondrial apoptosis can be induced by just a lack of antiapoptotic Bcl-2-like proteins or requires direct activators of the BH3-only proteins including Bid and Bim, we studied the molecular requisites for platelet apoptosis induced by Bcl-xL deficiency. Severe thrombocytopenia induced by thrombocyte-specific Bcl-xL knock-out was fully rescued in a Bak and Bax double knock-out background but not with single knock-out of either one. In sharp contrast, deficiency of either Bid, Bim, or both did not alleviate thrombocytopenia in Bcl-xL knock-out mice. An in vitro study revealed that ABT-737, a Bad mimetic, induced platelet apoptosis in association with a conformational change of the amino terminus, translocation from the cytosol to mitochondria, and homo-oligomerization of Bax. ABT-737-induced Bax activation and apoptosis were also observed in Bid/Bim-deficient platelets. Human platelets, upon storage, underwent spontaneous apoptosis with a gradual decline of Bcl-xL expression despite a decrease in Bid and Bim expression. Apoptosis was attenuated in Bak/Bax-deficient or Bcl-xL-overexpressing platelets but not in Bid/Bim-deficient platelets upon storage. In conclusion, platelet lifespan is regulated by a fine balance between anti- and proapoptotic multidomain Bcl-2 family proteins. Despite residing in platelets, BH3-only activator proteins Bid and Bim are dispensable for Bax activation and mitochondrial apoptosis.
Keywords: Apoptosis, Caspase, Cell Death, Mitochondrial Apoptosis, Mouse Genetics, Oncogene, Platelet, BH3-only Protein, Bcl-2 Family, Platelet Storage
Introduction
Platelets are unique blood cells that do not have a nucleus but contain mitochondria and have the daily job of handling hemostasis and thrombosis (1). They are produced from megakaryocytes and once released into circulation can function for about 10 days in humans and 4–5 days in mice (2). They are then thought to be destroyed by the reticuloendothelial system. Regarding the mechanism that controls their lifespan, several studies have observed a decrease in mitochondrial membrane potential, caspase activation, and phosphatidylserine exposure in platelets, leading to the conclusion that platelets undergo apoptotic cell death (3–5). It has been demonstrated that platelets contain several apoptosis-related proteins such as Bcl-2 family proteins and a variety of caspase family proteins (3–7). Recently, Mason et al. (8) reported that knock-out of a single allele of the bcl-x gene results in mild thrombocytopenia, which is ameliorated in a Bak knock-out background. We have also reported previously that thrombocyte-specific homozygous Bcl-xL knock-out mice show marked thrombocytopenia (9). These findings established the critical role of Bcl-2 family proteins in regulating platelet apoptosis and lifespan. Platelets may be the simplest model for the study of Bcl-2 biology with physiological relevance because they neither perform de novo protein synthesis nor undergo proliferation.
The proapoptotic multidomain Bcl-2 family proteins Bak and Bax serve as effector molecules for the mitochondrial pathway of apoptosis. Upon activation, they form pores by homo-oligomerization at the mitochondrial outer membrane through which apoptogenic factors such as cytochrome c are released into the cytosol (10). Currently, three models for regulation of Bak/Bax-dependent mitochondrial apoptosis by Bcl-2 family proteins have been proposed (11–15). One, referred to as the indirect model or displacement model, argues that Bak and Bax are constitutively active and are neutralized by binding to at least one or more antiapoptotic members of the multidomain Bcl-2 family proteins including Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and Bfl-1/A1. BH33-only proteins such as Bad, Bid, Bim, Noxa, and Puma bind to the antiapoptotic Bcl-2 proteins to unleash Bak and Bax (15). The second model, referred to as the direct model, argues that Bak and Bax are inactive by default and require activator proteins to function. Among BH3-only proteins, Bid and Bim are classified as activator proteins with the others classified as sensitizer proteins because only Bid and Bim have been demonstrated to directly activate Bak and Bax (16, 17). In this model, Bid and Bim are sequestered by the antiapoptotic Bcl-2 family proteins, and the sensitizer BH3-only proteins bind to the antiapoptotic Bcl-2 proteins to liberate Bid and Bim so they can directly engage Bak and Bax (14). The third model, referred to as the embedded together model, argues that BH3-only activator proteins can recruit not only Bax but also antiapoptotic Bcl-2 proteins to mitochondrial membranes. Although membrane-bound Bax can form oligomers, membrane-bound antiapoptotic Bcl-2 proteins function as a dominant-negative Bax by competitively binding to Bax (12, 18).
In the physiological setting, genetic studies have revealed a functional relationship between BH3-only activator proteins and multidomain Bcl-2 family proteins. For instance, fatal polycystic kidney disease and lymphopenia caused by loss of Bcl-2 are ameliorated in a Bim knock-out background (19). Similarly, we reported previously that spontaneous hepatocyte apoptosis caused by hepatocyte-specific deficiency of Bcl-xL or Mcl-1 is alleviated by Bid deficiency (20, 21). These studies indicated that Bid or Bim is apparently involved in apoptotic phenotypes induced by lack of an antiapoptotic Bcl-2 family protein. However, it had not been established whether or not these direct activators are required for Bak/Bax activation, leading to mitochondrial apoptosis.
In the present study, we explored the molecular requisites for platelet apoptosis induced by Bcl-xL deficiency. We observed complete recovery from severe thrombocytopenia in Bcl-xL knock-out mice with a Bak and Bax double knock-out background, confirming that Bcl-xL deficiency causes apoptotic cell death through a Bak/Bax-dependent mitochondrial apoptosis machinery. Deficiency of either Bid, Bim, or both did not alleviate thrombocytopenia in Bcl-xL knock-out mice. An in vitro study revealed that pharmacological inhibition of antiapoptotic Bcl-2 family proteins sufficiently activated Bax protein to cause apoptosis even in Bid/Bim-deficient platelets. Our current study indicates that Bak/Bax can be activated by neutralization of antiapoptotic Bcl-2 family proteins for the execution of apoptotic cell death without involvement of the BH3-only direct activator proteins Bid and Bim in specific cellular contexts.
EXPERIMENTAL PROCEDURES
Mice
Mice carrying a bcl-x gene with two loxP sequences at the promoter region and a second intron (bcl-xflox/flox) (22) and heterozygous pf4-Cre transgenic mice expressing the Cre recombinase gene under the regulation of the promoter of the platelet factor 4 gene (23) have been described previously. Thrombocyte-specific Bcl-xL knock-out mice (bcl-xflox/flox pf4-Cre) (9) and systemic Bid knock-out mice (24) also have been described previously. We purchased C57BL/6J mice from Charles River (Osaka, Japan) and systemic Bim knock-out mice, systemic Bak knock-out mice, systemic Bax knock-out mice, and conditional Bak/Bax double knock-out mice (bak−/−baxflox/flox) from The Jackson Laboratory (Bar Harbor, ME). We generated thrombocyte-specific Bcl-xL/Bid double knock-out mice (bid−/−bcl-xflox/flox pf4-Cre), Bcl-xL/Bim double knock-out mice (bim−/−bcl-xflox/flox pf4-Cre), Bcl-xL/Bid/Bim triple knock-out mice (bid−/−bim−/−bcl-xflox/flox pf4-Cre), Bcl-xL/Bak double knock-out mice (bak−/−bcl-xflox/flox pf4-Cre), Bcl-xL/Bax double knock-out mice (bax−/−bcl-xflox/flox pf4-Cre), Bcl-xL/Bak/Bax triple knock-out mice (bak−/−baxflox/floxbcl-xflox/flox pf4-Cre), and Bak/Bax double knock-out mice (bak−/−baxflox/flox pf4-Cre) by mating the strains. We also generated systemic Bid/Bim double knock-out mice (bid−/−bim−/−) by mating the strains. Heterozygous HA-hBcl-xL transgenic mice expressing human Bcl-xL gene under the regulation of the CAG promoter were generated according to a procedure described previously (25) using a hemagglutinin-tagged human bcl-xL expression plasmid, pcDNA3HAbcl-xL (26). Mice were maintained in a specific pathogen-free facility and treated with humane care under approval from the Animal Care and Use Committee of Osaka University Medical School.
Hematological Analyses
Blood was collected from the inferior vena cava of mice. Complete blood cell counts were determined using an automated cell counter (Sysmex, Kobe, Japan).
Platelet Isolation, Storage, and Preparation of Lysates
Platelets were isolated as described previously (9). Briefly, whole blood collected from mice or healthy donors was mixed with ¼ volume of citrate-phosphate-dextrose (Sigma-Aldrich). Platelet-rich plasma was obtained by centrifugation at 100 × g for 15 min at room temperature without braking. To avoid mechanical aggregation of platelets by centrifugation, platelet-rich plasma was incubated with 1 μm prostaglandin E1 (Sigma-Aldrich) and 1 unit/ml apyrase (Sigma-Aldrich) (27). Next, platelets were isolated by centrifugation at 200 × g at room temperature for 15 min. Washed platelets were resuspended in modified Tyrode's buffer (5 mm HEPES, 137 mm NaCl, 2.7 mm KCl, 0.4 mm NaH2PO4·2H2O, 2.8 mm dextrose, pH 7.4) and left standing for 30 min before use. In some experiments, platelet-rich plasma or platelet suspension was stored under continuous gentle agitation in an incubator at 25 °C for the indicated time. For preparation of cell lysates, the platelet pellet was obtained by centrifugation at 200 × g at room temperature for 10 min after incubation with 1 μm prostaglandin E1 (Sigma-Aldrich) for 10 min and lysed in lysis buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1× protease inhibitor mixture (Nacalai Tesque, Kyoto, Japan), 1× phosphatase inhibitor mixture (Nacalai Tesque), phosphate-buffered saline, pH 7.4) unless otherwise indicated. The platelet lysates were cleared by centrifugation at 10,000 × g at 4 °C for 15 min. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce). We confirmed that incubation with prostaglandin E1 did not affect the caspase-3/7 activity of isolated platelet supernatant (data not shown).
In Vitro ABT-737 Experiment
ABT-737, provided by Abbott Laboratories (Abbott Park, IL), was dissolved with DMSO. Platelets were treated with 10 μm ABT-737 or DMSO for the indicated times.
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) Assay
The MTS assay is a colorimetric assay for measuring the ability of living cells to reduce the uncolored MTS substrate to purple formazan. In platelets, this activity is directly related to cellular viability (4, 5). The MTS assay was performed with a cell proliferation kit (CellTiter 96 AQueous, Promega, Tokyo, Japan) according to the manufacturer's protocol. Upon addition of MTS solution, the reaction plate was incubated at 37 °C for 4 h, and then the absorbance was read at 490 nm with a plate reader (Bio-Rad).
Caspase-3/7 Activity
Serum or platelet supernatant caspase-3/7 activity was measured with a luminescent substrate assay for caspase-3 and caspase-7 (Caspase-Glo assay, Promega) according to the manufacturer's protocol.
Western Blot Analysis
Equal amounts of protein lysates were electrophoretically separated using SDS-PAGE and transferred onto PVDF membrane unless otherwise indicated. For immunodetection, the following antibodies were used: rabbit polyclonal antibody to Bcl-xL (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal antibody to Bid, rabbit polyclonal antibody to Bax, rabbit polyclonal antibody to cleaved caspase-3, rabbit polyclonal antibody to Bim, rabbit polyclonal antibody to Puma, rabbit polyclonal antibody to Bcl-2, rabbit polyclonal antibody to Bcl-w, rabbit polyclonal antibody to cytochrome c oxidase IV (Cell Signaling Technology, Beverly, MA), rabbit polyclonal antibody to Bak, rabbit polyclonal antibody to Bax (Millipore, Billerica, MA), rabbit polyclonal antibody to GAPDH (Trevigen, Gaithersburg, MD), rabbit polyclonal antibody to Bim (Assay Designs, Ann Arbor, MI), and mouse monoclonal antibody to β-actin (Sigma-Aldrich).
Isolation of Mitochondria-rich and Cytosolic Fractions
Platelet homogenates were prepared by repeated freeze-and-thaw methods (28). Briefly, platelets in isolation buffer (225 mm mannitol, 75 mm sucrose, 0.1 mm EGTA, 1 mg/ml fatty acid-free BSA, 10 mm HEPES-KOH, 1× proteinase inhibitor mixture, 1× phosphatase inhibitor mixture, pH 7.4) were frozen in liquid nitrogen for 1 min and then thawed at 37 °C for 3 min. This freeze-and-thaw sequence was repeated for two more cycles, and then the samples were centrifuged at 700 × g for 10 min at 4 °C. The supernatant was further centrifuged at 15,000 × g for 10 min at 4 °C. The pellet was regarded as the mitochondria-rich fraction, and the supernatant was the cytosolic fraction.
Immunoprecipitation
Platelets (1.0 × 108) were lysed in HNC buffer (25 mm HEPES/Na, 300 mm NaCl, 2% CHAPS, 1× protease inhibitor mixture, 1× phosphatase inhibitor mixture, pH 7.5) and immunoprecipitated using mouse monoclonal antibody to Bax (clone 6A7) (Abcam, Cambridge, MA) with an immunoprecipitation kit (Dynabeads Protein G, Invitrogen). Control immunoprecipitations were performed using mouse control IgG (Abcam).
Detection of Bax Oligomerization
Bax oligomerization was detected as described previously (29). Briefly, 5.0 × 107 platelets were lysed with HNC buffer. Next, ∼50 mg of platelet lysates was incubated with 5 mm bismaleimidohexane (Pierce) and 5 mm bis(sulfosuccinimidyl) suberate (Pierce) for 30 min at room temperature. To quench cross-linkers, the lysates were incubated with 100 mm Tris-HCl, pH 7.5 for 15 min at room temperature. Bax oligomers were detected by Western blot using rabbit polyclonal antibody to Bax (Cell Signaling Technology).
Statistical Analysis
All data are expressed as mean ± S.D. Statistical analyses were performed by unpaired Student's t test or by one-way analysis of variance. When analyses of variance were applied, differences in the mean values among the groups were examined by Scheffe post hoc correction. p < 0.01 was considered statistically significant.
RESULTS
Thrombocytopenia Induced by Bcl-xL Deficiency Is Dependent on Proapoptotic Effector Proteins Bax and Bak
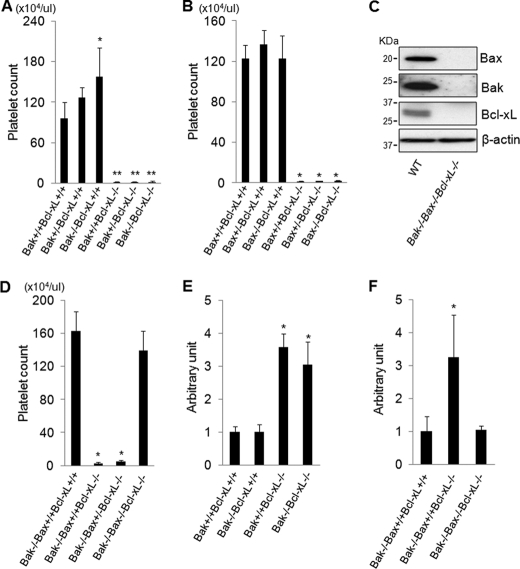
Previous research has reported that the mild thrombocytopenia caused by heterozygous Bcl-xL knock-out is prevented in a Bak knock-out background (8). We therefore first examined whether the severe thrombocytopenia seen in the thrombocyte-specific homozygous Bcl-xL knock-out mice (9) could also be prevented by loss of Bak. Bcl-xL and Bak double knock-out mice were generated by mating thrombocyte-specific Bcl-xL knock-out mice and systemic Bak knock-out mice. Bcl-xL and Bak double knock-out mice were born at the expected Mendelian frequency, but unexpectedly, their platelet count did not show any difference from that of the thrombocyte-specific Bcl-xL knock-out mice (Fig. 1A). Among Bcl-2 family proteins, not only Bak but Bax is also a well recognized proapoptotic effector protein. Therefore, we next generated Bcl-xL and Bax double knock-out mice by mating thrombocyte-specific Bcl-xL knock-out mice and systemic Bax knock-out mice. Bcl-xL and Bax double knock-out mice were also born at the expected Mendelian frequency, and their platelet count also was not different from that of the thrombocyte-specific Bcl-xL knock-out mice (Fig. 1B). To investigate whether the Bak/Bax-dependent mitochondrial apoptotic pathway is actually involved in thrombocytopenia caused by Bcl-xL deficiency, we generated Bcl-xL, Bak, and Bax triple knock-out mice by mating Bcl-xL and Bak double knock-out mice with thrombocyte-specific Bax knock-out mice because systemic Bak and Bax double knock-out mice usually die as neonates (30). Triple knock-out mice were born at the expected Mendelian frequency and did not show any protein expression of Bcl-xL, Bak, and Bax in their platelets on examination by Western blotting (Fig. 1C). The platelet count of the triple knock-out mice was almost normal and not significantly different from that of systemic Bak knock-out mice, which served as a control for this mating (Fig. 1D). These findings clearly demonstrated that the severe thrombocytopenia induced by thrombocyte-specific Bcl-xL knock-out was fully dependent on Bak/Bax. Serum caspase-3/7 activity, monitored by specific cleavage of the Ac-DEVD-p-nitroanilide substrate, was significantly higher in thrombocyte-specific Bcl-xL knock-out mice than control littermates (Fig. 1E), suggesting platelet apoptosis in the knock-out mice. Caspase activation in the Bcl-xL knock-out mice was not alleviated in a Bak knock-out background (Fig. 1E) but was diminished with a Bak and Bax double knock-out background (Fig. 1F), suggesting that Bcl-xL deficiency caused platelet apoptosis through a Bak/Bax-dependent mitochondrial pathway. These results also implied that either Bak or Bax was sufficient to induce apoptosis in Bcl-xL-deficient platelets.
FIGURE 1.
Thrombocytopenia induced by Bcl-xL deficiency is dependent on Bcl-2 effector proteins Bak and Bax. Bcl-xL+/+ and Bcl-xL−/− stand for bcl-xflox/flox without pf4-Cre and bcl-xflox/flox with pf4-Cre, respectively. Bak+/+, Bak+/−, and Bak−/− stand for bak+/+, bak+/−, and bak−/−, respectively. WT stands for wild type. A, platelet counts of the offspring from mating of bak+/−bcl-xflox/flox pf4-Cre mice and bak+/−bcl-xflox/flox mice (more than four mice per group; *, p < 0.01 versus all other groups; **, p < 0.01 versus Bcl-xL+/+ groups). B, platelet counts of the offspring from mating of bax+/−bcl-xflox/flox pf4-Cre mice and bax+/−bcl-xflox/flox mice (more than five mice per group; *, p < 0.01 versus Bcl-xL+/+ groups). Bax+/+, Bax+/−, and Bax−/− stand for bax+/+, bax+/−, and bax−/−, respectively. C, Western blot of platelet lysates for the expression of Bcl-xL, Bak, and Bax. D, platelet counts of the offspring from mating of bak−/−baxflox/+bcl-xflox/flox pf4-Cre mice and bak−/−baxflox/+bcl-xflox/flox mice (more than eight mice per group; *, p < 0.01 versus Bak−/−Bax+/+Bcl-xL+/+ group and Bak−/−Bax−/−Bcl-xL−/− group. Bax+/+, Bax+/−, and Bax−/− stand for bax+/+, baxflox/+ with pf4-Cre, and baxflox/flox with pf4-Cre, respectively. E, serum caspase-3/7 activity of the offspring from mating of bak+/−bcl-xflox/flox pf4-Cre mice and bak+/− bcl-xflox/flox mice (n = 5 or 6/group; *, p < 0.01 versus Bcl-xL+/+ group). F, serum caspase-3/7 activity of the offspring from mating of bak−/− baxflox/+bcl-xflox/flox pf4-Cre mice and bak−/−baxflox/+bcl-xflox/flox mice (n = 8/group; *, p < 0.01 versus all). Bax+/+, Bax+/−, and Bax−/− stand for bax+/+, baxflox/+ with pf4-Cre, and baxflox/flox with pf4-Cre, respectively.
ABT-737 Treatment Provokes Bak/Bax-dependent Apoptosis in Platelets
To investigate the molecular mechanisms of Bak/Bax-dependent platelet apoptosis provoked by a lack of antiapoptotic Bcl-2 proteins, we conducted an in vitro study using ABT-737, a Bad mimetic, which antagonizes the antiapoptotic function of Bcl-xL, Bcl-2, and Bcl-w by binding to the hydrophobic groove of these proteins (31). Western blot revealed that these antiapoptotic Bcl-2 proteins existed in platelets (Fig. 2A), and ABT-737 has already been reported to cause apoptosis in platelets in both in vivo and in vitro settings (7, 8). We first examined whether ABT-737-induced platelet apoptosis was executed via the Bak/Bax-dependent mitochondrial pathway. In platelets isolated from wild-type mice, administration of ABT-737 caused cleavage of caspase-3 (Fig. 2B). Supernatants of ABT-737-treated platelets showed marked elevation of caspase-3/7 activity (Fig. 2C). In addition, platelet cellular viability, which can be assessed by MTS assay (3, 4), decreased upon ABT-737 treatment (Fig. 2D). On the other hand, although expression of targeted antiapoptotic Bcl-2 proteins was not different between platelets from wild-type mice and Bak/Bax double knock-out mice (Fig. 2A), ABT-737 treatment neither caused caspase activation nor impaired cellular integrity in Bak/Bax-deficient platelets (Fig. 2, B–D). These findings demonstrated that ABT-737 caused platelet apoptosis via the Bak/Bax-dependent mitochondrial pathway. Interestingly, unlike what was reported previously (8), Bak deficiency could alleviate neither caspase activation nor loss of cellular viability in ABT-737-treated platelets (Fig. 2, B–D), offering evidence of the redundancy of Bak and Bax proteins in executing apoptosis in platelets under inhibition of these antiapoptotic Bcl-2 proteins.
FIGURE 2.
ABT-737 treatment provokes Bak/Bax-dependent apoptosis in platelets. WT, Bak−/−Bax−/−, and Bak−/− stand for wild type, bak−/−baxflox/flox with pf4-Cre, and bak−/−, respectively. A, Western blot of platelet lysates for the expression of Bak, Bax, Bcl-xL, Bcl-2, and Bcl-w. B, platelets (3.0 × 107) were incubated with 10 μm ABT-737 or vehicle for 2 h at room temperature. A Western blot of platelet lysates for the expression of cleaved caspase-3 is shown. C and D, platelets (2.0 × 106) were incubated with 10 μm ABT-737 or vehicle for 2 h at room temperature. C, caspase-3/7 activity of platelet supernatant (n = 4/group). D, MTS assay (n = 5/group). RLU, relative light units.
ABT-737 Treatment Causes Bax Activation in Platelets
After ABT-737 treatment of the platelets, we next examined the activation status of the Bax protein in these platelets. In general, Bax activation is divided into sequential steps. When subjected to a variety of apoptotic stimuli, the Bax protein first undergoes a conformational change such as exposure of the amino terminus. This active form is translocated from the cytosol to the mitochondria. Finally, mitochondrial Bax undergoes self-oligomerization, leading to permeabilization of the outer mitochondrial membrane (32). We found that upon addition of ABT-737 to platelets the Bax protein underwent a conformational change as demonstrated by Western blotting upon immunoprecipitation with an antibody that specifically recognizes the amino terminus of the Bax protein (33) (Fig. 3A). In addition, upon ABT-737 treatment, the Bax protein was translocated from the cytosol to the mitochondria (Fig. 3B) and then underwent homo-oligomerization (Fig. 3C). These findings indicated that inhibition of antiapoptotic Bcl-2 proteins in platelets caused Bax activation, promoting Bak/Bax-dependent mitochondrial apoptosis followed by caspase activation.
FIGURE 3.
ABT-737 treatment causes Bax activation in platelets. A–C, platelets (1.0 × 108) isolated from C57BL/6J mice were incubated with 10 μm ABT-737 or vehicle for 2 h at room temperature. A, Western blot of platelet lysates for the expression of Bax after immunoprecipitation (IP) using mouse antibody that specifically recognizes activated Bax (6A7) or mouse control IgG (active Bax exposes an amino-terminal epitope (amino acids 12–24) that is recognized by 6A7). B, Western blot for the expression of Bak, Bax, CoxIV (cytochrome c oxidase IV), and GAPDH after cellular fractionation of the platelet lysates. C, Western blot for the expression of Bax after incubation of the platelet lysates with or without protein cross-linkers (5 mm bismaleimidohexane and 5 mm bis(sulfosuccinimidyl) suberate). IB, immunoblot.
Thrombocytopenia Induced by Bcl-xL Deficiency Does Not Require BH3-only Activator Proteins Bid and Bim
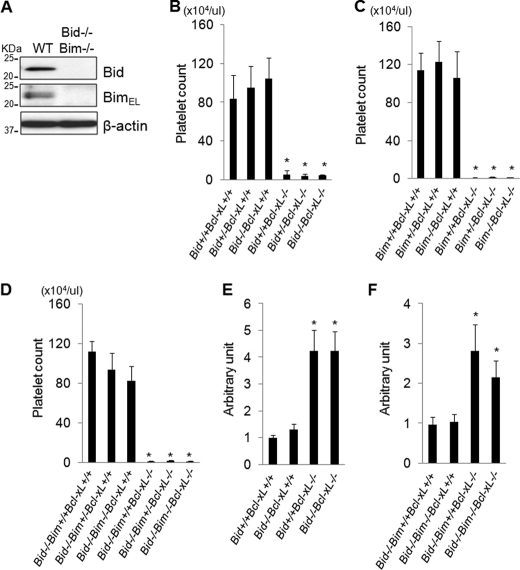
We explored whether Bak/Bax-dependent platelet apoptosis induced by Bcl-xL deficiency requires the direct activator proteins Bid and Bim. Western blot revealed that Bid and Bim were both present in platelets (Fig. 4A). We generated Bcl-xL/Bid double knock-out mice and Bcl-xL/Bim double knock-out mice by mating thrombocyte-specific Bcl-xL knock-out mice with systemic Bid knock-out mice or Bim knock-out mice, respectively. These double knock-out mice showed massive thrombocytopenia that was not alleviated at all compared with that of thrombocyte-specific Bcl-xL knock-out mice (Fig. 4, B and C). It was possible that, in Bcl-xL-deficient platelets, the existence of either Bid or Bim was sufficient to activate Bak/Bax directly, leading to platelet apoptosis in these double knock-out mice. We then generated Bcl-xL, Bid, and Bim triple knock-out mice by mating Bcl-xL/Bid double knock-out mice with Bcl-xL/Bim double knock-out mice. These triple knock-out mice still showed massive thrombocytopenia without any difference of platelet count compared with that of Bcl-xL/Bid double knock-out mice (Fig. 4D). These findings clearly demonstrated that BH3-only activator proteins Bid and Bim were dispensable for the severe thrombocytopenia induced by thrombocyte-specific Bcl-xL deletion in vivo. In addition, caspase activation in thrombocyte-specific Bcl-xL knock-out mice was not alleviated even in the Bid and Bim double knock-out background (Fig. 4, E and F), suggesting that the lack of Bcl-xL required neither Bid nor Bim to trigger Bak/Bax-dependent platelet apoptosis.
FIGURE 4.
Thrombocytopenia induced by Bcl-xL deficiency does not require BH3-only activator proteins Bid and Bim. Bcl-xL+/+ and Bcl-xL−/− stand for bcl-xflox/flox without pf4-Cre and bcl-xflox/flox with pf4-Cre, respectively. Bid+/+, Bid+/−, and Bid−/− stand for bid+/+, bid+/−, and bid−/−, respectively. Bim+/+, Bim+/−, and Bim−/− stand for bim+/+, bim+/−, and bim−/−, respectively. WT and Bid−/−Bim−/− stand for wild type and bid−/−bim−/−, respectively. A, Western blot of platelet lysates for the expression of Bid and BimEL. B, platelet counts of the offspring from mating of bid+/−bcl-xflox/flox pf4-Cre mice and bid+/−bcl-xflox/flox mice (more than five mice per group; *, p < 0.01 versus Bcl-xL+/+ groups). C, platelet counts of the offspring from mating of bim+/−bcl-xflox/flox pf4-Cre mice and bim+/−bcl-xflox/flox mice (more than seven mice per group; *, p < 0.01 versus Bcl-xL+/+ groups). D, platelet counts of the offspring from mating of bid−/−bim+/−bcl-xflox/flox pf4-Cre mice and bid−/−bim+/−bcl-xflox/flox mice (more than five mice per group; *, p < 0.01 versus Bcl-xL+/+ groups). E, serum caspase-3/7 activity of the offspring from mating of bid+/−bcl-xflox/flox pf4-Cre mice and bid+/−bcl-xflox/flox mice (n = 4–6/group; *, p < 0.01 versus Bcl-xL+/+ groups). F, serum caspase-3/7 activity of the offspring from mating of bid−/−bim+/−bcl-xflox/flox pf4-Cre mice and bid−/−bim+/−bcl-xflox/flox mice (n = 4–6/group; *, p < 0.01 versus Bcl-xL+/+ groups).
Bax Activation and Subsequent Apoptotic Cell Death Provoked by ABT-737 Can Proceed in Absence of Bid and Bim
To investigate whether Bax can be activated by inhibition of antiapoptotic Bcl-2 proteins even in the absence of Bid and Bim, we isolated platelets from Bid and Bim double knock-out mice. A Western blot study confirmed that neither Bid nor Bim existed in platelets of the double knock-out mice (Fig. 4A) and showed that Puma protein, another putative direct activator (13), was not detected in platelets of either wild-type mice or Bid/Bim double knock-out mice (Fig. 5A). The expression of antiapoptotic Bcl-2 proteins including Bcl-xL, Bcl-2, and Bcl-w was unchanged between these mice (Fig. 5A). Upon ABT-737 treatment, the Bax protein in Bid/Bim-deficient platelets could undergo conformational change (Fig. 5B), translocation from the cytosol to the mitochondria (Fig. 5C), and homo-oligomerization (Fig. 5D). These results clearly demonstrated that ABT-737-induced Bax activation did not require the direct activator proteins Bid and Bim. Upon ABT-737 treatment of Bid/Bim-deficient platelets, cleavage of caspase-3 and elevation of caspase-3/7 activity were both observed (Fig. 5, E and F), and the MTS assay demonstrated that platelet cellular viability was also impaired (Fig. 5G). These findings indicated that Bid and Bim were dispensable for Bak/Bax-dependent platelet apoptosis provoked by inhibition of antiapoptotic Bcl-2 proteins.
FIGURE 5.
Bax activation and subsequent apoptotic cell death provoked by Bcl-xL deficiency can proceed in absence of Bid and Bim. A, Western blot of platelet lysates for the expression of Puma, Bcl-2, Bcl-w, and Bcl-xL. WT and Bid−/−Bim−/− stand for wild type and bid−/−bim−/−, respectively. B–E, platelets (1.0 × 108) isolated from Bid/Bim double knock-out mice were incubated with 10 μm ABT-737 or vehicle for 2 h at room temperature. B, Western blot for the expression of Bax after immunoprecipitation (IP) using mouse antibody that specifically recognizes activated Bax (6A7) or mouse control IgG. C, Western blot for the expression of Bak, Bax, CoxIV (cytochrome c oxidase IV), and GAPDH after cellular fractionation of the platelet lysates. D, Western blot for the expression of Bax after incubation of the platelet lysates with protein cross-linkers (5 mm bismaleimidohexane and 5 mm bis(sulfosuccinimidyl) suberate). E, Western blot of platelet lysates for the expression of cleaved caspase-3. F and G, platelets (2.0 × 106) isolated from Bid/Bim double knock-out mice were incubated with 10 μm ABT-737 or vehicle for 2 h at room temperature. F, caspase-3/7 activity of platelet supernatant (n = 4/group). G, MTS assay (n = 5/group). IB, immunoblot; RLU, relative light units.
Spontaneous Apoptotic Cell Death in Stored Human Platelets Occurs with Decline of Bcl-xL Despite Decrease in Bid and Bim
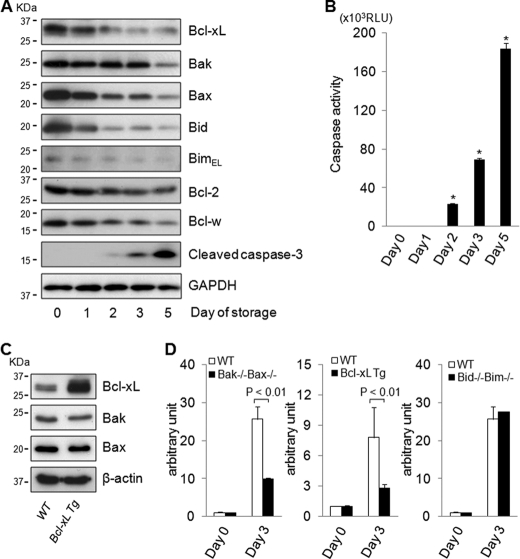
In stored human platelets, phosphatidylserine exposure increases with caspase-3 activation (4, 5), which leads to spontaneous platelet apoptosis, but the exact molecular mechanism of this process remains elusive. This led us to examine the profile of Bcl-2 family proteins in human platelets during the course of storage. In stored platelets, cleaved caspase-3 gradually increased (Fig. 6A) and caspase-3/7 activity rose simultaneously (Fig. 6B), indicating that the platelets steadily underwent apoptotic cell death with storage time. Regarding the Bcl-2 family protein profile, although expression of Bcl-xL and Bax proteins gradually decreased with time, the decrease in Bak expression occurred at a later time point (Fig. 6A). As for BH3-only direct activator proteins, Bid and Bim expression also decreased with time (Fig. 6A). To examine the involvement of Bcl-2 family proteins in spontaneous apoptosis in stored platelets, caspase-3/7 activity was measured in platelets from wild-type mice, Bak/Bax double knock-out mice, Bcl-xL transgenic mice, and Bid/Bim double knock-out mice upon storage. A Western blot revealed that Bcl-xL protein increased in platelets isolated from Bcl-xL transgenic mice compared with wild-type mice, whereas expression of effector proteins Bak and Bax did not differ between them (Fig. 6C). Although wild-type platelets showed elevation of the caspase-3/7 activity upon storage, it was significantly lower in Bak/Bax-deficient platelets than in wild-type platelets (Fig. 6D). These findings indicated that Bak/Bax-dependent mitochondrial apoptosis played an important role in the execution of spontaneous apoptosis in stored platelets. Furthermore, caspase activation was alleviated in Bcl-xL-overexpressing platelets compared with wild-type platelets upon storage (Fig. 6D), suggesting an antiapoptotic function of Bcl-xL in stored platelets. On the other hand, caspase-3/7 activity increased in Bid/Bim-deficient platelets and was not different from that in wild-type platelets (Fig. 6D), suggesting that direct activator proteins Bid and Bim are dispensable for the spontaneous platelet apoptosis upon storage.
FIGURE 6.
Spontaneous apoptotic cell death in stored platelets occurs with decline of Bcl-xL despite decrease in Bid and Bim. A and B, platelet-rich plasma derived from a healthy volunteer was stored for the indicated time course. A, Western blot of stored platelet lysates for the expression of Bcl-xL, Bak, Bax, Bid, BimEL, Bcl-w, Bcl-2, cleaved caspase-3, and GAPDH. Equal numbers of platelets were loaded per sample. B, caspase-3/7 activity of supernatant derived from platelet-rich plasma (n = 4/group; *, p < 0.01 versus all other groups). C, Western blot of platelet lysates derived from Bcl-xL transgenic mice for the expression of Bcl-xL, Bak, and Bax. WT and Bcl-xL Tg stand for wild-type mice and Bcl-xL transgenic mice, respectively. D, platelets derived from C57BL/6J mice, Bak/Bax double knock-out mice, Bcl-xL transgenic mice, and Bid/Bim double knock-out mice were stored for the indicated time course. Caspase-3/7 activity of stored platelet supernatant was assessed and is presented as the -fold induction compared with freshly isolated platelet supernatant (n = 4/group). WT, Bak−/−Bax−/−, and Bid−/−Bim−/− stand for wild-type, bak−/−baxflox/flox with pf4-Cre, and bid−/−bim−/− mice, respectively. Bcl-xL Tg stands for Bcl-xL transgenic mice. RLU, relative light units.
DISCUSSION
In the mitochondrial pathway, apoptotic cell death is dependent on activation of the proapoptotic effector proteins Bak and Bax. Cells lacking both Bak and Bax are resistant to multiple apoptotic stimuli (34). Genetic studies have revealed that Bax or Bak single knock-out mice have less pronounced phenotypes compared with Bak/Bax double knock-out mice, which display various severe defects during development, indicating the redundancy of their involvement in apoptosis (30, 35). With regard to the mitochondrial apoptosis machinery in platelets, the involvement of Bax seemed to be less critical because platelet numbers in Bax knock-out mice were normal in contrast to the thrombocytosis displayed in Bak knock-out mice (30, 35). However, our in vitro study revealed that ABT-737 could provoke apoptosis even in Bak-deficient platelets. Moreover, our in vivo studies have clearly demonstrated that either Bax or Bak was sufficient to cause platelet apoptosis in the absence of Bcl-xL, indicating that Bax and Bak are redundant and equivalently important for the mitochondrial apoptosis in platelets.
In support of the displacement model, co-immunoprecipitation studies revealed complexes of Bak with a variety of antiapoptotic proteins (36). However, the major concern with this model is that Bax is presumed to exist mainly in a cytosolic fraction as a monomer (37). Thus, Bax activation might not be controlled by displacement (38). Unlike Bak activation, sequential steps are necessary for Bax activation such as a conformational change, mitochondrial translocation, and homo-oligomerization. Recent reports have revealed the mechanism of how activator proteins Bid and Bim are directly involved in these steps and initiate Bax activation (39, 40). In the present study, we showed that all the serial steps of Bax activation can adequately proceed without the involvement of the activator proteins Bid and Bim in vitro. Moreover, Bak/Bax-dependent mitochondrial apoptosis could be fully executed by inhibition of antiapoptotic Bcl-2-like proteins even if the direct activator proteins Bid and Bim did not exist. Similar results have been presented by Willis et al. (15), who showed that embryonic fibroblasts from Bid and Bim double knock-out, when infected with retrovirus expressing BH3 sensitizer proteins, could undergo apoptosis in vitro. Based on their results, they claimed that the Bax protein may be constitutively active and inhibited through binding to antiapoptotic Bcl-2-like proteins for cells to survive. However, in our in vitro study, we could not detect physiological interaction between Bax and Bcl-xL in platelets. Therefore, it is difficult to evaluate whether Bak and/or Bax is active or inactive at the default state in platelets. On the other hand, genetically modified mice clearly showed that retrieval of direct activator proteins could not prevent caspase activation and thrombocytopenia induced by the lack of Bcl-xL. These findings demonstrated, for the first time, in vivo evidence that direct activator proteins Bid and Bim were dispensable for apoptosis execution provoked by the loss of antiapoptotic Bcl-2-family proteins.
Because ABT-737 can bind to and neutralize Bcl-2, Bcl-w, and Bcl-xL, all of which are present in platelets (Figs. 2A and 6A), it is difficult to directly conclude that the in vitro results from our ABT-737 study exactly reflect our in vivo results obtained from Bcl-xL deletion. However, in addition to reports that neither systemic Bcl-w knock-out nor Bcl-2 knock-out mice exhibit any phenotypes with respect to platelet counts (41–43), our in vivo results of massive thrombocytopenia seen in thrombocyte-specific Bcl-xL knock-out mice indicated that the antiapoptotic role of Bcl-2 and Bcl-w in platelets was apparently less important than that of Bcl-xL. Even if Bcl-2 and Bcl-w were involved in our in vitro results, our present results clearly demonstrated that neither Bid nor Bim is required for Bax activation and following mitochondrial apoptosis by inhibition of antiapoptotic Bcl-2 family proteins. Regarding the other antiapoptotic members of the Bcl-2 family, systemic A1a knock-out mice were not reported with any phenotype with respect to platelet counts (44). Mcl-1 is a rapid turnover protein and could not be detected in platelets (supplemental Fig. 1). Therefore, Bcl-xL may be the main antiapoptotic Bcl-2 family protein with functional significance in platelets. This simplicity may explain why Bid and Bim deficiency failed to ameliorate the phenotype of Bcl-xL knock-out in platelets in contrast to other scenarios in which Bid or Bim is apparently indispensable (19–21). Fatal polycystic kidney disease and lymphopenia observed in Bcl-2 knock-out mice are ameliorated in a Bim knock-out background (19). In this case, lymphocytes and other cell lineages may possess Bcl-2 and other antiapoptotic Bcl-2 proteins such as Mcl-1 (45). Hepatocyte apoptosis observed in hepatocyte-specific knock-out of Mcl-1 or Bcl-xL is ameliorated in a Bid knock-out background (20, 21). In this case, hepatocytes clearly have two critical antiapoptotic Bcl-2 family proteins, Bcl-xL and Mcl-1, and Bid may switch binding partners from one to the other in the case of deficiency of either protein. Bid and Bim could regulate the rheostat balance between antiapoptotic and proapoptotic Bcl-2 family proteins, which may become irrelevant if none of the antiapoptotic Bcl-2 family proteins are present.
Although among the BH3-only proteins Bid and Bim are recognized as the putative direct activators, Puma, one of the other BH3-only proteins, has been reported to have the ability to interact directly with effector proteins (13). However, a recent report has pointed out that Puma is a sensitizer protein, which indirectly activates Bak or Bax (46). Hence, its actual mechanism of action in apoptosis remains obscure and disputed. Importantly, in contrast to thymocyte tissue, a Western blot did not show a detectable amount of Puma protein in platelets (Fig. 5A), indicating that it might not be involved in the platelet apoptosis machinery. However, we could not exclude the possibility that other proteins may function as alternative direct activators in the absence of Bid and Bim, leading to Bax activation and mitochondrial apoptosis in platelets upon inactivation of antiapoptotic Bcl-2 family proteins.
In stored platelets, because of the lack of de novo protein synthesis, each protein may gradually decrease in relation to its half-life. Our current results showed that the decline of Bcl-xL and Bax protein was much faster than that of Bak protein, and the disruption of the balance between anti- and proapoptotic multidomain Bcl-2 proteins seemed to be associated with apoptosis in stored human platelets. In fact, upon storage, caspase activation was weakened in Bak/Bax-deficient or Bcl-xL-overexpressing platelets compared with wild-type platelets. Taken together with these findings, the balance between anti- and proapoptotic multidomain Bcl-2 family proteins seems to dictate the cellular fate of the life and death of stored platelets. Similar degradation of the Bcl-2 family proteins should occur in circulation, which may explain why Bak knock-out mice displayed mild thrombocytosis in vivo (Fig. 1A). On the other hand, spontaneous apoptosis occurred in stored platelets despite the absence of activator proteins Bid and Bim. Although in most physiological contexts cellular death is an active decision made by regulating BH3-only proteins, our present findings suggest that activator proteins Bid and Bim were dispensable for Bak/Bax-dependent spontaneous apoptosis in stored platelets.
How anti- and proapoptotic Bcl-2 family proteins interact to maintain cellular integrity and to command cellular survival and death is one of the most important issues that remain to be clearly determined. Although their networks seem to vary depending on the cellular context, our present findings provide an in vivo example indicating that the absence of antiapoptotic Bcl-2-like proteins can induce activation of the effector protein Bax, leading to apoptosis without the involvement of the activator proteins Bid and Bim.
Acknowledgments
We sincerely thank Radek Skoda (University Hospital Basel) and Lothar Hennighausen (National Institutes of Health) for providing the pf4-Cre mice and floxed bcl-x mice, respectively. We also thank Abbott Laboratories for providing ABT-737.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- BH3
- Bcl-2 homology domain 3
- Pf4
- platelet factor 4
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
REFERENCES
- 1. Holmsen H. (1989) Ann. Med. 21, 23–30 [DOI] [PubMed] [Google Scholar]
- 2. Ault K. A., Knowles C. (1995) Exp. Hematol. 23, 996–1001 [PubMed] [Google Scholar]
- 3. Bertino A. M., Qi X. Q., Li J., Xia Y., Kuter D. J. (2003) Transfusion 43, 857–866 [DOI] [PubMed] [Google Scholar]
- 4. Li J., Xia Y., Bertino A. M., Coburn J. P., Kuter D. J. (2000) Transfusion 40, 1320–1329 [DOI] [PubMed] [Google Scholar]
- 5. Perrotta P. L., Perrotta C. L., Snyder E. L. (2003) Transfusion 43, 526–535 [DOI] [PubMed] [Google Scholar]
- 6. Vanags D. M., Orrenius S., Aguilar-Santelises M. (1997) Br. J. Haematol. 99, 824–831 [DOI] [PubMed] [Google Scholar]
- 7. Zhang H., Nimmer P. M., Tahir S. K., Chen J., Fryer R. M., Hahn K. R., Iciek L. A., Morgan S. J., Nasarre M. C., Nelson R., Preusser L. C., Reinhart G. A., Smith M. L., Rosenberg S. H., Elmore S. W., Tse C. (2007) Cell Death Differ. 14, 943–951 [DOI] [PubMed] [Google Scholar]
- 8. Mason K. D., Carpinelli M. R., Fletcher J. I., Collinge J. E., Hilton A. A., Ellis S., Kelly P. N., Ekert P. G., Metcalf D., Roberts A. W., Huang D. C., Kile B. T. (2007) Cell 128, 1173–1186 [DOI] [PubMed] [Google Scholar]
- 9. Kodama T., Takehara T., Hikita H., Shimizu S., Li W., Miyagi T., Hosui A., Tatsumi T., Ishida H., Tadokoro S., Ido A., Tsubouchi H., Hayashi N. (2010) Gastroenterology 138, 2487–2498 [DOI] [PubMed] [Google Scholar]
- 10. Chipuk J. E., Green D. R. (2008) Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams J. M., Cory S. (2007) Curr. Opin. Immunol. 19, 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leber B., Lin J., Andrews D. W. (2010) Oncogene 29, 5221–5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim H., Rafiuddin-Shah M., Tu H. C., Jeffers J. R., Zambetti G. P., Hsieh J. J., Cheng E. H. (2006) Nat. Cell Biol. 8, 1348–1358 [DOI] [PubMed] [Google Scholar]
- 14. Willis S. N., Adams J. M. (2005) Curr. Opin. Cell Biol. 17, 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willis S. N., Fletcher J. I., Kaufmann T., van Delft M. F., Chen L., Czabotar P. E., Ierino H., Lee E. F., Fairlie W. D., Bouillet P., Strasser A., Kluck R. M., Adams J. M., Huang D. C. (2007) Science 315, 856–859 [DOI] [PubMed] [Google Scholar]
- 16. Kuwana T., Bouchier-Hayes L., Chipuk J. E., Bonzon C., Sullivan B. A., Green D. R., Newmeyer D. D. (2005) Mol. Cell 17, 525–535 [DOI] [PubMed] [Google Scholar]
- 17. Letai A., Bassik M. C., Walensky L. D., Sorcinelli M. D., Weiler S., Korsmeyer S. J. (2002) Cancer Cell 2, 183–192 [DOI] [PubMed] [Google Scholar]
- 18. Billen L. P., Kokoski C. L., Lovell J. F., Leber B., Andrews D. W. (2008) PLoS Biol. 6, e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bouillet P., Cory S., Zhang L. C., Strasser A., Adams J. M. (2001) Dev. Cell 1, 645–653 [DOI] [PubMed] [Google Scholar]
- 20. Hikita H., Takehara T., Kodama T., Shimizu S., Hosui A., Miyagi T., Tatsumi T., Ishida H., Ohkawa K., Li W., Kanto T., Hiramatsu N., Hennighausen L., Yin X. M., Hayashi N. (2009) Hepatology 50, 1972–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hikita H., Takehara T., Shimizu S., Kodama T., Li W., Miyagi T., Hosui A., Ishida H., Ohkawa K., Kanto T., Hiramatsu N., Yin X. M., Hennighausen L., Tatsumi T., Hayashi N. (2009) Hepatology 50, 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takehara T., Tatsumi T., Suzuki T., Rucker E. B., 3rd, Hennighausen L., Jinushi M., Miyagi T., Kanazawa Y., Hayashi N. (2004) Gastroenterology 127, 1189–1197 [DOI] [PubMed] [Google Scholar]
- 23. Tiedt R., Schomber T., Hao-Shen H., Skoda R. C. (2007) Blood 109, 1503–1506 [DOI] [PubMed] [Google Scholar]
- 24. Yin X. M., Wang K., Gross A., Zhao Y., Zinkel S., Klocke B., Roth K. A., Korsmeyer S. J. (1999) Nature 400, 886–891 [DOI] [PubMed] [Google Scholar]
- 25. Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. (1980) Proc. Natl. Acad. Sci. U.S.A. 77, 7380–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takehara T., Takahashi H. (2003) Cancer Res. 63, 3054–3057 [PubMed] [Google Scholar]
- 27. Feinstein M. B., Fraser C. (1975) J. Gen. Physiol. 66, 561–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaiyarit S., Thongboonkerd V. (2009) Anal. Biochem. 394, 249–258 [DOI] [PubMed] [Google Scholar]
- 29. Yamagata H., Shimizu S., Nishida Y., Watanabe Y., Craigen W. J., Tsujimoto Y. (2009) Oncogene 28, 3563–3572 [DOI] [PubMed] [Google Scholar]
- 30. Lindsten T., Ross A. J., King A., Zong W. X., Rathmell J. C., Shiels H. A., Ulrich E., Waymire K. G., Mahar P., Frauwirth K., Chen Y., Wei M., Eng V. M., Adelman D. M., Simon M. C., Ma A., Golden J. A., Evan G., Korsmeyer S. J., MacGregor G. R., Thompson C. B. (2000) Mol. Cell 6, 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oltersdorf T., Elmore S. W., Shoemaker A. R., Armstrong R. C., Augeri D. J., Belli B. A., Bruncko M., Deckwerth T. L., Dinges J., Hajduk P. J., Joseph M. K., Kitada S., Korsmeyer S. J., Kunzer A. R., Letai A., Li C., Mitten M. J., Nettesheim D. G., Ng S., Nimmer P. M., O'Connor J. M., Oleksijew A., Petros A. M., Reed J. C., Shen W., Tahir S. K., Thompson C. B., Tomaselli K. J., Wang B., Wendt M. D., Zhang H., Fesik S. W., Rosenberg S. H. (2005) Nature 435, 677–681 [DOI] [PubMed] [Google Scholar]
- 32. Chipuk J. E., Moldoveanu T., Llambi F., Parsons M. J., Green D. R. (2010) Mol. Cell 37, 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsu Y. T., Youle R. J. (1997) J. Biol. Chem. 272, 13829–13834 [DOI] [PubMed] [Google Scholar]
- 34. Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knudson C. M., Tung K. S., Tourtellotte W. G., Brown G. A., Korsmeyer S. J. (1995) Science 270, 96–99 [DOI] [PubMed] [Google Scholar]
- 36. Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., Huang D. C. (2005) Genes Dev. 19, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antonsson B., Montessuit S., Sanchez B., Martinou J. C. (2001) J. Biol. Chem. 276, 11615–11623 [DOI] [PubMed] [Google Scholar]
- 38. Leber B., Lin J., Andrews D. W. (2007) Apoptosis 12, 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gavathiotis E., Suzuki M., Davis M. L., Pitter K., Bird G. H., Katz S. G., Tu H. C., Kim H., Cheng E. H., Tjandra N., Walensky L. D. (2008) Nature 455, 1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lovell J. F., Billen L. P., Bindner S., Shamas-Din A., Fradin C., Leber B., Andrews D. W. (2008) Cell 135, 1074–1084 [DOI] [PubMed] [Google Scholar]
- 41. Print C. G., Loveland K. L., Gibson L., Meehan T., Stylianou A., Wreford N., de Kretser D., Metcalf D., Köntgen F., Adams J. M., Cory S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 12424–12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ross A. J., Waymire K. G., Moss J. E., Parlow A. F., Skinner M. K., Russell L. D., MacGregor G. R. (1998) Nat. Genet. 18, 251–256 [DOI] [PubMed] [Google Scholar]
- 43. Veis D. J., Sorenson C. M., Shutter J. R., Korsmeyer S. J. (1993) Cell 75, 229–240 [DOI] [PubMed] [Google Scholar]
- 44. Hamasaki A., Sendo F., Nakayama K., Ishida N., Negishi I., Nakayama K., Hatakeyama S. (1998) J. Exp. Med. 188, 1985–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dzhagalov I., Dunkle A., He Y. W. (2008) J. Immunol. 181, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jabbour A. M., Heraud J. E., Daunt C. P., Kaufmann T., Sandow J., O'Reilly L. A., Callus B. A., Lopez A., Strasser A., Vaux D. L., Ekert P. G. (2009) Cell Death Differ. 16, 555–563 [DOI] [PubMed] [Google Scholar]