Abstract
We report here the molecular cloning, characterization, and catalytic mechanism of a novel glycosphingolipid-degrading β-N-acetylgalactosaminidase (β-NGA) from Paenibacillus sp. TS12 (NgaP). Consisting of 1034 putative amino acid residues, NgaP shares no sequence similarity with known proteins. Recombinant NgaP, expressed in Escherichia coli, cleaved the nonreducing terminal β-GalNAc residues of gangliotriaosylceramide and globotetraosylceramide. The enzyme hydrolyzed para-nitrophenyl-β-N-acetylgalactosaminide ∼100 times faster than para-nitrophenyl-β-N-acetylglucosaminide. GalNAc thiazoline, an analog of the oxazolinium intermediate and potent inhibitor for enzymes adopting substrate-assisted catalysis, competitively inhibited the enzyme. The Ki of the enzyme for GalNAc thiazoline was 1.3 nm, whereas that for GlcNAc thiazoline was 46.8 μm. Comparison of the secondary structure with those of known enzymes exhibiting substrate-assisted catalysis and point mutation analysis indicated that NgaP adopts substrate-assisted catalysis in which Glu-608 and Asp-607 could function as a proton donor and a stabilizer of the 2-acetamide group of the β-GalNAc at the active site, respectively. These results clearly indicate that NgaP is a β-NGA showing substrate-assisted catalysis. This is the first report describing the molecular cloning of a β-NGA adopting substrate-assisted catalysis.
Keywords: Enzyme Catalysis, Enzyme Mechanisms, Enzyme Structure, Glycolipids, Protein Sequence
Introduction
O-Glycoside hydrolases, which hydrolyze the O-glycosidic linkage between two or more carbohydrates or between a carbohydrate and a non-carbohydrate moiety, have been classified into 118 glycoside hydrolase (GH)2 families based on amino acid similarity but not substrate specificity (1).
β-Hexosaminidase (β-HEX; EC 3.2.1.52) is an enzyme that hydrolyzes nonreducing terminal β-GlcNAc and β-GalNAc residues in oligosaccharides, glycoproteins, glycosaminoglycans, and glycosphingolipids (GSLs). A large number of β-HEXs are grouped into GH20 and clan GH-K (clans are classified by three-dimensional structure) (2). Among the β-HEXs, an enzyme that hydrolyzes the nonreducing terminal β-GalNAc residue much faster than the β-GlcNAc residue is named β-N-acetylgalactosaminidase (β-NGA; EC 3.2.1.53). β-NGA activities have been detected in bovine brain (3), rat brain (4), and bacteria (5); however, β-NGA has not yet been fully characterized. Recently, a nucleocytoplasmic neutral β-HEX (named β-HEX D) was cloned from humans and mice (6) as a homolog of Caenorhabditis elegans β-HEX (7). β-HEX D, classified into the GH20 family with other known β-HEXs, was found to hydrolyze para-nitrophenyl-(pNP)-β-GalNAc four times faster than pNP-β-GlcNAc, suggesting β-HEX D to be a β-NGA, although its catalytic mechanism was not clarified.
Many GH family proteins hydrolyze substrates through one of two mechanisms: the inversion or retention of the anomeric configuration of the substrate (8). Inverting glycosidases employ a single-displacement mechanism in which two amino acids function as a general acid and a general base, although almost all retaining glycosidases hydrolyze substrates through a double-displacement mechanism in which two amino acids function as a general acid/base and a nucleophile.
Although β-HEX is considered a retaining hydrolase, it hydrolyzes substrates through substrate-assisted catalysis in which the carbonyl oxygen of the C-2 acetamide group of the substrate behaves as a catalytic nucleophile, and therefore, only one amino acid is required as a proton donor from the protein side (9).
Previously, we described the isolation and identification of a soil bacterium, Paenibacillus sp. TS12, that efficiently degrades various GSLs when added to cultures by producing a series of exoglycosidases (10). Subsequently, the molecular cloning of the TS12 glucocerebrosidase (11) and two β-HEXs (12), all of which are capable of hydrolyzing GSLs, was described.
In this study, we report the molecular cloning, characterization, and catalytic mechanism of a novel GSL-degrading β-NGA (tentatively named NgaP) of Paenibacillus sp. TS12. This study revealed that the sequence of NgaP is not shared by any known GH family proteins and that NgaP specifically cleaves the nonreducing terminal β-GalNAc residue through substrate-assisted catalysis.
EXPERIMENTAL PROCEDURES
Materials
Escherichia coli strains DH5α and BL21(DE3) and Pyrobest DNA polymerase were purchased from Takara Bio Inc. Plasmids pET23a and pBluescript II SK(+) were from Novagen and Stratagene, respectively. Restriction enzymes, T4 DNA ligase, globotetraosylceramide (Gb4Cer),3 and globotriaosylceramide (Gb3Cer) were from Wako Pure Chemical Inc. Ltd. The crude ganglioside mixture was prepared from bovine brain as described previously (13), and GM2, asialo-GM1 (GA1), asialo-GM2 (GA2; gangliotriaosylceramide), lactosylceramide (LacCer), and glucosylceramide (GlcCer) were prepared from the crude ganglioside mixture using GSL-degrading enzymes from Paenibacillus sp. TS12. Precoated Silica Gel 60 TLC plates were purchased from Merck. 4-Methylumbelliferyl (4MU)-β-GalNAc and various pNP-glycopyranosides were from Sigma. All other reagents were of the highest purity available.
Construction of a Genomic DNA Library of Paenibacillus sp. TS12
Genomic DNA was prepared from Paenibacillus sp. TS12 as described (14) and partially digested with Sau3AI. The Sau3AI fragments (2–10 kilobase pairs) were gel-purified and ligated to BamHI-digested pBluescript II SK (+) DNA. The plasmids were used for the transformation of E. coli DH5α.
Expression Cloning of the Gene Encoding β-NGA
E. coli DH5α cells transformed with the plasmids containing Paenibacillus DNA fragments were seeded (∼400 colonies/9.2-cm plate) on LB agar plates supplemented with 100 μg/ml ampicillin and incubated at 37 °C for 16 h. Colonies were transferred from the plates onto nylon membranes (Biodyne A), which were then incubated with 0.3 mm 4MU-β-GalNAc in 200 μl of 10 mm sodium acetate buffer (pH 5.5). Following incubation at 37 °C for 30 min, positive colonies, visualized under a UV transilluminator, were picked out with a sterilized toothpick and transferred into 5 ml of LB medium. Following incubation at 37 °C for 16 h with shaking, cells were harvested by centrifugation, suspended in 500 μl of 10 mm sodium acetate buffer (pH 5.5), and lysed by sonication. The cell lysate was centrifuged at 8000 × g for 10 min, and the supernatant obtained was used as the crude enzyme solution. The activity of β-NGA was measured using GA2 as the substrate as described below. The positive clone obtained was designated pNgaP.
DNA Sequencing and Sequence Analysis
Nucleotide sequences were determined by the dideoxynucleotide chain termination method with a BigDye terminator ver.3 cycle sequencing ready reaction kit and a Model 377 DNA sequencer (Applied Biosystems). Computer analyses were performed using DNASIS, and the homology search of deduced amino acid sequences was performed using the DDBJ DNA Data Bank of Japan.
Construction of Expression Vectors
The following primers were used for PCR: UNgaP (5′-ATA GGA TCC ATG GTG AAT AGA AAA CAG AAG ACA-3′) and LNgaP (5′-ATT CTC GAG TTC AAT AAT TTT TTT GGC GAT TTC-3′). UNgaP and LNgaP contained a BamHI site (underlined) and an XhoI site (double-underlined), respectively. PCR was performed in 50 μl of reaction mixture containing each primer at 0.2 μm, 50 ng of template DNA (pNgaP), 0.2 mm dNTPs (dATP, dCTP, dGTP, and dTTP), and 2 units of Pyrobest DNA polymerase using a TPersonal 48 thermal cycler (Biometra) for 30 cycles (each consisting of denaturation at 98 °C for 10 s and annealing/extension at 68 °C for 2.5 min). PCR products were extracted from 0.7% agarose gel, and the amplified products were digested with BamHI and XhoI. The BamHI/XhoI fragments were cloned into BamHI/XhoI-digested pET23a. The recombinant plasmid was designated pETNgaP.
Expression and Purification of Recombinant β-NGA
E. coli BL21 cells transformed with pETNgaP (or recombinant mutant plasmids) were grown at 25 °C for 16 h in 5 ml of medium A (LB medium containing 100 μg/ml ampicillin) with shaking. The culture was transferred into a 2-liter flask containing 1 liter of medium A and incubated at 25 °C for 16 h with shaking. isopropyl β-d-thiogalactopyranoside was added to the culture at a final concentration of 0.1 mm to cause transcription. After an additional 3 h at 25 °C, cells were harvested by centrifugation and suspended in 50 ml of buffer A (25 mm Tris-HCl (pH 7.5) containing 150 mm NaCl, 5 mm β-mercaptoethanol, and 50 mm imidazole) containing a protease inhibitor mixture (Roche Applied Science). After sonication, cell debris was removed by centrifugation at 8000 × g for 10 min, and the supernatant obtained was loaded on a HisTrap HP column (5 ml; GE Healthcare) pre-equilibrated with buffer A. The column was washed with 50 ml of buffer A, and β-NGA was eluted with 25 mm Tris-HCl (pH 7.5) containing 150 mm NaCl, 5 mm β-mercaptoethanol, and 100 mm imidazole and then with 25 mm Tris-HCl (pH 7.5) containing 150 mm NaCl, 5 mm β-mercaptoethanol, and 300 mm imidazole. The active fractions were pooled, dialyzed against 10 mm Tris-HCl (pH 7.5), and used for characterization of the enzyme.
Protein Assay and Polyacrylamide Gel Electrophoresis
Protein content was determined by the bicinchoninic acid method (Pierce) or SDS-PAGE using bovine serum albumin as the standard. SDS-PAGE was carried out according to the method of Laemmli (15). The proteins on the SDS-polyacrylamide gel were visualized by staining with Coomassie Brilliant Blue.
Western Blotting
After separation by 10% SDS-PAGE, the proteins were transferred onto a PVDF membrane using a semidry blotter (Bio-Rad). The membrane was then incubated with anti-polyhistidine tag mouse IgG monoclonal antibody (Invitrogen) for 6 h at room temperature. The bands were visualized with HRP-labeled anti-mouse IgG antibody and a peroxidase staining kit (Nacalai Tesque).
Enzyme Assay
The activity of β-NGA was measured by two methods. In the first assay with pNP-β-GalNAc as the substrate, the reaction mixture contained 100 nmol of pNP-β-GalNAc and an appropriate amount of the enzyme in 100 μl of 25 mm sodium acetate buffer (pH 6.0). Following incubation at 37 °C for a specified period, the reaction was stopped by the addition of 100 μl of 1 m NaOH, and absorbance was measured at 405 nm. One unit of the enzyme was defined as the amount that catalyzed the release of 1 μmol of p-nitrophenol/min from pNP-β-GalNAc under the conditions used. In the second assay with GA2 or Gb4Cer as the substrate, the reaction mixture contained 5 nmol of GA2 or Gb4Cer and an appropriate amount of the enzyme in 20 μl of 25 mm sodium acetate buffer (pH 6.0) containing 0.2% (w/v) taurodeoxycholate. Following incubation at 37 °C for a given period, the reaction was stopped by heating in a boiling water bath for 5 min. The sample was dried with a Savant SpeedVac SC110 concentrator, and the residue was dissolved in 10 μl of chloroform/methanol (2:1, v/v) and applied to a TLC plate, which was then developed with chloroform/methanol and 0.02% CaCl2 (5:4:1, v/v/v). The remaining substrate (GA2 or Gb4Cer) and released product (LacCer or Gb3Cer) after incubation with the enzyme were visualized with orcinol/H2SO4 (16) and quantified by a Shimadzu CS-9300PC chromatic scanner with the reflection mode set at 540 nm.
pH-kcat/Km Profile
The pH profile was measured using pNP-β-GalNAc as the substrate under the conditions described in the legend of Fig. 2. pKa and pKb values were calculated using GraFit (17).
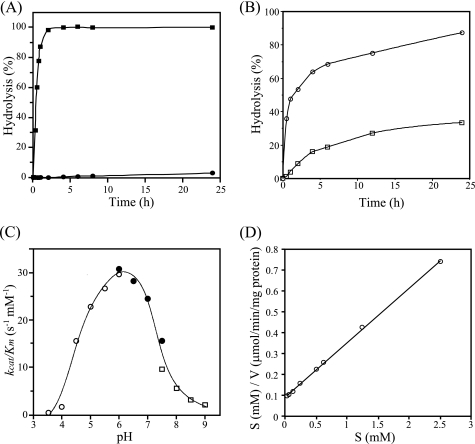
FIGURE 2.
Specificity and general properties of NgaP. A, time course for the hydrolysis of pNP-β-HexNAc by NgaP. Aliquots of 50 nmol of pNP-β-GalNAc (■) and pNP-β-GlcNAc (●) were incubated with 1 milliunit of the enzyme at 37 °C for the periods indicated in 100 μl of 25 mm acetate buffer (pH 6.0). The hydrolysis of pNP-substrates was examined as described under “Experimental Procedures.” B, time course of the hydrolysis of GSLs by NgaP. Aliquots of 5 nmol of GA2 (○) and Gb4Cer (□) were incubated with 10 milliunits of the enzyme at 37 °C for the times indicated in 20 μl of 25 mm acetate buffer (pH 6.0). The hydrolysis of GSLs was examined as described under “Experimental Procedures.” C, pH-kcat/Km profile of wild-type NgaP. The reaction mixture containing different amounts of pNP-β-GalNAc (12.5–200 nmol) and an appropriate amount of enzyme in 100 μl of various buffers (25 mm) was incubated at 37 °C for 30 min. ○, acetate buffer (pH 3.5–6.0); ●, phosphate buffer (pH 6.0–7.5); □, Tris-HCl buffer (pH 7.5–9.0). The kcat/Km values were determined using the Hanes-Woolf plot. D, Hanes-Woolf plot for the action of NgaP. The concentration of pNP-β-GalNAc was varied as indicated, and the incubation time was 30 min. Values are means of triplicate determinations.
Construction of Asp-607 and Glu-608 Mutants
Mutagenesis was performed using KOD polymerase (Toyobo Co.), with the following oligonucleotide primers: D607E, 5′-ACC TAT ATG GCT AAT GAA GAG AGA GCG CTG AAC-3′ and 5′-GTT CAG CGC TCT CTC TTC ATT AGC CAT ATA GGT-3′; D607N, 5′-ACC TAT ATG GCT AAT AAT GAG AGA GCG CTG AAC-3′ and 5′-GTT CAG CGC TCT CTC ATT ATT AGC CAT ATA GGT-3′; E608D, 5′-TAT ATG GCT AAT GAT GAC AGA GCG CTG AAC GAC-3′ and 5′-GTC GTT CAG CGC TCT GTC ATC ATT AGC CAT ATA-3′; and E608Q, 5′-TAT ATG GCT AAT GAT CAA AGA GCG CTG AAC GAC-3′ and 5′-GTC GTT CAG CGC TCT TTG ATC ATT AGC CAT ATA-3′ (locations of mutations are underlined). PCR products were digested with DpnI and introduced into E. coli DH5α. The recombinant mutant plasmids were designated pETD607E, pETD607N, pETE608D, and pETE608Q.
Determination of Ki Values
The Ki values of NgaP for GalNAc thiazoline and GlcNAc thiazoline were determined by the Morrison equation (18) and Dixon plots (19), respectively.
RESULTS
Molecular Cloning, Sequencing, and Alignment of β-NGA of Paenibacillus sp. TS12
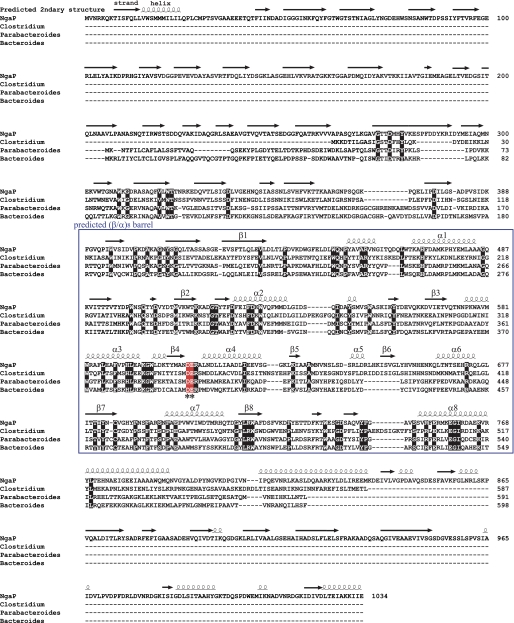
To isolate the gene encoding β-NGA of Paenibacillus sp. TS12, expression screening was performed using pNP-β-GalNAc as the substrate as described under “Experimental Procedures.” As a result, one positive clone was obtained and designated pNgaP. The ORF of pNgaP was 3102 bp long, encoding 1034 amino acids, and the gene product was named NgaP (supplemental Fig. 1A). The molecular weight and pI of NgaP were estimated to be 114,727 and 4.74, respectively, from the deduced amino acid sequence. A hydrophobic region, possibly a signal peptide, was found at the N terminus of NgaP (supplemental Fig. 1B). The deduced amino acid sequence of NgaP showed no significant similarity to the GH20 β-HEXs and other members of the GH family. Furthermore, the specific sequence conserved in all GH20 β-HEXs cloned to date (7), (His/Asn)-X-Gly-(Ala/Cys/Gly/Met)-Asp-Glu-(Ala/Ile/Leu/Val) (the catalytic glutamate residue is underlined), was not conserved in NgaP. On the other hand, 29–32% sequence similarity was found for hypothetical proteins of Clostridium perfringens (20), Parabacteroides distasonis (21), and Bacteroides thetaiotaomicron (22) (Fig. 1).
FIGURE 1.
Sequence alignment of NgaP and other unknown proteins and predicted secondary structure of NgaP. NgaP is aligned with other unknown proteins. Residues conserved in all of the proteins are shown in red. The alignment was carried out with ClustalW (31). The predicted secondary structural elements are indicated above the alignment. The putative (β/α)8-barrel region is shown in a blue box. The putative essential carboxylic amino acid residues (*) are indicated below the sequence.
Expression and Purification of Recombinant NgaP
NgaP was expressed in E. coli strain BL21(DE3) and purified from the cell lysate. The final preparation gave a single protein band corresponding to a molecular mass of 115 kDa on SDS-PAGE with Coomassie Brilliant Blue staining (supplemental Fig. 2A, lane 3), consistent with the molecular mass estimated from the deduced amino acid sequence. This band corresponded exactly to the band obtained by Western blotting using anti-polyhistidine tag monoclonal antibodies (supplemental Fig. 2B).
Substrate Specificity and Enzymatic Properties of Recombinant NgaP
The specificity of NgaP was examined using various pNP-glycopyranosides as substrates and 1 milliunit of the purified recombinant enzyme. Recombinant NgaP hydrolyzed pNP-β-GalNAc but not pNP-β-GlcNAc, pNP-α-GalNAc, or other pNP-glycosides tested under the conditions used. pNP-β-GlcNAc was found to be hydrolyzed by NgaP very slowly when the amount of enzyme was increased, but at <1% of the rate of the hydrolysis of pNP-β-GalNAc (Table 1). The time course for the hydrolysis of pNP-β-GalNAc and pNP-β-GlcNAc also indicated the strict specificity of the enzyme for the C-4 configuration of β-HexNAc, i.e. pNP-β-GalNAc was completely hydrolyzed by the enzyme after 2 h, whereas <5% of pNP-β-GlcNAc was hydrolyzed even after 24 h (Fig. 2A). These results clearly indicate that NgaP is a β-NGA, whose specificity for the C-4 configuration of β-HexNAc is extremely rigid. It is worth noting that such strict specificity has not been observed in any other β-HEXs, including β-Hex D (6).
TABLE 1.
Substrate specificity of recombinant NgaP
pNP-glycosides (50 nmol) were incubated with the enzyme at 37 °C for 30 min in 100 μl of 25 mm sodium acetate buffer (pH 6.0). Values are the means of triplicate determinations. ND, not determined. 10 nmol of each GSL was incubated at 37 °C for 24 h with 10 milliunits of the enzyme in 20 μl of 25 mm sodium acetate (pH 6.0). Values are the means of duplicate determinations. GalCer, galactosylceramide.
| pNP-substrate | Hydrolysis |
||
|---|---|---|---|
| 1 milliunit of NgaP | 3 milliunits of NgaP | 30 milliunits of NgaP | |
| % | |||
| pNP-β-GalNAc | 55.2 | 100 | 100 |
| pNP-β-GlcNAc | 0 | 0.851 | 2.84 |
| pNP-β-Gal | ND | ND | 0 |
| pNP-β-Glc | ND | ND | 0 |
| pNP-β-Fuc | ND | ND | 0 |
| pNP-β-Xyl | ND | ND | 0 |
| pNP-α-GalNAc | ND | ND | 0 |
| pNP-α-GlcNAc | ND | ND | 0 |
| pNP-α-Gal | ND | ND | 0 |
| pNP-α-Glc | ND | ND | 0 |
| pNP-α-Fuc | ND | ND | 0 |
| pNP-α-Xyl | ND | ND | 0 |
| GSL | Structure | Relative activity |
|---|---|---|
| % | ||
| GA2 | GalNAcβ1–4Galβ1–4Glcβ1–1′Cer | 100 |
| GM2 | GalNAcβ1–4(NeuAcα2–3)Galβ1–4Glcβ1–1′Cer | 0 |
| GA1 | Galβ1–3GalNAcβ1–4Galβ1–4Glcβ1–1′Cer | 0 |
| GM1a | Galβ1–3GalNAcβ1–4(NeuAcα2–3)Galβ1–4Glcβ1–1′Cer | 0 |
| Gb4Cer | GalNAcβ1–3Galα1–4Galβ1–4Glcβ1–1′Cer | 39.6 |
| Gb3Cer | Galα1–4Galβ1–4Glcβ1–1′Cer | 0 |
| LacCer | Galβ1–4Glcβ1–1′Cer | 0 |
| GlcCer | Glcβ1–1′Cer | 0 |
| GalCer | Galβ1–1′Cer | 0 |
| Sulfatide | HSO3-3Galβ1–1′Cer | 0 |
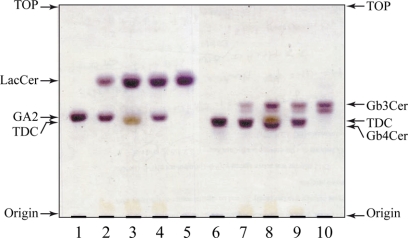
Paenibacillus sp. TS12 was isolated as a GSL-degrading bacterium (10), and the glycosidases produced by TS12 were found to hydrolyze GSLs (11, 12). Thus, to elucidate the specificity of the enzyme, various GSLs were tested as substrates. GA2 (GalNAcβ1–4Galβ1–4Glcβ1–1′Cer) and Gb4Cer (GalNAcβ1-3Galα1–4Galβ1–4Glcβ1–1′Cer) were hydrolyzed by NgaP, generating LacCer (Galβ1–4Glcβ1–1′Cer) and Gb3Cer (Galα1–4Galβ1–4Glcβ1–1′Cer), respectively. GM2, GA1, GM1a, Gb3Cer, LacCer, GlcCer, galactosylceramide, and sulfatide were completely resistant to hydrolysis by the enzyme (Table 1). It is noteworthy that NgaP hydrolyzed GA2 and Gb4Cer in the absence of detergents (Fig. 3, lanes 2 and 7). However, the addition of taurodeoxycholate and Triton X-100 at a concentration of 0.2% (w/v) increased the hydrolysis of GA2 by NgaP by 8- and 3-fold, respectively, compared with that in the absence of detergents (Fig. 3, lanes 3 and 4). Activation of the hydrolysis of substrates by detergents was not observed when pNP-β-GalNAc was used as the substrate instead of GSLs, indicating that detergents are required to solubilize the GSL substrates but not enzymes.
FIGURE 3.
Hydrolysis of GA2 and Gb4Cer by recombinant NgaP. TLC shows the hydrolysis of GA2 and Gb4Cer. Aliquots of 5 nmol of GA2 or Gb4Cer were incubated with 10 milliunits of the enzyme at 37 °C for 30 min or 6 h in 20 μl of 25 mm sodium acetate buffer (pH 6.0) containing 0.2% detergent. The hydrolysis of GA2 or Gb4Cer was examined as described under “Experimental Procedures.” Lane 1, GA2 marker; lane 2, NgaP + GA2; lane 3, NgaP + GA2 + 0.2% taurodeoxycholate (TDC); lane 4, NgaP + GA2 + 0.2% Triton X-100; lane 5, LacCer marker; lane 6, Gb4Cer marker; lane 7, NgaP + Gb4Cer; lane 8, NgaP + Gb4Cer + 0.2% taurodeoxycholate; lane 9, NgaP + Gb4Cer + 0.2% Triton X-100; lane 10, Gb3Cer marker.
The time course for the hydrolysis of GA2 and Gb4Cer by NgaP in the absence of detergent is shown in Fig. 2B. GA2 was hydrolyzed much faster than Gb4Cer by NgaP, suggesting that the enzyme prefers the GalNAcβ1–4 linkage over the GalNAcβ1–3 linkage. The activity of NgaP was maximal at pH ∼6.0 when pNP-β-GalNAc (Fig. 2C) or GA2 (data not shown) was used as the substrate. The pH-kcat/Km profile indicated that the pKa and pKb values were 4.7 and 7.3, respectively (Fig. 2C). The activity was strongly inhibited by Cu2+, Ni2+, and Hg2+ at 5 mm. The Km and kcat values of NgaP for pNP-β-GalNAc were 0.35 mm and 7.3 s−1 protein, respectively (Fig. 2D and Table 2).
TABLE 2.
Kinetic parameters of wild-type NgaP and mutants
The reaction mixture containing different amounts of pNP-β-GalNAc (3–250 nmol) was incubated with 0.5 milliunits of the enzyme at 37 °C for 30 min in 100 μl of 25 mm sodium acetate buffer (pH 6.0). Values are the means of triplicate determinations. ND, no products were detected.
| Enzyme | Km | kcat | kcat/Km | Relative kcat/Km |
|---|---|---|---|---|
| mm | s−1 | s−1mm−1 | ||
| WT | 0.35 | 7.3 | 21.0 | 100 |
| D607E | 1.74 | 0.10 | 0.06 | 0.28 |
| D607N | ND | ND | ND | ND |
| E608D | 0.20 | 0.38 | 1.9 | 9.0 |
| E608Q | 0.09 | 0.08 | 0.88 | 4.2 |
Exploring the Catalytic Mechanism of NgaP by Analysis of Secondary Structure
As shown in Table 1, the substrates with a C-2 acetamide group (such as pNP-β-GalNAc, GA2, and Gb4Cer) at the nonreducing end were hydrolyzed by NgaP, but the substrates without an acetamide group (such as pNP-β-Gal, GA1, and Gb3Cer) were not. It is known that some enzymes capable of hydrolyzing substrates with a C-2 acetamide group (such as β-GalNAc and β-GlcNAc) adopt the substrate-assisted mechanism (23–26). Thus, it was suggested that the carbonyl oxygen of the C-2 acetamide group of the substrate might behave as a catalytic nucleophile, i.e. NgaP could adopt the substrate-assisted mechanism like β-HEX. Here, common features of the active-site residue of the enzymes that adopt substrate-assisted catalysis were extracted from GH18, GH20, GH56, and GH84 (23–26). Supplemental Fig. 3 shows ribbon diagrams of the (β/α)x-barrel around the substrate-binding pocket and secondary structures of typical enzymes showing the substrate-assisted catalytic mechanism. The proton donor residues of the GH18, GH20, GH56, and GH84 enzymes (Glu-140, Glu-323, Glu-113, and Asp-298, respectively) exist in the loop just after strand β4 of the (β/α)8-barrel in GH18, GH20, and GH84 or strand β3 of the (β/α)7-barrel in GH56 (supplemental Fig. 3A). Additionally, an aspartate residue is found one or two residues before the proton donor residue (supplemental Fig. 3B). The Asp residue was reported to determine the orientation of the 2-acetamide group of the substrate, GlcNAc or GalNAc (27). Although the sequence similarity of GH18, GH20, GH56, and GH84 is very low, the mechanism for recognition of the substrate by these enzymes appears to be very similar (supplemental Fig. 3). We thus predict that the region where Asp is adjacent to Glu on the (β/α)8-barrel could form an active site in these enzymes.
Next, we searched for the active site of NgaP. First, using the deduced amino acid sequence of NgaP, the secondary structure of the enzyme was constructed with the PSIPRED Protein Structure Prediction Server (Fig. 1). As a result, Asp-607 and Glu-608 in the putative (β/α)8-barrel emerged as candidates on the loop after strand β4. To elucidate whether Asp-607 and Glu-608 are important for the reaction of NgaP, four mutants in which Asp-607 was replaced with Glu (D607E) or Asn (D607N) and Glu-608 was replaced with Asp (E608D) or Gln (E608Q) were constructed by site-directed mutagenesis. Table 2 shows the Km, kcat, and kcat/Km values of wild-type NgaP and the four mutants for pNP-β-GalNAc as a substrate. In mutant D607E, the Km was increased by 5-fold, whereas the kcat was decreased to 1.4% compared with the wild-type enzyme. The activity of mutant D607N was completely abolished. In mutants E608D and E608Q, the Km values were decreased to about 57% and 26%, and the kcat values were decreased to about 5.2% and 1.1%, respectively. The kcat/Km values of the D607E, E608D, and E608Q mutants were decreased to 0.28, 9.0, and 4.2%, respectively, compared with the wild-type enzyme. These results indicate that Asp-607 and Glu-608 of NgaP are integral for the hydrolysis of the terminal β-GalNAc residue of pNP-GalNAc.
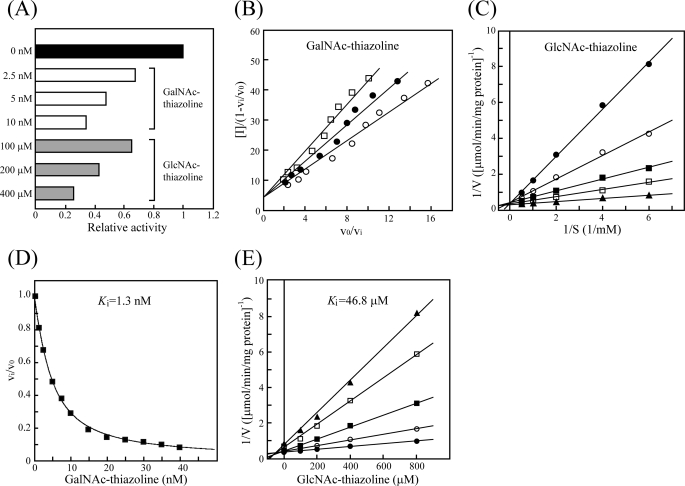
Inhibition of NgaP by GalNAc Thiazoline and GlcNAc Thiazoline
Comparison of the secondary structure of NgaP with that of known enzymes adopting substrate-assisted catalysis and point mutation analysis strongly suggested that the enzyme exhibits substrate-assisted catalysis. Thus, we examined whether GalNAc thiazoline and GlcNAc thiazoline inhibit the activity of NgaP. GlcNAc thiazoline ((3aR,5R,6S,7R,7aR)-5-(hydroxymethyl)-2-methyl-5,6,7,7a-tetrahydropyrano[3,2-d]thiazole-6,7-diol), a structural analog of the oxazolinium intermediate, is known to be a potent inhibitor of enzymes adopting substrate-assisted catalysis, such as β-HEX (28). The inhibition of GalNAc thiazoline was observed at a nanomolar range; in contrast, that of GlcNAc thiazoline was observed at a micromolar range (Fig. 4A). Thus, we employed a Henderson plot (29) and a Lineweaver-Burk plot (30) for inhibition models of the enzyme by GalNAc thiazoline and GlcNAc thiazoline, respectively. GalNAc thiazoline and GlcNAc thiazoline were found to competitively inhibit the enzymatic activity of NgaP (Fig. 4, B and C). The Ki values of the enzyme for GalNAc thiazoline and GlcNAc thiazoline were calculated by the Morrison equation (18) and Dixon plot (19), respectively. As a result, it was found that the Ki of NgaP for GalNAc thiazoline was 1.3 nm, whereas that for GlcNAc thiazoline was 46.8 μm (Fig. 4, D and E), indicating the inhibition of NgaP by GalNAc thiazoline to be 36,000 times stronger than that by GlcNAc thiazoline. These results clearly indicate that NgaP is a β-NGA exhibiting substrate-assisted catalysis.
FIGURE 4.
Inhibition of NgaP activity by GalNAc thiazoline and GlcNAc thiazoline. A, aliquots of 50 nmol of pNP-β-GalNAc were incubated with 17 nm enzyme at 37 °C for 30 min in 100 μl of 25 mm acetate buffer (pH 6.0). The concentrations of GalNAc thiazoline used were 2.5, 5, and 10 nm (white bars), and those of GlcNAc thiazoline used were 100, 200, and 400 μm (gray bars). The type of inhibition was determined using the Henderson plot (B) and Lineweaver-Burk plot (C) with GalNAc thiazoline and GlcNAc thiazoline, respectively. The reaction mixture containing different amounts of pNP-β-GalNAc and 17 nm enzyme was incubated at 37 °C for 30 min in 100 μl of 25 mm acetate buffer (pH 6.0). B, the concentrations of GalNAc thiazoline used were 5∼40 nm, and those of pNP-β-GalNAc used were 0.25 mm (○), 0.5 mm (●), and 0.75 mm (□). C, the concentrations of GlcNAc thiazoline used were 800 μm (●), 400 μm (○), 200 μm (■), 100 μm (□), and 0 μm (▴). The Ki values of NgaP for GalNAc thiazoline (D) and GlcNAc thiazoline (E) were determined by the Morrison equation (18) and Dixon plots (19), respectively. D, aliquots of 50 nmol of pNP-β-GalNAc were incubated with 17 nm enzyme at 37 °C for 30 min in 100 μl of 25 mm acetate buffer (pH 6.0). The Vi/V0 ratio was plotted against inhibitor concentration. E, the concentrations of pNP-β-GalNAc used were 2 mm (●), 1 mm (○), 0.5 mm (■), 0.25 mm (□), and 0.167 mm (▴). The reaction mixture containing different amounts of pNP-β-GalNAc, 17 nm enzyme, and different amounts of inhibitor in 100 μl of 25 mm acetate buffer (pH 6.0) was incubated at 37 °C for 30 min. Values are means of triplicate determinations.
DISCUSSION
Paenibacillus sp. TS12 was found to decompose various GSLs added to culture (10). For example, polysialogangliosides, GSLs with several NeuAc residues, were sequentially degraded in TS12 culture as follows: polysialogangliosides → GM1a → GA1 → GA2 → LacCer → GlcCer → Cer. This may indicate that Paenibacillus sp. TS12 produces a series of GSL-degrading exoglycosidases, such as sialidase, β-galactosidase, β-HEX, and glucocerebrosidase. Three enzymes that hydrolyze 4MU-β-GalNAc were expression-cloned using a TS12 genomic library. Two of the three were found to hydrolyze not only 4MU-β-GalNAc but also 4MU-β-GlcNAc efficiently, indicating that they are β-HEXs (12). The sequences of the two enzymes were homologous to those of GH20 β-HEXs. The remaining enzyme, tentatively designated NgaP in this study, hydrolyzed 4MU-β-GalNAc, but not 4MU-β-GlcNAc, under the conditions used.
NgaP hydrolyzed the nonreducing terminal GalNAc residue of GA2, but not the internal GalNAc residue of GA1 (Table 1), indicating that NgaP is an exo-type glycosidase. NgaP hydrolyzed GA2 more rapidly than Gb4Cer, suggesting that the GalNAcβ1–4 linkage is more susceptible to NgaP than the GalNAcβ1–3 linkage. NgaP hydrolyzed not only 4MU-β-GalNAc but also pNP-β-GalNAc. However, the enzyme did not hydrolyze pNP-α-GalNAc, pNP-β-GlcNAc, or pNP-β-Gal. Thus, NgaP appears to strictly recognize the 1-OH, 4-OH, and C-2 acetamide groups of the terminal sugar at the nonreducing end.
NgaP is not likely to belong to any existent GH families because its deduced amino acid sequence shows no similarity to any GH family member, including β-HEX. This is quite remarkable because almost all O-glycoside hydrolases reported so far have been classified into a GH family based on amino acid similarity. Thus, we propose a new group, which includes NgaP and hypothetical proteins of C. perfringens (GenBankTM accession number EDS79974.1), P. distasonis (accession number ABR44152.1), and B. thetaiotaomicron (accession number AAO77857.1) showing high sequence similarity to NgaP but whose functions are unknown. It remains to be clarified whether these hypothetical proteins have β-NGA activity.
Through x-ray structural analyses of various glycosidases, information about the reaction mechanism of the enzymes has been accumulated. GH families are further grouped into “clans,” which display the same folding and catalytic mechanisms. The structures of the GH18, GH20, GH56, and GH84 proteins have been uncovered. As a result, GH18 and GH20 were placed in clan GH-K, whereas GH56 and GH84 were not. However, all glycosidases belonging to these four GH families hydrolyze substrates with a C-2 acetamide group (such as β-GlcNAc and β-GalNAc) through substrate-assisted catalysis. Comparison of the amino acid sequences of these enzymes revealed that the arrangement of important residues around the active site is remarkably similar (supplemental Fig. 3A). Because NgaP hydrolyzes the β-GalNAc linkage at the nonreducing end, NgaP may use substrate-assisted catalysis like β-HEX. In substrate-assisted catalysis, the C-2 acetamide group of the substrate behaves as a nucleophile, the proton donor is present on the loop just after strand β of the (β/α)x-barrel, and the Asp residue is present one or two residues before the proton donor. This Asp residue was found to assist the correct orientation of the 2-acetamide group at the active site (27). Williams et al. (27) reported the structure of two Asp variants (D313N and D313A) of a GH20 β-HEX from Streptomyces plicatus in a complex with β-GlcNAc. According to their data, the 2-acetamide group of β-GlcNAc was rotated in the D313N mutant, and consequently, the carbonyl oxygen of the 2-acetamide group could not function as a nucleophile during the formation of an oxazolinium ion intermediate (27). This result indicates that Asp-313 is crucial for the substrate-assisted catalysis of β-HEX. Although the structure of NgaP has yet to be solved, we predicted the position of the proton donor (Glu-608) and crucial Asp residue (Asp-607) based on the observation of Williams et al. (27). The results of point mutations of these two amino acids are consistent with the prediction. GalNAc thiazoline competitively inhibited NgaP, and the inhibition was 36,000-fold that by GlcNAc thiazoline. Collectively, these results indicate that NgaP is a β-NGA that adopts substrate-assisted catalysis in which Glu-608 and Asp-607 could function as a proton donor and a stabilizer of the 2-acetamide group of β-GalNAc at the active site, respectively. X-ray crystal analysis of NgaP would provide more precise information about the catalytic mechanism of this novel glycosidase.
Acknowledgments
We thank Dr. S. G. Withers and Dr. K. A. Stubbs for providing HexNAc thiazolines. We also thank Drs. K. Takegawa, N. Okino, and Y. Kakuta (Kyushu University) for critical reading of the manuscript.
This work was supported in part by Grant-in-aid for Basic Research 21380066 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
The structures of GSLs are presented in Table 1.
- GH
- glycoside hydrolase
- β-HEX
- β-hexosaminidase
- GSL
- glycosphingolipid
- β-NGA
- β-N-acetylgalactosaminidase
- pNP
- para-nitrophenyl
- Gb4Cer
- globotetraosylceramide
- Gb3Cer
- globotriaosylceramide
- GA1
- asialo-GM1
- GA2
- asialo-GM2
- LacCer
- lactosylceramide
- GlcCer
- glucosylceramide
- 4MU
- 4-methylumbelliferyl.
REFERENCES
- 1. Henrissat B. (1991) Biochem. J. 280, 309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Henrissat B., Davies G. (1997) Curr. Opin. Struct. Biol. 7, 637–644 [DOI] [PubMed] [Google Scholar]
- 3. Frohwein Y. Z., Gatt S. (1967) Biochemistry 6, 2775–2782 [DOI] [PubMed] [Google Scholar]
- 4. Izumi T., Suzuki K. (1983) J. Biol. Chem. 258, 6991–6999 [PubMed] [Google Scholar]
- 5. Tanaka A., Ozaki S. (1997) J. Biochem. 122, 330–336 [DOI] [PubMed] [Google Scholar]
- 6. Gutternigg M., Rendić D., Voglauer R., Iskratsch T., Wilson I. B. (2009) Biochem. J. 419, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gutternigg M., Kretschmer-Lubich D., Paschinger K., Rendić D., Hader J., Geier P., Ranftl R., Jantsch V., Lochnit G., Wilson I. B. (2007) J. Biol. Chem. 282, 27825–27840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies G., Henrissat B. (1995) Structure 3, 853–859 [DOI] [PubMed] [Google Scholar]
- 9. Mark B. L., Vocadlo D. J., Knapp S., Triggs-Raine B. L., Withers S. G., James M. N. (2001) J. Biol. Chem. 276, 10330–10337 [DOI] [PubMed] [Google Scholar]
- 10. Sumida T., Sueyoshi N., Ito M. (2002) Appl. Environ. Microbiol. 68, 5241–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sumida T., Sueyoshi N., Ito M. (2002) J. Biochem. 132, 237–243 [DOI] [PubMed] [Google Scholar]
- 12. Sumida T., Ishii R., Yanagisawa T., Yokoyama S., Ito M. (2009) J. Mol. Biol. 392, 87–99 [DOI] [PubMed] [Google Scholar]
- 13. Kanfer J. N. (1969) Methods Enzymol. 14, 660–664 [Google Scholar]
- 14. Saito H., Miura K. I. (1963) Biochim. Biophys. Acta 72, 619–629 [PubMed] [Google Scholar]
- 15. Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 16. Svennerholm L. (1956) J. Neurochem. 1, 42–53 [DOI] [PubMed] [Google Scholar]
- 17. Leatherbarrow R. J. (1990) Trends Biochem. Sci. 15, 455–458 [DOI] [PubMed] [Google Scholar]
- 18. Morrison J. F. (1969) Biochim. Biophys. Acta 185, 269–286 [DOI] [PubMed] [Google Scholar]
- 19. Dixon M. (1953) Biochem. J. 55, 170–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shimizu T., Ohtani K., Hirakawa H., Ohshima K., Yamashita A., Shiba T., Ogasawara N., Hattori M., Kuhara S., Hayashi H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J., Mahowald M. A., Ley R. E., Lozupone C. A., Hamady M., Martens E. C., Henrissat B., Coutinho P. M., Minx P., Latreille P., Cordum H., Van Brunt A., Kim K., Fulton R. S., Fulton L. A., Clifton S. W., Wilson R. K., Knight R. D., Gordon J. I. (2007) PLoS Biol. 5, 1574–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu J., Bjursell M. K., Himrod J., Deng S., Carmichael L. K., Chiang H. C., Hooper L. V., Gordon J. I. (2003) Science 299, 2074–2076 [DOI] [PubMed] [Google Scholar]
- 23. van Aalten D. M., Komander D., Synstad B., Gåseidnes S., Peter M. G., Eijsink V. G. H. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 8979–8984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lemieux M. J., Mark B. L., Cherney M. M., Withers S. G., Mahuran D. J., James M. N. (2006) J. Mol. Biol. 359, 913–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marković-Housley Z., Miglierini G., Soldatova L., Rizkallah P. J., Müller U., Schirmer T. (2000) Structure 8, 1025–1035 [DOI] [PubMed] [Google Scholar]
- 26. Dennis R. J., Taylor E. J., Macauley M. S., Stubbs K. A., Turkenburg J. P., Hart S. J., Black G. N., Vocadlo D. J., Davies G. J. (2006) Nat. Struct. Mol. Biol. 13, 365–371 [DOI] [PubMed] [Google Scholar]
- 27. Williams S. J., Mark B. L., Vocadlo D. J., James M. N., Withers S. G. (2002) J. Biol. Chem. 277, 40055–40065 [DOI] [PubMed] [Google Scholar]
- 28. Knapp S., Vocadlo D., Gao Z., Kirk B., Lou J., Withers S. G. (1996) J. Am. Chem. Soc. 118, 6804–6805 [Google Scholar]
- 29. Henderson P. J. (1972) Biochem. J. 127, 321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lineweaver H., Burk D. (1934) J. Am. Chem. Soc. 56, 658–666 [Google Scholar]
- 31. Thompson J. D., Higgins D. G., Gibson T. J. (1994) Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]