Abstract
Evidence suggests that the C-terminal truncation of α-synuclein is equally important as aggregation of α-synuclein in Parkinson disease (PD). Our previous results showed that an endopeptidase, matrix metalloproteinase-3 (MMP3), was induced and activated in dopaminergic (DA) cells upon stress conditions. Here, we report that MMP3 cleaved α-synuclein in vitro and in vivo and that α-synuclein and MMP3 were co-localized in Lewy bodies (LB) in the postmortem brains of PD patients. Incubation of α-synuclein with the catalytic domain of MMP3 (cMMP3) resulted in generation of several peptides, and the peptide profiles of WT α-synuclein (WTsyn) and A53T mutant (A53Tsyn) were different. Combined analysis using mass spectrometry and N-terminal determination revealed that MMP3 generated C-terminally truncated peptides of amino acids 1–78, 1–91, and 1–93 and that A53Tsyn produced significantly higher quantities of these peptides. Similar sizes of peptides were detected in N27 DA cells under oxidative stress and RNA interference to knock down MMP3-attenuated peptide generation. Co-overexpression of cMMP3 with either WTsyn or A53Tsyn led to a reduction in Triton X-100-insoluble aggregates and an increase in protofibril-like small aggregates. In addition, overexpression of the 1–93-amino acid peptide in the substantia nigra led to DA neuronal loss without LB-like aggregate formation. The results strongly indicate that MMP3 digestion of α-synuclein in DA neurons plays a pivotal role in the progression of PD through modulation of α-synuclein in aggregation, LB formation, and neurotoxicity.
Keywords: Cell Death, Matrix Metalloproteinase, Neurodegeneration, Parkinson Disease, Synuclein, Lewy Body, MMP3
Introduction
The epidemiological as well as animal studies suggest that environmental factors, genetic predispositions, and the interplay between the two factors are implicated in the PD3 pathogenesis (1), although the underlying molecular mechanisms remain largely unknown. α-Synuclein is a major component of Lewy bodies (LB), the pathological hallmark of Parkinson disease (2). As expression of three mutant forms of α-synuclein, A53T, A30P, and E46K, in the brain (3–5) and increased copy number by duplication or triplication of the wild-type α-synuclein gene (6–8) have been reported in the early onset familial cases of PD, the pathophysiological role of these mutants of α-synuclein has been a target of extensive investigations in PD research. The ability of α-synuclein to aggregate and form fibrillar deposits has been shown to play a central role in its pathology. The facts that the presence of C-terminally truncated α-synuclein in LB of sporadic PD and LB dementia (9, 10), the existence of various lengths of the truncated forms of α-synuclein in brain (10, 11), and the formation of C-terminally truncated α-synuclein in A53T transgenic mice that show motor symptoms (12) implicate the probable involvement of endopeptidases in cleaving α-synuclein in brains. In addition, about 15% of α-synuclein in LB are truncated forms, and incomplete degradation of α-synuclein produced highly amyloidogenic fragments (9, 13). Recent reports showed that matrix metalloproteinases were able to cleave α-synuclein, and MMP3 was the most efficient enzyme among them in cleaving α-synuclein into several fragments (14). Several other proteinases that include calpain, neurosin, and proteasome 20 S are known to cleave α-synuclein in DA neurons and are localized in or around LB where α-synuclein is localized (10, 15–18). However, the role of cleaved peptides is still obscure.
Increasing lines of evidence have linked matrix metalloproteinases to the pathogenesis of neurodegenerative diseases such as Alzheimer and Parkinson disease (19–22). Our previous studies demonstrated that MMP3 plays a crucial role in degeneration of dopaminergic neurons in substantia nigra (22, 23). We have also demonstrated recently that the active MMP3 accumulated in the cytoplasm of DA cells under various stress conditions was responsible for DJ-1 degradation and abolished antioxidant property of DJ-1 (24).
We investigated the following: (i) whether active MMP3 cleaves α-synuclein in DA neuronal cells under stress conditions; (ii) whether MMP3-mediated cleavage of WT and mutant α-synucleins yields different sizes and quantities of peptides; (iii) whether differentially cleaved peptides have their specific pathophysiological properties, and (iv) whether cleaved peptides play significant roles in DA neuronal degeneration in vivo. Here, we present evidence that MMP3-mediated α-synuclein cleavage plays a pivotal role in the pathogenesis of PD.
EXPERIMENTAL PROCEDURES
Materials
Recombinant α-synuclein peptides (WTsyn, A53Tsyn, and A30Psyn) were purchased from rPeptide, LLC (Bogart, GA). Fetal bovine serum (FBS) and cell culture reagents were bought from Invitrogen. Nisobutyl-N-(4-methoxyphenylsulfonyl)glycyl hydroxamic acid (NNGH) was purchased from Biomol International (Plymouth Meeting, PA). The recombinant catalytic domain of MMP3 protein was obtained from Calbiochem. Goat polyclonal anti-MMP3 antibody and rabbit polyclonal α-synuclein antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-FLAG antibody was obtained from Sigma. Secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). NuPAGE Tricine-ready gels (10–20% polyacrylamide), prestained SDS-PAGE standards (broad range), MMP3 siRNA and control constructs, and Lipofectamine 2000 were from Invitrogen. Primers were synthesized at Invitrogen. The Flp-InTM T-RexTM system for tetracycline-inducible cell line establishment was purchased from Invitrogen. First strand DNA synthesis kit was purchased from Roche Applied Science. PCR master mix was from Promega (Madison, WI). All other chemicals were reagent grade and were bought from Sigma or Merck.
Human Substantia Nigra Materials and Immunohistochemistry
Paraffin-embedded, formalin-fixed brain tissues from nine PD samples and six normal controls were obtained from the New York Brain Bank at Colombia University. History was obtained by retrospective chart analysis at the time of autopsy or collected prospectively. Postmortem intervals were less than 24 h in most cases (Table 1). Control cases had no recognizable pathology in the brain, and PD cases had diagnostic pathology (disappearance of DA neurons and Lewy bodies) on hematoxylin and eosin-stained slides. Immunohistochemistry was performed using MMP3 antibody that reacts with the C-terminal hemopexin domain of the human MMP3 (Santa Cruz Biotechnology) at 1:50 dilution or α-synuclein antibody (Santa Cruz Biotechnology). Paraffin-embedded human brain sections were incubated in citrate buffer at 95 °C for 20 min for antigen retrieval. Staining was completed using the tyramide signal amplification biotin system (PerkinElmer Life Sciences). We used the Vectastain Elite ABC kit from Vector Laboratories (Burlingame, CA) at 1:200 followed by exposure to tyramide solution according to the manufacturer's instructions. To develop color, diaminobenzidine (brown) was used as the chromogen. Sections were lightly counterstained with hematoxylin. For antibody blocking, MMP3 antibody was pre-absorbed with a 5-fold high concentration of blocking peptide (Santa Cruz Biotechnology) in 500 μl of PBS at room temperature for 2 h.
TABLE 1.
List of postmortem human brain tissues
Nine PD samples and six normal controls were obtained from the New York Brain Bank at Colombia University. History was obtained by retrospective chart analysis at the time of autopsy or collected prospectively. Postmortem intervals were less than 24 h in most cases.
| Status | Case ID | Age/Sex | Postmortem interval | Diagnosis |
|---|---|---|---|---|
| h | ||||
| PD | T243 | 77/F | 11:50 | Parkinson disease, moderate |
| T227 | 76/F | 6:44 | Parkinson disease, severe | |
| T155 | 83/F | 11:27 | Parkinson disease, moderate | |
| T112 | 78/M | 9:04 | Parkinson disease, severe | |
| T306 | 80/F | 3:20 | Parkinson disease | |
| T177 | 84/F | 3:30 | Parkinson disease | |
| T127 | 89/M | 5:50 | Parkinson disease | |
| T72 | 81/M | 2:30 | Parkinson disease | |
| T9 | 74/M | 4:15 | Parkinson disease | |
| Control | T198 | 82/M | 3:50 | Normal |
| T206 | 49/M | 13:55 | Normal | |
| T230 | 87/M | 5:10 | Normal | |
| T346 | 84/M | 10:00 | Normal | |
| T52 | 80/M | Not available | Normal | |
| T110 | 62/M | Not available | Normal |
Digestion of Recombinant α-Synucleins by MMP3 and Protein Gel Electrophoresis
500 ng of each recombinant α-synuclein peptide was incubated with recombinant catalytic domain of human MMP3 (cMMP3) at various concentrations (250 ng/ml to 2 μg/ml) in 30 μl of PBS for various duration (5 min to 1 h). Reactions were mixed with loading buffer and were subjected to SDS-PAGE using 10–20% Tricine gels. Protein was detected by Coomassie Brilliant Blue staining.
Transient Transfection of α-Synuclein Tagged with FLAG (N-terminal) and Myc (C-terminal), MMP3 Knockdown by siRNA, and Western Blot Analysis
Human α-synuclein was cloned into the p3×FLAG-myc-CMVTM-23 expression vector (Sigma) for N- and C-terminal tagging with FLAG and Myc, respectively. Site-directed mutagenesis using PCR was used for constructing A53T mutation. For transient overexpression of tagged α-synuclein, SN4741 cells or N27 cells were plated onto 6-well plates at 5 × 105 cells per well 1 day prior to transfection. The next day, cell were transiently transfected with either FLAG-WTsyn-myc or FLAG-A53Tsyn-myc. Briefly, 1 μg of plasmid DNA was mixed with 4 μl of Lipofectamine 2000 (Invitrogen) in 100 μl of Opti-MEM for 20 min prior to addition in the culture. 4 h after incubation, culture medium was freshly exchanged. Cells were treated with various toxic stimuli after overnight incubation. For MMP3 inhibition, cells were treated with NNGH (100 μm) 30 min prior to each stimuli. Total protein lysates were prepared 24 h after toxic stimuli, and N- and C-terminal fragmentations were determined by Western blot analysis using anti-FLAG and anti-Myc antibodies, respectively. To knock down MMP3 in N27 cells, three siRNA constructs were purchased from Invitrogen (Stealth RNAi) and tested for knockdown efficiency. The sense and antisense oligonucleotides were annealed following the manufacturer's protocol to generate double-stranded siRNAs at the final concentration of 20 μm. N27 cells were grown to 80% confluency in 6-well culture plates and were subjected to co-transfection by incubation with 8 μl of 20 μm siRNAs (final concentration 40 nm) and 1 μg of α-synuclein plasmid in 100 μl of Opti-MEM containing 10 μl of Lipofectamine 2000. After 6 h of incubation, the culture medium was freshly changed, and cells were maintained for additional 30 h before analysis. Stealth RNAi with a similar GC content was used for negative control.
Co-expression of α-Synuclein and Catalytically Active MMP3 in COS Cells Using Adenovirus
Human WTsyn, A53Tsyn, MMP3 catalytic domain (amino acid 100–273, and inactive mutated one (E219A) were cloned into the pENTR/D TOPO vector (Invitrogen) by PCR and then subcloned into the pAd/CMV/V5-DEST, adenovirus-mediated expression vector by site-specific recombination using clonase (Invitrogen). These viral constructs were transfected into HEK293 cells to obtain recombinant viruses, followed by virus amplification and purification based on the manufacturer's protocol. Viral titers were determined by methods previously described (25). Because the N-terminal prodomain, which is responsible for inhibition of catalytic activity of MMP3, was removed, overexpression of the catalytic domain produced an enzymatically active MMP3.
Establishment of Tetracycline-inducible α-Synuclein Cell Lines
Human α-synuclein constructs (full-length, 1–78, 1–91, and 1–93 amino acids of WTsyn and A53Tsyn) were cloned into the pcDNA5/FRT/TO©TOPO®TA vector containing a tetracycline-inducible promoter (Invitrogen). To establish the tetracycline-inducible expression system, the Flp-InTM T-RExTM 293 cell line (Invitrogen), which contains a single Flp recombination target site and tetracycline repressor in their genome, was used. Integration of an expression vector containing each α-synuclein construct into the genome via Flp recombinase-mediated DNA recombination at the Flp recombination target site produced isogenic cell lines. Tetracycline (5 μg/ml)-regulatable expression of the relevant construct was tested by Western blot analysis using anti-V5 antibody.
Establishment of Adeno-associated Viral (AAV) Constructs and AAV Packaging
FLAG-tagged full-length A53T or A53T1–93 peptide were subcloned into the CMV-IRES-hrGFP/AAV2 (A53Tsyn/AAV or A53T1–93/AAV). Either empty vector, A53Tsyn/AAV, or A53T1–93/AAV was co-transfected with pHelper and pAAV-RC to HEK293 cells using a standard calcium phosphate method. After 72 h, the cells were harvested, and crude rAAV vector solutions were obtained by repeated freeze/thaw cycles. The cleared crude lysate was then applied on a heparin-agarose column (Sigma). After all the lysate went through the column, the matrix was washed twice with 25 ml of PBS (pH 7.4, 0.1 m NaCl). The virus was then eluted with 15 ml of PBS (pH 7.4, 0.4 m NaCl). The eluent was concentrated to about 1 ml with a Millipore Centriplus YM-30 centrifugal filter by centrifugation at 4000 relative centrifugal force for 15–40 min. To adjust the NaCl concentration to physiological levels, the filter device was refilled with PBS, pH 7.4, and the virus was concentrated to 250–300 μl again. After removal of the virus-containing solution, the membrane of the filter device was washed three times with 100 μl of PBS, pH 7.4, which was added to the main part of the recombinant AAV2. The fractions containing high titer rAAV vectors were collected and used for injection into animals. The number of rAAV genome copies was semiquantified by PCR within the CMV promoter region using primers 5′-GACGTCAATAATGACGTATG-3′ and 5′-GGTAATAGCGATGACTAATACG-3′.
Overexpression of Full-length A53T or A53T1–93 in the Rat SN by Stereotaxic Delivery of AAV Viral Particles
The experiments were carried out on rat, in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). All procedures were approved by the local Animal Care and Use Committee. Female Sprague-Dawley (SD) rats (Charles River; 8 weeks old at the time of the beginning of AAV expression, 2–3 per cage) were maintained in a temperature/humidity-controlled environment under a 12-h light/dark cycle with free access to food and water. All rats were allocated into two groups, full-length A53T/AAV and A53T1–93/AAV. Rats were deeply anesthetized (30 mg/kg ketamine and xylazine mixture, intraperitoneally) and placed in a rat stereotactic apparatus, a site in the right SN (coordinates: anteroposterior, −5.3 mm; mediolateral, +2.0 mm; dorsoventral, −5.8 mm) was selected to inject full-length A53T/AAV (n = 8) or A53T1–93/AAV (n = 8). For the negative control, AAV containing empty vector was injected into the contralateral side (coordinates: anteroposterior, −5.3 mm; mediolateral, −2.0 mm; dorsoventral, −5.8 mm). A total of 1 × 1011 genome copy/ml rAAV particles diluted in 2 μl of ice-cold sterilized phosphate-buffered saline (PBS) were used in every animal. 12 weeks later, the animals were sacrificed.
Data Analysis and Statistics
Statistical analysis was carried out with GraphPad Prism version 5 (GraphPad Software Inc., San Diego). Data are expressed as percentages of values obtained in control conditions and are presented as means ± S.E. of at least four individual experiments. Statistical analyses were performed using the Student's t test or one-way analysis of variance followed by Bonferroni's multiple comparison test. Values of p < 0.05 were considered significant.
RESULTS
MMP3 Is Co-localized with α-Synuclein in the LB in the SN of Postmortem Brains of PD Patients
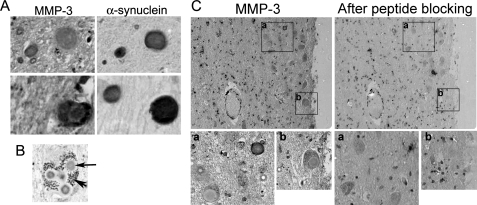
Two adjacent sections with 6-μm thickness of the SN area of postmortem brains of PD patients (Table 1) were stained with either MMP3 antibody or α-synuclein antibody. Although MMP3 was barely detected in control brain samples, it was localized in LB with strong staining in the intermediate zone where outer filaments start (Fig. 1, A and B); although its localization pattern in LB varied, it was mainly in the outer halo but was sometimes homogeneously distributed. Interestingly, MMP3 was not present in all LB that are α-synuclein-positive (Fig. 1A, lower panels). About 57% of Lewy bodies were co-stained with MMP3. MMP3 antibody preincubated with antigen-peptides failed to detect MMP3 in the adjacent sections (Fig. 1C). MMP3 was also localized in the cytoplasm. Whether MMP3 in the LB is catalytically active is unknown. MMP3 level was increased in the postmortem brain tissue of PD patients compared with control (supplemental Fig. 1).
FIGURE 1.
Immunostaining of MMP3 and α-synuclein in Lewy bodies in the SN of postmortem brains of PD patients. A, representative photomicrographs of adjacent sections of the SN tissues immunostained with MMP3 (left column) or α-synuclein (right column) show MMP3 staining in LB. B, representative photomicrograph of neuromelanin-positive dopaminergic neuron in the SN. Arrowhead indicates neuromelanin granules. Arrow indicates Lewy body stained with MMP3. Approximately 50 Lewy bodies stained with α-synuclein were analyzed. 57% of them were co-stained with MMP3. C, disappearance of MMP3 staining in LB after antibody absorption with blocking peptide. MMP3 antibody specificity was evaluated by preincubation with specific blocking peptide for 30 min. Right column, MMP3 immunostaining without blocking; left column, immunostaining after antibody preincubation with blocking peptide; lower panels, enlarged view of designated boxed areas a and b.
Differential Cleavage of WT α-Synuclein (WTsyn) and A53T Mutant (A53Tsyn) by cMMP3 and Generation of C-terminally Truncated Peptides
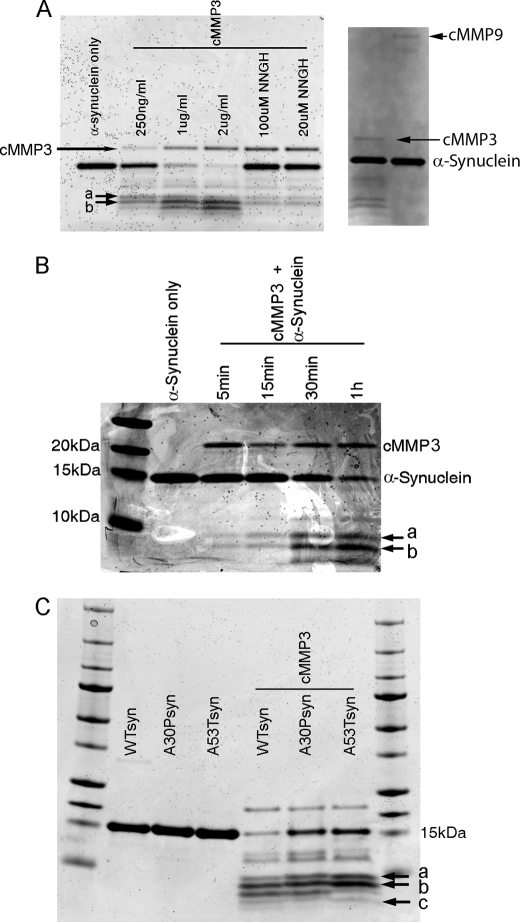
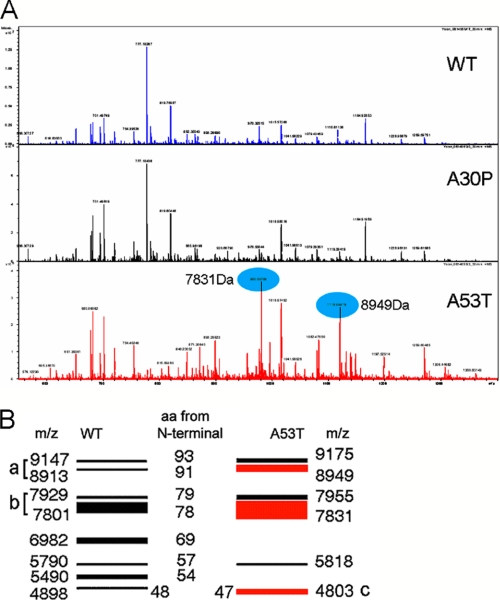
We first tested whether WTsyn was digested by the recombinant catalytic domain of MMP3 (cMMP3). WTsyn (500 ng) was mixed with various concentrations of cMMP3 (250 ng/ml to 2 μg/ml) for 60 min. Two distinguishable peptides (peptide-a and -b on gel) and several other peptides with higher molecular weight were generated by the lowest concentration of cMMP3 (250 ng/ml), and more peptides of lower molecular weight were produced by higher concentrations of cMMP3 (Fig. 2A). Preincubation of cMMP3 (2 μg/ml) with NNGH (20 or 100 μm), an MMP3 inhibitor, prevented α-synuclein digestion. MMP9 did not cleave α-synuclein (Fig. 2A), suggesting cleavage of α-synuclein was MMP3-specific. Cleavage of α-synuclein by cMMP3 over a period of time in the same conditions showed that two peptides (peptide-a and -b in Fig. 2B) were generated within 5 min, and more peptides were produced at a later time (30 min after incubation), but the intensity of peptide-a and -b remained strong, suggesting that peptide-a and -b were preferentially generated by cMMP3 as reported by others (14). To find differential cleavages of α-synuclein mutants by cMMP3, two human mutant recombinant α-synuclein variants (A30Psyn and A53Tsyn) in addition to WTsyn (1 μg each) were incubated with cMMP3 (1 μg/ml) for 1 h. Peptides generated from A53Tsyn by cMMP3 differed from those of WTsyn or A30Psyn, showing increased intensity of peptide-a and -b and a new appearance of an additional low molecular weight band (peptide-c) (Fig. 2C). The result suggests that conformational change caused by A53T mutation modifies MMP3-mediated digestion property. We also incubated β-synuclein with cMMP3, demonstrating differential fragment generation (supplemental Fig. 2). Fourier transform-ion cyclotron resonance mass spectrometry in conjunction with N-terminal determination using Edman degradation was used to identify the cleavage sites for peptide-a, -b, and -c in α-synuclein. The amino acid sequences of all three peptides started with a first amino acid of noncleaved α-synuclein, suggesting cMMP3 digested these α-synucleins from their C termini. The spectrometric pattern of peptides of A53Tsyn was different from those of WTsyn or A30Psyn (Fig. 3A). Based on the calculated masses and first amino acid of each peptide, the cleavage site of each peptide was determined. Peptide band-a consisted of two peptides, amino acids 1–93 and 1–91, and peptide band-b consisted of two peptides, amino acid 1–78 and 1–77. Peptide band-c was a single peptide of amino acids 1–47 (Fig. 3B). Two peptides, 1–91 and 1–78, in A53Tsyn digestion were distinguishable among others with their high intensity.
FIGURE 2.
Cleavage of WT (WTsyn) and mutant (A53Tsyn) recombinant α-synucleins by catalytic domain of MMP3 (cMMP3). A, cMMP3 dose-dependent digestion of WTsyn. WTsyn (500 ng) was incubated with various concentrations of cMMP3 (250 ng/ml and 1 and 2 μg/ml) at 37 °C for 1 h. NNGH (20 and 100 μm) was preincubated with cMMP3 (1 μg/ml) for 30 min prior to α-synuclein. WT recombinant α-synuclein was also incubated with cMMP9 (1 μg/ml). Cleaved peptides were detected by SDS-PAGE followed by Coomassie Blue staining. a and b indicate peptide bands generated at the lowest cMMP3 concentration (250 ng/ml). B, time course cleavage of WTsyn by cMMP3. Recombinant WTsyn (500 ng) was incubated with cMMP3 (1 μg/ml) at 37 °C for various durations (5, 15, and 30 min and 1 h), and cleaved peptides were detected by SDS-PAGE followed by Coomassie Blue staining. Note that peptide bands a and b (arrows) were most efficiently generated within 5 min. C, differential cleavage of A53Tsyn from WTsyn. cMMP3 (1 μg/ml) was added to recombinant WTsyn, A30Psyn, or A53Tsyn (500 ng each), and after 1 h of incubation, generated peptides were detected by SDS-PAGE followed by Coomassie Blue staining. a and b, bands most intensely stained in A53Tsyn; c, band exclusively generated in A53Tsyn.
FIGURE 3.
Mass spectrometric analysis of peptides generated from both WT and mutants form of α-synuclein by cMMP3 cleavage. A, after 30 min of incubation with cMMP3 (1 μg/ml), peptides from WTsyn, A30Psyn, and A53Tsyn (1 μg each) were analyzed by Fourier transform-ion cyclotron resonance mass spectrometry. Blue ovals represent two strong peaks generated exclusively in A53Tsyn. B, amino acid sequences and intensity determined accordingly with deconvoluted m/z values and N-terminal amino acids. Note that band a consists of two peptides (1–93 and 1–91 aa), and band b also consists of two (1–79 and 1–78 aa). Band c is one peptide of 1–47 aa, which is exclusively generated from A53T.
N-terminal Fragments of α-Synuclein Generated by MMP3 in Stressed DA Cells
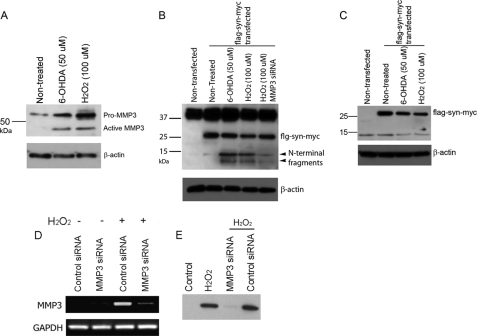
We sought to investigate whether MMP3-elicited α-synuclein fragmentation also occurs in DA cells after stress. Our previous studies demonstrated that expression and activity of MMP3 were increased in DA cells under stress conditions (22), and intracellular activation of MMP3 resulted in apoptosis of DA cells (23). We demonstrate here that the catalytically active form of MMP3 was induced in N27 cells, a rat DA cell line, after treatment with H2O2 and 6-hydroxydopamine (6-OHDA) (Fig. 4A). Proteasomal inhibition and the presence of rotenone also led to MMP3 activation (data not shown). Under these conditions, the generation of α-synuclein fragments was investigated using FLAG-WTsyn-myc (α-synuclein tagged with FLAG for N terminus and with Myc for C terminus) construct, because antibodies against the N terminus we tested were not effective. When FLAG-WTsyn-myc-overexpressed N27 cells were cultured in the presence of H2O2 (100 μm) or 6-OHDA (50 μm) for 24 h, tagged α-synuclein was fragmented (Fig. 4B). Molecular sizes of fragmented N-terminal peptides were equivalent to those obtained in in vitro cleavage of α-synuclein by cMMP3 (Fig. 4B). Anti-Myc antibody detected only full-length α-synuclein, suggesting that no C-terminal peptides were generated (Fig. 4C). To test whether MMP3 was responsible for this fragmentation, MMP3 was inhibited by siRNA-mediated knockdown prior to H2O2 (100 μm) treatment. Transfection of siRNA targeting MMP3 significantly reduced MMP3 in both mRNA and protein levels (Fig. 4, D and E). Knockdown of MMP3 using siRNA reduced H2O2-induced fragmented peptide generation (Fig. 4B). The results demonstrated that MMP3 cleavage of α-synuclein did occur in DA cells in stressful conditions.
FIGURE 4.
Stress-mediated MMP3 activation and generation of α-synuclein N-terminal fragments in dopaminergic cells. A, N27 cells were treated with either 6-OHDA (50 μm) or H2O2 (100 μm) for 24 h. Both pro- and active MMP3 in total lysates were detected by Western blot analysis. B and C, N27 cells were transiently transfected with FLAG-WTsyn-myc, and then 48 h later, cells were treated with 6-OHDA (50 μm) or H2O2 (100 μm) for 24 h. B, various N-terminal fragments were generated, and they were detected by anti-FLAG antibody. C, C-terminal fragment was determined using anti-Myc antibody. D and E, siRNA-mediated MMP3 knockdown efficiency was tested in N27 cells. N27 cells were transiently transfected with either control siRNA or siRNA targeting MMP3 (MMP3 siRNA) for 24 h and then cells were treated with H2O2 (100 μm) for 24 h. D, MMP3 mRNA level was determined by RT-PCR. E, MMP3 protein level was determined by Western blot analysis. Three independent experiments were performed, and representative photomicrographs are shown.
Co-overexpression of Active MMP3 and α-Synuclein Resulted in Decreased Insoluble Aggregates and Increased Formation of Protofibril-like Aggregates
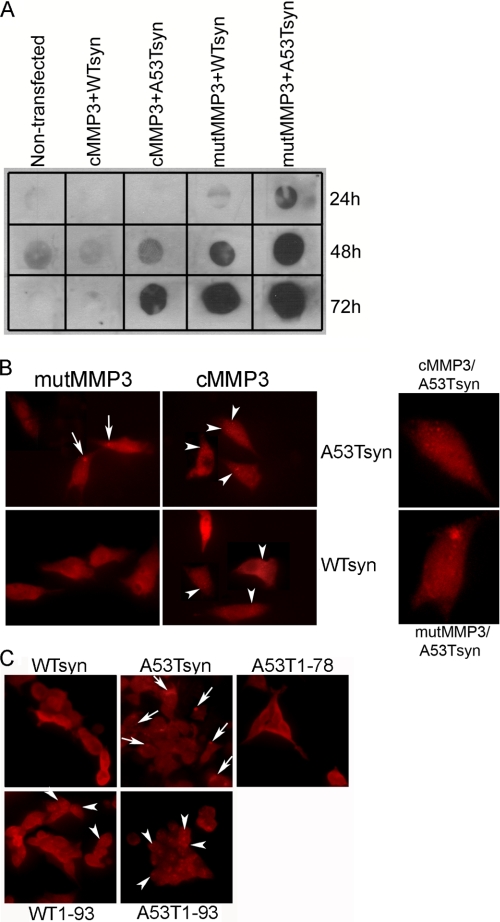
To characterize intracellular pathogenic molecular species of α-synuclein generated by MMP3 cleavage, we first tested co-overexpression of cMMP3 and α-synuclein into SN4741 cells by employing recombinant adenoviral transduction. Unfortunately, adenoviral constructs were highly toxic to SN4741 cells. As an alternative, COS cells that are known to be relatively resistant to adenoviral transduction were used (26). COS cells were co-transduced with adenovirus expressing WTsyn or A53Tsyn combined with one carrying cMMP3. Adenovirus expressing catalytically inactive mutant MMP3 (mutMMP3) was used as a negative control. Size exclusion filter assay was performed to characterize Triton X-100-insoluble α-synuclein aggregates at various time points (24, 48, and 72 h) after transduction. The result showed that A53Tsyn formed more insoluble aggregates than WTsyn, and co-overexpression with cMMP3 resulted in reduced formation of insoluble aggregates of α-synucleins (both WT and A53T) (Fig. 5A). Immunochemical staining of aggregates showed that overexpression of α-synuclein in COS cells produced two distinct types of aggregates, LB-like large perinuclear inclusions and small punctated aggregates scattered throughout the cytoplasm that are similar to those observed by others (27). The α-synuclein immunohistochemistry of the aggregates in COS cells co-overexpressing cMMP3 with WTsyn or A53Tsyn for 72 h after adenoviral transduction showed the following: 1) co-overexpression of full-length A53Tsyn and mutMMP3 resulted in perinuclear inclusion formation; 2) cMMP3 cleavage led to smaller A53Tsyn aggregates; 3) WTsyn did not form any visible inclusions at this time point; and 4) co-overexpression with cMMP3, however, produced small punctated aggregates in the cytoplasm (Fig. 5B).
FIGURE 5.
Decreased insoluble α-synuclein aggregates and increased protofibril-like small aggregate formation by MMP3. A, WTsyn and A53Tsyn were co-overexpressed either with cMMP3 or mutMMP3 (catalytically inactive construct) in COS cells for 24, 48, and 72 h. Triton X-100-insoluble aggregates were determined by filter trap assay. α-Synuclein was visualized using anti-α-synuclein antibody. B, representative photomicrograph of α-synuclein immunostaining in COS cells co-overexpressing either WTsyn or A53Tsyn with cMMP3 or mutMMP3 for 72 h. α-Synuclein was visualized using anti-α-synuclein antibody. Arrows indicate perinuclear inclusions; arrowheads indicate protofibril-like small punctate aggregates. Right panels show magnified images of a single cell. C, representative photomicrograph of α-synuclein immunostaining in tetracycline-inducible 293 cells expressing full-length WTsyn, A53Tsyn, and 1–93- and 1–78-amino acid peptides (WTsyn, A53Tsyn, WT1–93, A53T1–93, and A53T1–78, respectively). After 72 h of incubation with tetracycline, α-synuclein was visualized using anti-α-synuclein antibody. Arrows indicate perinuclear inclusions; arrowheads indicate protofibril-like small punctate aggregates.
To further characterize each α-synuclein fragment generated by MMP3 cleavage, tetracycline-inducible cell lines stably expressing FLAG-WTsyn, FLAG-A53Tsyn, and their MMP3-cleaved peptides (aa 1–78, 1–91, and 1–93 peptides of WTsyn and A53Tsyn) were established. Tetracycline inducibility was tested by measuring both mRNA and protein levels. Cells treated with 5 μg/ml tetracycline overnight reached maximum expressions. Interestingly, protein expression of aa 1–91 peptides of WTsyn and A53Tsyn (WT1–93 and A53T1–93, respectively) was less than others, although their mRNA levels were similar to others (supplemental Fig. 3). Immunocytochemistry using anti-FLAG antibody at 72 h after tetracycline treatment showed that, whereas full-length A53Tsyn formed inclusions near the nucleus, WTsyn1–93 and A53T1–93 yielded increased small aggregates throughout the cytoplasm (Fig. 5C). Full-length WTsyn and aa 1–78 of WTsyn and A53Tsyn showed diffuse cytoplasmic staining without aggregation (Fig. 5C).
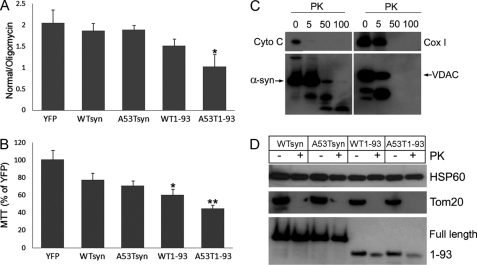
MMP3-cleaved α-Synuclein Fragments Localized into the Mitochondrial Matrix and A53T1–93 Decreased Mitochondrial O2 Consumption
An increasing body of evidence demonstrated that the interaction between α-synuclein and mitochondria is an important part in DA neurodegeneration (28–31). Our aim was to test whether MMP3-cleaved α-synuclein peptides are differentially localized in the mitochondria and whether they differentially affect mitochondrial functions. All fragments were localized into the mitochondria when α-synuclein species were induced with tetracycline (5 μg/m) for 5 days. However, only WT1–93 and A53T1–93 reduced mitochondrial O2 consumption, and A53T1–93 was more effective than WT1–93 in reducing O2 consumption (Fig. 6A). Here, YFP-expressing cells similarly treated were used as control. Growing cells in the galactose media, which forces cells to utilize mitochondria for ATP generation instead of glycolysis, led to the most significant cell death in cells expressing A53T1–93 (Fig. 6B).
FIGURE 6.
Localization of α-synucleins into the mitochondrial matrix and significant decrease in mitochondrial O2 consumption and survival by A53T1–93. Tetracycline-inducible 293 cells expressing various α-synuclein constructs, including full-length WT and A53T, 1–93-amino acid WT, and A53T (WTsyn, A53Tsyn, WT1–93, and A53T1–93, respectively), were incubated with tetracycline for 5 days. Tetracycline-inducible 293 cells overexpressing YFP were used as a control. A, mitochondrial O2 consumption was measured. n = 5. B, cell survival was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after culture in galactose media for 1 week. n = 5. C, mitochondrial fractions of cells overexpressing A53Tsyn were treated with various concentrations of proteinase K (PK) (0–100 μg/ml). Degradation of submitochondrial marker proteins and α-synuclein were detected by specific antibodies: Cyto C, cytochrome c (intermembranous space); α-syn, anti-FLAG antibody; VDAC (voltage-dependent anion channel) (outer membrane); Cox1 (inner membrane). D, after incubation of mitochondrial fractions with 5 μg/ml PK for 30, degradation of submitochondrial marker proteins and α-synuclein was detected by anti-Hsp60 antibody for matrix protein, anti-Tom20 antibody for outer membrane protei, and anti-FLAG antibody for α-synuclein. Data are shown as the mean ± S.D. Statistical analysis was performed using the Student t test. *, p < 0.05 versus YFP; **, p < 0.01 versus YFP.
Proteinase K (PK) protection assay was employed to determine submitochondrial localization of both full-length and 1–93-aa fragments. Optimal conditions that preserved the integrity of the mitochondrial inner membrane were determined in mitochondrial fractions of cells overexpressing full A53T after incubation with various PK concentrations. Proteins in all compartments were almost completely degraded at 50 and 100 μg/ml of PK. At a concentration of 5 μg/ml, voltage-dependent anion channel (integral protein in outer membrane) was partially digested; cytochrome c (inter-membrane space) was almost completely degraded, and Cox I (inner membrane) was slightly digested. At the same concentration, α-synuclein was protected from PK digestion (Fig. 6C). This suggests that PK is not able to digest matrix protein at this concentration. Thus, we used a 5 μg/ml concentration of PK to test other α-synuclein species. Because the voltage-dependent anion channel was not efficiently digested by PK, we used Tom20 as outer membrane marker. Although PK completely degraded Tom20, Hsp60 (matrix protein) was protected from PK digestion. At this concentration, full-length α-synucleins (WT and A53T) were not degraded and 1–93-aa fragments were slightly digested, suggesting that both forms are mainly localized into the matrix (Fig. 6D). Using anti-Akt antibody and anti-Tim-23 antibody for cytosolic and mitochondrial fractions, respectively, efficiency of mitochondrial fractionation was verified.
A53T1–93-mediated SNDA Neurodegeneration in Vivo
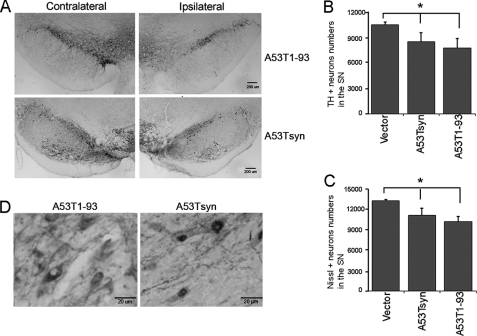
As described above, MMP3-cleaved α-synuclein, especially A53T1–93 resulted in cytotoxicity. This result is consistent with others demonstrating that application of MMP3-digested α-synuclein elicits increased cytotoxicity (14). To investigate toxicity of A53T1–93 in dopaminergic neurons in the nigrostriatal pathway, we overexpressed both full-length and A53T1–93 tagged with FLAG in the rat SN by stereotaxic delivery of rAAV carrying each construct into the SN. AAV harboring empty vector was injected at the same coordinate as control. AAV transduction efficiency was monitored by GFP, which was bicistronically expressed. Four weeks after AAV injection, about 75% of tyrosine hydroxylase (TH)-positive DA cells in the SN expressed GFP (supplemental Fig. 4). DA neuronal loss and α-synuclein aggregation were examined with anti-TH and anti-FLAG antibody, respectively, at 12 weeks post-AAV injection. Both full-length A53T and A53T1–93 caused degeneration of DA neurons in the SN (Fig. 7A, upper and lower panels). About 30% of TH-positive DA neuronal loss was observed in the SN overexpressing A53T1–93 compared with control (Fig. 7B). Nissl staining also showed a similar decrease in A53T1–93 (Fig. 7C). α-Synuclein staining using anti-FLAG antibody showed that LB-like inclusions were only formed by full-length A53T but not by A53T1–93 (Fig. 7D).
FIGURE 7.
Dopaminergic neurodegeneration in the substantia nigra induced by A53T1–93 α-synuclein. Either full-length A53Tsyn or A53T1–93 was overexpressed in the rat SN for 12 weeks. TH and α-synuclein were visualized by immunostaining. A, representative TH immunostaining in the SN (upper panels, full-length A53Tsyn; lower panels, A53T1–93; right, contralateral SN; left, ipsilateral SN). B, TH-positive DA neurons were stereologically counted, n = 8. C, Nissl-positive neurons were stereologically counted, n = 8. Statistical analysis was performed using one-way analysis of variance followed by Bonferroni's multiple comparison test. *, p < 0.01. D, A53T1–93 and A53Tsyn were visualized using anti-FLAG antibody in the SN. Although diffuse cytoplasmic distribution was observed in A53T1–93, LB-like aggregate was detected in dopaminergic neurons overexpressing full-length A53T.
DISCUSSION
Intracellular existence of matrix metalloproteinase and its activation under various conditions have been reported (23, 32, 33). In the previous study, we demonstrated that MMP3 is activated in DA cells treated with 1-methyl-4-phenylpyridinium, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-mediated DA neuronal degeneration in the mouse SN was largely attenuated in Mmp3 null mice (22). We have also shown that intracellularly activated MMP3 participated in apoptosis of DA cells in the presence of toxic stimuli (23). More recently, we demonstrated that active MMP3 digests DJ-1, abolishes its antioxidant function, and eventually leads to dopaminergic neuronal degeneration (24). These data suggest that MMP3 activity is not limited outside cells, but intracellular MMP3 is active as an endopeptidase and interacts with available substrates in cells. We also show herein MMP3 immunoreactivity in cytoplasmic inclusions, LB in nigral DA neurons of postmortem brain tissue from PD patients (Fig. 1). A majority of these LB where MMP3 is localized also contains α-synuclein. This observation led us to believe that intracellular interaction of active MMP3 with α-synuclein, probably cleaving α-synuclein, is possible. Serine protease neurosin also can cleave α-synuclein and co-localize with LB (16, 17). A number of studies suggests that inclusion body formation is a part of cellular protection mechanism by reducing diffuse distribution of toxic molecules (34, 35). Stress-induced intracellular MMP3 activity might be harmful to DA neurons, and thus cells actively limit its spread by trapping it within the inclusion bodies. Another explanation is that MMP3 might be passively localized with its substrates to LB. Whether MMP3 in LB is enzymatically active or it exists as proform remains to be elucidated.
Consistent with other studies (14, 36), we also observed here that MMP3 cleaved α-synuclein in vitro, generating several N-terminal fragments (Fig. 2). Other matrix metalloproteinases, including MMP-1,-2, and -9, were analyzed in the postmortem brain tissue from PD patients (20). They demonstrated that MMP9 is primarily found in neurons in the SN. In our study, we tested whether MMP9 also cleaves α-synuclein, but it did not digest α-synuclein, suggesting the specificity of MMP3 on α-synuclein cleavage (Fig. 2). Interestingly, mass spectrometry combined with N-terminal determination using Edman degradation identified that MMP3 generated 1–93, 1–91, and 1–78 peptides of both WTsyn and A53Tsyn, and A53Tsyn yielded more of these peptides (Fig. 3). The result may imply that structural alterations resulting from A53T mutation changes its interaction with MMP3.
α-Synuclein peptides that have equivalent size to ones generated by MMP3 in vitro was detected in DA cells treated with various toxic stimuli such as H2O2, rotenone, and 6-OHDA. Increased expression and intracellular activation of MMP3 was commonly observed under these stress conditions, and MMP3 inhibitions using siRNA resulted in decreased cleavage (Fig. 4). It has been demonstrated that C-terminally truncated peptide generation occurs spontaneously and preferentially in A53T transgenic mice (12). The size of those fragments range from 10 to 12 kDa and are correlated with neuropathological changes. Smaller N-terminal fragments observed in our conditions may result from MMP3 activation caused by toxic stimuli. Various protease-induced smaller peptide generations have been reported (15, 37). The results suggest that under stress conditions using toxic stimuli, MMP3-mediated proteolytic cleavage of α-synuclein may play a role in pathogenesis of PD.
MMP3 cleavage led to the change in aggregation property of α-synuclein. Although overexpression of full-length WTsyn in COS cells for 72 h did not form detectible inclusions, A53Tsyn resulted in perinuclear inclusion formations (Fig. 5B). Co-expressed with catalytically active MMP3 (cMMP3), both WTsyn and A53Tsyn preferentially formed diffuse punctated staining throughout the cytoplasm without large inclusions and showed a mild increase in toxicity. It has been shown that overexpression of WTsyn in COS cells results in two distinct types of aggregates, punctated small aggregates and large perinuclear inclusion bodies. Biochemical analysis defined small aggregates as the cellular equivalents of the protofibrils (27). It suggests that small punctated cytoplasmic staining induced by co-overexpression with cMMP3 can be the protofibril-like aggregates. However, large juxtanuclear inclusions were exclusively observed in COS cells overexpressing A53Tsyn but not WTsyn in this study. This discrepancy might result from different α-synuclein expression levels and incubation times. Similar to what we observed in COS cells co-expressing A53Tsyn and cMMP3, tetracycline-inducible 293 cells expressing A53T1–93 demonstrated increased small punctated aggregates over time (Fig. 5C). Consistent with our data, electron microscopic analysis on MMP3-digested α-synuclein in vitro showed spherical granular aggregate formations, and they were more toxic to cells than undigested ones, suggesting protofibril intermediate formation (14). Although devoid of two amino acids from the non-amyloid β component (non-Aβ component of Alzheimer disease amyloid, amino acid 61–95), A53T1–93 generated small protofibril-like aggregates. In fact, it has been shown that the 12-amino acid stretch (71VTGVTAVAQKTV82) in the middle of the hydrophobic non-Aβ component of Alzheimer disease amyloid region of human α-synuclein is necessary and sufficient for its fibrillation (38). As expected, the 1–78-aa fragment did not form any detectible aggregates. As shown in supplemental Fig. 1, tetracycline-inducible 293 cells harboring WT1–91 and A53T1–91 showed less protein levels than others, although its mRNA level is similar to others, implying protein instability. This was also observed in SN4741 cells transiently transfected with this construct. Molecular mechanism underlying this phenomenon might need further investigation.
It has been shown that mitochondrial complex I inhibition and oxidative stress can promote α-synuclein aggregation and increase toxicity (26, 39). A53T transgenic mice reveal its association with mitochondria and that catalytic activity of Cox-I in spinal cord is significantly reduced. Subsets of mitochondria contain α-synuclein and become dysmorphic. Another interesting mitochondrial pathology observed is mitochondrial DNA damage as seen by TUNEL assay (30). In this study, mitochondrial localization was not affected by MMP3-mediated α-synuclein cleavage (Fig. 6D). Mitochondrial O2 consumption, however, was decreased by the 1–93-aa fragment. A53T1–93 caused the most significant decrease in mitochondrial O2 consumption (Fig. 6A). Although it did not induce direct cell death under high glucose culture conditions, A53T1–93 led to significant cell death in culture medium containing galactose in which cells are forced to utilize mitochondria for ATP generation (Fig. 6B). A recent study identified the N-terminal 32 amino acids of human α-synuclein as a cryptic mitochondrial targeting sequence, and α-synuclein accumulation resulted in reduced complex I activity (40). This suggests that N-terminal fragments generated by MMP3 cleavage would translocate into mitochondria. Mitochondrial functional changes caused by each fragment, however, might be determined by its sequence and aggregation pattern.
Finally, we demonstrated that overexpression of A53T1–93 in the rat SNDA neurons led to neurodegeneration (Fig. 7). Interestingly, although only full-length A53Tsyn was able to form LB-like inclusions in the cytoplasm, both full-length and A53T1–93 overexpression resulted in DA neuronal degeneration. AAV2 was employed for neuron-specific delivery of full-length A53Tsyn or A53T1–93 constructs. Increasing reports have shown that AAV2-mediated gene transfer provides an effective means of achieving long term expression of target genes in nondividing cells such as neurons (41, 42). As shown in supplemental Fig. 2, more than 70% of TH-positive neurons were GFP-positive 4 weeks after AAV injection into the SN, indicating efficient expression in DA neurons. It has been demonstrated that AAV-mediated α-synuclein delivery into the SN resulted in DA neuronal degeneration in both rat and mouse PD models (43, 44). Transgenic mice overexpressing amino acid residues 1–130 of human α-synuclein developed selective dopaminergic neuronal loss in the substantia nigra pars compacta (45). Another study using transgenic mice carrying 1–120 residues of human α-synuclein demonstrated that this truncated form rendered dopaminergic cells more susceptible to oxidative stress (46). In our study, we failed to show the significant difference in DA neuronal death between A53T1–93 and full-length A53T at 12 weeks of overexpression. It might be necessary to investigate longer periods of overexpression to observe the difference. Because A531–93 causes significant mitochondrial toxicity, mitochondrion-focused long term in vivo experiments might be needed.
In summary, we have shown that active MMP3-mediated cleavage generates different pools of peptides from WTsyn and A53Tsyn, and it increases the propensity of protofibril-like aggregate formations. The 1–93-amino acid peptide that is produced more abundantly from A53Tsyn elicits mitochondrial toxicity. Overexpression of full-length and A53T1–93 in the rat SN similarly causes degeneration of SNDA neurons, whereas LB-like inclusions are exclusively formed by full-length A53Tsyn. The result provides a novel insight into the α-synuclein modification by activate MMP3 and its role in the pathogenesis of PD.
Acknowledgment
We thank Dr. Guhathakurta for the critical reading and revising of this manuscript.
This work was supported by Michael J. Fox Foundation Community Fast Track 2005 and National Research Foundation of Korea Grant KRF-E00081 funded by the Korean Government.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- PD
- Parkinson disease
- DA
- dopamine
- LB
- Lewy body
- NNGH
- nisobutyl-N-(4-methoxyphenylsulfonyl)glycyl hydroxamic acid
- rAAV
- recombinant adeno-associated virus
- SN
- substantia nigra
- aa
- amino acid
- TH
- tyrosine hydroxylase
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- 6-OHDA
- 6-hydroxydopamine.
REFERENCES
- 1. Thomas B., Beal M. F. (2007) Hum. Mol. Genet. 16, R183–R194 [DOI] [PubMed] [Google Scholar]
- 2. Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 3. Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez., Tortosa E., del Ser T., Muñoz D. G., de Yebenes J. G. (2004) Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 4. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 5. Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J. T., Schöls L., Riess O. (1998) Nat. Genet. 18, 106–108 [DOI] [PubMed] [Google Scholar]
- 6. Chartier-Harlin M. C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., Waucquier N., Defebvre L., Amouyel P., Farrer M., Destée A. (2004) Lancet 364, 1167–1169 [DOI] [PubMed] [Google Scholar]
- 7. Farrer M., Kachergus J., Forno L., Lincoln S., Wang D. S., Hulihan M., Maraganore D., Gwinn-Hardy K., Wszolek Z., Dickson D., Langston J. W. (2004) Ann. Neurol. 55, 174–179 [DOI] [PubMed] [Google Scholar]
- 8. Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. (2003) Science 302, 841. [DOI] [PubMed] [Google Scholar]
- 9. Baba M., Nakajo S., Tu P. H., Tomita T., Nakaya K., Lee V. M., Trojanowski J. Q., Iwatsubo T. (1998) Am. J. Pathol. 152, 879–884 [PMC free article] [PubMed] [Google Scholar]
- 10. Liu C. W., Giasson B. I., Lewis K. A., Lee V. M., Demartino G. N., Thomas P. J. (2005) J. Biol. Chem. 280, 22670–22678 [DOI] [PubMed] [Google Scholar]
- 11. Li W., West N., Colla E., Pletnikova O., Troncoso J. C., Marsh L., Dawson T. M., Jäkälä P., Hartmann T., Price D. L., Lee M. K. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2162–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee M. K., Stirling W., Xu Y., Xu X., Qui D., Mandir A. S., Dawson T. M., Copeland N. G., Jenkins N. A., Price D. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8968–8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell B. C., McLean C. A., Culvenor J. G., Gai W. P., Blumbergs P. C., Jäkälä P., Beyreuther K., Masters C. L., Li Q. X. (2001) J. Neurochem. 76, 87–96 [DOI] [PubMed] [Google Scholar]
- 14. Sung J. Y., Park S. M., Lee C. H., Um J. W., Lee H. J., Kim J., Oh Y. J., Lee S. T., Paik S. R., Chung K. C. (2005) J. Biol. Chem. 280, 25216–25224 [DOI] [PubMed] [Google Scholar]
- 15. Mishizen-Eberz A. J., Guttmann R. P., Giasson B. I., Day G. A., 3rd, Hodara R., Ischiropoulos H., Lee V. M., Trojanowski J. Q., Lynch D. R. (2003) J. Neurochem. 86, 836–847 [DOI] [PubMed] [Google Scholar]
- 16. Iwata A., Maruyama M., Akagi T., Hashikawa T., Kanazawa I., Tsuji S., Nukina N. (2003) Hum. Mol. Genet. 12, 2625–2635 [DOI] [PubMed] [Google Scholar]
- 17. Kasai T., Tokuda T., Yamaguchi N., Watanabe Y., Kametani F., Nakagawa M., Mizuno T. (2008) Neurosci. Lett. 436, 52–56 [DOI] [PubMed] [Google Scholar]
- 18. Alves da Costa C., Dunys J., Brau F., Wilk S., Cappai R., Checler F. (2006) J. Biol. Chem. 281, 9824–9831 [DOI] [PubMed] [Google Scholar]
- 19. Guo S., Wang S., Kim W. J., Lee S. R., Frosch M. P., Bacskai B. J., Greenberg S. M., Lo E. H. (2006) Brain Res. 1111, 222–226 [DOI] [PubMed] [Google Scholar]
- 20. Flex A., Gaetani E., Proia A. S., Pecorini G., Straface G., Biscetti F., Fioroni G., Sabusco A., Flore R., Tondi P., Pola P., Pola R. (2006) J. Gerontol. A Biol. Sci. Med. Sci. 61, 1065–1069 [DOI] [PubMed] [Google Scholar]
- 21. Lorenzl S., Albers D. S., Narr S., Chirichigno J., Beal M. F. (2002) Exp. Neurol. 178, 13–20 [DOI] [PubMed] [Google Scholar]
- 22. Kim Y. S., Choi D. H., Block M. L., Lorenzl S., Yang L., Kim Y. J., Sugama S., Cho B. P., Hwang O., Browne S. E., Kim S. Y., Hong J. S., Beal M. F., Joh T. H. (2007) FASEB J. 21, 179–187 [DOI] [PubMed] [Google Scholar]
- 23. Choi D. H., Kim E. M., Son H. J., Joh T. H., Kim Y. S., Kim D., Flint Beal M., Hwang O. (2008) J. Neurochem. 106, 405–415 [DOI] [PubMed] [Google Scholar]
- 24. Choi D. H., Hwang O., Lee K. H., Lee J., Beal F., Kim Y. S. (2011) Antioxid. Redox. Signal., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Carroll S. J., Hall A. R., Myers C. J., Braithwaite A. W., Dix B. R. (2000) BioTechniques 28, 408–410, 412 [DOI] [PubMed] [Google Scholar]
- 26. Lee H. J., Shin S. Y., Choi C., Lee Y. H., Lee S. J. (2002) J. Biol. Chem. 277, 5411–5417 [DOI] [PubMed] [Google Scholar]
- 27. Lee H. J., Lee S. J. (2002) J. Biol. Chem. 277, 48976–48983 [DOI] [PubMed] [Google Scholar]
- 28. Ding T. T., Lee S. J., Rochet J. C., Lansbury P. T., Jr. (2002) Biochemistry 41, 10209–10217 [DOI] [PubMed] [Google Scholar]
- 29. Hsu L. J., Sagara Y., Arroyo A., Rockenstein E., Sisk A., Mallory M., Wong J., Takenouchi T., Hashimoto M., Masliah E. (2000) Am. J. Pathol. 157, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin L. J., Pan Y., Price A. C., Sterling W., Copeland N. G., Jenkins N. A., Price D. L., Lee M. K. (2006) J. Neurosci. 26, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song D. D., Shults C. W., Sisk A., Rockenstein E., Masliah E. (2004) Exp. Neurol. 186, 158–172 [DOI] [PubMed] [Google Scholar]
- 32. Kwan J. A., Schulze C. J., Wang W., Leon H., Sariahmetoglu M., Sung M., Sawicka J., Sims D. E., Sawicki G., Schulz R. (2004) FASEB J. 18, 690–692 [DOI] [PubMed] [Google Scholar]
- 33. Eguchi T., Kubota S., Kawata K., Mukudai Y., Uehara J., Ohgawara T., Ibaragi S., Sasaki A., Kuboki T., Takigawa M. (2008) Mol. Cell. Biol. 28, 2391–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen L., Feany M. B. (2005) Nat. Neurosci. 8, 657–663 [DOI] [PubMed] [Google Scholar]
- 35. Arrasate M., Mitra S., Schweitzer E. S., Segal M. R., Finkbeiner S. (2004) Nature 431, 805–810 [DOI] [PubMed] [Google Scholar]
- 36. Levin J., Giese A., Boetzel K., Israel L., Högen T., Nübling G., Kretzschmar H., Lorenzl S. (2009) Exp. Neurol. 215, 201–208 [DOI] [PubMed] [Google Scholar]
- 37. Lindersson E., Beedholm R., Højrup P., Moos T., Gai W., Hendil K. B., Jensen P. H. (2004) J. Biol. Chem. 279, 12924–12934 [DOI] [PubMed] [Google Scholar]
- 38. Giasson B. I., Murray I. V., Trojanowski J. Q., Lee V. M. (2001) J. Biol. Chem. 276, 2380–2386 [DOI] [PubMed] [Google Scholar]
- 39. Betarbet R., Sherer T. B., MacKenzie G., Garcia-Osuna M., Panov A. V., Greenamyre J. T. (2000) Nat. Neurosci. 3, 1301–1306 [DOI] [PubMed] [Google Scholar]
- 40. Devi L., Raghavendran V., Prabhu B. M., Avadhani N. G., Anandatheerthavarada H. K. (2008) J. Biol. Chem. 283, 9089–9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mandel R. J., Burger C. (2004) Curr. Opin. Mol. Ther. 6, 482–490 [PubMed] [Google Scholar]
- 42. Björklund A., Kirik D., Rosenblad C., Georgievska B., Lundberg C., Mandel R. J. (2000) Brain Res. 886, 82–98 [DOI] [PubMed] [Google Scholar]
- 43. St Martin J. L., Klucken J., Outeiro T. F., Nguyen P., Keller-McGandy C., Cantuti-Castelvetri I., Grammatopoulos T. N., Standaert D. G., Hyman B. T., McLean P. J. (2007) J. Neurochem. 100, 1449–1457 [DOI] [PubMed] [Google Scholar]
- 44. Chung C. Y., Koprich J. B., Siddiqi H., Isacson O. (2009) J. Neurosci. 29, 3365–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wakamatsu M., Ishii A., Iwata S., Sakagami J., Ukai Y., Ono M., Kanbe D., Muramatsu S., Kobayashi K., Iwatsubo T., Yoshimoto M. (2008) Neurobiol. Aging 29, 574–585 [DOI] [PubMed] [Google Scholar]
- 46. Michell A. W., Tofaris G. K., Gossage H., Tyers P., Spillantini M. G., Barker R. A. (2007) Cell Transplant. 16, 461–474 [DOI] [PubMed] [Google Scholar]