Abstract
One of the most important characteristics of type 2 diabetes is insulin resistance, during which the patients normally experienced hyperinsulinism stress that would alter insulin signal transduction in insulin target tissues. We have previously found that early growth responsive gene-1 (Egr-1), a zinc finger transcription factor, is highly expressed in db/db mice and in the fat tissue of individuals with type 2 diabetes. In this report, we found that chronic exposure to hyperinsulinism caused persistent Erk/MAPK activity in adipocytes and enhanced insulin resistance in an Egr-1-dependent manner. An elevation in Egr-1 augmented Erk1/2 activation via geranylgeranyl diphosphate synthase (GGPPS). Egr-1-promoted GGPPS transcription increased Ras prenylation and caused Erk1/2 activation. The sustained activation of Erk1/2 resulted in the phosphorylation of insulin receptor substrate-1 at Serine 612. Phosphorylation at this site impaired insulin signaling in adipocytes and reduced glucose uptake. The loss of Egr-1 function, knockdown of GGPPS, or inhibition of Erk1/2 activity in insulin-resistant adipocytes restored insulin receptor substrate-1 tyrosine phosphorylation and increased insulin sensitivity. Our results suggest a new mechanism by which the Egr-1/GGPPS/Erk1/2 pathway is responsible for insulin resistance during hyperinsulinism. This pathway provides a new therapeutic target for increasing insulin sensitivity: inhibiting the function of Egr-1.
Keywords: Adipocyte, Diabetes, Insulin Resistance, MAP Kinases (MAPKs), Ras, Egr-1, GGPPS
Introduction
Insulin resistance prevents insulin-responsive tissues, such as adipose tissue, liver, and muscle, from reacting to insulin and taking up the glucose that circulates in plasma. When adipocytes and others become less sensitive and more resistant to the insulin, the pancreas increases secretion, and the level of insulin in the blood rises to maintain normal glucose levels (1). However, if insulin resistance worsens, the glucose levels will begin to rise, both of which are major symptoms of type 2 diabetes mellitus. Consequently, those with type 2 diabetes mellitus normally experience hyperinsulinism for many years before clinic characteristics occur (1, 2).
Upon insulin stimulation, the activated insulin receptor recruits and phosphorylates insulin receptor substrate-1 (IRS-1)4 at a tyrosine site (Tyr-Met-Xaa-Met (YMXM) or Tyr-Xaa-Xaa-Met (YXXM)), which activates the PI3K/Akt pathway and enhances glucose uptake and glycogenesis (3). However, IRS-1 is instead phosphorylated at several other sites in insulin-resistant rodents (e.g. Ser307, Ser612, Ser636, and Ser639) and type 2 diabetes patients (e.g. Ser312 and Ser616), all of which negatively regulate IRS-1 activity and block insulin signal transduction (4–10). It has been reported that insulin-stimulated Erk1/2 and JNK are able to modulate the phosphorylation of IRS-1. The activation of JNK can enhance the phosphorylation of IRS-1 on Ser307; this action not only impairs IRS-1 function by inhibiting its interaction with the insulin receptor but also promotes the degradation of the IRS-1 protein (11). Erk1/2 has also been shown to attenuate insulin signaling via Ser612 phosphorylation of IRS-1 (5). Thus, augmenting PI3K/Akt signaling and blocking MAPK signaling may prevent the development of insulin resistance and increase insulin sensitivity during stress; these pathways may therefore serve as targets for the development of pharmacological interventions.
In insulin-resistant patients, insulin target tissues cannot initiate glucose uptake because of their lack of response to insulin stimulation; however, insulin-resistant adipocytes have certain genes, such as mcp-1, fra-1, and egr-1, that can maintain sensitivity to insulin (9). Egr-1 (early growth response gene-1) is a member of the immediate early gene family characterized by zinc finger domains that recognize the highly conserved consensus GC-rich nucleotide sequences (GCG (G/T)GG GCG) (12, 13). Egr-1 can be transiently activated by many cytokines and hormones, including insulin, through the MAPK pathway (14, 15). In addition, as a transcription factor, Egr-1 can bind to target sequences and regulate the expression of many genes, such as TNF-α, PTEN, and SOCS-1 (16–18). Also, Egr-1 can exert its inhibitory effect on adipocyte differentiation in 3T3-L1 cells (19). In this study, we found that Egr-1 may augment Erk1/2 MAPK pathway signaling through its target gene geranylgeranyl diphosphate synthase (GGPPS). GGPPS is a branch point enzyme in the mevalonate pathway that catalyzes the synthesis of geranylgeranyl diphosphate (GGPP). GGPP and farnesyl pyrophosphate are used for the geranylgeranylation of proteins, such as Ras and Ras-related small GTP-binding proteins, with a CAAX motif at the C terminus (20).
We hypothesize that long term hyperinsulinism overactivates Egr-1 and causes sustained activation of MAPK in a GGPPS transcription-dependent manner. This results in an increase in serine phosphorylation of IRS-1 that leads to impaired PI3K/Akt activity followed by enhanced insulin resistance. This mechanism explains how hyperinsulinism enhances insulin resistance in type 2 diabetes mellitus. In this study, we provide in vivo and in vitro evidence that the Egr-1/GGPPS/Erk1/2 pathway is essential for the development of insulin resistance. Loss of Egr-1 function in the insulin-resistant adipocytes reduced the phosphorylation of IRS-1 on Ser612, thereby restoring the activity of IRS-1, the PI3K/Akt pathway, and glucose uptake. Our results suggest that the Egr-1/GGPPS/Erk1/2 pathway may be a promising target for the development of pharmacological inhibitors designed to prevent insulin resistance and increase insulin sensitivity in type 2 diabetes mellitus.
EXPERIMENTAL PROCEDURES
Materials
Anti-Erk1/2, anti-AKT, and antibodies to their phosphorylated forms were from Cell Signaling Technology; alkaline phosphatase-conjugated secondary antibody, anti-Egr-1, anti-IRS-1, and antibodies to its phosphorylated forms were from Santa Cruz Biotechnology; HRP-tagged secondary antibody was from Dako Cytomation; mouse recombinant TNF-α was from R & D; U0126 (a MEK1/2 inhibitor) and GGTI (a GGTase inhibitor) were from Sigma; 2-deoxy-d-[1-3H]glucose (200 μCi/mmol/liter) was from Amersham Biosciences; and insulin (40 IU/ml) was from Novolin. Human Egr-1 cDNA and dnEgr-1 (dominant negative Egr-1) with only zinc finger domains (96 amino acids) that could bind to the promoters of all the Egr-1 target genes including ggpps but had no transcription activity (21) were a gift from Prof. J. M. Baraban. Adenoviruses were constructed in our laboratory with the AdEasyTM system according to the manufacturer's protocol. To generate adenoviruses overexpressing Egr-1, dnEgr-1, the sequences of Egr-1 and dnEgr-1 were cloned from their original vectors to the pAdTrack-CMV vector. Human GGPPS cDNA was cloned by RT-PCR from the total RNA of HEK293 to the pAdTrack-CMV vector. The siRNAs purchased from Invitrogen were designed to target the following cDNA sequences: Egr-1, 5′-TCTCCCAGGACAATTGAAATTTGCT-3′; and scrambled, 5′-CCTACGCCACCAATTTCGT-3′. The target sequence of siEgr-1 was on the exon 2 of Egr-1 gene. To generate a siRNA/GGPPS adenovirus, a 19-nucleotide unique sequence targeting mouse GGPPS 5′-GGTGTCCCATCTGTCATTA-3′ and the scrambled sequence 5′-TTCTCCGAACGTGTCACGT-3′ were inserted into a pShuttle-H1 vector.
Animal Studies
Male BKs db/db mice were purchased from the Model Animal Research Center of Nanjing University. All of the mice were maintained on a 12-h light/dark cycle. All of the protocols for animal use were reviewed and approved by the Animal Care Committee of Nanjing University in accordance with Institutional Animal Care and Use Committee guidelines. The in vivo adenovirus administration experiment was performed as described previously (22). Briefly, BKs db/db mice (6 weeks old) were anesthetized prior to abdomen exposure. The adenoviral preparation (1 × 108 plaque-forming units in a volume of 20 μl) was injected at four points on each side of the epididymal fat tissue in db/db mice. The mice were maintained on a standard diet (65% carbohydrate, 4% fat, 24% protein). After 5 days, total protein was prepared from the fat pads for the Western blot analysis.
Generation of Adipose-specific GGPPS Deletion Mice and Primary Adipocyte Culture
The GGPPS genomic DNA was isolated from a 129SVJ mouse genomic library and used to construct the GGPPS targeting vector by standard techniques. Two LoxP sites were inserted into the flank of exons 3 and 4 of the GGPPS gene. GGPPS-LoxP-targeted mice were subsequently crossed with ap2-Cre transgenic mice to generate adipose-specific GGPPS knock-out mice. The transgenic lines (from ES cells of 129/Sv mouse strain) were back-crossed for at least six generations into the C57BL/6 background. Primary cultures of mice adipocytes were performed as described previously (23), with modifications. After a 24-h incubation in DMEM/F-12 with 10% fetal calf serum allowing attachment, the cells were washed in PBS and cultured in serum-free medium supplemented with agents (isobutylmethyl-xanthine, dexamethasone, insulin, transferrin, rosiglitazone, and triiodothyronine) that induce differentiation of preadipocytes to adipocytes. Triglyceride-storing adipocytes, representing 40–80% of cultured cells, are visible within 5–10 days. Adipocytes were maintained for an additional 4 days in DMEM/F-12 with 10% FCS before experiments.
3T3-L1 Adipocyte Differentiation
3T3-L1 cells were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China) and grown in DMEM supplemented with 10% FCS and 1% penicillin/streptomycin (growth medium). The cells were induced to differentiate into adipocytes according to previously described methods (24). To generate insulin-resistant adipocytes, the differentiated cells were treated for another 3 days with growth medium containing TNF-α (5 ng/ml).
Adenovirus Infection, siRNA Transfection, and Inhibitor Administration
The cells were infected with adenovirus at a multiplicity of infection of 200 in serum-free medium for 2 h; the medium was then supplemented with 2% FCS to maintain cell survival for another 48 h. Transfection of siRNA was performed using LipofectamineTM 2000 transfection reagent (Invitrogen) according to the manufacturer's protocol. For GGTI administration, the cells that had been infected with an adenovirus for 24 h were incubated in growth medium containing 540 nm GGTI for another 48 h. The administration of U0126 (10 μm) was performed 2 h before the cell lysates were collected.
Total RNA Preparation and Real Time RT-PCR
Total RNA from treated cells was extracted using TRIzol reagent (Invitrogen) and reversed transcribed with a PrimeScriptTM RT reagent kit (TaKaRa). The resulting cDNA was used for real time PCR performed on an ABI-7300 machine. SYBR-Green master mix was purchased from TAKARA. All of the quantitations were independent, conducted in triplicate, and normalized to an endogenous 18 S RNA control. The sequences of primer probes are as followed: GGPPS, 5′-TTTTGCATACACTCGACACACT-3′ and 5′-GGCCTCAATTTGTTTGTAGGCTT-3′; and 18 S, 5′-GTCTGTGATGCCCTTAGATG-3′ and 5′-AGCTTATGACCCGCACTTAC-3′.
Glucose Uptake
Glucose uptake in cells was performed as described previously (25). Briefly, the cells were pretreated with 1.5 μl of [3H]d-glucose (200 μCi/mmol/liter) and a final concentration of 1000 nm insulin. After 3 h, the reaction was terminated by washing the cells three times with ice-cold PBS. The cells were solubilized with 0.3 ml of 10 n KOH. Cold glycogen carrier was added to the lysates. Glycogen was precipitated with ethanol overnight and then centrifuged at 10,000 × g for 10 min. The pellets were resuspended in water and counted by scintillation counting (Beckman LS6500).
Western Blotting
Whole cell lysates were prepared according to previously reported standard protocols (26). For Western blotting, equal amounts of protein for each group were resolved by 10% SDS-PAGE and then transferred onto PVDF membranes (Bio-Rad, Richmond, CA). The membranes were then incubated with the appropriate primary antibody as indicated. Bound antibodies were visualized using alkaline phosphatase-conjugated or HRP-labeled secondary antibodies.
Immunoprecipitation
Immunoprecipitation was performed according to standard protocol (27). Pan-Ras antibody was used to form immune complex with Ras proteins of the cell lysates and immunoprecipitated down with protein A/G plus agarose; after a thorough wash, the samples were eventually subjected to Western blot analysis against K-Ras.
Ras Geranylgeranylation Measurements
The cells were infected with the indicated adenovirus, treated with the indicated inhibitor, lysed in 500 μl of lysis buffer, and centrifuged for 15 min at 18,000 × g. The total protein concentration was diluted to 1 mg/ml. Ras geranylgeranylation was measured as described previously (28). Briefly, an equal volume of 4% Triton X-114 was added to the supernatant to solubilize and fractionate the lipid-rich cell membrane. The aqueous upper phase containing enriched intracellular Ras, and the organic lower phase, which contained highly enriched membrane-associated Ras, was separated by 4% Triton X-114 at 37 °C for 5 min. Following the standard immunoprecipitation protocol, the collected samples were subjected to Western blot analysis.
RESULTS
In Adipocytes, Egr-1 Participated in Insulin Resistance Induced by Long Term Exposure to Insulin
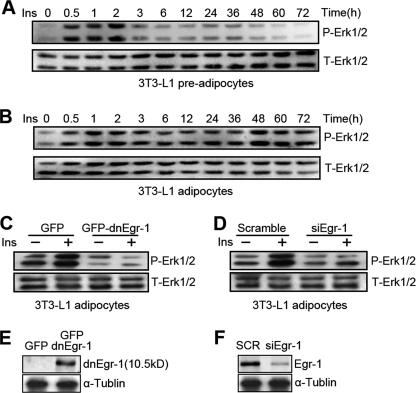
Before the patients are clinically diagnosed as type 2 diabetes mellitus, they are typically exposed to chronic hyperinsulinism stress, which occurs with a sustained, above normal level of insulin. To mimic in vivo chronic hyperinsulinism, we exposed preadipocytes that were used as controls and adipocytes to high insulin (1000 nm) for up to 72 h with fresh culture medium (plus insulin) provided every 12 h. Meanwhile, we compared the base-line change in the gene expression between the preadipocytes and adipocytes that were not pretreated with insulin (Fig. 1A). We found that insulin stimulation increased Egr-1 expression in as early as 60 min in both preadipocyte controls (Fig. 1B) and adipocytes (Fig. 1C), as expected. However, adipocytes and preadipocytes displayed completely different insulin response patterns after 24 h of insulin treatment. In adipocytes, the Egr-1 level increased continuously from 24 to 72 h of treatment (Fig. 1C), but in preadipocytes, Egr-1 expression was no longer responsive to insulin after 24 h (Fig. 1B). An analysis of insulin signaling showed that the tyrosine-phosphorylated form of IRS-1 (the active form) was still elevated after insulin treatment for 36 h in preadipocytes (Fig. 1B) but decreased to the basal level in adipocytes after 24 h of insulin exposure (Fig. 1C). Notably, the phosphorylation of IRS-1 on Serine 612 (the inactive form) increased over time in adipocytes, with a pattern that was similar to Egr-1 expression (Fig. 1C). Moreover, long term hyperinsulinism inhibited glucose uptake in adipocytes (Fig. 1D). However, this effect was reversed when we blocked the transcription activity of Egr-1 with a dnEgr-1 adenovirus (Fig. 1D). Collectively, these results suggest that long term exposure to excess insulin may trigger insulin resistance in adipocytes in an Egr-1-dependent manner in vitro.
FIGURE 1.
Egr-1 participates in the development of insulin resistance in adipocytes. A, to compare the base-line changes in genes expression between preadipocytes and mature adipocytes, these two kinds of cells were lysated at the indicated times without any insulin pretreatment. The cell lysates were Western blotted against P-IRS-1(Tyr), T-IRS-1, and Egr-1. B and C, to create a model of hyperinsulinism in vitro, preadipocytes (B) and adipocytes (C) were treated with insulin (Ins, 1000 nm) for the indicated times. To maintain the effect of insulin on the cells, insulin was added to the medium every 12 h. The cell lysates were Western blotted against P-IRS-1(Tyr), P-IRS-1(Ser612), and Egr-1. D, adipocytes were infected with dnEgr-1 adenovirus or GFP control adenovirus for 48 h and then stimulated with insulin (1000 nm) for 72 h. The cells were washed with fresh medium and acutely stimulated with insulin for 30 min and then assessed glucose uptake. *, p < 0.05, compared with the GFP group untreated with insulin; ##, p < 0.01, compared with the GFP group treated with insulin.
Long Term Insulin Stimulation in Adipocytes Caused Sustained Activation of Erk1/2 in an Egr-1-dependent Manner
The development of insulin resistance requires the activity of MAPK (Erk1/2) and the SAPKs (JNK and p38), which decrease insulin signaling by phosphorylating IRS-1 at serine sites (11, 29, 30). We found that insulin triggered a sustained increase in Erk1/2 from 48 to 72 h of treatment in adipocytes (Fig. 2B) but not in preadipocytes (Fig. 2A). This increase corresponds to the elevated Egr-1 expression observed in Fig. 1B. To determine whether Erk1/2 activity depended on Egr-1, we infected adipocytes with dnEgr-1 adenovirus or transfected them with Egr-1 siRNA. Insulin-stimulated Erk1/2 activity decreased significantly when the transcription activity of Egr-1 was inhibited (Fig. 2C) and when the expression of Egr-1 was knocked down (Fig. 2D). The efficiency of the dnEgr-1 adenovirus and the Egr-1 siRNA was determined (Fig. 2, E and F). These results indicate that hyperinsulinism-induced sustained Erk1/2 activation depends on the function of Egr-1.
FIGURE 2.
Sustained activation of Erk1/2 under long term insulin stimulation is dependent on Egr-1. A and B, preadipocytes (A) and adipocytes (B) were treated with insulin (Ins, 1000 nm) for the indicated times with changing fresh medium containing insulin every 12 h. The cell lysates were Western blotted against phosphorylated Erk1/2 (P-Erk1/2) and total Erk1/2 (T-Erk1/2). C and D, adipocytes were infected with GFP-dnEgr-1 adenovirus (C) or transfected with Egr-1 siRNA (siEgr-1) (D) for 48 h. The cells were treated with insulin for 3 h before the samples were Western blotted against P-Erk1/2 and total Erk1/2. E and F, adipocytes were infected with GFP-dnEgr-1 adenovirus (E) or transfected with Egr-1 siRNA (siEgr-1) (F) for 48 h. The cell lysates were Western blotted against Egr-1 and α-tubulin. The overexpressed dnEgr-1 is a zinc finger protein that can bind the promoters of Egr-1 target genes but has no transcriptional activity. It is a 10.5-kDa protein that can be detected with the Egr-1 antibody (Santa Cruz Biotechnology).
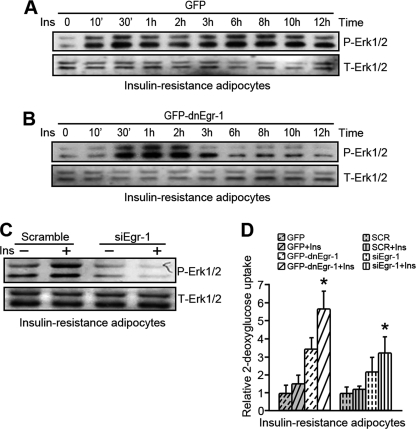
Insulin-resistant Adipocytes Exhibit Sustained Erk1/2 Activation When Exposed to Hyperinsulinism
As shown in normal adipocytes, insulin-resistant adipocytes also exhibited sustained Erk1/2 activation in response to insulin stimulation (Fig. 3A). A loss of Egr-1 transcriptional function inhibited the sustained Erk1/2 activity after insulin stimulation for 3 h (Fig. 3B). The results were further supported by an Egr-1 siRNA transfection experiment (Fig. 3C); Erk1/2 activity decreased significantly in response to insulin stimulation when Egr-1 expression was knocked down. Assessment of glucose uptake showed that inhibition of Egr-1 transcription activity or the knockdown of Egr-1 expression could increase insulin sensitivity in IR adipocytes (Fig. 3D). These results suggest that Egr-1-regulated sustained Erk1/2 activation maintains insulin insensitivity in IR adipocytes.
FIGURE 3.
Egr-1 is critical for sustained activation of Erk1/2 in response to long term insulin stimulation in insulin-resistant adipocytes. A and B, insulin-resistant adipocytes were infected with GFP-dnEgr-1 adenovirus (B) or GFP control adenovirus (A) for 48 h, followed by long term insulin (Ins) stimulation. The cell lysates were Western blotted against phosphorylated Erk1/2 (P-Erk1/2) and total Erk1/2 (T-Erk1/2). C, insulin-resistant adipocytes were transfected with Egr-1 siRNA (siEgr-1) for 48 h. The cells were treated with insulin for 3 h before the samples were collected and Western blotted against P-Erk1/2 and total Erk1/2. D, insulin-resistant adipocytes were infected with GFP-dnEgr-1 adenovirus or transfected with Egr-1 siRNA (siEgr-1) for 48 h. Insulin was administered to cells 30 min before measuring glucose uptake. *, p < 0.05, compared with the GFP group (plus insulin) or the scrambled group (plus insulin).
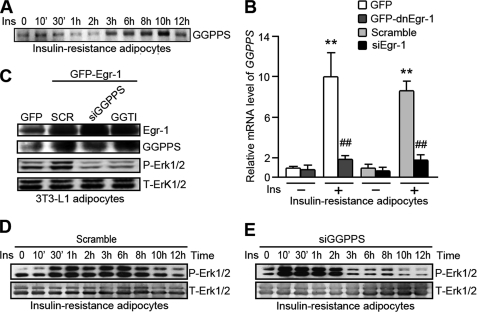
GGPPS, an Egr-1 Target Gene, Mediates Egr-1-regulated Erk1/2 Activation during Long Term Insulin Stimulation in IR Adipocytes
We previously reported that GGPPS is a target gene of Egr-1. GGPPS catalyzes the synthesis of isoprenoid moieties, such as GGPP (31, 32), which can be used for the geranylgeranylation of small GTP-binding proteins, including Ras and Ras-related proteins. The prenylation of Ras is generally considered essential for its membrane association and its ability to activate the downstream MAPK pathway (20, 33). We found that GGPPS could be increased by insulin stimulation in insulin-resistant adipocytes (Fig. 4A). A loss of Egr-1 function or knockdown of Egr-1 expression inhibited the expression of GGPPS in insulin-resistant adipocytes stimulated with insulin (Fig. 4B). To determine whether Egr-1 regulates Erk1/2 activity through GGPPS, we overexpressed exogenous Egr-1 in insulin-resistant adipocytes and introduced either GGPPS siRNA or GGTI (a GGTase inhibitor) before analyzing Erk1/2 phosphorylation. We found that exogenous Egr-1 greatly activated Erk1/2, whereas the disruption of GGPPS or the inhibition of GGTase function reversed the effect of Egr-1 overexpression (Fig. 4C). Long term exposure of insulin-resistant adipocytes to insulin revealed that when GGPPS expression was knocked down, insulin-induced sustained Erk1/2 activation after 2 h was inhibited (Fig. 4, D and E). These results suggest that Egr-1 regulates sustained Erk1/2 activity by promoting GGPPS expression in insulin-resistant adipocytes.
FIGURE 4.
GGPPS mediates the regulation of Erk1/2 activity by Egr-1 in insulin-resistant adipocytes. A, insulin-resistant adipocytes were treated with insulin (Ins, 1000 nm) for the indicated times with changing fresh medium containing insulin every 12 h. The cell lysates were Western blotted against GGPPS. B, insulin-resistant adipocytes were infected with GFP-dnEgr-1 adenovirus or transfected with Egr-1 siRNA (siEgr-1) for 48 h. After insulin treatment for 3 h, the cells were lysed, and total RNA was collected and subjected to quantitative PCR for GGPPS mRNA. C, adipocytes were infected with GFP-Egr-1 adenovirus and GGPPS siRNA (siGGPPS) adenovirus or treated with GGTI. After 48 h, the cells were Western blotted against Erk. D and E, IR adipocytes were infected with GGPPS siRNA adenovirus (E) or a scrambled control adenovirus (D) for 48 h and then subjected to long term insulin stimulation. The cell lysates were collected at the indicated times and subjected to Western blot analysis. **, p < 0.01, compared with the group untreated with insulin; ##, p < 0.01, compared with the group treated with insulin. P-Erk1/2, phosphorylated Erk1/2; T-Erk1/2, total Erk1/2.
Egr-1 Regulates Erk1/2 Activity via GGPPS-induced Ras Prenylation
Ras prenylation and membrane association are key steps for Ras-mediated activation of the Erk1/2-MAPK pathway. To determine whether Egr-1-activated GGPPS expression regulates Erk1/2 through Ras prenylation, we overexpressed exogenous Egr-1 or GGPPS in adipocytes or insulin-resistant adipocytes and then analyzed Ras prenylation and Erk1/2 activation. We found that Egr-1 or GGPPS overexpression increased Ras prenylation (down portion of Triton X-114 extraction) (Fig. 5A); however, the GGPPS knockdown or inhibition of GGTase activity reversed the effect of exogenous Egr-1 overexpression in adipocytes (Fig. 5A). Egr-1-stimulated Erk1/2 activation was also blocked by GGPPS knockdown and by inhibition of GGTase activity in adipocytes (Fig. 5B). In insulin-resistant adipocytes, a loss of Egr-1 function or GGPPS knockdown inhibited the Ras prenylation (Fig. 5C) and the Erk1/2 activity (Fig. 5D) induced by insulin. These results suggest that Egr-1 may promote positive feedback to regulate Ras/MAPK/Erk1/2 activation, which is dependent on GGPPS transcription and GGPPS-regulated Ras prenylation in both adipocytes and insulin-resistant adipocytes.
FIGURE 5.
Ras prenylation is crucial for Egr-1/GGPPS-promoted Erk1/2 activity. A and B, adipocytes were infected with Egr-1 or GGPPS adenovirus or disrupted prenylation by knockdown GGPPS or treated with GGTI for 48 h. The cells were subjected to Ras immunoprecipitation and prenylation measurement (A) or Western blotted to detect changes in Erk1/2 activity (B). C and D, IR adipocytes were infected with dnEgr-1 or GGPPS siRNA adenoviruses for 48 h. After insulin (Ins) treatment for 3 h, the cells were subjected to Ras immunoprecipitation and prenylation measurement (C) or Western blotted to detect changes in Erk1/2 activity (D). IP, immunoprecipitation; IB, immunoblot; P-Erk1/2, phosphorylated Erk1/2; T-Erk1/2, total Erk1/2.
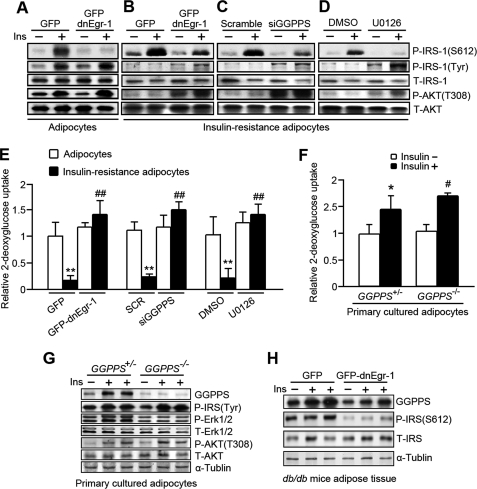
The Egr-1/GGPPS/Ras/Erk1/2 Pathway Enhances Insulin Resistance by Increasing IRS-1 Ser612 Phosphorylation
Erk1/2 has been shown to attenuate insulin-elicited signals by phosphorylating IRS-1 on Ser612 (5) and thereby inactivating it. To determine whether the Egr-1/GGPPS/Erk1/2 pathway is essential for the responses to insulin that occur during hyperinsulinism, we introduced a dnEgr-1 adenovirus (Fig. 6B), GGPPS siRNA (Fig. 6C), and U0126, a MAPK/Erk kinase inhibitor (Fig. 6D), into insulin-resistant adipocytes to block the components of this pathway. Meanwhile, we used the non-insulin-resistant adipocytes as control (Fig. 6A). The results show that insulin-stimulated phosphorylation of IRS-1 at Ser612 was significantly decreased by the inhibition of Egr-1 transcription activity (Fig. 6B), the knockdown of GGPPS expression (Fig. 6C), and the inhibition of Erk1/2 activation (Fig. 6D). In addition, tyrosine phosphorylation of IRS-1 (its active form) and Akt activation were also rescued after inhibition of the Egr-1/GGPPS/Erk1/2 pathway in insulin-resistant adipocytes (Fig. 6, B–D). Likewise, the ability of insulin-resistant adipocytes to take up glucose responding to insulin was also rescued when the Egr-1/GGPPS/Erk1/2 pathway was disrupted (Fig. 6E). We knocked out the GGPPS gene in the adipose tissue of mice and separated the adipocytes to expose them to insulin stimulation. Glucose uptake determination showed that GGPPS−/− adipocytes were more sensitive to insulin than GGPPS+/− adipocytes (Fig. 6F). Immunoblot analysis of GGPPS−/− adipocytes after insulin exposure also suggested that IRS-1 tyrosine phosphorylation was enhanced in GGPPS−/− adipocytes, and insulin signaling pathway had tilted to the PI3K/Akt pathway because Erk1/2 phosphorylation was decreased, and Akt phosphorylation was increased by GGPPS deletion (Fig. 6G). Additionally, to determine whether Egr-1 is required for GGPPS induction of IRS-1 inhibition in vivo, we disrupted the transcriptional activity of Egr-1 in the adipose tissue of the diabetic db/db mice by injecting dnEgr-1 adenovirus into the epididymal fat tissue (Fig. 6H). The immunoblot result shows that GGPPS expression in the adipose tissue was dependent on Egr-1, and the phosphorylated IRS-1 on Ser612, which was the inhibitory form of IRS-1, was decreased after loss of function of Egr-1. These data suggest that stimulation of the Egr-1/GGPPS/Erk1/2 pathway is crucial for the development of insulin resistance. Disruption of this pathway may increase insulin sensitivity; the Egr-1/GGPPS/Erk1/2 pathway may therefore be an effective target for the treatment of type 2 diabetes mellitus.
FIGURE 6.
The Egr-1/GGPPS/Erk1/2 pathway promotes insulin resistance by phosphorylating IRS-1 at Ser612. A–D, non-IR adipocytes and IR adipocytes were infected with GFP-dnEgr-1 (A and B) or GGPPS siRNA adenovirus for 48 h (C) or treated with U0126 for 2 h (D). Insulin treatment was performed 3 h, and the cells were subjected to Western blot analysis. E, non-IR adipocytes and IR adipocytes were infected with GFP-dnEgr-1 adenovirus or GGPPS siRNA for 48 h or treated with U0126 for 2 h. Insulin and [3H]d-glucose treatment was performed for 3 h. The cell lysates were assessed for glucose uptake. The relative glucose uptake of IR adipocyte was compared with normal adipocytes infected with GFP control adenovirus or scrambled adenovirus or treated with dimethyl sulfoxide (DMSO). F, glucose uptake measurement of the primary cultured adipocytes from GGPPSloxP/loxP aP2-Cre+/− (GGPPS−/−) and GGPPSwt/loxP aP2-Cre+/− (GGPPS+/−) mice. The primary cultured adipocytes were pretreated with insulin (1000 nm) and [3H]d-glucose (200 μCi/mmol/liter) for 3 h and subjected to glucose uptake measurement. G, the primary cultured adipocytes from the knock-out mice were pretreated with (1000 nm) for 3 h and subjected to Western blot. H, immunoblot analysis of the dnEgr-1-overexpressing db/db mice after insulin challenge (2 units/kg of body weight). The transcriptional activity of Egr-1 was inhibited in diabetic db/db mice by injecting the dnEgr-1 adenovirus into epididymal fat pads. All of the groups of animal experiments contained at least five mice. * and #, p < 0.05, compared with the groups untreated with insulin; **, p < 0.01, compared with the adipocytes; ##, p < 0.01, compared with the control group of IR adipocytes. P-Erk1/2, phosphorylated Erk1/2; T-Erk1/2, total Erk1/2.
DISCUSSION
Insulin activates two signaling pathways: PI3K/AKT and MAPK. Tyrosine-phosphorylated IRS-1 associates with the p85 subunit of PI3K and activates the PI3K/Akt pathway, which promotes glucose uptake and glycogenesis (3, 34). Insulin stimulation also activates MAPK (Erk1/2) and the SAPKs (JNK and p38), which result in cell growth (35, 36). These two signaling pathways, PI3K/AKT and MAPK, can both be triggered by insulin under normal physiological conditions. However, during insulin resistance development, the balance of PI3K/Akt and MAPK might be impaired by long term hyperinsulinism (37). The PI3K/AKT signal is disrupted by serine phosphorylation of IRS-1, whereas the MAPK pathway is still augmented and leads to insulin resistance (38). Benz et al. (39) have reported that Egr-1 can be induced by FGF-1 via increase of MAPK and inhibition of AKT/PKB in hippocampal neurons. They found that when the phosphorylated MAPK reached maximal activation, the phosphorylated AKT was at its lowest levels. This suggested an interaction between MEK-1/2 and PI3K during Egr-1 induction. However, it is still not clear what causes the imbalance between PI3K/AKT signaling and the MAPK pathway. In this study, our results suggest that Egr-1 and its target gene GGPPS may be involved in insulin resistance through the sustained activation of Erk/MAPK signaling during prolonged exposure to above normal level of insulin that resulted from insulin resistance. Such an exposure in adipocytes would decrease tyrosine phosphorylation of IRS-1 but increase serine phosphorylation, thereby further decreasing insulin sensitivity. This increase in the serine phosphorylation of IRS-1 depends on the sustained activation of Erk1/2 that is controlled by Egr-1-regulated GGPPS transcription. Our results provide a new mechanism by which the Egr-1/GGPPS/Erk1/2 pathway could impair insulin signaling: phosphorylation of IRS-1 at Serine 612.
GGPPS is a branch point enzyme in the mevalonate pathway that catalyzes the synthesis of GGPP. This isoprenoid may be used for Ras prenylation and then results in Ras membrane translocation and Ras-Raf-MAPK signaling pathway activation (20, 40). We found that Egr-1 may provide positive feedback to regulate Ras/MAPK/Erk1/2 activation through its target gene GGPPS under hyperinsulinism stress (Fig. 5). dnEgr-1 overexpression largely impaired GGPPS transcription induced by insulin in IR adipocytes. Furthermore, the overexpression of exogenous Egr-1 significantly increased Ras prenylation and Erk1/2 activation, which were highly dependent on Egr-1-promoted GGPPS transcription. The biological significance is that long term hyperinsulinism will stress insulin-sensitive tissues, such as fat, and evokes sustained activation of Erk1/2 by GGPPS-induced Ras prenylation. Sustained activation of Erk1/2 catalyzes the phosphorylation of IRS-1 at Serine 612 and blocks the interaction between IRS-1 and PI3K.
IRS-1, a key molecule in insulin signaling, has several phosphorylation sites: whereas tyrosine phosphorylation activates IRS-1, phosphorylation on Ser307 (4), Ser612 (5), and Ser636/639 (41) will inactivate this molecule. At a normal physiological level, insulin phosphorylates IRS-1 on tyrosine site (42). Phosphorylation on Ser307 and Ser636/639 primarily results from inflammation-induced JNK activation, which is the major cause of obesity-related insulin resistance (4, 43); phosphorylation on Ser612 is catalyzed by Erk1/2 (5). A normal physiological level of insulin can activate both PI3K/AKT and MAPK pathways through IRS-1 and Ras, respectively (44, 45). However, the PI3K/AKT pathway is inhibited, whereas the MAPK pathway remains active during insulin resistance and hyperinsulinism (30, 38); the balance between these two pathways is altered by an unknown mechanism. Our results indicate that the activation of Erk1/2 may be sustained by long term insulin stimulation in normal and insulin-resistant adipocytes. Erk1/2-induced phosphorylation of IRS-1 on Ser612 increased as tyrosine phosphorylation of IRS-1 was decreased by Egr-1 in a GGPPS-dependent manner. Additionally, glucose uptake in adipocytes was impaired by Egr-1-activated GGPPS transcription and sustained Erk1/2 activation. Our results suggest that this pathway could serve as a target for the development of pharmacological treatments for type 2 diabetes mellitus. As shown in this report, insulin signaling and glucose uptake were markedly restored when a dnEgr-1 adenovirus, GGPPS siRNA, and U0126 were introduced into insulin-resistant adipocytes.
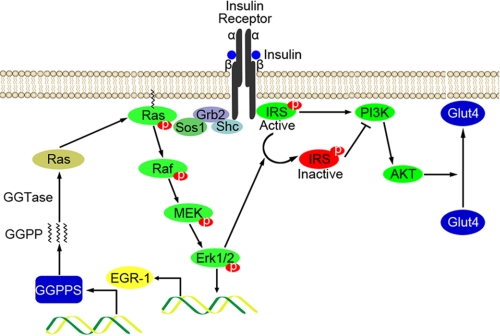
We showed for the first time that in insulin-resistant adipocytes, Egr-1 activates the MAPK pathway in response to insulin via a positive feedback mechanism involving a GGPPS-induced increase in Ras prenylation. The sustained activation of Erk1/2 in hyperinsulinism leads to the phosphorylation of IRS-1 on Ser612, which may inhibit the association of IRS-1 and PI3K (5, 43, 46) and thereby impair PI3K/AKT signaling and glucose metabolism (Fig. 7). In this way, hyperinsulinism leads to an imbalance in PI3K/AKT and MAPK signaling that eventually further enhances insulin resistance and finally results in type 2 diabetes mellitus. Collectively, our results suggest that Egr-1 may be a perfect target for the development of a pharmacological treatment for insulin resistance and type 2 diabetes mellitus.
FIGURE 7.
The model of Egr-1/GGPPS/Erk1/2 pathway regulating insulin resistance in hyperinsulinism. Egr-1 is greatly increased in hyperinsulinism and in insulin resistance through activation of the Erk1/2 MAPK pathway. The highly expressed Egr-1 can reactivate Erk1/2 by triggering GGPPS transcription and promoting Ras membrane association. Furthermore, the sustained activation of Erk1/2 can phosphorylate IRS-1 on Ser612 and inhibit its activity. Insulin resistance eventually results from impaired glucose uptake.
This work was supported by National Basic Research Program of China Grants 2009CB918703 and 2006CB943500 and National Natural Science Foundation of China Grant 30671086 awarded (to C.-J. L.). This work was also supported by National Natural Science Foundation of China Grant 30700394, Natural Science Foundation of Jiangsu Province of China Grant BK2007598, and Foundation of the Jiangsu Key Laboratory for Molecular and Medical Biotechnology Grant NMB09KF03 (to B. X.).
- IRS
- insulin receptor substrate
- GGPP
- geranylgeranyl diphosphate
- GGPPS
- GGPP synthase.
REFERENCES
- 1. Guilherme A., Virbasius J. V., Puri V., Czech M. P. (2008) Nat. Rev. Mol. Cell Biol. 9, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prentki M., Nolan C. J. (2006) J. Clin. Invest. 116, 1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wick K. R., Werner E. D., Langlais P., Ramos F. J., Dong L. Q., Shoelson S. E., Liu F. (2003) J. Biol. Chem. 278, 8460–8467 [DOI] [PubMed] [Google Scholar]
- 4. Aguirre V., Werner E. D., Giraud J., Lee Y. H., Shoelson S. E., White M. F. (2002) J. Biol. Chem. 277, 1531–1537 [DOI] [PubMed] [Google Scholar]
- 5. Bard-Chapeau E. A., Hevener A. L., Long S., Zhang E. E., Olefsky J. M., Feng G. S. (2005) Nat. Med. 11, 567–571 [DOI] [PubMed] [Google Scholar]
- 6. Emanuelli B., Eberlé D., Suzuki R., Kahn C. R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3545–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaneto H., Nakatani Y., Miyatsuka T., Kawamori D., Matsuoka T. A., Matsuhisa M., Kajimoto Y., Ichijo H., Yamasaki Y., Hori M. (2004) Nat. Med. 10, 1128–1132 [DOI] [PubMed] [Google Scholar]
- 8. Luan B., Zhao J., Wu H., Duan B., Shu G., Wang X., Li D., Jia W., Kang J., Pei G. (2009) Nature 457, 1146–1149 [DOI] [PubMed] [Google Scholar]
- 9. Sartipy P., Loskutoff D. J. (2003) J. Biol. Chem. 278, 52298–52306 [DOI] [PubMed] [Google Scholar]
- 10. Greene M. W., Sakaue H., Wang L., Alessi D. R., Roth R. A. (2003) J. Biol. Chem. 278, 8199–8211 [DOI] [PubMed] [Google Scholar]
- 11. Aguirre V., Uchida T., Yenush L., Davis R., White M. F. (2000) J. Biol. Chem. 275, 9047–9054 [DOI] [PubMed] [Google Scholar]
- 12. Hamilton T. B., Borel F., Romaniuk P. J. (1998) Biochemistry 37, 2051–2058 [DOI] [PubMed] [Google Scholar]
- 13. Silverman E. S., Collins T. (1999) Am. J. Pathol. 154, 665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hodge C., Liao J., Stofega M., Guan K., Carter-Su C., Schwartz J. (1998) J. Biol. Chem. 273, 31327–31336 [DOI] [PubMed] [Google Scholar]
- 15. Keeton A. B., Bortoff K. D., Bennett W. L., Franklin J. L., Venable D. Y., Messina J. L. (2003) Endocrinology 144, 5402–5410 [DOI] [PubMed] [Google Scholar]
- 16. Yao J., Mackman N., Edgington T. S., Fan S. T. (1997) J. Biol. Chem. 272, 17795–17801 [DOI] [PubMed] [Google Scholar]
- 17. Virolle T., Adamson E. D., Baron V., Birle D., Mercola D., Mustelin T., de Belle I. (2001) Nat. Cell Biol. 3, 1124–1128 [DOI] [PubMed] [Google Scholar]
- 18. Mostecki J., Showalter B. M., Rothman P. B. (2005) J. Biol. Chem. 280, 2596–2605 [DOI] [PubMed] [Google Scholar]
- 19. Boyle K. B., Hadaschik D., Virtue S., Cawthorn W. P., Ridley S. H., O'Rahilly S., Siddle K. (2009) Cell Death Differ. 16, 782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang F. L., Casey P. J. (1996) Annu. Rev. Biochem. 65, 241–269 [DOI] [PubMed] [Google Scholar]
- 21. Levkovitz Y., Baraban J. M. (2001) J. Neurosci. 21, 5893–5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tiraby C., Tavernier G., Lefort C., Larrouy D., Bouillaud F., Ricquier D., Langin D. (2003) J. Biol. Chem. 278, 33370–33376 [DOI] [PubMed] [Google Scholar]
- 23. Seboek D., Linscheid P., Zulewski H., Langer I., Christ-Crain M., Keller U., Müller B. (2004) J. Clin. Endocrinol. Metab. 89, 4833–4839 [DOI] [PubMed] [Google Scholar]
- 24. Kim S., Whelan J., Claycombe K., Reath D. B., Moustaid-Moussa N. (2002) J. Nutr. 132, 1135–1140 [DOI] [PubMed] [Google Scholar]
- 25. Wang Z., Lv J., Zhang R., Zhu Y., Zhu D., Sun Y., Zhu J., Han X. (2006) Biochem. Biophys. Res. Commun. 345, 976–983 [DOI] [PubMed] [Google Scholar]
- 26. Baron V., Duss S., Rhim J., Mercola D. (2003) Ann. N.Y. Acad. Sci. 1002, 197–216 [DOI] [PubMed] [Google Scholar]
- 27. Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, pp. 1486–1495, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 28. Goalstone M. L., Leitner J. W., Golovchenko I., Stjernholm M. R., Cormont M., Le Marchand-Brustel Y., Draznin B. (1999) J. Biol. Chem. 274, 2880–2884 [DOI] [PubMed] [Google Scholar]
- 29. Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 30. Fujishiro M., Gotoh Y., Katagiri H., Sakoda H., Ogihara T., Anai M., Onishi Y., Ono H., Abe M., Shojima N., Fukushima Y., Kikuchi M., Oka Y., Asano T. (2003) Mol. Endocrinol. 17, 487–497 [DOI] [PubMed] [Google Scholar]
- 31. Farnsworth C. C., Gelb M. H., Glomset J. A. (1990) Science 247, 320–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farnsworth C. C., Wolda S. L., Gelb M. H., Glomset J. A. (1989) J. Biol. Chem. 264, 20422–20429 [PMC free article] [PubMed] [Google Scholar]
- 33. Glomset J. A., Farnsworth C. C. (1994) Annual review of cell biology 10, 181–205 [DOI] [PubMed] [Google Scholar]
- 34. Biddinger S. B., Kahn C. R. (2006) Annu. Rev. Physiol. 68, 123–158 [DOI] [PubMed] [Google Scholar]
- 35. Begum N., Song Y., Rienzie J., Ragolia L. (1998) Am. J. Physiol. Cell Physiol. 275, C42. [DOI] [PubMed] [Google Scholar]
- 36. Weng L. P., Smith W. M., Brown J. L., Eng C. (2001) Hum. Mol. Genet. 10, 605–616 [DOI] [PubMed] [Google Scholar]
- 37. Samuelsson A. M., Bollano E., Mobini R., Larsson B. M., Omerovic E., Fu M., Waagstein F., Holmäng A. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H787–H796 [DOI] [PubMed] [Google Scholar]
- 38. Cusi K., Maezono K., Osman A., Pendergrass M., Patti M. E., Pratipanawatr T., DeFronzo R. A., Kahn C. R., Mandarino L. J. (2000) J. Clin. Invest. 105, 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benz A. H., Shajari M., Peruzki N., Dehghani F., Maronde E. (2010) Br. J. Pharmacol. 160, 1621–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kainou T., Kawamura K., Tanaka K., Matsuda H., Kawamukai M. (1999) Biochem. Biophys. Acta 1437, 333–340 [DOI] [PubMed] [Google Scholar]
- 41. Moeschel K., Beck A., Weigert C., Lammers R., Kalbacher H., Voelter W., Schleicher E. D., Häring H. U., Lehmann R. (2004) J. Biol. Chem. 279, 25157–25263 [DOI] [PubMed] [Google Scholar]
- 42. Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. (1991) Nature 352, 73–77 [DOI] [PubMed] [Google Scholar]
- 43. Paz K., Hemi R., LeRoith D., Karasik A., Elhanany E., Kanety H., Zick Y. (1997) J. Biol. Chem. 272, 29911–29918 [DOI] [PubMed] [Google Scholar]
- 44. Bae S. S., Cho H., Mu J., Birnbaum M. J. (2003) J. Biol. Chem. 278, 49530–49536 [DOI] [PubMed] [Google Scholar]
- 45. Eringa E. C., Stehouwer C. D., van Nieuw Amerongen G. P., Ouwehand L., Westerhof N., Sipkema P. (2004) Am. J. Physiol. Heart Circ. Physiol. 287, H2043–H2048 [DOI] [PubMed] [Google Scholar]
- 46. Tzatsos A., Kandror K. V. (2006) Mol. Cell. Biol. 26, 63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]