Abstract
The mechanisms that control cardiac contractility are complex. Recent work we conducted in vertebrate skeletal muscle identified a new state of myosin, the super-relaxed state (SRX), which had a very low metabolic rate. To determine whether this state also exists in cardiac muscle we used quantitative epi-fluorescence to measure single nucleotide turnovers by myosin in bundles of relaxed permeable rabbit ventricle cells. We measured two turnover times—one compatible with the normal relaxed state, and one much slower which was shown to arise from myosin heads in the SRX. In both skeletal and cardiac muscle, the SRX appears to play a similar role in relaxed cells, providing a state with a very low metabolic rate. However, in active muscle the properties of the SRX differ dramatically. We observed a rapid transition of myosin heads out of the SRX in active skeletal fibers, whereas the population of the SRX remained constant in active cardiac cells. This property allows the SRX to play a very different role in cardiac muscle than in skeletal muscle. The SRX could provide a mechanism for decreasing the metabolic load on the heart, being cardioprotective, particularly in time of stress such as ischemia.
Introduction
Understanding the metabolic rate of the myocardium and its control is an active area of investigation. The work produced and energy used in a single twitch can vary over a large range, influenced by muscle length, protein phosphorylation, and oxygen availability (for review, see (1)). During times of stress, ischemia, or hypoxia, the myocardium can rapidly downregulate output and energy use to very low levels (2). The major element controlling active force is exerted through calcium binding to the thin filament. A wide variation in force is produced by variation in the amount of calcium released into the cell interior. However, there has been increasing evidence that force can also be modulated by the thick filament (for review, see (3)). The structure of the cardiac thick filament is known to undergo dynamic changes produced by alterations in protein phosphorylation, and these changes have been associated with variation in cardiac output and energy utilization (3–9). Here we investigate what we believe is a new state of cardiac myosin, the super-relaxed state (SRX), and discuss the potential physiological function and importance of this state.
We have recently used quantitative epi-fluorescence to measure single nucleotide turnovers in relaxed skeletal muscle fibers and identified what to our knowledge is a new relaxed state of myosin in skeletal muscle, the SRX (10). This state has an ATP turnover rate that is an order-of-magnitude smaller than that normally observed in chemically skinned skeletal muscle fibers or purified myosin. Although it has long been recognized that there are multiple, attached force-generating actomyosin chemomechanical states in the active cycle, this was, to our knowledge, the first identification of relaxed states with such widely disparate kinetic properties and represents a paradigm shift in the description of relaxed muscle. Myosin heads are still relaxed in the classical sense; they are detached from actin and not generating force. However, their ATP turnover can differ dramatically according to which subpopulation within the relaxed state that they occupy. Furthermore, resting energy utilization can be modulated via perturbation of the relative fractions of the two relaxed states.
This work extends these studies to resting cardiac muscle cells, as well as to active skeletal and cardiac muscle cells. We show that there is an SRX in resting cardiac muscle with properties similar to those seen in resting skeletal fibers. However, here we extend these studies to active muscle, and in active muscles, the characteristics of this state differ between the two types of muscle. We demonstrate that the properties of the cardiac SRX would allow it to serve as a modulator of cardiac energy utilization and contractility, and that in particular it could be involved in decreasing metabolic rate in both the normally functioning myocardium and during times of hypoxic or other stress.
Materials and Methods
Fiber preparations and solutions
Rabbit cardiac ventricle muscle was harvested and cut into strips (diameter ∼2 mm, length ∼6 mm) and attached to sticks under low tension. Then the cells were chemically skinned as described in the Supporting Material and stored at −20°C. Removal of the membranes allows modulation of the chemical milieu surrounding a relatively intact myofilament array.
The cardiac muscle strips were manually dissected into small bundles of cells having a diameter between 40 and 80 μm, and mounted in a simple flow cell as described in the Supporting Material. Sarcomere lengths varied from 1.9–2.2 μm. Temperature was 26 ± 1°C.
Solutions
The basic rigor buffer contained 120 mM KAcetate, 5 mM MgAcetate2, 5 mM EGTA, 2.5 mM K2HPO4, 2.5 mM KH2PO4, and 50 mM 3-(n-morpholino)propanesulfonic acid, pH 6.8, with daily fresh addition of 2 mM DTT. Nucleotides were added to the stated concentrations.
Basic protocol
The ATPase turnover was measured by means of the replacement of the fluorescent nucleotides, 2′(3′)-O-n-methylanthraniloyl-ATP (mantATP), by nonfluorescent nucleotides or vice versa. The fluorescent-nucleotide mantATP has a long history as an ATP analog used to measure kinetic rates in myosin (11–13). In the standard experiment, the mounted cells were first washed with rigor solution to remove any residual nucleotide from the skinning process. Then, the cells were incubated for several minutes with the fluorescent nucleotides, mantATP or mantADP, until maximum fluorescence was reached (incubation phase). Measurement of tension of bundles of cardiac cells in 250 μM mantATP or 4 mM ATP showed that the fibers were relaxed, tension <2% of fully activated (see the Supporting Material). This shows that the activation of relaxed fibers seen at higher temperatures has not occurred during the incubation phase (14). The fluorescence of mant-nucleotides increases when bound to the myosin nucleotide pocket (11–13). Subsequently, the fluorescent nucleotides were chased by nonfluorescent nucleotides (chase phase).
The intensities initially obtained in the incubation phase were used to normalize the data obtained during the chase phase. Fibers were incubated for 2 min in the incubation phase before the chase. Between each run, the cell preparation was washed with rigor buffer several times to remove all nucleotides before another run. Up to three runs could be done on a single bundle of cells without deterioration, e.g., <10% change in populations or time constants. Runs were separated by a rigor wash for at least 6 min to remove free and bound nucleotides. In the reverse experiment, nonfluorescent nucleotides are chased by fluorescent nucleotides. The cells were first incubated with ATP or ADP and then chased by mantATP or mantADP. The data were normalized to the level obtained when the cells were incubated in only mantATP or mantADP in another run on the same bundle.
Determination of fractions of slowly and rapidly cycling cross-bridges in relaxed muscle
Fluorescence intensity, I, as a function of time, t, was fit using a two-exponential fit of the form
where P1 and P2 are the relative initial fractions in the two states and T1 and T2 are the time constants for the life of the state. The equation for the fits to intensity increase was obtained by subtracting the expression on the right-hand side of the above equation from 1. Fitting was done using a nonlinear least-squares algorithm in KaleidaGraph Version 3.6 (Synergy Software, Reading, PA). The fit also defined the 95% confidence limits.
Apparatus
Fluorescence images were acquired on a model No. TE2000 microscope (Nikon, Melville, NY) with a CoolSNAP HQ2 camera (Photometrics, Tucson, AZ). The average fluorescence intensities within small rectangular areas, 15–30 μm × 20–40 μm, were measured and fiber fluorescence was determined by subtracting the background intensity from the fiber intensity. The data were fit to a two-exponential decay or rise, as described in the Supporting Material. The fit also defined the 95% confidence limits. The fluorescence images of nucleotides bound to the cells was visualized using a spinning disk confocal spectrometer (Nikon).
Results
Measuring single nucleotide turnovers in permeable cardiac cells
We determined the ATP turnover rate in permeable bundles of rabbit ventricle muscle by observing the fluorescence intensity of mantATP using quantitative epi-fluorescence microscopy. MantATP binds to myosin with high affinity, functioning similarly to ATP (11,13). Small bundles of cells were observed in a flow cell that allowed nucleotide solutions to be rapidly exchanged (see Fig. S1 and Fig. S2 in the Supporting Material). Two protocols were used.
In the first protocol, cells were incubated with a relaxing solution containing 250 μM mantATP, and chased by another relaxing solution containing 4 mM ATP. The fluorescent intensity of the muscle decreases with time as mant-nucleotide was released and replaced by excess ATP (see Fig. 1). The fluorescence intensity decayed rapidly during the first ∼30 s followed by a slower decay over several minutes.
Figure 1.

Changes in fluorescence intensity during the chase phase of the single nucleotide turnover experiments are shown for mantATP chased by ATP (O), and for ATP chased by mantATP (●). In both experiments the intensity changes in two phases—a rapid phase with a time constant of ∼10–15 s and a slow phase with a time constant of ∼140 s. The symmetry between the two experiments shows that the single nucleotide turnover measurements are the same for mantATP replacing ATP and for ATP replacing mantATP. The data were fit with a double-exponential function (solid lines). Parameters of the fits to the data shown here are provided in the Supporting Material.
In the second protocol, cells were incubated with 4 mM ATP and chased with 250 μM mantATP. Fluorescence intensity increased as mantATP replaced ATP (see Fig. 1). An important observation from Fig. 1 is that the populations and time constants of the two fluorescent phases were not changed when the order of the nucleotides in the incubation and chase were switched. This shows that the nucleotide release rates were not significantly influenced by the use of the mant-labeled nucleotides. The results were also unchanged when nucleotide concentrations were varied (see the Supporting Material).
The fluorescence data could be fit by a sum of two exponentials, one with a short time constant, ∼12–25 s, and a second with a time constant that is much longer, ∼140 s (see Table 1). The fast component represented ∼65% of the total muscle fluorescence. This component is composed of multiple processes. The diffusion of free nucleotides out of the muscle bundle of the diameters used here occurs in ∼10–15 s (15). The release of nonspecifically bound nucleotides is, presumably, also fast. The release of nucleotides by normally relaxed myosin heads occurs with a time constant of ∼27 s (16), All of these processes are occurring together and are not resolvable by the present protocol; however, they contribute to the complexity of the decay, as discussed in the paragraph below. The slow component represented ∼28% of total muscle fluorescence.
Table 1.
Parameters for the two-exponential fit to the data for cardiac muscle
| Incubation | Chase | N | P1 | T1 (s) | P2 | T2 (s) |
|---|---|---|---|---|---|---|
| mantATP | ATP | 64 | 0.66 ± 0.01 | 14 ± 1 | 0.27 ± 0.01 | 144 ± 10 |
| ATP | mantATP | 9 | 0.63 ± 0.03 | 12 ± 1 | 0.29 ± 0.02 | 138 ± 21 |
| mantADP | ADP | 15 | 0.83 ± 0.01 | 10 ± 1 | 0.09 ± 0.01 | 261 ± 49 |
| ADP∗ | mantATP | 17 | 0.92 ± 0.02 | 9 ± 1 | 0.04 ± 0.02 | 75 ± 16 |
| mantATP +ATP† | ATP | 8 | 0.51 ± 0.06 | 17 ± 4 | 0.08 ± 0.01 | 123 ± 12 |
| mantATP | ADP | 17 | 0.74 ± 0.02 | 12 ± 1 | 0.19 ± 0.02 | 76 ± 10 |
| mantATP | ATP/Ca | 22 | 0.64 ± 0.04 | 26 ± 2 | 0.26 ± 0.02 | 228 ± 22 |
First two columns give the nucleotides in the initial incubation and the chase solutions. The third column gives number of experiments averaged. P1 and P2 are the magnitudes and T1 and T2 are the time constants for the first and second phases of the two-exponential function that was fit to the data (see Materials and Methods). Errors are standard errors of the mean.
Similar results were obtained in ADP or in rigor (in the absence of nucleotides), or when the nucleotide in the chase was 250 μM mantADP, and these results are included in this analysis.
The data were normalized to the cell fluorescence in the absence of competition with ATP.
A closer inspection of the fit to the data shows that the processes involve more than two exponential components. A fit to the data of Fig. 5 B involving three exponential processes produced χ-squared values that were little changed. These fits produced one rapid time constant, ∼20 s, which accounted for one-half the decay. Thus, the rapid component was little changed. It produced two slow components, one with a time constant, 50–100 s and one with a longer time constant of ∼400 s. Similar fits to other data produced similar results. It is clear that the release of nucleotides is more complex than can be explained by a two-exponential fit. However the basic conclusion, that a fraction of the nucleotides is released quickly, largely over by 30 s, while a second fraction is released more slowly, requiring several minutes is unchanged. Thus, we have chosen to fit the data with a two-exponential decay.
Figure 5.

SRX is present in active cardiac muscle but not active skeletal muscle. Muscles were relaxed in mantATP and were chased with either a relaxing solution containing 4mM ATP (red) or an activating solution containing an additional 3 mM CaCl2 in the presence of 5 mM EGTA, pCa = 5.7 (blue). (A) In skeletal fibers, the fluorescence decays slowly in a relaxing solution (upper curve), but quickly in an activating solution (lower curve). This shows that in activated skeletal fibers the myosin heads transition rapidly out of the SRX. (B) In cardiac muscles, the decay of fluorescence is not changed between chase with relaxing and activating solutions. This shows that myosin heads in the SRX remain in that state when adjacent myosin heads are interacting with actin and generating force. Parameters of the fits to the data shown here are provided in the Supporting Material.
Demonstration that the slow nucleotide turnover arises from nucleotides released by myosin
Below we describe experiments that argue that the fluorescent component with a long time constant arises from the slow release of nucleotides by myosin heads in the SRX. These include the demonstration that
-
1.
The slow component is not seen if the muscle is not relaxed during the incubation.
-
2.
Competition by ATP decreases its magnitude.
-
3.
The magnitude of the specifically bound nucleotides is similar to the concentration of myosin heads.
-
4.
The fluorescence of nucleotides bound to the muscle during the chase is found mainly in the myosin-containing A-bands of the sarcomere.
The slow release of nucleotides was not observed when the muscle was not relaxed in the initial incubation. When a muscle was incubated in ADP, the myosin•ADP head attached to the actin filament forming cross-bridges that are strongly bound to actin (17). Thus incubating the cells with mantADP and chasing with ADP, allowed the determination of nucleotide turnover rate in a nonrelaxed state (see Fig. 2). The fraction of fluorescence decay with a slow time constant was much smaller, 0.09, than seen when the muscle was relaxed in both incubation and chase phases. A similar control experiment was done with the inverse experiment with the same result (see Table 1).
Figure 2.

Comparison of three conditions is shown: 1), incubation in 250 μM mantATP chased by 4mM ATP (●); 2), incubation in 250 μM mantATP chased by 4 mM ADP (○); and 3), incubation in 250 μM mantADP chased by 4 mM ADP (▵). All of the data were obtained from the same muscle bundle. Parameters of the fits to the data shown here are provided in the Supporting Material.
The specificity of mantATP bound in the cell bundle was determined by competition with ATP. Cells were incubated with a relaxing solution containing 250 μM mantATP plus varying concentrations of ATP. The measured fluorescence intensity as a function of added [ATP] is shown in Fig. 3 and fit by an equation describing the competition of two substrates for a single site on an enzyme. At high concentrations of ATP, ATP occupies all specific binding sites. Thus, the observed fluorescence intensity arises from nonspecific binding of mantATP. Mant-nucleotides bound nonspecifically to the cell bundle account for 52 ± 3% of the total fluorescence in the absence of competition with ATP. This is slightly higher than the 40% observed in skeletal psoas tissue (10). The affinity of mantATP for myosin is 1.5 ± 0.3 times higher than that of ATP. This is similar to that observed in skeletal fibers and in agreement with solution studies showing tighter binding of mantATP to myosin or actomyosin (10,13).
Figure 3.
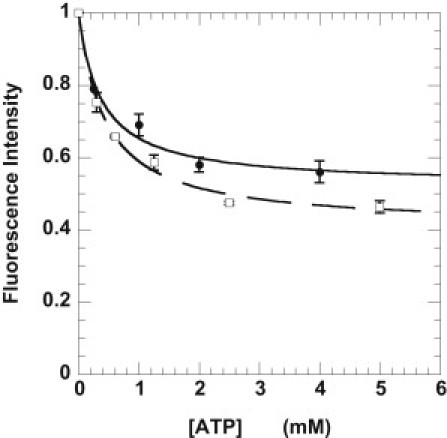
Determination of the fraction of mant nucleotides bound specifically to the ATP binding sites in both skeletal muscle fibers (□, dashed line) and cardiac cells (●, solid line). The cells were incubated in 250 μM mantATP with varied concentrations of ATP. The fluorescence obtained was normalized to the fluorescence in the absence of added ATP. The data were fit to a simple competition model. (Solid and dashed lines) Intensity = INS + (1 − INS)/([ATP]•KappATP /[mATP]•KappmATP + 1), where INS is the intensity due to nonspecific binding. This defines the fraction of mant-nucleotides nonspecifically bound to the cell bundles, and the ratio of the apparent affinities of the two nucleotides for the myosin binding site, Kapp. The equation was derived assuming that both nucleotides are present in excess of their Km values, which is true for the conditions used. The nonspecific fraction was 0.41 ± 0.02 for skeletal fibers (data from Stewart et al. (10)) and 0.52 ± 0.03 for cardiac cells. The ratio of the affinities of ATP and mantATP was 0.56 ± 0.07 for skeletal fibers and 0.67 ± 0.18 for cardiac cells.
The slow component of fluorescence decay in a subsequent chase with ATP was also greatly reduced by addition of 4 mM ATP to the incubation (see Table 1). The observed magnitude of the second component of the fit, P2 = 0.08, is only a little greater than that expected from the remaining fraction of specifically bound nucleotides, 4%, under these conditions. These results show that the component of nucleotides released slowly arises from the specifically bound nucleotides. The absolute concentration of mant-nucleotides bound to the muscle was determined as described previously (10). The concentration of nucleotides bound specifically to the fiber, 144 ± 7 μM (mean ± SE, n = 10), was similar to the concentration of myosin heads, 157 μM (18). Assuming that all specific binding is due to mant-nucleotides bound to myosin, the observed amplitude of the slow component, 0.28, indicates that 58% of the myosin heads in the relaxed muscle are in the SRX.
The location of the fluorescence bound to the muscle was determined by spinning disk confocal microscopy. Confocal microscopy was used because the depth of field of the epi-fluorescence microscope is short, making it difficult to resolve individual sarcomeres in the image. Fig. 4 shows that the fluorescence observed during the chase was found largely in bright bands perpendicular to the axis of the muscle. The width of the bright bands was 1.5 ± 0.2 μm, and did not vary as the sarcomere length varied from 2.2 to 3.5 μm. Their length and lack of change with sarcomere length, show that these bands correspond to the sarcomeric A-bands, which contain the myosin filaments. Thus, a large fraction of the mant-nucleotides are bound to myosin during the chase phase.
Figure 4.

Bundle of cardiac cells visualized by spinning-disk confocal microscopy after 1.5 min in the chase phase. The cells were incubated in a solution containing 125 μM mantATP for 2 min then chased with a solution containing 4 mM ATP for 1.5 min before taking the image. The horizontal stripes are ∼1.5 μm in width, close to the length of the A-band in cardiac muscles, showing that much of the fluorescence intensity remaining in the muscle is associated with the A-band. The sarcomere length is 2.1 μm. The bar represents 10 μm.
We conclude that an SRX exists in cardiac muscles that has many similarities to the one previously identified in skeletal muscle. In both muscle types, the release of fluorescence occurs with two components—one rapid, and one with a time constant much longer than that of the normal relaxed state. A variety of experiments show that the fluorescence component with a long time-constant arises from release of mant-nucleotides from myosin.
Myosin turnover rates and the SRX in active muscles
Despite the similarities between skeletal and cardiac muscles, there were several experimental protocols in which the cardiac results differed from the skeletal. These involved situations in which a fraction of myosin heads were in the SRX while adjacent myosin heads were strongly bound to actin, either in active muscle or in rigor-ADP states. When the muscle was initially relaxed in 250 μM mantATP and chased with 4 mM ADP, a portion of the myosin heads are initially in the SRX while other heads have bound ADP and are forming rigor-ADP bonds with actin. In this case a slow decay of fluorescence was observed, although with reduced amplitude, 0.19 ± 0.02, and time constant, 74 ± 10 s. This observation is very different from that in skeletal muscle where a chase with 4 mM ADP resulted in virtual elimination of the slow component (10).
Thus, in cardiac tissue the release of nucleotides by myosin heads in the super relaxed state is affected to a lesser extent by rigor-ADP states of adjacent myosin heads than is observed in skeletal fibers. In skeletal tissue, it was suggested that myosin heads in the super relaxed state were destabilized by the interaction of adjacent myosin heads with actin to provide for a rapid recruitment of all myosin heads in activated fibers; however, this hypothesis was not tested directly (10).
This explanation was tested directly by measuring the properties of the super relaxed state in partially activated skeletal and cardiac muscles. Muscles were partially activated in 1.8 μM Ca2+ (pCa = 5.7). The force generated in this solution was 57 ± 4% (mean ± SE, n = 15) of that achieved by full activation for cardiac fibers, and was 56 ± 3% (mean ± SE, n = 14) for skeletal fibers (see Fig. S3). At full activation, the cardiac fibers generated 40 ± 8 kN/m2, which is in line with other measurements of maximal cardiac force when adjusted for the 45% inhibition of tension due to 5 mM phosphate in the experimental buffer (19,20). Activation was rapid for cardiac fibers. The time to half-activation depended on the diameter of the fiber, but was ∼4 ± 1 s for fibers with a diameter of 80 μm, which was the maximum used here. Thus, activation of the fibers had been achieved well before measurement of the time course of the SRX. Partial activation was used to minimize artifacts due to muscle movements that complicated the analysis of fully activated muscles. This caused minimal movement of muscles that were held at both ends. In partially activated skeletal fibers, the fluorescence intensity decayed rapidly during the chase (see lower trace in Fig. 5 A).
This observation shows that myosin heads initially in the SRX were rapidly (faster than the temporal resolution of our experiments, ∼20 s) recruited into states with a high nucleotide turnover rate. This experiment provides direct evidence for the hypothesis put forward by Stewart et al. (10) that cooperative interactions between myosin heads in skeletal fibers would lead to full activation of the thick filament. In dramatic contrast, in partially activated cardiac muscles, the decay of fluorescence intensity was little changed from that in a chase with a relaxing solution, showing that myosin heads remain in the SRX during partial activation (see Fig. 5 B). These observations demonstrate a fundamental difference in the cooperativity between myosin heads in super relaxed filaments from skeletal and cardiac muscles, and, as discussed below, demonstrates that this state plays different roles in the two types of muscle.
Discussion
Our major new observation is that the state of myosin in relaxed cardiac muscle is not the single monolith that had been previously assumed. Instead there are two subpopulations of relaxed cross-bridges (normal-relaxed and the SRX) with ATP-turnover rates that differ by approximately a factor of 5. The data indicate that the SRX in cardiac muscles has many similarities to that previously identified in skeletal muscle. In both muscle types, the release of fluorescence in the basic experiment occurs with two components—one rapid and compatible with nucleotide turnover by the normal relaxed state, and one with a time constant much longer than that of the relaxed state. The nucleotide turnover time in the cardiac SRX, 138–144 s, is >5 times longer than in the normal relaxed state, ∼27 s (16). The fraction of the total muscle fluorescence associated with the slow component is similar in the two muscle types. The SRX only occurs when muscles are relaxed in the initial incubation phase. Thus, we conclude that an SRX occurs in both skeletal and cardiac muscles.
A structural hypothesis for the mechanism of the inhibition of myosin ATPase activity
The fact that super-relaxed cross-bridges are seen only in relaxed cell bundles and not in isolated myosin implies the involvement of a structural component associated with the filament array in the downregulation of the ATPase rate for the SRX. Electron microscopy has identified a structural motif in which interacting myosin heads are helically ordered along the backbone of the myosin thick filament (21–23). In some types of muscles, in which activation is controlled through myosin rather than the thin filament, myosin heads in this structural motif release nucleotides very slowly (24,25).
Of particular note, this same structural motif with reduced ATPase has recently been identified in mammalian skeletal myosin and cardiac thick filaments, leading to the hypothesis that this motif is common to many muscle types (26,27). Thus, we suggest that the inhibition of nucleotide turnover by myosin heads in the SRX in cardiac muscles is caused by binding of the myosin heads to the core of the thick filament. A similar hypothesis for the inhibition of skeletal myosin ATPase activity has been proposed (10). The ordered structure of the cardiac thick filament is dynamic, and can be modulated by several known factors, including phosphorylation of the myosin regulatory light chain and myosin binding protein-C (28–30). Accepting the structural hypothesis for inhibition of the myosin ATPase activity, these factors would also affect the population of the SRX.
Why would incorporation of the myosin into an array where they are bound to the core of the thick filament provide such a large inhibition in ATP turnover rate?
Inhibition of myosin ATP turnover by mechanical immobilization has been shown to occur in myosin precipitates (31), and in myosin immobilized on SiO2 surfaces (32). Myosin in crystals is also incapable of nucleotide hydrolysis (33). When bound to the core of the thick filament, myosin heads are immobilized by multiple contacts with the core of the filament and with adjacent heads. It is possible that prevention of the large changes in conformation that occur during the nucleotide cycle is inhibiting the cycle.
Possible roles of the SRX in cardiac muscle
The most significant difference between the cardiac and skeletal SRX states is that cross-bridges remain in the cardiac SRX even under conditions of partial activation. This contrasts with skeletal muscle where rapid recruitment of force-generating cross-bridges is required for normal physiological function. The rapid turnover of nucleotides observed in skeletal fibers during chase with ADP or ATP plus calcium (shown in Fig. 5 A) is attributed to cooperative interactions between myosin heads in the thick filament. Myosin heads that are interacting strongly with actin induce adjacent heads in the SRX to transition out of that state and to commence interacting with actin as well. This mechanism provides for the ability of a skeletal fiber to achieve full activation in a few milliseconds. A cooperative disordering of the thick filament has been observed during the activation of frog skeletal muscle fibers stretched to partial overlap (34). During activation, all of the myosin heads rapidly leave their binding sites on the core of the thick filament and become disordered, although only half of them are overlapped by the actin filaments. Myosin heads in the overlap region must have acted cooperatively through the thick filament to disorder heads that were not capable of interacting with actin. This cooperative interaction is much weaker or missing in cardiac filaments. The nature of this cooperativity is a strong determinant of the function of this state. In skeletal muscle, the population of the SRX is appreciable only in relaxed muscle, where it plays a role in determining the metabolic rate. In cardiac cells, the SRX can provide an additional modulatory mechanism on the active muscle, influencing the recruitment of force-producing cross-bridges out of the relaxed states to produce the more graded cardiac mechanical output.
We note that an alternative possibility to explain the apparent difference in cooperativity between skeletal and cardiac muscle, is via changes in the thin filament. Addition of high concentrations of ADP to resting skeletal or cardiac muscle produces isometric tension via a cooperative activation of the thin filament (17). The Hill coefficient for skeletal muscle (coefficient of 3) shows slightly more cooperativity than that for cardiac muscle (coefficient of 2). Thus, cooperative interactions occurring through the thin filaments may also play a role.
Although the SRX has been proposed to play a role in adaptive thermogenesis in skeletal muscle (10), modulation of the population of the SRX to produce thermogenesis in cardiac tissue is an unlikely function. Skeletal muscle may be an optimal tissue for adaptive thermogenesis, because of its low basal rate and its unique reserve metabolic capacity. The continuous pumping activity of the heart produces a large metabolic load on the cardiac tissue, and any additional load to produce thermogenesis would be deleterious. Instead, we suggest that the SRX could play a role in regulating the energy use of the cardiac muscle cell. By sequestering a portion of the myosin heads into a state where they do not interact with actin and they have a very low rate of energy utilization, the SRX could provide a very energy efficient mechanism of regulating myocardial output.
By effectively parking myosin heads on the thick filament in the SRX, where they consume very little energy, the overall metabolic rate of the myocardium could be optimally reduced. Unlike skeletal muscle, these heads would not be recruited with each heart beat, but would remain in the SRX as other heads generated force, as shown in Fig. 5 B. This could play a significant role in regulating the energy utilization of the myocardium during normal operation. Supporting this concept are data showing that dephosphorylation of the myosin regulatory light chain, known to stabilize the array of heads bound to the thick filament, decreases output in living cardiac muscle (35,36). However, the SRX would likely play a more major role in stressful conditions. When exposed to stressful conditions the heart can induce mechanisms to shut down energy-using processes early during ischemia and in states that are cardioprotective, known as myocardial hibernation or stunning (1,2,9,37). Although considerable work has been done in identifying factors that modulate basic cellular energy utilization, little attention seems to have been paid to defining factors that modulate the underlying, energy-intensive actomyosin interaction as a cardioprotective mechanism.
Does the SRX play a role during the functioning of the normal heart?
The data shown in Fig. 5 B demonstrate that the population of the SRX is not changed by the presence of activation by calcium. Thus, the SRX provides a mechanism for sequestering a portion of the myosin heads into a quiescent state. The population of the SRX would not depend on the activation level of the cell but on other factors that affect the structure of the thick filament. In addition to providing a mechanism exerting another level of control on force generation, the SRX provides a mechanism that is more energy-efficient than is the normal relaxed state.
How large is the difference between reducing the cardiac output by sequestering myosin heads in the SRX relative to leaving them in the normal relaxed state?
To calculate this difference, we assume that the activity of myosin in the normal relaxed state is the same as that of purified rabbit ventricular myosin, 0.037 s−1. The enthalpy released by ATP hydrolysis if all of the myosin heads were in the normal relaxed state in rabbit cardiac muscle would be ∼0.4 W/kg. The basal rate of the myocardium is quite variable but has been measured in rabbit whole heart to be 2.3 W/kg (38). Thus, the ATPase of myosin in the relaxed state would represent 17% of the total metabolism. If these heads were sequestered into the SRX, most of this energy utilization would be saved. Thus, shifting populations between the normal relaxed state and the SRX could make a small but still significant contribution to the energy utilization of the resting heart.
The metabolic rate of the activated myocardium is of course much higher and varies with the strength of the contractile activity. The SRX will play a more prominent role at lower levels of activity. The metabolic rate of rabbit myocardium during isovolumic activations at one-quarter of the maximum rate is ∼10 W/kg (39,40). The metabolic rate of normally relaxed myosin would amount to 4–8% of the total metabolic rate of the functioning myocardium. Again, if the relaxed heads were in the SRX this amount of energy utilization could be saved. Thus, these calculations show that the SRX could make a small contribution to the efficiency of the working myocardium. Although small, any increase in cardiac efficiency is advantageous to the organism.
Summary
We identify what we believe to be a new state of myosin in cardiac muscle with a very low ATP turnover rate. This state could play a role in decreasing the metabolic load of the myocardium. The mechanism proposed here for cardiac muscle suggests that therapeutic interventions that increase the population of the SRX would be cardioprotective during times of stress. They may also be useful in preserving organs for transplant.
Acknowledgments
Data for this study were acquired at the Nikon Imaging Center at University of California, San Francisco/QB3. The authors thank Dr. Kurt Thorn and Ms. Alice Myo Thwin for their generous help in using the microscopes, Ms. Kathy Franks-Skiba for technical help, and Dr. Ger Stienen and Dr. Edward Pate for comments on the manuscript.
This work was supported by the U.S. Public Health Service under grant No. HL32145.
Supporting Material
References
- 1.Gibbs C.L. Cardiac energetics: sense and nonsense. Clin. Exp. Pharmacol. Physiol. 2003;30:598–603. doi: 10.1046/j.1440-1681.2003.03878.x. [DOI] [PubMed] [Google Scholar]
- 2.Depre C., Vatner S.F. Cardioprotection in stunned and hibernating myocardium. Heart Fail. Rev. 2007;12:307–317. doi: 10.1007/s10741-007-9040-3. [DOI] [PubMed] [Google Scholar]
- 3.Moss R.L., Fitzsimons D.P. Regulation of contraction in mammalian striated muscles—the plot thick-ens. J. Gen. Physiol. 2010;136:21–27. doi: 10.1085/jgp.201010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colson B.A., Bekyarova T., Moss R.L. Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circ. Res. 2008;103:244–251. doi: 10.1161/CIRCRESAHA.108.178996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kooij V., Boontje N., van der Velden J. Protein kinase C α and ɛ phosphorylation of troponin and myosin binding protein C reduce Ca2+ sensitivity in human myocardium. Basic Res. Cardiol. 2010;105:289–300. doi: 10.1007/s00395-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine R.J., Yang Z., Sweeney H.L. Structural and functional responses of mammalian thick filaments to alterations in myosin regulatory light chains. J. Struct. Biol. 1998;122:149–161. doi: 10.1006/jsbi.1998.3980. [DOI] [PubMed] [Google Scholar]
- 7.Sadayappan S., Gulick J., Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ. Res. 2005;97:1156–1163. doi: 10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong C.W., Stelzer J.E., Moss R.L. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ. Res. 2008;103:974–982. doi: 10.1161/CIRCRESAHA.108.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Velden J., Narolska N.A., Stienen G.J. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc. Res. 2006;69:876–887. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Stewart M.A., Franks-Skiba K., Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. USA. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremo C.R., Neuron J.M., Yount R.G. Interaction of myosin subfragment 1 with fluorescent ribose-modified nucleotides. A comparison of vanadate trapping and SH1-SH2 cross-linking. Biochemistry. 1990;29:3309–3319. doi: 10.1021/bi00465a023. [DOI] [PubMed] [Google Scholar]
- 12.Hiratsuka T. New ribose-modified fluorescent analogs of adenine and guanine nucleotides available as substrates for various enzymes. Biochim. Biophys. Acta. 1983;742:496–508. doi: 10.1016/0167-4838(83)90267-4. [DOI] [PubMed] [Google Scholar]
- 13.Woodward S.K., Eccleston J.F., Geeves M.A. Kinetics of the interaction of 2′(3′)-O-(n-methylanthraniloyl)-ATP with myosin subfragment 1 and actomyosin subfragment 1: characterization of two acto-S1-ADP complexes. Biochemistry. 1991;30:422–430. doi: 10.1021/bi00216a017. [DOI] [PubMed] [Google Scholar]
- 14.Ranatunga K.W. Thermal stress and Ca-independent contractile activation in mammalian skeletal muscle fibers at high temperatures. Biophys. J. 1994;66:1531–1541. doi: 10.1016/S0006-3495(94)80944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys. J. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smitherman T.C., Johnson R.S., Richards E.G. Acute thyrotoxicosis in the rabbit: changes in cardiac myosin, contractility, and ultrastructure. Biochem. Med. 1979;21:277–298. doi: 10.1016/0006-2944(79)90083-8. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda N., Fujita H., Ishiwata S. Regulatory roles of MgADP and calcium in tension development of skinned cardiac muscle. J. Muscle Res. Cell Motil. 1998;19:909–921. doi: 10.1023/a:1005437517287. [DOI] [PubMed] [Google Scholar]
- 18.Barsotti R.J., Ferenczi M.A. Kinetics of ATP hydrolysis and tension production in skinned cardiac muscle of the guinea pig. J. Biol. Chem. 1988;263:16750–16756. [PubMed] [Google Scholar]
- 19.Ebus J.P., Stienen G.J. ATPase activity and force production in skinned rat cardiac muscle under isometric and dynamic conditions. J. Mol. Cell. Cardiol. 1996;28:1747–1757. doi: 10.1006/jmcc.1996.0164. [DOI] [PubMed] [Google Scholar]
- 20.Kentish J.C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J. Physiol. 1986;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodhead J.L., Zhao F.Q., Padrón R. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 22.Craig R., Woodhead J.L. Structure and function of myosin filaments. Curr. Opin. Struct. Biol. 2006;16:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Alamo L., Wriggers W., Padrón R. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J. Mol. Biol. 2008;384:780–797. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vibert P., Craig R. Structural changes that occur in scallop myosin filaments upon activation. J. Cell Biol. 1985;101:830–837. doi: 10.1083/jcb.101.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cross R.A., Jackson A.P., Bagshaw C.R. Active site trapping of nucleotide by smooth and non-muscle myosins. J. Mol. Biol. 1988;203:173–181. doi: 10.1016/0022-2836(88)90100-3. [DOI] [PubMed] [Google Scholar]
- 26.Jung H.S., Komatsu S., Craig R. Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol. Biol. Cell. 2008;19:3234–3242. doi: 10.1091/mbc.E08-02-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoghbi M.E., Woodhead J.L., Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc. Natl. Acad. Sci. USA. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine R.J., Kensler R.W., Sweeney H.L. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys. J. 1996;71:898–907. doi: 10.1016/S0006-3495(96)79293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kensler R.W., Harris S.P. The structure of isolated cardiac myosin thick filaments from cardiac myosin binding protein-C knockout mice. Biophys. J. 2008;94:1707–1718. doi: 10.1529/biophysj.107.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine R., Weisberg A., Winegrad S. Multiple structures of thick filaments in resting cardiac muscle and their influence on cross-bridge interactions. Biophys. J. 2001;81:1070–1082. doi: 10.1016/S0006-3495(01)75764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Highsmith S., Duignan K., Cooke R. Reversible inactivation of myosin subfragment 1 activity by mechanical immobilization. Biophys. J. 1998;74:1465–1472. doi: 10.1016/S0006-3495(98)77858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Persson M., Albet-Torres N., Balaz M. Heavy meromyosin molecules extending more than 50 nm above adsorbing electronegative surfaces. Langmuir. 2010;26:9927–9936. doi: 10.1021/la100395a. [DOI] [PubMed] [Google Scholar]
- 33.Bauer C.B., Holden H.M., Rayment I. X-ray structures of the apo and MgATP-bound states of Dictyostelium discoideum myosin motor domain. J. Biol. Chem. 2000;275:38494–38499. doi: 10.1074/jbc.M005585200. [DOI] [PubMed] [Google Scholar]
- 34.Haselgrove J.C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J. Mol. Biol. 1975;92:113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- 35.Dias F.A., Walker L.A., Wolska B.M. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J. Mol. Cell. Cardiol. 2006;41:330–339. doi: 10.1016/j.yjmcc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Scruggs S.B., Hinken A.C., Solaro R.J. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J. Biol. Chem. 2009;284:5097–5106. doi: 10.1074/jbc.M807414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casey T.M., Arthur P.G. Hibernation in noncontracting mammalian cardiomyocytes. Circulation. 2000;102:3124–3129. doi: 10.1161/01.cir.102.25.3124. [DOI] [PubMed] [Google Scholar]
- 38.Gibbs C.L., Loiselle D.S. Cardiac basal metabolism. Jpn. J. Physiol. 2001;51:399–426. doi: 10.2170/jjphysiol.51.399. [DOI] [PubMed] [Google Scholar]
- 39.Coulson R.L. Energetics of isovolumic contractions of the isolated rabbit heart. J. Physiol. 1976;260:45–53. doi: 10.1113/jphysiol.1976.sp011503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonald R.H., Jr. Myocardial heat production: its relationship to tension development. Am. J. Physiol. 1971;220:894–900. doi: 10.1152/ajplegacy.1971.220.4.894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


