Abstract
A new mechanism of cell-cell communication was recently proposed after the discovery of tunneling nanotubes (TNTs) between cells. TNTs are membrane protrusions with lengths of tens of microns and diameters of a few hundred nanometers that permit the exchange of membrane and cytoplasmic constituents between neighboring cells. TNTs have been reported to mediate intercellular Ca2+ signaling; however, our simulations indicate that passive diffusion of Ca2+ ions alone would be inadequate for efficient transmission between cells. Instead, we observed spontaneous and inositol trisphosphate (IP3)-evoked Ca2+ signals within TNTs between cultured mammalian cells, which sometimes remained localized and in other instances propagated as saltatory waves to evoke Ca2+ signals in a connected cell. Consistent with this, immunostaining showed the presence of both endoplasmic reticulum and IP3 receptors along the TNT. We propose that IP3 receptors may actively propagate intercellular Ca2+ signals along TNTs via Ca2+-induced Ca2+ release, acting as amplification sites to overcome the limitations of passive diffusion in a chemical analog of electrical transmission of action potentials.
Cells have long been known to employ gap junctions and synapses to communicate with their neighbors. A recent study (1) described a new route of cell-cell communication via tunneling nanotubes (TNTs; membrane protrusions, a few hundred nanometers in diameter, that physically link cell bodies over distances of tens of micrometers). These membrane tubes have been observed in diverse cell types in vitro and in vivo, contain F-actin, and are characteristically distinct from other cellular protrusions in that they lack contact to the substratum. TNTs have been shown to transfer membrane-bound components such as lipids and proteins between cells, to permit transfer of organelles such as mitochondria, and to facilitate intercellular transfer of pathogens such as bacteria, HIV-1, and prion (2). TNTs have also been shown to mediate transmission of intercellular Ca2+ signals (3,4) in a manner that is analogous to the well-established intercellular transmission of Ca2+ waves via gap junctions but enables transmission between cells that are not in intimate contact.
To investigate whether passive diffusion of Ca2+ ions along TNTs might be sufficient to enable cell-cell communication, we simulated diffusion between two cells connected by TNTs of different radii and lengths containing 100 μM immobile cytosolic Ca2+ buffer (see Fig. S1 in the Supporting Material). At 10 s after a large (10 μM) step increase in cytosolic free [Ca2+] in one cell, the Ca2+ flux (current) from the end of a 30 μm TNT with a typical diameter of 200 nm was <1 fA. Given that openings of a single inositol trisphosphate receptor (IP3R) channel Ca2+ passing a current of ∼100 fA (5) generally fail to trigger Ca2+-induced Ca2+ release (CICR) (6), passive diffusion alone appears to be inadequate for robust intercellular transmission of Ca2+ signals via TNTs.
We therefore looked for evidence of active Ca2+ signaling within TNTs by employing cultured SH-SY5Y neuroblastoma and HEK cell lines in which we had previously characterized Ca2+ signaling mechanisms. TNTs were present in both cell types and displayed properties consistent with those previously reported (2). They were suspended in the medium above the base of the imaging dish (Fig. 1 A), contained F-actin but little or no tubulin (Fig. 1 B), attained lengths as great as 70 μm, and allowed interchange of mitochondria between cells, demonstrating cytosolic continuity between the TNT and cell body (Movie S1). Of note, in the context of Ca2+ signaling, the TNTs contained extensions of the endoplasmic reticulum (ER; Fig. 1 C) and expressed type 1 IP3Rs along their length (Fig. 1 D). The IP3R channel mediates liberation of Ca2+ ions sequestered in the ER, and its opening is promoted by IP3 and cytosolic Ca2+, leading to regenerative CICR (7) that may remain localized as Ca2+ puffs or propagate as a Ca2+ wave (8).
Figure 1.
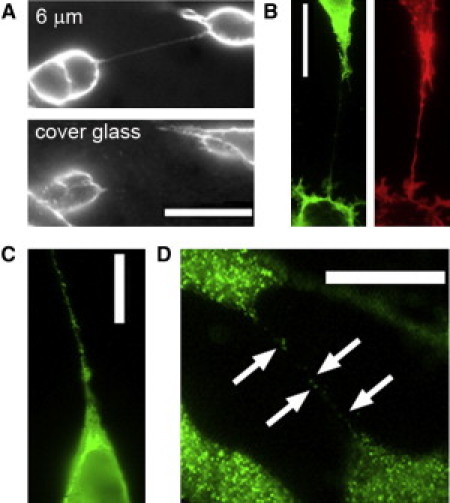
TNTs between cultured SH-SY5Y cells. (A) Cells stained with the fluorescent membrane dye Di-8-ANEPPQ showing a TNT suspended ∼6 μm above the coverglass. Images were obtained focused at the coverglass (bottom) and 6 μm higher (top). (B) Immunofluorescence staining for tubulin (green) and F-actin (red, phalloidin 647). (C) SH-SY5Y cell transfected 24 h previously to express ER-GFP showing the presence of ER in a TNT. (D) Immunofluorescence staining of type 1 IP3Rs along a TNT. All scale bars are 20 μm. Images are representative of ≥8 TNTs.
To investigate whether TNTs display localized Ca2+ signals independently of their connected cells, we incubated cells with membrane permeant-esters of the Ca2+ indicator Fluo-4, caged iIP3, and the slow Ca2+ buffer EGTA (9). Fig. 2 A illustrates a TNT that was initially quiescent but generated recurring localized transient fluorescence Ca2+ signals after the iIP3 was photoreleased by a UV flash that illuminated the entire imaging field. These localized fluorescence signals had a mean amplitude ΔF/F0 of 0.43 ± 0.05, mean duration (at half-maximal amplitude) of 68 ± 25 ms, and spatial spread (full width at half-maximal amplitude) of 1.1 ± 0.11 μm (n = 7 sites). Except for being essentially constrained along one spatial dimension, local Ca2+ events in TNTs thus closely resemble the IP3-mediated Ca2+ puffs that arise from discrete clusters of IP3R within the bodies of many cell types (6,8). Moreover, local events in TNTs were spaced 4–6 μm apart (Fig. 2 A), in similarity to the distribution of puff sites in the cell body (6).
Figure 2.
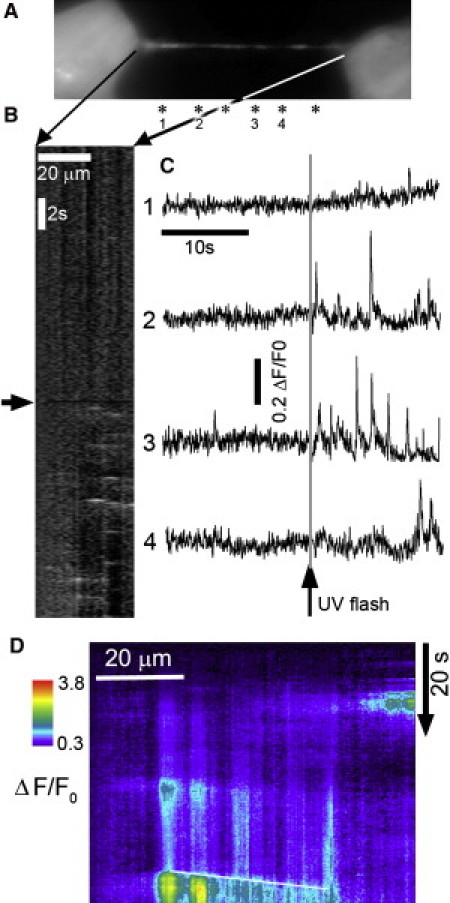
IP3-evoked (A–C) and spontaneous (D) local Ca2+ events along TNTs. (A) Monochrome image shows resting fluo-4 fluorescence in a TNT bridging two HEK cells. Asterisks indicate locations of local Ca2+ events. (B) Linescan image derived by measuring Ca2+-dependent fluorescence along the TNT (abscissa) as a function of time (ordinate). The arrow indicates when a photolysis flash was delivered. (C) Numbered traces show fluorescence signals measured from corresponding regions of the TNT marked in A. (D) Linescan image from a TNT bridging SH-SY5Y cells illustrating spontaneous local Ca2+ signals and their coordination to generate a propagating Ca2+ wave (marked by the white diagonal line).
We also observed spontaneous Ca2+ events along TNTs (n = 16) even without photorelease of IP3 and in cells that were not loaded with caged iIP3. Fig. 2 D shows an instance in which Ca2+ signals initially remained localized but a localized event subsequently triggered a regenerative wave of Ca2+ that propagated in a saltatory manner across neighboring release sites with a velocity of ∼8 μm s−1. The localized signals persisted in the absence of external Ca2+ (medium with zero added Ca2+ and 1 mM EGTA), indicating that they involve a liberation of intracellular Ca2+ and not an influx of extracellular Ca2+. Moreover, SH-SY5Y and HEK293 cells lack ryanodine receptors (RyRs; the other major class of ER Ca2+ release channels), and signals were inhibited by 20 mM caffeine, an IP3R antagonist but RyR agonist (9/12 TNTs with spontaneous events in control: 3/14 after caffeine). Thus, the spontaneous events also appear to primarily involve IP3Rs rather than RyRs, and may arise because of endogenous basal IP3 within TNTs.
The observation of active, local IP3-mediated Ca2+ signals within TNTs suggests that they may facilitate Ca2+ wave propagation between cells. We investigated this possibility by focusing a small (∼8 μm diameter) UV spot onto one cell of a pair bridged by a TNT to evoke Ca2+ liberation. This technique achieved a highly selective stimulation of the illuminated cell in that closely adjacent cells (not connected via TNTs) failed to respond, whereas in our hands an approach using local mechanical stimulation was confounded by intercellular Ca2+ waves mediated by extracellular release of ATP. As shown in Fig. 3, local uncaging of iIP3 evoked a strong Ca2+ signal in the leftmost cell; however, although this produced a rapid spread of Ca2+ down the TNT, the connected cell failed to respond for ∼20 s. We then observed two successive, transient local responses within the TNT (region 2 in Fig. 3 B). These responses were associated with a clear increase in Ca2+ fluorescence in the second cell. This evoked a global regenerative response in that cell, which subsequently back-propagated into the TNT. Another example of robust cell-cell communication of global Ca2+ signals (n = 8) is illustrated in Movie S2. In other cases (n = 17; e.g., Movie S3), we observed small Ca2+ increases and local puffs in connected cells, although we also observed instances in which there was no detectable response (n = 22; e.g., Movie S4). Intercellular communication of Ca2+ signals persisted in Ca2+-free medium (n = 4), ruling out the possibility that this was mediated via synaptic transmission.
Figure 3.
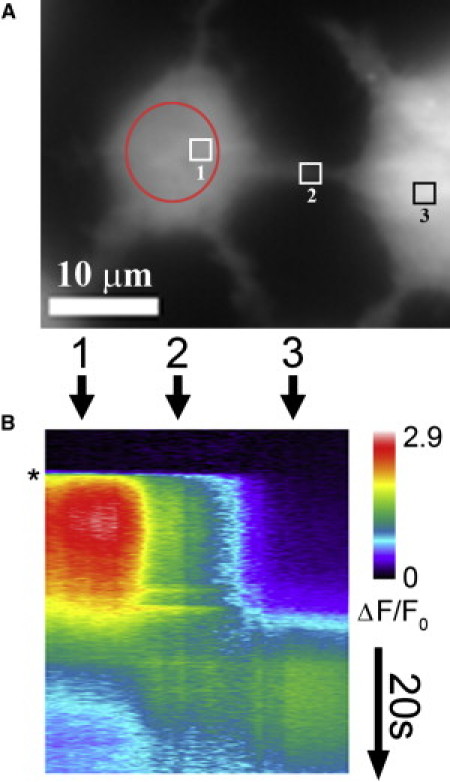
Active Ca2+ release in TNTs promotes intercellular transmission of Ca2+ signals. (A) Resting fluorescence image showing a TNT bridging adjacent SH-SY5Y cells. The red circle indicates the UV photolysis spot. Numbered regions correspond to regions in B. (B) Linescan image of Ca2+ signals in each cell and along the TNT. The asterisk indicates when a photolysis flash was delivered.
In conclusion, our results demonstrate the presence of functional IP3R Ca2+ release channels along the length of TNTs between cultured SH-SY5Y and HEK cells. Through successive cycles of Ca2+ release, diffusion, and CICR, these channels may serve as active amplification sites to promote propagation of Ca2+ signals along TNTs. Opening of IP3R channels absolutely requires IP3 as well as Ca2+ (7). Our simulations (Fig. S2) indicate that passive flux of IP3 (which diffuses faster than Ca2+ (10)) from a stimulated cell could rapidly elevate [IP3] within a TNT so as to allow active wave propagation, but would only slowly raise [IP3] throughout the enormously larger volume of a connected cell. This may explain why connected cells often gave only small Ca2+ signals or failed to respond. Nevertheless, active transmission of Ca2+ waves along TNTs may be an efficient physiological mechanism for communication across a network of connected cells that are primed by a small basal elevation of cytosolic [IP3]. We further speculate that other cell types may use an analogous mechanism mediated by RyRs that exhibit CICR without requiring a further second messenger.
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM 40871 to I.P., and GM65830 to I.P. and J.S.) and the National Natural Science Foundation of China (30970970), and the Specialized Research Fund for the Doctoral Program of Higher Education (20090121110028 to J.S.).
Supporting Material
References and Footnotes
- 1.Rustom A., Saffrich R., Gerdes H.H. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 2.Davis D.M., Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 3.Hase K., Kimura S., Ohno H. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 2009;11:1427–1432. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- 4.Watkins S.C., Salter R.D. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Vais H., Foskett J.K., Daniel Mak D.O. Unitary calcium current through recombinant type 3 inositol triphosphate receptor channels under physiological ionic conditions. J. Gen. Physiol. 2010;136:687–700. doi: 10.1085/jgp.201010513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith I.F., Wiltgen S.M., Parker I. Ca2+ puffs originate from preestablished stable clusters of inositol trisphosphate receptors. Sci. Signal. 2009;2:ra77. doi: 10.1126/scisignal.2000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foskett J.K., White C., Mak D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao Y., Choi J., Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J. Physiol. 1995;482:533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith I.F., Wiltgen S.M., Parker I. Localization of puff sites adjacent to the plasma membrane: Functional and spatial characterization of calcium signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45:65–76. doi: 10.1016/j.ceca.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allbritton N.L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


