Abstract
B-A transition and DNA condensation are processes regulated by base sequence and water activity. The constraints imposed by interhelical interactions in condensation compromise the observation of the mechanism by which B and A base-stacking modes influence the global state of the molecule. We used a single-molecule approach to prevent aggregation and mechanical force to control the intramolecular chain association involved in condensation. Force-extension experiments with optical tweezers revealed that DNA stretches as B-DNA under ethanol and spermine concentrations that favor the A-form. Moreover, we found no contour-length change compatible with a cooperative transition between the A and B forms within the intrinsic-force regime. Experiments performed at constant force in the entropic-force regime with magnetic tweezers similarly did not show a bistable contraction of the molecules that could be attributed to the B-A transition when the physiological buffer was replaced by a water-ethanol mixture. A total, stepwise collapse was found instead, which is characteristic of DNA condensation. Therefore, a low-humidity-induced change from the B- to the A-form base-stacking alone does not lead to a contour-length shortening. These results support a mechanism for the B-A transition in which low-humidity conditions locally change the base-stacking arrangement and globally induce DNA condensation, an effect that may eventually stabilize a molecular contour-length reduction.
Introduction
Double-stranded (ds) DNA has a significant ability to change its structural levels of organization (1,2) from a random-coiled polymer with polymorphic secondary structure to general networks of fibrous aggregates (3), which can lead to tight particles with toroidal or rodlike geometry (4–7). This structural variability has been observed to take place through changes in the dsDNA environmental conditions, such as the humidity level (8–10), ion activity (8,11,12) and temperature (13,14). Dehydration of dsDNA by the addition of nonpolar cosolvents, such as alcohols, has a remarkable influence on the organization of the polymer. In fact, the collapse of dsDNA molecules in water-alcohol mixtures and its eventual precipitation has been long observed (6). Specifically, low-polarity environments make DNA-solvent interactions less favorable and promote electrostatic interactions, thus facilitating both DNA condensation and aggregation. Physiological binding of counterions to DNA, such as polyamines (3,15,16), has the local effect of competing for the available water molecules, thus reducing the water activity in the vicinity of the dsDNA polymer surface (17,18) and inducing DNA condensation (19) and stabilization of compact forms of DNA (20,21).
DNA secondary structure and its transitions among its different forms (22) have constituted a central problem in molecular and structural biology. The transition between B-DNA and A-DNA (B-A transition) is characterized locally by a change in base-stacking arrangement and globally by a reduction of the rise/basepair from the canonical 3.38 Å (B-DNA) to 2.56 Å (A-DNA) (2). The latter process should therefore be amenable to observation in real time by measuring the contour length of the polymer or the extension when the polymer is subjected to force. The B-A transition is experimentally induced in solution by the addition of alcohol. Multivalent cations have also been used to promote the B-A transition with lower critical alcohol concentration (11), and even in aqueous solutions for appropriate DNA sequences (23,24). The sequence of nucleotide bases is also implicated in DNA polymorphism (25). In particular, it has been reported that G·C-rich regions of dsDNA are more A-philic than A·T-rich regions, which are resistant to adopting the A form even at very low humidity (14,26–29). It has also been proven that the mechanochemical stability of particular sequences of DNA plays a relevant role in both transcription and molecular recognition (30) and is very likely to influence transition mechanics between different forms of DNA. The conditions that induce the B-A transition are paradigmatically within the ranges that also strongly induce both DNA aggregation and condensation (3,6,19,31,32). In fact, it has been suggested that DNA electrostatic aggregation and condensation stabilize the A form by establishing lateral intermolecular and intramolecular associations, respectively, between DNA helices (29,32–36). This synergistic effect is further enhanced in the presence of multivalent cations, which may subsequently help packaging processes (37,38). An alternative view in which the B-A transition proceeds without aggregation or condensation has also been reported (39,40). A kinetic study reconciling the two alternatives shows a fast initial B-A transition followed by a slower aggregation process (41).
Here, we study the B-A transition at the single-molecule level in water-ethanol mixtures to understand the role of condensation/aggregation. Single-molecule experiments give access to the global conformation of individual molecules, and force allows the control of intrahelical interactions. Therefore, this analysis complements the structural information on the base-stacking modes and sheds light on the mechanism by which B-A transition influences the elastic properties of the polymer. We use spermine to analyze the effect of condensing multivalent counterions and two linearized plasmids that differ in their proportion of G·C content to analyze the effect of sequence. After characterizing the bulk secondary structure of these two plasmids in water-ethanol mixtures by circular dichroism (CD), we use optical tweezers (OT) to examine the existence of a cooperative force-induced B-A transition in the intrinsic-force regime (42,43).In this regime (force range ∼3–65 pN (44)), DNA is stretched beyond its so-called contour length, which is the length of the molecule as derived from the number of basepairs multiplied by the rise/basepair in the absence of stress. OT experiments show that for the conditions that induce A-DNA in bulk, no cooperative contour-length change relative to that of B-DNA exists in the intrinsic-force regime. The entropic-force regime, which entails molecular deviations due to thermal fluctuations but without elastic extension (F ∼ 0–3 pN (44)), was further studied with magnetic tweezers (MT), which allows real-time collapsing events to be tracked in different solutions with the same molecule at constant force (45). The analysis with MT shows that when ethanol is present, DNA collapse is the main effect and it drives the entire molecule to a condensed state. The absence of a contour-length change due to the B-A transition and the role that intramolecular interactions may have in the condensed state are discussed.
Materials and Methods
DNA samples and solutions
Two kinds of DNA molecules that differed in G·C content were used: an ∼8-kbp dsDNA molecule with 48% G·C, obtained from pBACgus11 plasmid (Novagen, Darmstadt, Germany) and an ∼5.7-kbp dsDNA molecule with 70% G·C made from piJ702 plasmid purified from Streptomyces (46). Circular plasmids were linearized by digestion with HindIII (pBACgus11) and NdeI (piJ702) and purified using the Qiagen polymerase chain reaction (PCR) purification kit (Qiagen, Venlo, The Netherlands) and phenol, respectively. These DNA substrates were 8041 bp (pBACgus11) and 5724 bp (piJ702) and were ready to be used in CD experiments. For OT and MT experiments, the plasmids were cleaved with BamHI and HindIII (pBACgus11) and BamHI and XhoI (piJ702), which gave DNA fragments of 8022 bp (pBACgus11) and 5682 bp (piJ702). Cohesive ends were ligated to two labeled DNA handles at each end of the DNA molecule. The DIG handle was a 400-bp PCR product made by using digoxigenin-11-dUTP (Roche, Basel, Switzerland) and was ligated to the BamHI end of both DNAs. The other end was labeled with two 20-bp complementary oligos containing two biotins in the same strand at the 5′ end. The biotin-labeled end of the molecules was thus linked to the streptavidin-coated bead by only one of the two strands, and as a result, the molecules were torsionally unconstrained for the MT and OT experiments. Ligation was performed with T4 DNA ligase (Roche). All restriction enzymes were purchased from New England Biolabs (Ipswich, MA). DNA substrates were stored in TE buffer (10 mM Tris-HCl and 1 mM EDTA, pH 7.5). Experiments were performed in TE buffer containing either 1 mM or 80 mM NaCl. These buffers were mixed with ethanol (≥99.9% purity; Merck, Darmstadt, Germany) to prepare either low- or high-salt buffer-ethanol mixtures, respectively. All mixtures were sonicated before use. Spermine tetrahydrochloride (Sigma, St. Louis, MO) was used without further purification in stock solutions with deionized water.
Methods
We used CD to analyze the secondary structure of complete linearized plasmid DNAs and to find the conditions for which they presented the A-form base-stacking arrangement (see Supporting Material). Force-extension experiments with single molecules were carried out with the OT. In these experiments, contour length variations that may arise as a consequence of a low-humidity-induced B-A structural transition were measured as changes in DNA extension in ethanol relative to that in buffer at 20 pN, where the largest shifts in extension are found. Constant-force experiments were carried out with magnetic tweezers. The description of these methods is detailed in the accompanying article (47). All experiments were carried out at room temperature, 23 ± 1°C.
Results
Ethanol has been widely used to promote the B-A transition in solution, because it reduces water activity around the DNA molecules. In these conditions, CD spectroscopy is the method most frequently used for characterizing the different DNA base-stacking conformations and its cooperative transitions (22). We first used CD to find the conditions for which our two DNA substrates experience the B-A transition (see Supporting Material). In agreement with former literature (8,9,48), measurements of pBACgus11 (48% G·C) showed characteristic B-DNA CD spectra in both buffer and buffer-ethanol mixtures at <70% ethanol concentration and a characteristic A-DNA CD signal at concentrations >80% (Fig. S1, A and B, in the Supporting Material). Our previous experiments with linearized piJ702 DNA (70% G·C) showed A-form CD spectra in aqueous solution due to its high content of G·C basepairs (47). As expected, the A-type CD signal of this DNA is maintained in buffer-ethanol mixtures of decreasing water activity, because the addition of ethanol increases the stabilization of the A-type base-stacking (see Fig. S1, C and D). In light of observations in the literature, we decided to investigate whether the DNA local structural differences measured by CD spectroscopy have a reflection in the individual but global behavior of the molecules subjected to force. We used OT first to characterize the force-extension behavior of the polymer, and then MT to perform constant-force experiments in the entropic region.
Force-extension experiments with optical tweezers
We examined with optical tweezers the existence of a cooperative force-induced B-A transition. dsDNA mechanically overstretches at forces of ∼65 pN, and therefore, if it takes place at all, the B-A transition can only occur below these forces. The mechanical response in low-humidity conditions was determined by filling the fluid chamber with a buffer-ethanol mixture at different ethanol concentrations. Solutions were flowed into the chamber by means of a fluidics system that consisted of glass microdispensers and polyethylene tubes connected to the solution reservoirs, as described elsewhere (42). With this method, buffers and ethanol mixtures can be gently exchanged at a controlled rate with a single DNA molecule remaining linked between the beads, thus allowing experiments in different solutions with the same molecule. A dramatic defocusing of the laser spot due to the change in refractive index was observed as the new solution was completely refilling the chamber, thus allowing control of the exchanging process. The force measured by a light-momentum sensor calibrated from first principles does not depend on the refractive index of the solution and hence, successive refills of the fluid chamber only require refocusing of the laser beams in the new medium with no further recalibration of the setup (49).
Fig. 1 A outlines the experimental procedure used. We first performed a complete stretch-relax cycle of an individual dsDNA molecule in buffer (Fig. 1 A, left; Fig. 1, B and C, and Fig. 2; black lines). The buffer was then replaced by a buffer-ethanol mixture (Fig. 1 A, center). The molecule was extended to nearly its full contour length to prevent condensation from being triggered by the proximity of the different helical-chain domains during the exchanging process. A tension of ∼4 pN was sufficient to keep the DNA polymer highly oriented, and this was controlled by separation of the beads. Once the fluidic chamber was completely refilled with the ethanolic solution, the molecule was stretched and relaxed again (Fig. 1 A, right). This second stretch-release cycle in a buffer-ethanol mixture is plotted in red (Figs. 1, B and C, and 2).
Figure 1.
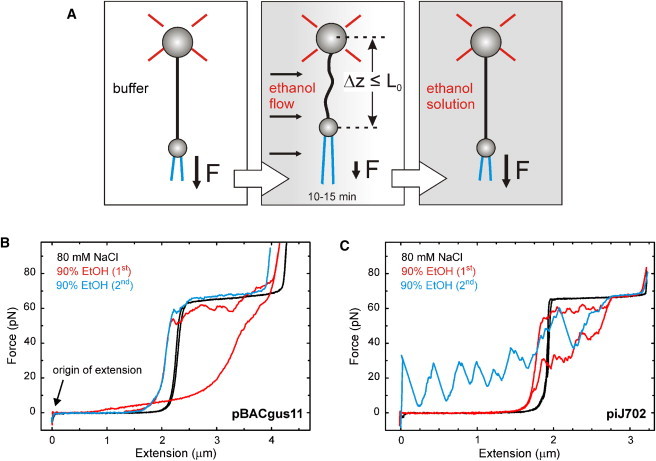
Force-extension characterization of DNA in ethanolic solutions by optical tweezers. (A) Diagram showing the phases of the experimental procedure. OT are used to trap one microsphere and measure the forces acting on it while the other microsphere is held by a micropipette. At the start of a measurement (left), a single DNA molecule is stretched in buffer by moving the micropipette, thus registering its elastic response in aqueous buffer. Then, initial buffer is gently exchanged at a controlled rate by a buffer-ethanol mixture (middle). During the exchanging process (10–15 min), the molecule is maintained at an extension near its contour length to prevent strong condensation or unspecific interactions with the beads. Finally, the same molecule is stretched in the ethanolic solution (right), so the mechanical response in low-humidity conditions is determined. (B and C) Force-extension characterization of pBACgus11 (B) and piJ702 (C) in the presence of ethanol. An individual dsDNA molecule is first stretched and relaxed in 80 mM NaCl TE buffer (black line) with subsequent cycles (red and blue lines) performed in a mixture of the same buffer with 90% ethanol. Stretch and release curves are shown in the same color. Pulling speed, 500 nm/s.
Figure 2.
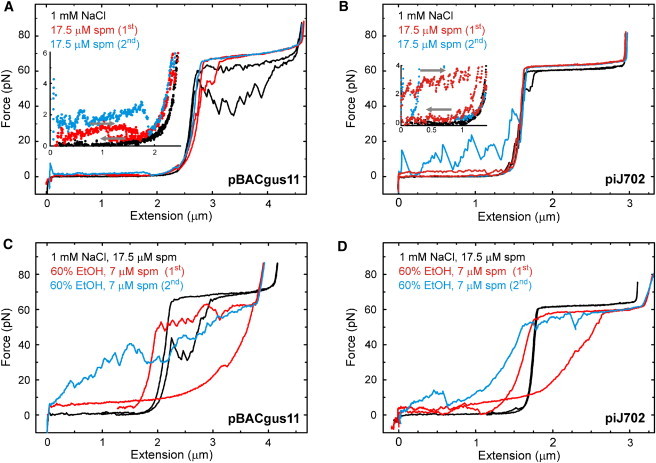
Force-extension curves of pBACgus11 (A and C) and piJ702 (B and D) in the presence of spermine (A and B) and ethanol and spermine (C and D). An individual dsDNA molecule is first stretched and relaxed in TE buffer (black line; [NaCl] and [spermine], as displayed in the legends), with subsequent cycles (red and blue lines) performed in a mixture of the same buffer with ethanol (% concentration as displayed in the legends). Stretch and release curves are shown in the same color. (A and B, insets) Zooms of the low-force region to show the strength of the condensation plateaus, with gray arrows marking the stretch and relax paths. Pulling speed, 500 nm/s. spm, spermine.
The elastic behavior of individual molecules at 90% ethanol concentration is shown in Fig. 1, B and C. In these conditions, the CD spectrum is characteristic of A-DNA for both substrates (see Fig. S1). However, no cooperative force-induced B-A transition is present in either the stretching or the relaxation traces of any of the two plasmids in the ethanolic solution. The presence of the ethanol causes instead a strong hysteresis in the relaxation trace, which indicates the melting of the two strands by fraying due to the lower dielectric constant of the new solution. These experiments represented in Fig. 1, B and C, were performed for different ethanol concentrations from 10% to 100% (n > 1000 molecules) with the same qualitative effects: absence of a transition in the intrinsic-force regime and large hysteresis area in the stretch-relax cycle due to melting of DNA strands (data not shown). Mixtures of ethanol with a low-salt buffer (1 mM NaCl) were also tested, with the same results (data not shown). Note that a cooperative change in extension from an A-form to a B-form contour length should extend the DNA molecule by 24% in the stretching curve (from 2.56 Å/bp in the A-form, to 3.38 Å/bp in the B-form), which would produce a change in extension of ∼700 nm for pBACgus11 and of ∼500 nm for piJ702 plasmid, clearly within the resolution of our OT instrument in the intrinsic-force regime (<10 nm) (49).
To analyze the structural state of the molecules in the intrinsic-force regime in buffer-ethanol mixtures, we measured their extension relative to that in buffer for each molecule (Figs. 1 and 2, red and black lines, respectively). To set a relative origin of extension for each molecule, we gently made the beads collide at relaxation. The collision of the bead is characterized by a sudden decay in force at zero extension in the curves of Figs. 1 and 2 (see Fig. 1 B, arrow). The contour length of each molecule in buffer-ethanol mixture was then obtained by subtracting the measured shift in extension with respect to the canonical B-DNA contour length (3.38 Å/bp × (number of basepairs)). Table 1 summarizes the rise/basepair values (l0) of high-G·C DNA and balanced A·T/G·C DNA from these comparative experiments. As shown in the table, the mean rise/basepair for both DNAs in all conditions tested is similar to that of B-DNA (3.38 Å) and significantly different from that of A-DNA (2.56 Å). Finally, it is also important to note that, at the overstretching force, the curves always displayed a reversible, cooperative transition to an extended form that is ∼70% longer than B-DNA, as observed in all natural DNAs to date independent of the type of DNA or the buffer conditions used (43,44,50). Together, these data show that DNA stretches as B-DNA for forces above the entropic regime. The analysis of the force-extension curves at low force reveals the effects of condensation upon the total relaxation of the molecule (F ∼ 0 pN). To show them, we stretched again the molecules in these conditions (Figs. 1 and 2, blue lines). At zero force, molecules are free to condense, and these stretching curves often then showed sudden jumps in force due to the violent release of the polymer from the collapsing interactions (51,52). Periodic force jumps or so-called stick-release patterns that indicate a higher organization have been shown to appear in the presence of polycations in aqueous solution (51,52). Here, we find that such stick-release patterns are also induced by high ethanol concentrations (>80%). Fig. 2 B (blue line) shows a stick-release pattern in aqueous buffer with spermine, i.e., in conditions similar to those used previously (51,52). The analysis of the decondensation peak-to-peak distance periodicity of piJ702 in ethanol, both with and without spermine, is detailed in Fig. S2 and was between 150 and 250 nm. This is in support of the idea of unraveling collapsed structures, such as coiled DNA or toroidal supercoiling, as described elsewhere (51,52). Periodic stick-release patterns for pBACgus11 in ethanol, however, were not so marked for the loading rate used. This indicates that the influence of condensing intramolecular interactions is more significant in the presence of a high-G·C-content DNA.
Table 1.
Rise/basepair of DNA with different G·C content in buffer and low-humidity conditions as determined with optical tweezers
|
l0, rise/bp (Å/bp) |
||
|---|---|---|
| Solution condition | pBACgus11 (48% G·C) | piJ702 (70% G·C) |
| Buffer∗ | 3.38 ± 0.07 (13) | 3.38 ± 0.15 (22) |
| 30% EtOH | 3.37 ± 0.11 (6) | 3.36 ± 0.16 (12) |
| 90% EtOH | 3.24 ± 0.27 (18) | 3.27 ± 0.35 (18) |
| 60% EtOH + 7 μM spm4+ | 3.28 ± 0.15 (9) | 3.11 ± 0.21 (14) |
Results are reported as the mean ± SD. Numbers in parentheses indicate the population. spm, spermine.
Force-extension experiments in aqueous buffer with spermine (Fig. 2, A and B) reveal a plateau at entropic forces, 1–2 pN (pBACgus11) and ∼3 pN (piJ702) (Fig. 2, A and B, insets). This condensation effect is again stronger for piJ702 plasmid, since the plateau appears at a higher force. In the presence of 60% ethanol and 7 μM spermine, the condensation effect is more dramatic and similar for both DNAs, since the force at the condensation plateau was ∼6 pN (Fig. 2, C and D, red lines). However, condensation plateaus were not detected for DNA in spermine-free solutions within our force resolution (0.1 pN) (see Fig. 1, B and C). In contrast to the violent unfolding of the polymer, which generates the stick-release pattern, the appearance of a smooth, low-force-equilibrated condensation plateau suggests that spermine induces a finer organization through lateral interactions between DNA segments in the collapsed, globular structure (51,52). In light of this interpretation, our experiments suggest that the concerted action of low water activity and polycations in the dsDNA molecule stabilizes a condensed structure that exhibits a short-range organization presumably withstood by lateral interactions between basepairs.
Magnetic tweezers study of real-time condensation dynamics
To better explore the entropic-force region in a search for any global signature of the B-A transition, and to study the dynamics of the condensation process, we used MT, which in addition offers better resolution at low forces than OT. In the experimental configuration, a single DNA molecule is subjected to a constant force between one bead and a glass surface (see Fig. 3 A). Standard MT experiments monitor the distance between the bead and the surface as a function of time while the buffer is being exchanged for a buffer-ethanol mixture. In these conditions, the effect of condensation can be measured as changes in extension (Fig. 3 A). The event of a cooperative B-A transition should be characterized by a defined and reproducible change in extension, certainly <24%. Our fluid system allows relatively fast exchange of buffers, so that new buffer conditions are achieved within seconds.
Figure 3.
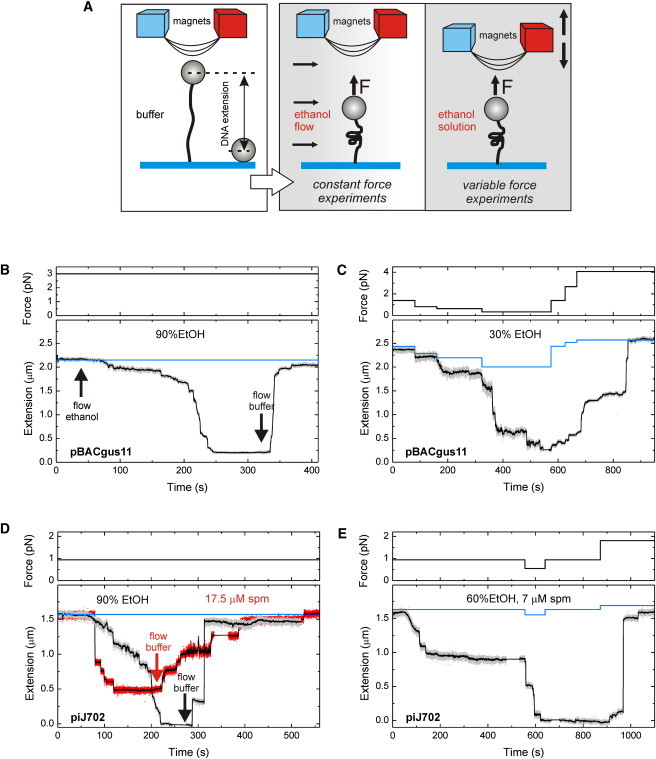
Measurements of DNA extension under the influence of ethanol and spermine using magnetic tweezers. (A) Diagram showing the two experimental approaches used. A single DNA molecule is tethered between a superparamagnetic bead at one end and a glass surface at the other end (left). DNA extension is measured while flowing a buffer-ethanol mixture in constant-force experiments (middle) or while changing the force in the presence of ethanol (right). (B–E) Real-time tracking of pBACgus11 extension (upper row) and piJ702 extension (lower row) in the presence of ethanol and/or spermine, showing the extension of the molecule (lower) as well as the force acting on it as a function of time (upper). Blue lines indicate the expected DNA extension free of condensing agents for each force. Average over 20 data points are shown as black traces. [NaCl], [spermine], and % ethanol are displayed in the legends. spm, spermine.
We first tethered a single pBACgus11 molecule that contains ∼50% G·C at a constant force of 3 pN. Then, a buffer that contains a high concentration of ethanol (90%) was introduced into the fluid chamber while the extension of the DNA molecule was monitored in real time (Fig. 3 B). At this force and ethanol concentration, the extension of the DNA changed from 2.2 μm to 300 nm within ∼200 s as a consequence of depletion of water molecules by ethanol and DNA condensation. The condensation curve showed features that we reproduced in subsequent experiments performed under the same conditions: 1), an initial slow decrease in extension punctuated by short and discrete drops of tens of nanometers; and 2), a gradual increase in the length of the drops ending up in large and fast collapsing events. Our condensation data never showed a plateau corresponding to a stable B-A transition, which should reproducibly occur for 3 pN at 1.64 μm, according to a decrease in contour length of 24% (from 3.38 Å/bp in B-form to 2.56 Å/bp in A-form). The condensation process was reversible upon introduction of the original noncondensing buffer (see Fig. 3 B, arrow). In MT experiments, the force applied to the bead acts against condensation and favors recovering the original length of the DNA when the condensing buffer is exchanged. As a consequence, the speed of condensation was found to be quite dependent on the applied force. Fig. 3 B clearly shows how the original length of the DNA is recovered within seconds in contrast with the condensation process, which took hundreds of seconds.
We next studied whether DNA could be decondensed upon the action of force. From previous experiments, we found that the condensing force under high ethanol concentration was larger than that applied by the magnets. Therefore, to decrease the condensing force, we decided to use a much lower concentration of ethanol. Fig. 3 C shows a time trace in a buffer containing 30% ethanol. At forces >1 pN, we could not identify any condensation effect, as data (gray trace) overlapped with the expected length measured in the absence of ethanol (blue line). At a force of 0.35 pN, the bead approached the surface in a manner similar to that observed in Fig. 3 B, with a reduction in length of ∼90%. Further decrease of the force triggered full condensation of DNA. Then we tested whether it was possible to revert to the full length of the DNA construct by increasing the force. Indeed, condensation was reversible upon increase of the pulling force; the starting length of the DNA was obtained at 4 pN (Fig. 3 C). Similar to previous experiments, we could not identify any stable plateau corresponding to A-form DNA that could be reproduced from molecule to molecule.
The role of base content in condensation dynamics is not well known. Phasing of A·T dinucleotides was shown to affect the persistence length and rigidity of DNA (53) and our previous experiments based on CD spectra showed that DNA with high G·C content can adopt an A-type base-stacking arrangement. Our work (47), based on measurements of the contour length, showed that high-G·C-content DNA maintains the overall B-form but that the structural differences arising from the nucleotide content affect the persistence length of the molecule at zero force and the stretch modulus at high forces. However, it is not known whether high-G·C-content DNA has a tendency to change its extension in dehydration conditions. For these reasons, we decided to extend the work done on pBACgus11 plasmid to the piJ702 plasmid, which, as mentioned, contains 70% G·C content, and to study curves of condensation under different ethanol and spermine conditions.
High-G·C-content DNA showed a behavior similar to that of 50%-G·C DNA under typical condensation conditions. A single piJ702 DNA molecule was tethered and kept at a constant force of 1 pN (Fig. 3 D). We then introduced a buffer containing 90% ethanol and measured the distance between bead and surface in real time (gray trace). Traces shared features previously seen in Fig. 3 B. Condensation curves with ethanol showed a gradual decrease in extension in the beginning, as well as small drops in extension. Note that the duration of the entire condensation process is shorter than in Fig. 3 B. This is possibly due to the effect of the large force in Fig. 3 B that slowed down the condensation process. As expected, condensation was reversible upon insertion of buffer without ethanol and the original contour length was recovered. It is interesting to note that a similar experiment done in a buffer containing 17.5 μM spermine yielded a rather different condensation curve (Fig. 3 D, red trace). Spermine dramatically speeded up the condensation process and introduced large drops in the trace. The shape of the trace, containing large drops, suggested a different condensation mechanism if we compared this trace with those obtained with ethanol. The fact that spermine facilitates condensation is in agreement with previous OT experiments where condensation effects became more evident in the presence of this polyamine (Fig. 2, B and D).
In a manner similar to that seen in Fig. 3 C, condensation of piJ702 DNA could be favored by lowering the stretching force or, alternatively, abolished by increasing the force (Fig. 3 E). Under the conditions used in this experiment (60% ethanol, 7 μM spermine), condensation stalled at ∼65% of the total length at ∼1 pN. Condensation resumed by lowering the pulling force down to 0.5 pN, and the DNA molecule collapsed, dragging the bead toward the surface in two large steps. This process could be reverted by increasing the force up to 2 pN. Then again, we could not identify any stable plateau corresponding to A-form DNA; instead, we observed the noncooperative features of condensation.
Discussion
The secondary structure of DNA at low humidity and the global helical arrangement can be influenced by the presence of condensation or aggregation. The B-A transition stemming from a change in base-stacking arrangement may accommodate a global change in contour-length independent of condensation or aggregation or may proceed with the interplay of condensation/aggregation. We used conditions for which DNA adopts the A-form base-stacking arrangement, as measured by CD (Fig. S1).We then analyzed how these conditions influence the global conformational state of DNA by using single-molecule experiments. This approach prevented the effect of intermolecular aggregation and the use of force allowed us to control condensation. In the analysis of the force-extension curves, if a specific base-stacking configuration could alone promote a global helical structure, B-DNA contour length would be expected to be favored by force, since A-DNA possesses a shorter rise/residue (2). Therefore, a sudden change in extension should have appeared at a critical force in the curves. We never observed such bistability. Moreover, the measurements revealed no contour-length values compatible with an A-form rise/basepair.
In this regard, Fig. 4 shows a diagram of the mean rise/basepair (l0) in comparison to published bulk data for A and B conformational families. Contour-length changes that arise as a consequence of low-humidity conditions were detected by measuring the change in DNA extension in ethanol relative to that in buffer. These measurements were performed in the intrinsic-force regime (see Materials and Methods) where the DNA double helix is kept highly oriented. In these conditions, changes in extension provide a measurement of the change in contour length. The rise/residue data were calculated from the contour length scaled to the number of basepairs, and it is therefore an average rise/basepair over the molecular length. These experiments in which the same molecule is stretched in both A- and B-stabilizing conditions and with A- and B-philic sequences did not show any trend in mean l0. In addition, the extension of the overstretching plateau always complied with a length of 0.7 × (B-DNA contour length) (43,44,50), independent of the DNA substrate or buffer conditions used. Taken together, all these data are consistent with a global DNA structure, typical of B-form, which is not affected overall by the local base-stacking arrangement. Some molecules presented contour-length changes (significantly shorter than that of the B-A transition) that were uniformly distributed around the B-form contour length. These minor changes should be understood in terms of deviations from B-like elasticity as a consequence of the highly denaturing conditions imposed by the ethanolic mixtures.
Figure 4.
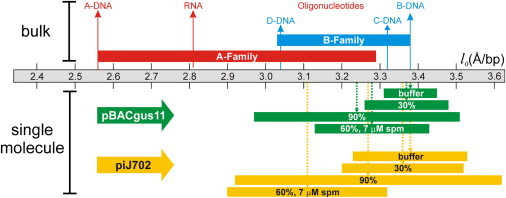
Diagram comparing single-molecule measurements of the mean rise/residue to those in bulk for B and A conformational families and their main members: A-DNA and dsRNA belonging to the A family and B-, C-, and D-DNA belonging to the B family (bulk data from Saenger (2)). For the single-molecule data (which correspond to Table 1), vertical arrows represent average values of at least six individual experiments (each with a different molecule) and bar lengths represent 2SD. The rise/basepair values in buffer are assigned to the corresponding canonical B-form, whereas the SD values in buffer are those measured from adsorbed molecules on mica with atomic force microscopy (see Hormeño et al. (47)). Labeled bars represent mixtures of ethanol at 30% and 90% concentration, respectively, with 80 mM NaCl TE buffer, and mixtures of ethanol at 60% concentration with 1 mM NaCl TE buffer and 7 μM spermine final concentration.
The force-extension curves also revealed characteristic features of DNA condensation at low forces. We followed the effect of ethanol on the DNA extension in real time by using MT in constant-force mode. We confirmed that the conditions that induce the A-type base-stacking do globally produce condensation of the single molecule. However, we did not find any signature of the B-A transition, which should appear as a definite and reproducible change in extension of the molecules. These data strengthen the idea that the overall contour length is not affected by the specific base-stacking arrangement (47), here induced by low humidity, and that condensation prevails over the B-A transition in the global conformation of DNA. The fact that a cooperative B-A shortening was obtained with aggregated molecules under stress (12,54–57), and not at the single-molecule level (this work and (47)), suggests that interhelical interactions may stabilize a global contraction of the molecule promoted by a B-A transition at the basepair level. Consistent with this view, former CD measurements on DNA restriction fragments showed that a high ethanol concentration produces a fast B-A transition signal followed by a slow aggregation process (41). In fact, the highly organized condensation in dehydrated DNA fibers that produce crystalline matrices may explain an eventual reduction in rise/basepair in x-ray diffraction measurements (2).
The A-type base-stacking arrangement, although not leading to shorter molecules, may be distorted in the presence of force, as was shown in the study of sequence-induced A-DNA (47). Recent numerical predictions (58) can be used to infer the force at which the A-form base-stacking arrangement in water-ethanol mixtures should change to B-form. The free-energy difference for the B→A conformational transition for the Dickerson's dodecamer calculated by Noy et al. (58) in 85% ethanol, ΔGB→A(85% EtOH) = −0.8 kcal/mol, is much lower than that for B→A in water, ΔGB→A (H2O) = 11.4 kcal/mol. We can express these energies in units of kBT/residue at T = 25°C to compare with the energy of the thermal fluctuations: ΔGB→A (H2O) = 1.60 kBT/residue and ΔGB→A(85% EtOH) = −0.11 kBT/residue. According to these calculations, although the stability of a basepair in B-DNA with respect to that in A-DNA in water is on the order of the base-stacking free energies (59), the stability of a basepair in A-DNA with respect to that in B-DNA in 85% ethanol is well below those energies. This means that in ethanolic conditions, A-form base-stacking is weakly stable, i.e., strongly subjected to thermal fluctuations. This energy estimation, together with our experimental data, supports the idea that interhelical interactions are required to reinforce A-DNA global stability. To further discuss these calculations, we can use them to infer an approximate force for which the A-form base-stacking is destabilized in our experiments. In the presence of an external stretching force, f, the intrinsic free energy is augmented by the work needed to pull the bead against the external force, fΔl, where Δl is the change in the length of the molecule. The transition should occur when the two energy terms are balanced, provided that we approach the transition force, f∗, at a near-equilibrium loading-rate: ΔG + f∗Δl = 0. We obtain a transition force of f∗ ∼ 5 pN, which is within the force range of our measurements. According to our experiments, we observe condensation at these forces, and therefore this theoretical prediction agrees with phase coexistence between condensation and A-form.
Distortions from B-type base-stacking which do not entail significant changes in the axial direction, as that expected for the B-A transition, may therefore be present in the entropic-force regime at low humidity. The single-molecule approach employed did not provide structural information at the basepair level, i.e., it did not give access to configurational deviations from the B-form base-stacking. It did, however, measure changes in the contour length, which represent basepair-averaged variations in the rise/basepair. This information allowed us to conclude that a change in local arrangement induced by ethanol does not compromise the global state of the molecule, as represented by its B-form contour length and elasticity.
Conclusions
We studied the mechanical stability of single DNA molecules in water-ethanol mixtures to investigate the interplay of condensation in the B-A transition and in the global conformation of the molecules. We used two DNA substrates differing in both G·C content and base-stacking configuration in aqueous buffer, as characterized in the accompanying article (47). Specifically, we used a high-G·C-content DNA with A-type base-stacking and a DNA with balanced nucleotide composition and B-type base-stacking. Our experiments show that a local change in base-stacking arrangement induced by low water activity does not lead to a global change in the contour length of the DNA molecule, a result that is consistent with previous measurements in which A-DNA was induced by sequence (47). Specifically, a local B-A transition in secondary structure, as monitored by CD spectroscopy, does not promote a decrease in length of the DNA molecule, since both OT and MT measured typical B-form values of the mean rise/basepair for both DNA substrates. The fact that condensation coexists with the A-form base-stacking conformation at low water activity suggests that a global rearrangement of the DNA molecule through interhelical chain associations may ultimately explain the decrease in rise/basepair measured by x-ray crystallography (2). Specifically, our experiments suggest that the A-form of DNA (secondary structure) under low humidity conditions is weakly promoted at the basepair level by its sequence (primary structure) and environmental conditions, and that it is ultimately stabilized by condensation (tertiary structure).
Acknowledgments
C. Bustamante and S. B. Smith are acknowledged for discussion and help in setting up the optical tweezers laboratory. G. Montoya is acknowledged for help with CD experiments. F. Malpartida and T. Cuesta are acknowledged for piJ702 plasmid DNA purification.
This work was supported by grants from the Spanish Ministry of Science and Innovation (CSD2007-00010, BFU2010-15703/BMC, and BFU2008-02328/BMC) and the Comunidad de Madrid (S-0505/MAT/0283). S.H. acknowledges a scholarship from Consejería de Educación de la Comunidad de Madrid and the European Social Fund. J.R.A.-G. acknowledges a former individual fellowship from the International Human Frontier Science Program Organization and a Ramón y Cajal contract from the Spanish Ministry of Science and Innovation. Work in the F.M.-H. laboratory is supported by a Starting Grant from the European Research Council (no. 206117) and a grant from the Spanish Ministry of Science and Innovation (no. FIS2008-0025).
Supporting Material
References
- 1.Calladine, C. R., H. R. Drew, …, A. A. Travers. 2004. Understanding DNA. The molecule and how it works. Elsevier. Academic Press.
- 2.Saenger W. Springer-Verlag; New York: 1984. Principles of nucleic acid structure. [Google Scholar]
- 3.Arscott P.G., Ma C., Bloomfield V.A. DNA condensation by cobalt hexaammine (III) in alcohol-water mixtures: dielectric constant and other solvent effects. Biopolymers. 1995;36:345–364. doi: 10.1002/bip.360360309. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y., Spisz T.S., Hoh J.H. Ethanol-induced structural transitions of DNA on mica. Nucleic Acids Res. 1999;27:1943–1949. doi: 10.1093/nar/27.8.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widom J., Baldwin R.L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J. Mol. Biol. 1980;144:431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- 6.Lang D. Collapse of single DNA molecules in ethanol. J. Mol. Biol. 1969;46:209. doi: 10.1016/0022-2836(69)90069-2. [DOI] [PubMed] [Google Scholar]
- 7.Hud N.V., Downing K.H. Cryoelectron microscopy of lambda phage DNA condensates in vitreous ice: the fine structure of DNA toroids. Proc. Natl. Acad. Sci. USA. 2001;98:14925–14930. doi: 10.1073/pnas.261560398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanov V.I., Minchenkova L.E., Poletayev A.I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12:89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- 9.Brahms J., Mommaerts W.F. A study of conformation of nucleic acids in solution by means of circular dichroism. J. Mol. Biol. 1964;10:73–88. doi: 10.1016/s0022-2836(64)80029-2. [DOI] [PubMed] [Google Scholar]
- 10.Girod J.C., Johnson W.C., Jr., Maestre M.F. Conformation of deoxyribonucleic acid in alcohol solutions. Biochemistry. 1973;12:5092–5096. doi: 10.1021/bi00749a011. [DOI] [PubMed] [Google Scholar]
- 11.Minyat E.E., Ivanov V.I., Schyolkina A.K. Spermine and spermidine-induced B to A transition of DNA in solution. J. Mol. Biol. 1979;128:397–409. doi: 10.1016/0022-2836(79)90094-9. [DOI] [PubMed] [Google Scholar]
- 12.Rupprecht A., Piškur J., Lahajnar G. Mechanochemical study of conformational transitions and melting of Li-, Na-, K-, and CsDNA fibers in ethanol-water solutions. Biopolymers. 1994;34:897–920. doi: 10.1016/s0006-3495(94)80857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albiser G., Lamiri A., Premilat S. The A-B transition: temperature and base composition effects on hydration of DNA. Int. J. Biol. Macromol. 2001;28:199–203. doi: 10.1016/s0141-8130(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 14.Usatyi A.F., Shlyakhtenko L.S. Melting of DNA in ethanol-water solutions. Biopolymers. 1974;13:2435–2446. doi: 10.1002/bip.1974.360131204. [DOI] [PubMed] [Google Scholar]
- 15.Bauer C., Wang A.H.J. Bridged cobalt amine complexes induce DNA conformational changes effectively. J. Inorg. Biochem. 1997;68:129–135. doi: 10.1016/s0162-0134(97)00083-4. [DOI] [PubMed] [Google Scholar]
- 16.Kankia B.I., Buckin V., Bloomfield V.A. Hexamminecobalt(III)-induced condensation of calf thymus DNA: circular dichroism and hydration measurements. Nucleic Acids Res. 2001;29:2795–2801. doi: 10.1093/nar/29.13.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saenger W., Hunter W.N., Kennard O. DNA conformation is determined by economics in the hydration of phosphate groups. Nature. 1986;324:385–388. doi: 10.1038/324385a0. [DOI] [PubMed] [Google Scholar]
- 18.Subirana J.A., Soler-Lopez M. Cations as hydrogen bond donors: a view of electrostatic interactions in DNA. Annu. Rev. Biophys. Biomol. Struct. 2003;32:27–45. doi: 10.1146/annurev.biophys.32.110601.141726. [DOI] [PubMed] [Google Scholar]
- 19.Bloomfield V.A. DNA condensation by multivalent cations. Biopolymers. 1997;44:269–282. doi: 10.1002/(SICI)1097-0282(1997)44:3<269::AID-BIP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Gosule L.C., Schellman J.A. Compact form of DNA induced by spermidine. Nature. 1976;259:333–335. doi: 10.1038/259333a0. [DOI] [PubMed] [Google Scholar]
- 21.Raspaud E., Chaperon I., Livolant F. Spermine-induced aggregation of DNA, nucleosome, and chromatin. Biophys. J. 1999;77:1547–1555. doi: 10.1016/S0006-3495(99)77002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov V.I., Minchenkova L.E., Schyolkina A.K. The B to A transition of DNA in solution. J. Mol. Biol. 1974;87:817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- 23.Xu Q., Shoemaker R.K., Braunlin W.H. Induction of B-A transitions of deoxyoligonucleotides by multivalent cations in dilute aqueous solution. Biophys. J. 1993;65:1039–1049. doi: 10.1016/S0006-3495(93)81163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson H., Wang A.H. Neomycin, spermine and hexaamminecobalt (III) share common structural motifs in converting B- to A-DNA. Nucleic Acids Res. 1996;24:676–682. doi: 10.1093/nar/24.4.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leslie A.G., Arnott S., Ratliff R.L. Polymorphism of DNA double helices. J. Mol. Biol. 1980;143:49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- 26.Becker M.M., Wang Z. B-A transitions within a 5 S ribosomal RNA gene are highly sequence-specific. J. Biol. Chem. 1989;264:4163–4167. [PubMed] [Google Scholar]
- 27.Borovok N., Molotsky T., Kotlyar A. Poly(dG)-poly(dC) DNA appears shorter than poly(dA)-poly(dT) and possibly adopts an A-related conformation on a mica surface under ambient conditions. FEBS Lett. 2007;581:5843–5846. doi: 10.1016/j.febslet.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Nara-Inui H., Akutsu H., Kyogoku Y. Alcohol induced B-A transition of DNAs with different base compositions studied by circular dichroism. J. Biochem. 1985;98:629–636. doi: 10.1093/oxfordjournals.jbchem.a135319. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura Y., Torigoe C., Tsuboi M. An A-form poly(dG).poly(dC) in H2O solution. Biopolymers. 1985;24:1841–1844. doi: 10.1002/bip.360240913. [DOI] [PubMed] [Google Scholar]
- 30.Arnott S., Selsing E. Letter: the structure of polydeoxyguanylic acid with polydeoxycytidylic acid. J. Mol. Biol. 1974;88:551–552. doi: 10.1016/0022-2836(74)90502-6. [DOI] [PubMed] [Google Scholar]
- 31.Lang D., Taylor T.N., Gray D.M. Dehydrated circular DNA: electron microscopy of ethanol-condensed molecules. J. Mol. Biol. 1976;106:97–107. doi: 10.1016/0022-2836(76)90302-8. [DOI] [PubMed] [Google Scholar]
- 32.Piskur J., Rupprecht A. Aggregated DNA in ethanol solution. FEBS Lett. 1995;375:174–178. doi: 10.1016/0014-5793(95)01206-t. [DOI] [PubMed] [Google Scholar]
- 33.Herbeck R., Yu T.J., Peticolas W.L. Effect of cross-linking on the secondary structure of DNA I. Cross-linking by photodimerization. Biochemistry. 1976;15:2656–2660. doi: 10.1021/bi00657a027. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman S.B., Pheiffer B.H. A direct demonstration that the ethanol-induced transition of DNA is between the A and B forms: an x-ray diffraction study. J. Mol. Biol. 1979;135:1023–1027. doi: 10.1016/0022-2836(79)90526-6. [DOI] [PubMed] [Google Scholar]
- 35.Subirana J.A., Chiva M., Mayer R. X-ray diffraction studies of complexes of DNA with lysine and with lysine-containing peptides. In: Srinivasan R., Yathindra N., Subramanian E., editors. Biomolecular Structure, Conformation, Function and Evolution. Pergamon Press; London: 1980. pp. 431–440. [Google Scholar]
- 36.Mazur A.K. Electrostatic polymer condensation and the A/B polymorphism in DNA: Sequence effects. J. Chem. Theory Comput. 2005;1:325–336. doi: 10.1021/ct049926d. [DOI] [PubMed] [Google Scholar]
- 37.Schnell J.R., Berman J., Bloomfield V.A. Insertion of telomere repeat sequence decreases plasmid DNA condensation by cobalt (III) hexaammine. Biophys. J. 1998;74:1484–1491. doi: 10.1016/S0006-3495(98)77860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reich Z., Ghirlando R., Minsky A. Secondary conformational polymorphism of nucleic acids as a possible functional link between cellular parameters and DNA packaging processes. Biochemistry. 1991;30:7828–7836. doi: 10.1021/bi00245a024. [DOI] [PubMed] [Google Scholar]
- 39.Zavriev S.K., Minchenkova L.E., Ivanov V.I. On the flexibility of the boundaries between the A-form and B-form sections in DNA molecule. Nucleic Acids Res. 1978;5:2657–2663. doi: 10.1093/nar/5.7.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potaman V.N., Bannikov Y.A., Shlyachtenko L.S. Sedimentation of DNA in ethanol-water solutions within the interval of B to A transition. Nucleic Acids Res. 1980;8:635–642. doi: 10.1093/nar/8.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillen W., Wells R.D. Circular dichroism studies of the B goes to A conformational transition in seven small DNA restriction fragments containing the Escherichia coli lactose control region. Nucleic Acids Res. 1980;8:5427–5444. doi: 10.1093/nar/8.22.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hormeño S., Arias-Gonzalez J.R. Exploring mechanochemical processes in the cell with optical tweezers. Biol. Cell. 2006;98:679–695. doi: 10.1042/BC20060036. [DOI] [PubMed] [Google Scholar]
- 43.Bustamante C., Bryant Z., Smith S.B. Ten years of tension: single-molecule DNA mechanics. Nature. 2003;421:423–427. doi: 10.1038/nature01405. [DOI] [PubMed] [Google Scholar]
- 44.Smith S.B., Cui Y., Bustamante C. Overstretching B-DNA: the elastic response of individual double-stranded and single-stranded DNA molecules. Science. 1996;271:795–799. doi: 10.1126/science.271.5250.795. [DOI] [PubMed] [Google Scholar]
- 45.Besteman K., Van Eijk K., Lemay S.G. Charge inversion accompanies DNA condensation by multivalent ions. Nat. Phys. 2007;3:641–644. [Google Scholar]
- 46.Katz E., Thompson C.J., Hopwood D.A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J. Gen. Microbiol. 1983;129:2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- 47.Hormeño S., Ibarra B., Carrascosa J.L., Valpuesta J.M., Moreno-Herrero F. Mechanical properties of high GC-content DNA with A-type base-stacking. Biophys. J. 2011;100:1996–2005. doi: 10.1016/j.bpj.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray D.M., Ratliff R.L., Vaughan M.R. Circular dichroism spectroscopy of DNA. Methods Enzymol. 1992;211:389–406. doi: 10.1016/0076-6879(92)11021-a. [DOI] [PubMed] [Google Scholar]
- 49.Smith S.B., Cui Y., Bustamante C. Optical-trap force transducer that operates by direct measurement of light momentum. Methods Enzymol. 2003;361:134–162. doi: 10.1016/s0076-6879(03)61009-8. [DOI] [PubMed] [Google Scholar]
- 50.Cluzel P., Lebrun A., Caron F. DNA: an extensible molecule. Science. 1996;271:792–794. doi: 10.1126/science.271.5250.792. [DOI] [PubMed] [Google Scholar]
- 51.Baumann C.G., Bloomfield V.A., Block S.M. Stretching of single collapsed DNA molecules. Biophys. J. 2000;78:1965–1978. doi: 10.1016/S0006-3495(00)76744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murayama Y., Sakamaki Y., Sano M. Elastic response of single DNA molecules exhibits a reentrant collapsing transition. Phys. Rev. Lett. 2003;90:018102. doi: 10.1103/PhysRevLett.90.018102. [DOI] [PubMed] [Google Scholar]
- 53.Moreno-Herrero F., Seidel R., Dekker N.H. Structural analysis of hyperperiodic DNA from Caenorhabditis elegans. Nucleic Acids Res. 2006;34:3057–3066. doi: 10.1093/nar/gkl397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albiser G., Harmouchi M., Premilat S. Influence of a mechanical tension on the B-A and B-C conformational transitions in DNA fibres. J. Biomol. Struct. Dyn. 1988;6:359–366. doi: 10.1080/07391102.1988.10507718. [DOI] [PubMed] [Google Scholar]
- 55.Fornells M., Campos J.L., Subirana J.A. Changes of conformation of DNA produced by mechanical forces. J. Mol. Biol. 1983;166:249–252. doi: 10.1016/s0022-2836(83)80012-6. [DOI] [PubMed] [Google Scholar]
- 56.Harmouchi M., Albiser G., Premilat S. Effect of a mechanical tension on the hydration of DNA in fibres. Biochem. Biophys. Res. Commun. 1992;188:78–85. doi: 10.1016/0006-291x(92)92352-x. [DOI] [PubMed] [Google Scholar]
- 57.Schultz J., Rupprecht A., Lahajnar G. A mechanochemical study of MgDNA fibers in ethanol-water solutions. Biophys. J. 1994;66:810–819. doi: 10.1016/s0006-3495(94)80857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noy A., Pérez A., Orozco M. Theoretical study of large conformational transitions in DNA: the B<—>A conformational change in water and ethanol/water. Nucleic Acids Res. 2007;35:3330–3338. doi: 10.1093/nar/gkl1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.SantaLucia J., Jr. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proc. Natl. Acad. Sci. USA. 1998;95:1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


