Abstract
The genus Protocrea is redefined, based on holotype and fresh specimens of its type species P. farinosa, using morphology of teleomorph and anamorph and phylogenetic analyses of rpb2 sequences. Data based on currently available specimens suggest the existence of three well defined and three still unnamed species. Apart from the type, P. farinosa, none of the species originally included are accepted in the genus. Species of Protocrea are characterized by perithecia formed in or on a subiculum, bicellular ascospores that disarticulate at the septum while still in the ascus and by anamorphs belonging to Gliocladium sensu stricto. For Hypocrea farinosa sensu auct. the new species H. decipiens is introduced. Hypocrea pallida is recognized as a species of Protocrea. It is closely related to P. farinosa, morphologically, phylogenetically and by habit. Protocrea illinoënsis is described here as the sister taxon of P. farinosa found in the USA. All species are polyporicolous, with the principal hosts Skeletocutis nivea for P. farinosa and P. illinoënsis, and species of Oligoporus/Tyromyces for P. pallida. In addition to hosts the main differences among these species are a stronger (orange) pigmentation of perithecia and subiculum in P. pallida and a violaceous KOH reaction in P. pallida and P. illinoënsis. P. farinosa is known only from Europe with certainty and P. illinoënsis only from the USA, while P. pallida is probably cosmopolitan. Putative synonymy of some similar species is discussed.
Keywords: Ascomycetes, Gliocladium, Hypocrea, Hypocreales, ITS, LSU, morphology, phylogeny, rpb2, sequence analysis, systematics, tef1
INTRODUCTION
Hypocrea typically consists of a teleomorph, the stroma of which is usually no more than a few millimeters in diameter and includes primarily pseudoparenchymatous tissue, bicellular ascospores that disarticulate at the septum and a Trichoderma Pers. anamorph with green or, less frequently, colorless (white in mass) conidia. However several described species vary from this stereotype in having effused stromata or a subiculum and/or having acremonium-, verticillium- or gliocladium-like anamorphs. Segregate genera have been proposed for some of these species, including Protocrea and Arachnocrea Moravec, but in the absence of critical study, the paucity of specimens and the lack of cultures that elucidate anamorphs while providing molecular phylogenetic information correct application of these generic names and assignment of species has been haphazard.
Petch (1937) erected the genus Protocrea including Hypocrea farinosa Berk. & Broome, H. delicatula Tul. and H. stipata (Lib.) Fuckel. He did not indicate a type species. Moravec (1956) lectotypified the genus with P. farinosa, while retaining P. delicatula and removing P. stipata to his new genus Arachnocrea, the latter characterized by disarticulating biconical ascospores. Põldmaa (2000) provided molecular phylogenetic evidence that the type species of Arachnocrea, A. stipata, having a verticillium-like anamorph (Põldmaa 1999), is distinct from Hypocrea, including H. pallida. Hypocrea delicatula in contrast belongs to Hypocrea, based on gene sequences and a verticillium-like (Trichoderma sect. Hypocreanum) anamorph (W. Jaklitsch, unpubl) and will be treated elsewhere. Overton et al (2006b) epitypified Protocrea, but as we will demonstrate this epitypification was based on misidentified material and must be overturned.
Another common species similar to P. farinosa, producing perithecia in a subiculum and a Gliocladium anamorph, and occurring on polypores is Hypocrea pallida. Based on LSU ribosomal DNA sequences, the species was found to fall outside Hypocrea in the Hypocreaceae, but no generic redisposition has been suggested for it (Rehner and Samuels 1994, Põldmaa et al 1999, Põldmaa 2000).
The main objectives of this study are (i) the correct interpretation, conceptual definition and description of the genus Protocrea and its type species P. farinosa, (ii) to determine the phylogenetic position of Protocrea and ‘Hypocrea’ pallida and (iii) to describe the fungus interpreted as Hypocrea farinosa by recent authors as a new species of Hypocrea.
MATERIALS AND METHODS
Isolates and specimens
Isolates and GenBank accession numbers for ITS, LSU, rpb2 and tef1 sequences in this study are listed (Table I). Isolates given as C.P.K. (Kubicek) are those maintained in the collection of the Institute of Chemical Engineering, Vienna University of Technology, those listed as G.J.S. are those maintained at the USDA-ARS, Systematic Mycology and Microbiology Laboratory, Beltsville, Maryland, and those given as TFC are maintained at the University of Life Sciences, Tartu, Estonia. Representative isolates have been deposited at the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS). Specimens were deposited in the Herbarium of the Institute of Botany, University of Vienna, Austria (WU), in the U.S. National Fungus Collections, Beltsville, Maryland (BPI), or the Estonian University of Life Sciences, Tartu, Estonia (TAA).
Table I.
Isolates and accession numbers for ITS, rpb2, tef1, and LSU sequences used in this study. All sequences with accession numbers starting with EU7 were obtained and submitted in this study, all others retrieved from GenBank
| Name | Strain | ITS | rpb2 | tef1 | LSU |
|---|---|---|---|---|---|
| Aphysiostroma stercorarium | ATCC 62321 | EF469103 | AF543792 | ||
| Arachnocrea stipata | TFC 97-43 | EU710770 | AF160227 | ||
| Cladobotryum cubitense | G. Arnold i1361 | EU710771 | AJ583470 | ||
| Cordyceps militaris | OSU 93623 | AY545732 | AF327374 | ||
| Hypocrea americana | G.J.S. 92-93 | DQ835455 | |||
| Hc. americana | AFTOL-ID 52 | AY544649 | |||
| Hc. avellanea | C.T.R. 77-155 | AF545562 | EU710767 | ||
| Hc. decipiens | G.J.S. 96-281 | EU716330 | |||
| Hc. decipiens | G.J.S. 91-101 | DQ835520 | |||
| Hc. lutea | G.J.S. 89-129 | AF545517 | U00739 | ||
| Hc. rufa | CBS 119325 | EU711362 | |||
| Hc. rufa | G.J.S. 89-127 | AY489726 | |||
| Hc. virens | G.J.S. 01-287 | EU341804 | |||
| Hc. virens | CBS 249.59 | AF399252 | |||
| Hypomyces chrysospermus | TFC 93-63 | EU710772 | AF160233 | ||
| Hp. lactifluorum | TAA 171006 | EU710773 | |||
| Hp. lactifluorum | TAA 170476 | EU710768 | |||
| Hp. rosellus | TFC 95-105 | EU710774 | |||
| Hp. rosellus | TFC 96-159 | AF160242 | |||
| Hp. semitranslucens | TFC 03-23 | EU710775 | |||
| Hp. semitranslucens | TFC 96-35 | AJ459303 | |||
| Hp. stephanomatis | G.J.S. 88-50 | AF545566 | AF160243 | ||
| Hp. subiculosus | TFC 97-166 | EU710776 | |||
| Hp. subiculosus | TFC 07-19 | AM779861 | |||
| Hp. tremellicola | TFC 97-50 | EU710777 | |||
| Hp. tremellicola | G.J.S. 90-36 | HTU17427 | |||
| Protocrea farinosa | CBS 139.92 | EU703919 | EU703934 | EU703885 | |
| P. farinosa | C.P.K. 2408 | EU703887 | |||
| P. farinosa | C.P.K. 2416 | EU703888 | |||
| P. farinosa | CBS 121551 | EU703910 | EU703935 | EU703889 | |
| P. farinosa | C.P.K. 2453 | EU703911 | EU703936 | EU703890 | |
| P. farinosa | C.P.K. 2459 | EU703912 | |||
| P. farinosa | CBS 121554 | EU703913 | EU703942 | ||
| P. farinosa | C.P.K. 2468 | EU703891 | |||
| P. farinosa | C.P.K. 2472 | EU703914 | EU703892 | ||
| P. farinosa | C.P.K. 2491 | EU703915 | |||
| P. farinosa | C.P.K. 2853 | EU703916 | |||
| P. farinosa | C.P.K. 2866 | EU703893 | |||
| P. farinosa | C.P.K. 3144 | EU703917 | EU703938 | EU703894 | |
| P. farinosa | C.P.K. 3146 | EU703918 | EU703939 | ||
| P. farinosa | TFC 96-85 | EU703920 | EU703937 | EU703895 | |
| P. farinosa | TFC 97-168 | EU703921 | EU703941 | EU703896 | EU754022 |
| P. farinosa | TFC 06-23 | EU703909 | EU703940 | EU703886 | |
| Protocrea pallida | CBS 121552 | EU703922 | EU703944 | EU703897 | |
| P. pallida | CBS 120648 | EU703923 | EU703946 | EU703898 | |
| P. pallida | CBS 298.78 | EU703924 | EU703947 | EU703899 | |
| P. pallida | CBS 299.78 | EU703925 | EU703948 | EU703900 | |
| P. pallida | G.J.S. 90-27 | EU703926 | EU703950 | EU703901 | |
| P. pallida | TFC 99-209 | EU703927 | EU703949 | EU703902 | EU710769 |
| P. pallida | TFC 99-238 | EU703928 | EU703945 | EU703903 | |
| P. pallida | G.J.S. 89-83 | AY015636 | |||
| Protocrea illinoënsis | G.J.S. 94-54 | EU703929 | EU703951 | EU703904 | |
| P. illinoënsis | TFC 96-98 | EU703930 | EU703952 | EU703905 | |
| Protocrea sp. 1 | TFC 06-24 | EU703931 | EU703943 | EU703906 | |
| Protocrea sp. 2 | G.J.S. 95-193 | EU703932 | EU703953 | EU703907 | |
| Protocrea sp. 3 | TFC 99-217 | EU703933 | EU703954 | EU703908 | |
| Sphaerostilbella aureonitens | TFC 96-77 | EU710778 | AF160246 | ||
| Sph. berkeleyana | CBS 102308 | DQ522465 | U00756 | ||
| Sph. broomeana | TFC 03-25 | EU710779 | |||
| Sph. broomeana | TFC 97-11 | AF160231 | |||
| Sporophagomyces chrysostomus | TFC 97-192 | EU710780 | |||
| Sp. chrysostomus | TFC 96-192 | AF160234 | |||
| Simplicillium lamellicola | CBS 116.25 | DQ522462 | AF339552 | ||
| Torrubiella wallacei | CBS 101237 | EF469119 | AY184967 |
Single-ascospore isolates (C.P.K., CBS strains) were prepared from fresh specimens of Hypocrea stromata as described by Jaklitsch et al (2006), or a mass of ascospores was isolated onto 2% MEA (TFC strains). Cultures designated G.J.S. were isolated with the use of a micromanipulator on cornmeal agar (Difco) supplemented with 2% dextrose (CMD).
Growth characterization
Strains were cultivated on CMD (cornmeal agar, Sigma, St Louis, Missouri) supplemented with 2% (w/v) dextrose), PDA (potato-dextrose agar, Merck, Darmstadt, Germany) and 2% MEA (malt extract agar, Merck), all in distilled water. Growth took place at 25 C (alternating 12 h cool white fluorescent light and 12 h darkness).
Morphological observations
Structures of the anamorph were examined, measured and photographed on a compound microscope from cultures grown on CMD or PDA at 25 C on the plates under low magnification and after mounting in 3% KOH. Dry stromata were rehydrated overnight by water vapor in a TLC chamber at room temperature, imbedded in Tissue-Tek O.C.T. Compound 4583 (Sakura Finetek Europe B.V., Zoeterwoude, The Netherlands) and sectioned 12 μm thick with a freezing microtome. Sections were measured and photographed in lactic acid, asci and ascospores in 3% KOH. Measurements of asci, ascospores and anamorph characters are reported as maxima and minima in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses. Nomarski differential interference contrast (DIC) was used for observations and measurements. Colors were determined and cited according to Kornerup and Wanscher (1981).
DNA extraction, PCR amplifications and sequencing
Mycelium for DNA extraction was grown on MEA covered by sterile cellophane. Genomic DNA was extracted with the Plant DNeasy Minikit (QIAgen GmbH, Hilden, Germany) according to the manufacturer’s instructions. A region of nuclear rDNA, containing the ITS1 and 2 regions, was amplified by PCR with the primer combinations SR6R and LR1 (White et al 1990) or with ITS4 (White et al 1990) and ITS1F (Gardes and Bruns 1993). LSU rDNA was amplified with the latter primer in combination with LR5 (http://www.biology.duke.edu/fungi/mycolab/primers.htm). A 1.3 kb fragment of the tef1 gene encoding translation elongation factor 1 alpha was amplified with the primer pair EF1728F and TEF1LLErev (Jaklitsch et al 2005, 2006). This fragment includes the fourth and the fifth introns and a part of the last large exon. A 0.9 kb fragment of RNA polymerase II subunit B (rpb2) was amplified with the primer pair fRPB2-5f and fRPB2-7cr (Liu et al 1999). PCR products either were purified with the QIAquick Kit (QIAGEN) according to the manufacturer’s instructions or with an enzymatic PCR cleanup (Werle et al 1994). For the latter 20 μL PCR reactions were digested with 10 u exonuclease I (Fermentas, St Leon-Rot, BRD) and 2 u calf intestine alkaline phosphatase (Fermentas) for 45 min at 37 C, followed by an enzyme deactivation step at 85 C for 15 min. DNA was cycle-sequenced with the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington) with the same primers as in PCR, or with ITS4 and ITS5 (White et al 1990) for ITS, or with the internal primers 5′-CCGTGA(T/C)TT CATCAAGAACATG-3 and 5′-TTGGCAGTGTCCATCTT GTTG-3′ for tef1, and an automated DNA sequencer (ABI Genetic Analyzers, Applied Biosystems).
Molecular phylogenetic analyses
Sequence fragments were analyzed and assembled with SeqMan Pro (Lasergene, DNASTAR, Madison, Wisconsin) or Sequencher 4.7 (Gene Codes, Ann Arbor, Michigan). DNA sequences were submitted to GenBank. Alignments were performed with MAFFT v 6.240 (Katoh et al 2005), followed by manual adjustments with Genedoc 2.6 (Nicholas et al 1997).
Maximum parsimony (MP) analyses were conducted in PAUP* 4.0b10 (Swofford 2002) with 10000 heuristic searches with random taxon addition sequences and tbr branch swapping. The confidence of branching was assessed with bootstrap resampling (bs): 1000 replicates, each with 100 random taxon addition sequences. Gaps were treated as missing data.
Bayesian inference of phylogeny was performed with LSU rDNA and rpb2 sequences of 25 members of the Hypocreaceae and with the combined dataset of 19 Protocrea sequences using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001, Ronquist and Huelsenbeck 2003). In both cases the GTR + I + G DNA substitution model chosen by the hLRT information criterion of the program MrModeltest 2.2 (Nylander 2004) was applied. The Markov chains Monte Carlo (MCMC) were run with 4 000 000 generations, applying default values of other prior settings. The first 400 000 generations without reaching a stable likelihood score were discarded, leaving residual trees for computing the consensus trees and Bayesian posterior probability (pp) values.
Analyses were conducted separately with different numbers of Protocrea sequences obtained in this study for ITS rDNA, rpb2 and tef1. For 19 isolates all three genes were sequenced; these were combined into one matrix that was subjected to MP and Bayesian analyses. A separate analysis on generic relationships involved LSU rDNA and rpb2 sequences, 11 and 3 of which respectively were obtained in this study (Table I).
RESULTS
Morphological findings and observations
Examination of the holotype of Hypocrea farinosa and of 20 freshly collected European specimens revealed that P. farinosa occurs on resupinate to semipileate polypores (mainly Skeletocutis, rarely Bjerkandera, ?Trametes). Perithecia are typically gregarious, immersed in a white to yellowish KOH-negative mycelium (subiculum), without any trace of a pseudoparenchymatous development. No anamorph is present on the holotype specimen but a Gliocladium anamorph formed in cultures derived from fresh collections. The holotype specimens of Hypocrea tomentosa Berk. and H. nebulosa Massee, both from Tasmania, are morphologically indistinguishable from the holotype of H. farinosa and are regarded as putative synonyms.
Doi (1972) identified a wood-inhabiting fungus collected in Japan as Protocrea farinosa. According to his description, the perithecia are immersed in a subiculum. This fungus formed an acremonium (cephalosporium)-like anamorph. No fungal host was indicated. This has become the concept of Protocrea farinosa. Two additional species with similar traits were described, P. latissima Mercuri & Ranalli (1976) and P. seminuda Yoshim. Doi (1978).
Overton et al (2006b) identified specimens from the USA and France as P. farinosa, following Doi’s (1972) concept, but did not examine Doi’s material. They epitypified the species with a specimen from France (BPI 747356, culture G.J.S. 97-207) and cited several additional collections from Canada, Japan and the USA. Their molecular phylogenetic analysis was based on rpb2 and ‘LEtef1’ (large exon of tef1) sequences of three strains, but they did not include sequences of the epitype strain nor did they deposit a culture of the latter in an international culture collection. We sequenced tef1 introns 4 and 5 of the proposed epitype collection, G.J.S. 97-207 (subsequently deposited by the current authors as CBS 121307). Its tef1 intron 4 sequence is nearly identical to that deposited by Overton et al for G.J.S. 91-101, thus confirming conspecificity of the French strain with the American isolates cited by Overton et al (2006b). The ITS sequences of all isolates identified by them as H. farinosa, including G.J.S. 97-207, and the additional collection G.J.S. 96-281, obtained in this study, are identical. Their molecular-phylogenetic analysis placed the species in Hypocrea among species that have anamorphs in Trichoderma sect. Hypocreanum Bissett [Samuels et al (2006), Citrina and Megalocitrina Clades of Jaklitsch et al (2006)]. Thus Protocrea was placed in synonymy with Hypocrea.
The specimen selected by Overton et al (2006b) as epitype (BPI 747356) is not conspecific with the holotype of Hypocrea farinosa. This specimen does not occur on a polypore; it has a thin pseudoparenchymatous stroma below perithecia, and its anamorph is acremonium-verticillium-like, not Gliocladium. The specimens misidentified as H. farinosa differ from Protocrea farinosa in having a light yellow to light brown, thin, extensive pseudoparenchymatous subperithecial stroma, in a persistent orange-brown KOH reaction, which is not violaceous as in P. pallida, and in having an acremonium/verticillium-like anamorph. When the epitype was selected the holotype of H. farinosa was not studied; instead reference was made to Rossman et al (1999), and no reason was given for the need of an epitype or for the selection of that specimen. For these reasons we reject the epitypification of H. farinosa proposed by Overton et al (2006b). Overton et al (2006a, b) described several species having effused stromata and anamorphs in Trichoderma sect. Hypocreanum, including such well known species as H. pulvinata, H. citrina and H. sulphurea. However as was shown by Overton et al (2006b) this fungus is distinct from any of those species. It superficially resembles H. delicatula, but in H. delicatula the perithecia are formed in a subiculum, the ostioles are typically darker than the perithecium, ascospores are smaller and a distinct reaction in KOH is lacking. We conclude that the specimens characterized as H. farinosa by Overton et al (2006b) represent an undescribed species of Hypocrea, which we name below as H. decipiens. Hypocrea decipiens occurs on bark, on wood previously used for the cultivation of Lentinula edodes or its bag cultures, on Stereum spp., a Hymenochaete sp., and Phellinus gilvus.
Molecular phylogeny
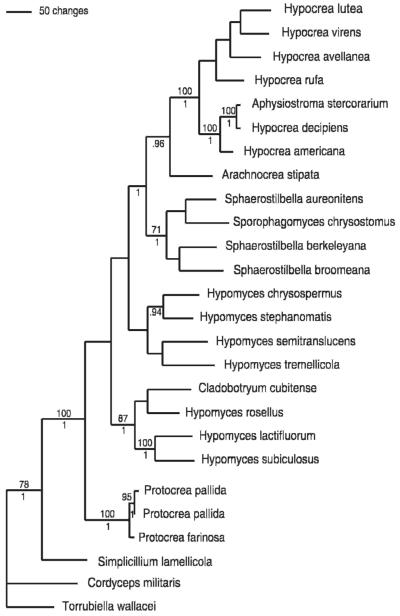
To infer the phylogenetic relationships of the species treated in this study within the phylogenetic framework of the Hypocreaceae we performed MP and Bayesian analyses including LSU rDNA and rpb2 sequences of representatives of the major genera. Three species of Cordycipitaceae were selected as outgroup based on recent, more wide-scale phylogenetic analyses (Sung et al 2007, Zhang et al 2006). The analyses of the matrix comprising 26 sequences and 1833 characters (458 informative) resulted in one most parsimonious tree (Fig. 1). The Bayesian tree (not shown), on which some of the terminal clades were unresolved, has the same topology as the MP tree. High support is assigned to the ingroup, while relationships of several of the larger clades remain ambiguous, especially in bootstrap analyses. The sequences of Protocrea farinosa and P. pallida form a strongly supported clade with unsupported sister group relationship to the rest of the ingroup. The monophyly of Hypocrea (including Aphysiostroma Barrasa et al) and the Sphaerostilbella (Henn.) Sacc. & D. Sacc - Sporophagomyces K. Põldmaa & Samuels clade however is well supported. Arachnocrea forms a sister group of the Hypocrea clade, these together appearing as the sister clade of Sphaerostilbella and Sporophagomyces. One of the two groups of Hypomyces (Fr.) Tul. species includes a clade of four agaricicolous and aphyllophoricolous species, comprising also the type species, H. lactifluorum, and a branch joining the boleticolous H. chrysospermus and H. stephanomatis, a parasite of the discomycete Humaria hemisphaerica (Pyronemataceae, Pezizales).
Fig. 1.
Phylogenetic relationships in the Hypocreaceae inferred from LSU rDNA and rpb2 sequences. The single most parsimonious tree (length = 2603, CI = 0.32, HI = 0.68) is shown respectively with bootstrap values ≥70% and Bayesian posterior probabilities ≥0.94 above and below the branches.
Delimitation of species in Protocrea along with revealing their phylogenetic relationships relied upon separate as well as combined analyses of ITS rDNA, tef1 and rpb2 sequences. The two strains G.J.S. 95-193 and TFC 99-217 (Protocrea spp.) observed as basal in the analyses of the more inclusive rpb2 dataset of Hypocreaceae (not shown), were used as outgroup in all four datasets.
The ITS1-5.8S-ITS2 region appeared to be the least informative of the three gene regions. Among the 25 isolates sequenced only 19 characters were informative, with the number dropping to 10 when excluding the most divergent strains treated as the outgroup with 100% bs support. The only supported subclade of the ingroup comprised the two European strains of P. pallida (CBS 120648 and CBS 121552) with TFC 06-24 as their unsupported sister group. The two strains, described below as P. illinoënsis, form a sister group of these three strains. It is noteworthy that the NCBI BLAST analysis for ITS sequences of Protocrea strains obtained in this study did not provide a single sequence of Hypocrea/Trichoderma in the list of best matches despite the numerous accessions of the latter in GenBank.
Partial sequences of the rpb2 gene, obtained for 22 strains formed a matrix of 1077 characters, 83 of which appeared to be informative. The MP search resulted in six equally parsimonious trees (CI 0.78). The matrix of the partial tef1 gene data comprising 23 sequences (only G.J.S. 95-193 used as outgroup) and 1290 characters (64 informative) yielded 12 trees (CI 0.8).
The two consensus trees (not shown) computed from both sets of trees agree in recognizing two well supported larger clades. One of these clades contains all strains of P. pallida included in this research. The structure of this group, partially unresolved in both cases, differs considerably. While most of the North American strains are joined into one clade based on rpb2 sequences, TFC 99-238 appears as the sister group of the moderately supported subclade of the two European strains (CBS 120648, CBS 121552) or basal to the whole pallida-clade. In the consensus of tef1 sequences however TFC 99-238 constitutes the sister group of the clade comprising North American strains with the exception of G.J.S. 90-27. Together with the two strains from Europe the latter forms the remaining unresolved part of the subclade.
In the consensus trees of rpb2 and tef1 datasets, all strains in addition to the outgroup and P. pallida form respectively the other larger clade with 65 and 74 bs support. The monophyly of the European strains recognized as P. farinosa is strongly supported (99% bs for the 9 rpb2 and 92% for 12 tef1 sequences). Their further subdivisions are difficult to compare due to different sets of strains sequenced for each gene but do not coincide with their geographical distribution patterns in either case. In both consensus trees the two strains from North America, described as the new species below, form a well supported clade (rpb2: 100% bs, tef1: 89% bs). Strain TFC 06-24 from Estonia is placed either as a sister group of the new species or of the larger clade including also P. farinosa, with low support in both cases.
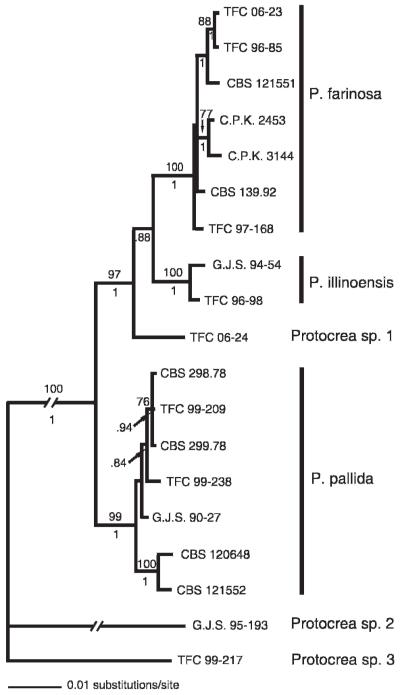
All three genes were sequenced for 19 strains and were combined into one dataset of 2878 characters out of which 163 were informative. The Bayesian analyses resulted in a tree with high posterior probability values assigned to most of the clades (Fig. 2). The topology of the consensus of 48 trees obtained with the maximum parsimony algorithm (not shown, CI 0.76) is in agreement with the Bayesian tree on which the respective bootstrap values are mapped; only some of the intermediate branches are collapsed on the former. Seven strains recognized as P. pallida form a strongly supported clade. While the branch of two European strains receives maximum support, the larger subclade comprising North American strains is much less supported. The residual strains analyzed fall into a well supported clade with TFC 06-24 as the basal lineage. The strongly supported clades of P. illinoënsis and P. farinosa reveal a moderately supported sister group relationship.
Fig. 2.
Phylogenetic relationships of members of Protocrea inferred from Bayesian analysis of a combined dataset of ITS rDNA, rpb2 and tef1 sequences. A 50% majority rule consensus tree is shown with bootstrap support values ≥70%, obtained respectively from a MP analysis, and Bayesian posterior probabilities ≥0.94 above and below the branches.
Separate and combined analyses of ITS, rpb2 and tef1 sequences clearly distinguish two collections from the USA (G.J.S. 95-193 and TFC 99-217) from the rest. Sequence variation between these two strains exceeds that observed among the other strains of Protocrea. Considering also their morphology, these two strains, used as outgroup in trees of Protocrea, are considered to represent two different species.
DELIMITATION OF SPECIES
Analyses of the three genes sequenced for various numbers of strains of Protocrea are generally in agreement with our morphological observations on species delimitation. The rpb2 and tef1 sequences provide good support for the recognition of six species, P. farinosa, P. illinoënsis and P. pallida as well as three discrete Protocrea species represented by TFC 06-24 (Protocrea sp. 1), G.J.S. 95-193 (Protocrea sp. 2) and TFC 99-217 (Protocrea sp. 3).
Morphological differences among Protocrea species characterized here are presented under notes for the respective species. In cultures of P. farinosa a yellow pigment is formed consistently on CMD, while cultures of P. pallida typically remain unpigmented on CMD or sometimes develop yellow spots only after several weeks. The shape of conidia in P. farinosa (on CMD) is more variable (with ellipsoidal often being predominant) than in P. pallida, where the oblong to suballantoid is predominant. Conidiophores are wider at their bases in P. farinosa than in P. pallida. On PDA much more Gliocladium heads and many fewer solitary phialides generally are formed in P. pallida than in P. farinosa. In P. illinoënsis pigmentation on CMD is inconsistent and conidia range from ellipsoidal to cylindrical but not suballantoid. Its conidiophores are twice as long as in P. farinosa and P. pallida, with walls often becoming swollen, forming an undulating sheath covering the stipe. The anamorphs of all the three species are morphologically similar to the type species of Gliocladium, G. penicillioides Corda, the anamorph of Sphaerostilbella aureonitens (Tul. & C. Tul.) Seifert, Samuels & W. Gams (Seifert 1985).
Collections TFC 06-24, G.J.S. 95-193 and TFC 99-217, each of which can be considered to represent a distinct species, are either too meager to serve as type specimens or do not provide morphological features for delimitation from other species recognized in Protocrea. Therefore we leave them unnamed in the hope that they will be recollected. Their teleomorphs are very similar to those of other Protocrea species described in this study. In G.J.S. 95-193 (USA, Indiana, on a resupinate polypore) and TFC 99-217 (USA, Maryland, on Skeletocutis cf. amorpha [Fr.] Kotl. & Pouzar) the teleomorph is scarce, with scattered perithecia as found in P. farinosa and P. illinoënsis and partly overmature or covered with other fungi. In TFC 06-24, collected in Estonia, perithecia form a continuous yellowish layer on basidiomata of Skeletocutis odora (Peck ex Sacc.) Ginns. A clear KOH reaction was observed only in G.J.S. 95-193. While cultures of TFC 06-24 do not differ from those of P. farinosa, those of the other two strains exhibit recognizable differences: G.J.S. 95-193 grows much more slowly on all media compared to all other strains cultured in this study. In TFC 99-217 the conidiophores of the Gliocladium anamorph were much less developed and it was the only strain to form abundant globose chlamydospores in a terminal position on lateral branches of submerged hyphae.
We did not observe any differences in morphology or colony characters between the American and European collections of P. pallida, despite their moderately supported segregation based on ITS, rpb2 and combined sequence data (Fig. 2). Insofar as the host has been determined most of the American specimens occur on Tyromyces chioneus (Fr.) P. Karst. while the European collections occur on Oligoporus (including Postia) spp., mainly O. tephroleucus (Fr.) Gilb. & Ryvarden and O. balsameus (Peck) Gilb. & Ryvarden. These genera are superficially similar, although separated phylogenetically (Yao et al 1999). Molecular and host data may point to a process of allopatric speciation that eventually might lead to the recognition of two distinct species. However T. chioneus also occurs in Europe and possibly future European collections on this host will permit alternative conclusions.
TAXONOMY
Protocrea Petch, J. Bot. 75:217-230. 1937.
Ascomata perithecial, immersed in or erumpent from a continuous or discontinuous, widely effuse subiculum on polypores. Asci containing 2-celled ascospores disarticulating at the septum into globose to oblong or wedge-shaped cells within asci. Anamorphs: Gliocladium Corda sensu stricto.
Type species: P. farinosa (Berk. & Broome) Petch (lectotype fide Moravec 1956)
KEY TO THE SPECIES OF protocrea:
| 1. Perithecia bright orange or yellow, immersed in a compact white to yellow or orange subiculum, KOH+ pink, violaceous to red; on soft and fleshy polypores, chiefly on Oligoporus and Tyromyces spp., presumably cosmopolitan . . . . . . . . . . . . . | P. pallida |
| 1. Perithecia usually not bright orange, scattered or embedded in a scarce and arachnoid or compact, cottony, white to yellowish subiculum, KOH+ or KOH−, on Skeletocutis spp.; Europe or USA. . . . . . | 2 |
| 2. Perithecia white, ivory, amber, luteous to olivaceous, KOH− . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 3 |
| 2. Perithecia amber to orange-yellow, KOH+ . . . . . . . | 4 |
| 3. Perithecia white, amber, luteous, olivaceous; on S. nivea, Europe . . . . . . . . . . . . . . . . | P. farinosa |
| 3. Perithecia ivory, on Skeletocutis sp., USA. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | Protocrea sp. 3 |
| 4. KOH reaction weak, pale purplish; on Skeletocutis odora, Europe . . . . . . . . . . . . . . . . . . . | Protocrea sp. 1 |
| 4. KOH+ distinctly purple to violaceous; USA. . . . . . . | 5 |
| 5. Perithecia mostly immersed in compact subiculum; on S. nivea . . . . . . . . . . . . . . . . . . . . . . | P. illinoënsis |
| 5. Perithecia scattered; on Skeletocutis sp. . | Protocrea sp. 2 |
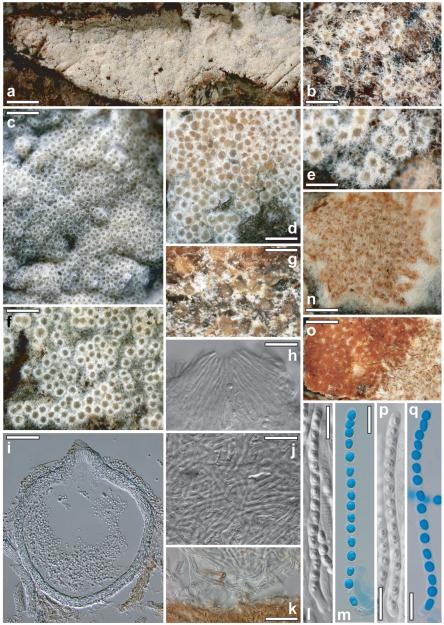
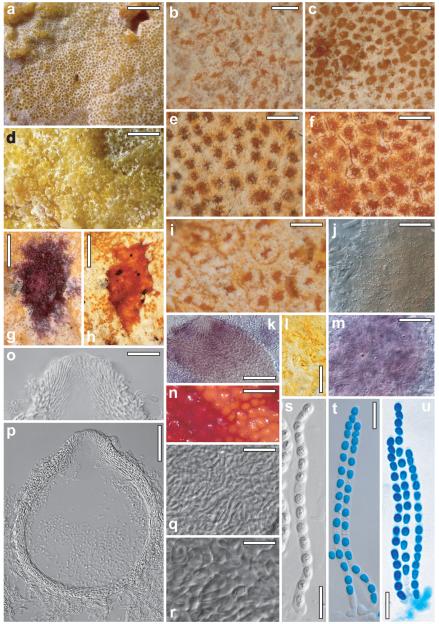
Protocrea farinosa (Berk. & Broome) Petch, J. Bot. 75:219. 1937. Figs. 3 a–m
Fig. 3.
Teleomorphs of Protocrea farinosa (a–m), Hypocrea nebulosa (g), H. decipiens (n–q). a. habit of fresh stroma on the hymenium of Skeletocutis nivea. b. Dry reduced stroma of holotype (scattered perithecia). c, d, f. Fresh stromata. e, g, n, o. Dry stromata (o. H. decipiens: orange perithecia in 3% KOH). h. Ostiolum. i. Perithecium in section. j. Subiculum hyphae on perithecial surface. k. Subiculum on host. l, m, p, q. Asci with ascospores (l, p. in KOH; m, q. in cotton blue/lactic acid). a, i, k. WU 28433; b. K(M) 48950; c. WU 28444; d, f. WU 28450; e, h, j. WU 28434; g. K(M) 126750; l. WU 28432; m. WU 28435; n–q. BPI 747356. Bars: a = 7 mm; b = 1 mm; c = 2 mm; d, f = 0.8 mm; e = 0.5 mm; g = 0.3 mm; h, j, k = 20 μm; i = 40 μm; l, m, p, q = 10 μm; n, o = 0.7 mm.
≡ Hypocrea farinosa Berk. & Broome, British Fungi n.692, Ann. Mag. Nat. Hist. Ser. 2, 7: 186. 1851
?= Hypocrea tomentosa Berk., Flora Tasmaniae II:278. 1859.
?= Hypocrea nebulosa Massee, Kew Bull. 1898:130. 1898.
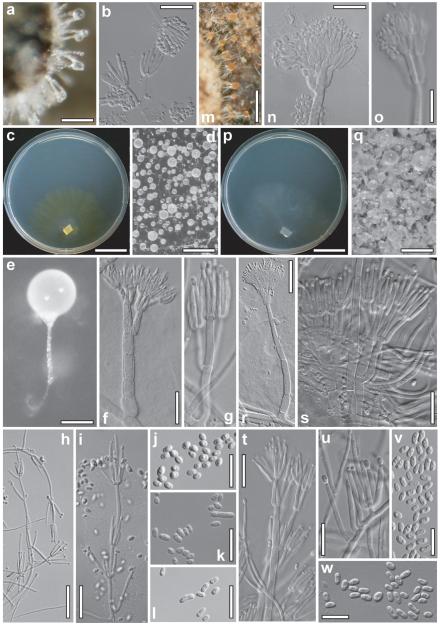
Anamorph: Gliocladium sp. Figs. 5a–l
Fig. 5.
Anamorphs of Protocrea farinosa (a–l) and P. pallida (m–w). a, b, m, n, o. Conidial heads and conidiophores on natural substrate. c, p. Colony on CMD (13d, 25 C). d, q. Conidial heads (d. on CMD, 20 d, 25 C; q. on PDA, 6 d, 25 C). e. Conidiophore with conidial head on CMD (20 d, 25 C). f, r. Conidiophores on CMD (25 C; f. 10 d, r. 7 d). g, h, i, s, t, u. Conidiophores on PDA (25 C; g, h, i. 8 d; s, t, u. 6 d). j, k, l, v, w. Conidia. a, b, C.P.K. 2408 (WU 28431); c, f, k. C.P.K. 3144 (WU 28445); d, e. C.P.K. 2853 (WU 28440); g, h, i. C.P.K. 3169 (WU 28450); j, l. CBS 121551 (WU 28433); m, n. Lectotype (Ellis 1880, NY); o. Ellis 1885 (FH 221.67); p, u, v. CBS 121552 (WU 28451); q, r, s, t, w. CBS 120648 (WU 28452). Bars: a = 80 μm; b, f, i, k, l, n, o, s, u = 15 μm; c, p = 22 mm; d = 0.3 mm; e = 50 μm; g, j, v, w = 10 μm; h = 35 μm; m, q = 0.2 mm; r = 25 μm; t = 20 μm.
Perithecial subiculum (‘stroma’) (2–)5–45(−83) × (1–)4–19(−35) mm (n = 40), 0.1–0.4 mm high, widely effused across hymenium of the host, dimensions depending on size of the polypore; typically numerous perithecia densely aggregated in a monostichous lawn, immersed in the more or less continuous, compact and well developed, white to yellowish, more rarely brownish to rust, subiculum with farinose surface, or perithecia scattered to gregarious and individually surrounded by a fringe of white to cream-yellowish hyphae (Fig. 3b, e, f, g), some free and glabrous in older material. Subiculum (Fig. 3j, k) generally well developed when young, reduced in older material, cottony or forming strands of hyaline hyphae (1.5–)2.0–3.0(−5.0) μm (n = 60) wide, loosely arranged around, below or less commonly, on perithecia; sometimes lacking in aged specimens. Perithecia little projecting (max ca. one-third free), loosely attached to subicular hyphae or situated directly on or within uppermost levels of the pores of the host, generally easily detachable. Perithecia sphaeroid, smooth, shiny, often appearing flat from above, collapsed discoidal when dry, sometimes laterally compressed when old, visible part (32–)60–155(−260) μm diam (n = 207); hyaline, amber, yellowish, pale ochraceous, light to dark olivaceous, ocher-brownish to rust, sometimes nearly black, sometimes nearly orange in the stereo microscope. Ostioles inconspicuous, central, broadly papillate to blunt and short conical, concolorous with perithecial body or slightly lighter. Overall color whitish, grayish-white, cream to yellowish, pale yellow to pale brownish, 3A2, 4A2–4, (4B4), sometimes dull brown-orange 5CD4 when older. Spore deposits white to yellowish. Perithecial subiculum after reconstitution in water pale, whitish to pale yellowish 2A2 to 3–4A2–3; unchanged or darkening and turning brown (determined by the brown surface of the host) in 3% KOH, but no distinct reaction noted, also yellow subiculum not reacting with KOH, perithecia becoming globose with conical ostioles. In section minute colorless to yellowish granules seen, unchanged in KOH. Ostioles (Fig. 3h) (27–)40–62(−66) μm long, projecting (5–)18–42(−48) μm, (11–)14–33(−47) μm (n = 38) wide at apex, of narrow cylindrical vertical elements, 1–3 μm wide, with convergent periphyses within. Perithecia (Fig. 3i) (130–)150–210(−248) μm high, (130–)150–230(−280) μm (n = 50) wide, height including ostioles (170–)190–240(−265) μm (n = 30), in section after reconstitution in water (sub)globose to slightly depressed, with at most a slight violaceous tint in KOH, but no discoloration seen. Peridium (10–)12–19(−26) μm (n = 99) wide at base and sides, directly merging with ostiolum in upper part, hyaline, inner and principal layer of tightly packed, thin, refractive, thick-walled, parallel or interwoven hyphae, outer layer of minute, more isodiametric cells. Asci (Fig. 3l, m) (50–)62–76(−91) × (3.0–)3.4–4.0(−5.0) μm, including a stipe (1–)4–13(−21) μm (n = 168) long, cylindrical, with 16 part-spores, apex thickened to 1.0–1.5 μm, often with a flat ring below thickening; stipe variable, base thickened or not. Ascospores hyaline, finely spinulose, cells dimorphic, but often with little difference in shape and size, 1 to several guttules per cell, distal cell (2.5–)3.0–3.5(−4.5) × (2.2–)2.5–3.0(−3.5) μm, l/w (0.9–)1.0–1.3(−1.5) (n = 270), subglobose to ellipsoidal (to oblong), proximal cell (2.8–)3.4–4.2 (−5.0) × (1.7–)2.2–2.6(−3.0) μm, l/w (1.1–)1.3–1.8(−2.2) (n = 270), oblong, ellipsoidal to wedge-shaped (to subglobose).
Holotype
Perithecial subiculum on a strongly decomposed resupinate polypore. Perithecial groups extending 2–45 × 1–12 mm; perithecia (Fig. 3b) solitary to gregarious, collapsed cupulate, yellowish to ocher, visible part 50–200(−260) μm diam, free and superficial, or partly immersed in loose, arachnoid, whitish to cream subiculum. No pseudoparenchymatous structures present. Asci and ascospores as in freshly collected material, some ascospores brown and collapsed.
Cultures and anamorph
On CMD (Fig. 5c) growth slow, colony radius typically less than 20 mm after 1 wk at 25 C, mycelium covering plate entirely after ca. 3–6 wk. Colony hyaline, more or less circular or margin becoming lobate, finely concentrically zonate, mycelium dense, of narrow hyphae, turning pale to bright yellow, 2–3A3–6, after ca. 1(−2) wk in fresh isolates, entirely, in the center or in irregular spots; surface becoming slightly farinose or downy and whitish by conidiation; no chlamydospores noted; odor none to slightly mushroomy after ca. 1 mo.
Conidiation starting after 3–7 d; after 7–10 d at 25 C effuse, colorless, typically first on aerial hyphae <1 mm long, with phialides solitary or divergent to parallel in small whorls in irregular configurations, forming minute conidial heads; followed by the formation of short, erect, mononematous Gliocladium conidiophores. Gliocladium conidiophores (Fig. 5f) discrete, loosely to densely disposed, 60–160 μm long (including phialides), erect, straight, simple, of a stipe (main axis) with 1 apical penicillus, the latter branched on 1–3(−4) levels; typically asymmetrically branched on the lowest level, symmetrically at 1–2(−3) points above, with (1–)4–6 branches on each level, bearing conidial heads on dense whorls of phialides. Stipe thick-walled (1.0–1.5 μm), smooth to verruculose, of 3–6 cells, to ca. 80 μm long, ca. 7–9 μm wide at the base, gradually attenuated upward, 4–5 μm below first branching level, branches ca. 3–5 μm wide, in narrow angles to nearly parallel, terminal branches including origins of phialides (1.5–)2.5–3.0(−3.5) μm wide; fourth level branch, if present, arising from a third level next to a whorl of phialides. Terminal branches each with 1 head of numerous, densely packed phialides and conidia; heads (Fig. 5d, e) globose, variable in size, to ca. 130 μm diam (confluent and eventually to ca. 250 μm diam after ca. 30 d), wet, mucous, colorless to milky white under stereomicroscope. Conidiophores sometimes branched at base. Phialides (4–)6–11(−15) × 1.5–2.0(−2.5) μm, (0.9–)1.0–1.7(−2.5) μm wide at base, l/w (2.7–)3.7–5.7(−8.3) (n = 90), to ca. 4–6 in minute heads, densely aggregated in large numbers in larger heads, parallel, typically narrowly lageniform, subulate to lanceolate, symmetrical, straight, inaequilateral and curved on sides of whorls. Conidia (Fig. 5j) (2.2–)2.5–3.5(−5.0) × (1.2–)1.7–2.5(−3.2) μm, l/w (1.1–)1.3–1.7(−2.2) (n = 90), hyaline, smooth, variable in shape, narrowly ellipsoidal, subglobose to oval, oblong to suballantoid, with 1 to several guttules, basal abscission scar indistinct or projecting and pointed.
On PDA mycelium covering Petri dish after ca. 3–5 wk; aerial hyphae frequent, forming strands, surface becoming white and downy to hairy, central surface granular, condensed; yellow pigment not formed or appearing around plug or in irregular spots. Conidiation (after 6–10 d at 25 C) effuse, loosely disposed or dense in varying areas, on simple, erect conidiophores (Fig. 5g–i) mostly ca. 60–100(−300) μm long, ca. 3–4 μm wide at base, terminally 1.5–2.0 μm wide, origins of phialides slightly thickened, 2.5–3.0 μm; with various combinations of phialides, solitary and in whorls of up to ca. 10, parallel or divergent on the same conidiophore; conidia forming wet, watery to milky white heads to ca. 100 μm diam. Side branches of conidiophores sometimes branched on 1–3 levels, each branch with terminal whorl of phialides. Phialides (Fig. 5g) (6–)11–26(−44) × (1.5–)1.7–2.1(−2.5) μm, l/w (3.5–)5–14(−31); (0.7–)1.2–2.0(−2.5) μm (n = 90) wide at base, typically long, narrow, subulate or widest slightly above base, straight, curved in lateral parts of whorls; a tendency of increased length noted when solitary. Conidia (Fig. 5k, l) (2.5–)3.2–6.7(−12.0) × (1.7–)2.0–3.0(−3.5) μm, l/w (1.3–)1.4–2.6(−4.6) (n = 93), hyaline, smooth, oblong, cylindrical to suballantoid or ellipsoidal, with fine guttules, scar indistinct or projecting and truncate; the smaller and more ellipsoidal conidia formed in whorls of phialides, longer and oblong to cylindrical on solitary phialides.
Anamorph on natural substrate (Fig. 5a, b); often, particularly in young stages, present in marginal areas of the perithecial subiculum, sometimes evenly scattered on its whole surface. Conidiophores unbranched, smooth, with a clavate conidial head ca. 15–35 μm long and wide at the apex. Phialides (8.5–)9.0–12.5(−14.0) × 1.5–2.0 μm, (0.7–)1.0–1.6(−2.0) μm wide at base, l/w 5.5–6.7 (n = 10), in dense terminal whorls to 6. Conidia (2.0–)2.5–4(−5.0) × (1.3–)1.4–2.2(−3.0) μm, l/w (1.3–)1.5–2.1(−2.4) (n = 30), hyaline, smooth, subglobose, ellipsoidal to oblong.
Habitat
on the hymenium of Skeletocutis spp., particularly S. nivea (Jungh.) Jean Keller, occasionally on other polypores (e.g. Bjerkandera, ?Trametes).
Known distribution
with certainty only known from Europe, collected in Austria, Denmark, Estonia, France, Germany, Netherlands, Slovenia, United Kingdom.
Holotype
UNITED KINGDOM. Northamptonshire: Milton, King’s Cliff, on a strongly decomposed, resupinate polypore, K(M) 48950. Epitype designated here to establish the correct relationship of teleomorph, anamorph, culture and gene sequences: AUSTRIA. Kärnten: Klagenfurt Land, St Margareten im Rosental, Drau-Auen, MTB 9452/1, elev. 410 m, 46°32′51″N, 14°24′31″E, on Skeletocutis nivea (on Alnus incana and Fraxinus excelsior), holomorph, 30 Oct 2005, H. Voglmayr and W. Jaklitsch, W.J. 2879 (WU 28433, culture CBS 121551 = C.P.K. 2429).
Other specimens examined
AUSTRIA. Burgenland: Mattersburg, Rosaliengebirge, Starenbühl, MTB 8264/3, elev. 350 m, 47°42′39″N, 16°22′55″E, on Skeletocutis nivea/Carpinus betulus, Protocrea parasitized by Nematogonum ferrugineum, 22 Sep 2007, W. Jaklitsch, W.J. 3169 (WU 28447, culture C.P.K. 3155). Kärnten: Klagenfurt Land, Lanzendorf, Sablatnig-Moor, MTB 9453/2, elev. 475 m, 46°34′12″N, 14°36′28″E, on Skeletocutis nivea/Fraxinus excelsior, polypore strongly decomposed, soc. coelomycete and black rhizomorphs, teleomorph, 24 Sep 2006, W. Jaklitsch and H. Voglmayr, W.J. 2984 (WU 28438, culture C.P.K. 2472). – St Margareten im Rosental, Sabosach, MTB 9452/3, elev. 550 m, 46°32′20″N, 14°24′33″E, on Skeletocutis nivea/Corylus avellana, teleomorph, 09 Jul 2007, W. Jaklitsch, W.J. 3115 (WU 28444). Niederösterreich: Hagenbrunn, Bisamberg, accessed from Wolfsbergen-Siedlung, MTB 7664/3, elev. 280 m, 48°19′17″N, 16°23′33″E, on Skeletocutis nivea/Carpinus, soc. hyphomycetes, 07 Oct 2007, W. Jaklitsch, W.J. 3178 (WU 28449). – Mauerbach, Friedhofstraße, MTB 7763/1, elev. 310 m, 48°15′26″N, 16°10′20″E, on Skeletocutis nivea/Carpinus betulus, soc. hyphomycetes, holomorph, 14 Oct 2007, W. Jaklitsch, W.J. 3183 (WU 28450, culture C.P.K. 3169). – Melk, Schönbühel-Aggsbach, Aggsteingraben, at walking path to the castle ruin Aggstein, MTB 7658/4, elev. 380 m, on Skeletocutis nivea/Fraxinus excelsior, soc. coelomycete and green Trichoderma sp., holomorph, 14 Oct 2006, H. Voglmayr and I. Krisai-Greilhuber, W.J. 3024 (WU 28439, culture C.P.K. 2491). – Mödling, Wienerwald, Wöglerin, MTB 7862/4, elev. 490 m, on Skeletocutis nivea/Fraxinus excelsior, soc. hyphomycetes, holomorph, 06 Oct 2007, H. Voglmayr and W. Jaklitsch, W.J. 3177 (WU 28448, culture C.P.K. 3166). Vienna: 2nd district, Prater, northeast from Lusthaus beside the church Maria Grün, elev. 155 m, 48°11′40″N, 16°26′32″E, on Skeletocutis nivea/Fraxinus excelsior, polypore old, soc. various hyphomycetes, holomorph, 08 Dec 2006, H. Voglmayr, W.J. 3057 (WU 28442, culture C.P.K. 2866). – 19th district, Himmelstraße, MTB 7763/2, elev. 350 m, 48°15′41″N, 16°19′11″E, on Skeletocutis nivea/Fraxinus excelsior, 18 Mar 2007, W. Jaklitsch, W.J. 3075 (WU 28443). – 22nd district, Lobau, at Panozzalacke, MTB 7865/1, elev. 150 m, 48°11′06″N, 16°29′20″E, on Skeletocutis nivea/Fraxinus excelsior, soc. hyphomycete, teleomorph, 18 Nov 2006, W. Jaklitsch, W.J. 3038 (WU 28440, culture C.P.K. 2853). Oberösterreich: Schärding, St Willibald, between Patrichsham und Unterholzen, MTB 7648/3, elev. 410 m, 48°21′00″N 13°41′14″E, on Skeletocutis nivea/Fraxinus excelsior, soc. hypho-, coelomycetes, holomorph, 02 Sep 2006, H. Voglmayr, W.J. 2967, (WU 28435, culture C.P.K. 2459). DENMARK. Lolland: Fuglsang Storskov, Lögnor, on Skeletocutis nivea/Fagus sylvatica, soc. hyphomycete, holomorph, 31 Jul 2005, T. Læssøe, TL-12208 (WU 28431, culture C.P.K. 2408). Sjælland: Jægersberg Dyrehave (N from Copenhagen), on Skeletocutis nivea/Fagus sylvatica, holomorph, 31 Aug 2005, T. Læssøe, TL 12356 (WU 28432, culture C.P.K. 2416). ESTONIA. Pärnumaa County: Nigula Nature Reserve, on Skeletocutis amorpha, 17 Sep 1996, K. Põldmaa (TAA 169600, culture TFC 96-85 = G.J.S. 96-248). Saaremaa County: Viidumäe Nature Reserve, on Skeletocutis nivea, 11 Aug 2006, K. Põldmaa (K.P. 18, culture TFC 06-23). Võrumaa County: Paganamaa, on Trametes versicolor (?), K. Põldmaa (TAA 169906, culture TFC 97-168). FRANCE. Ariège: Saint-Girons, Rimont, on Skeletocutis nivea, J. Fournier, 31 Nov 1998 (BPI 842454). GERMANY. Niedersachsen: Landkreis Goslar, Seesen, Kurpark, elev. 240 m, 51°53′02″N, 10°12′33″E, on Skeletocutis nivea/Fraxinus excelsior, soc. hyphomycetes, 27 Aug 2006, W. Jaklitsch and H. Voglmayr, W.J. 2954 (WU 28434, culture C.P.K. 2453). NETHERLANDS. Utrecht, De Uithof, small mixed deciduous forest between sport park Olympos, botanical garden and motorway crossing Rijnsweerd, elev. 0 m, on Skeletocutis nivea, soc. coelomycete, holomorph, 18 Nov 2006, H. Voglmayr, W.J. 3045 (WU 28441). SLOVENIA. Vipava: Mount Nanos massif, Sanabor, close to the village at the roadside, elev. 560 m, 45°51′23″N 13°59′21″E, on Skeletocutis nivea/Corylus avellana, soc. Hyphodontia sp., hyphomycetes, green Trichoderma, teleomorph, 23 Sep 2006, W. Jaklitsch and H. Voglmayr, W.J. 2975 (WU 28436, culture CBS 121554 = C.P.K. 2467). Logatec, close to the motorway exit, elev. 480 m, 45°54′35″N 14°15′02″E, on Skeletocutis nivea/Corylus avellana, soc. various fungi, holomorph (little anamorph), 23 Sep 2006, H. Voglmayr and W. Jaklitsch, W.J. 2979 (WU 28437, culture, C.P.K. 2468). UNITED KINGDOM. Leicestershire: Stamford, Tixover, elev. ca. 50 m, 52°35′48″N, 0°34′28″W, on Skeletocutis nivea/Fraxinus excelsior, soc. Mycoacia uda, teleomorph, 08 Sep 2007, W. Jaklitsch, W.J. 3144 (WU 28445, culture C.P.K. 3144). Warwickshire: Stratford-on-Avon, Bannam’s Wood, elev. ca. 90 m, 52°16′27″N, 1°50′13″W, on Skeletocutis nivea/Crataegus monogyna, holomorph, 10 Sep 2007, W. Jaklitsch, W.J. 3149 (WU 28446, culture C.P.K. 3146).
Notes
P. farinosa is easily recognizable on its typical host, Skeletocutis nivea. This species is common in Europe, although inconspicuous due to the perithecial color varying from white, over luteous to dark olivaceous.
The next two species, described from Tasmania, Australia, are morphologically indistinguishable from P. farinosa. A description of the holotypes is provided:
Hypocrea nebulosa Massee, Kew Bull. 1898:130. 1898.
Holotype
AUSTRALIA. Tasmania: locality not known, on a semiresupinate polypore, holomorph, L. Rodway 494 (although Rodway 484, probably erroneously, cited in the protologue) (K[M] 126750!). Little material, of one part with continuous white to yellowish subiculum with immature perithecia entirely immersed, pale amber to ocher. Second part (Fig. 3 g) of densely aggregated, collapsed, flat, discoidal perithecia, pale ocher to brown with olivaceous tones, appearing gelatinous, surrounded by fringes of whitish mycelium. Visible part of perithecia (80–)115–205(−236) μm diam (n = 18) in face view. Peridium hyaline in KOH, hyphal to coarsely pseudoparenchymatous in face view. Ostioles (15–)16–30(−39) μm (n = 20) in face view, inconspicuous to papillate or pointed, concolorous with perithecial body. Subicular hyphae (2–)2.5–4.5(−6) μm (n = 30), hyaline, KOH−. Asci (70–)75–98(−107) × (4.0–)4.2–4.7(−5) μm, including a stipe (6–)9–20(−24) μm (n = 10) long, cylindrical, apex with a flat ring and thickened to ca. 1 μm, base thickened. Ascospores hyaline, finely spinulose, cells dimorphic, distal cell (3.0–)3.2–3.8(−4.0) × 2.5–3.2(−3.7) μm, l/w (1.0–)1.1–1.3(−1.4) (n = 30), (sub)globose to ellipsoidal, proximal cell (3.3–)3.5–4.3(−4.8) × (2.0–)2.4–3.0(−3.2) μm, l/w (1.2–)1.4–1.7(−2.1) (n = 30), oblong, ellipsoidal to nearly wedge-shaped. Both parts KOH−; no anamorph seen.
Hypocrea tomentosa Fries in Berkeley, Hooker’s Fl. Tasman. 2:278. 1860 [16 Aug 1859].
Holotype
AUSTRALIA. Tasmania: Fl. Tasman. 2:273. 1860 (K, herb. Berk.!)
Teleomorph effuse, of numerous crowded, off white to pale luteous perithecia in or on a white subiculum of smooth, thin-walled hyphae ca. 3 μm wide. Perithecia easily removed from the subiculum, collapsed cupulate, 350–375 μm diam (in crush mounts), with a small central ostiolar papilla. Peridium smooth, of intertwined hyphae in face view, ca. 15 μm wide in section, of compressed, ellipsoidal to fusoid cells ca. 5 × 2 μm. No thick-walled or clavate elements seen in the ostiolum. Asci cylindrical, 55–72 × 3.2–5.2 μm (n = 23). Apex slightly thickened, with a ring. Ascospores hyaline, spinulose, cells dimorphic; distal cell (2.5–)3.0–4.0(−4.5) × (2.2–)2.5–3.2(−3.7) μm, subglobose, proximal cell (3.0–)3.2–4.0(−4.5) × (1.7–)2.0–2.5(−4.0) μm, wedge-shaped. Ascospore deposits white. No reaction to KOH seen.
Notes
Whether H. nebulosa and H. tomentosa are in fact synonyms of P. farinosa can be ascertained only by material freshly collected in Tasmania. Anamorphs and gene sequences are necessary to solve this question.
The second species recognized in the genus Protocrea is P. pallida, described below:
Protocrea pallida (Ellis & Everh.) Jaklitsch, K. Põldmaa & Samuels, comb. nov.
MycoBank MB 512131 Figs. 4a–u
Fig. 4.
Teleomorph of Protocrea pallida. a, d. Fresh stroma on the hymenium of Oligoporus tephroleucus. b, c, e, f, i. Dry stromata. g, h, k, m, n. Reactions in 3% KOH (g. fresh stroma. h. dry stroma, k. microscopic, perithecium, m. microscopic, subiculum, n. after reconstitution of dry stroma in water). j. granules in subiculum. l. subiculum in cotton blue/lactic acid after treatment with 3% KOH. o. Ostiole. p. Perithecium in section. q, r. Peridium in face view (q. partly covered by subiculum hyphae). s, t, u. Asci with ascospores (s. in KOH; t, u. in cotton blue/lactic acid). a, c, d, g, h, l, m, n, o, p, t. WU 28451; b, j, q, r, s. Lectotype (Ellis 1880, NY); e, k, l. Epitype C.T.R. 67-51 (NY); f, u. holotype of Hypocrea aurantiaca (NY). i. G.J.S. 89-83. Bars: a = 3 mm; b, c, e, f, i, n = 0.5 mm; d = 1.3 mm; g, h = 1.7 mm; j, o = 20 μm; k = 40 μm; l, r–u = 10 μm; m, q = 15 μm; p = 50 μm.
≡ Hypocrea pallida Ellis & Everh., J. Mycol. 1886:65. 1886.
= Hypocrea aurantiaca Peck, Ann. Rep. New York State Mus. 51:295. 1898.
Anamorph: Gliocladium sp. Figs. 5m–w
Perithecial subiculum 2–62 × 1–28 mm (n = 22), 0.15–0.5 mm thick, size dependent on the host, indeterminate, without a defined margin, effuse, forming diverse patches or covering entire hymenium of the host, with numerous, densely crowded, less commonly widely spaced, orange perithecia immersed in a single layer in the subiculum. Perithecia slightly (to ca. one-third) projecting, rarely free, yellow to orange when fresh (Fig. 4a, d), bright orange (bright yellow, ochraceous, orange-brown) when dry (Fig. 4b, c, e, f, i), shiny, globose and ca. 140–315 μm diam in face view when fresh; when dry typically collapsed cupulate, with thick margins sometimes surrounded by separated ring-like subiculum, sometimes laterally collapsed; sometimes translucent through subiculum; visible part (40–)90–185(−250) μm diam (n = 205) in face view. Ostioles invisible or inconspicuous, (8–)13–40(−87) μm diam (n = 143) in face view when dry, central, papillate, apex rounded or acute conical, outline circular or laterally compressed, concolorous with perithecial body, shiny. Surface hairy, floccose to cottony when young, later farinose, finely granulose by slightly projecting perithecia, or tubercular to irregular depending on the surface of the polypore hymenium. Subiculum generally well developed and compact when young, white to yellow or orange, often reduced to lacking in older or overmature material, sometimes present on upper side of polypore. Overall color yellowish, orange to golden yellow 5A–B4–7 to more yellow or grayish-orange 6A–B5–6 when fresh, when dry whitish to pale yellowish or pale orange when young (subiculum well developed), later yellow to (grayish-)orange (subiculum reduced or itself yellow to orange), mostly 4A3–4(−6), 5A–B3–6, more intense and darker in certain specimens, (5B6–8) to 6B5–6 (H. aurantiaca holotype), or even brown-red 8C–D6–8 (BPI 631554). Spore deposits white to yellowish. Sometimes (e.g. several specimens collected by C.T. Rogerson) only subiculum and anamorph formed (with or without few perithecia), the polypore stained yellow by the fungus. Hymenium of the host light colored, or yellow to orange, discolored by the Protocrea, sometimes dark due to other hyphomycetes.
Macroscopic reaction to 3% KOH in fresh (moist) material bright pink, magenta to violaceous (Fig. 4g), 14–15E6–8, red when dry (Fig. 4h). Microscopic reaction to 3% KOH (Fig. 4k, m) seen in all specimens examined, but only seen in at least partly yellow, not in pure white subiculum: bright violaceous spots formed in various areas of the subiculum and frequently appearing in apical parts of perithecia, apparently excreted by subicular hyphae, extracellular, dissolved and in part localized in minute globose granules, subiculum hyphae and peridia themselves remaining colorless. Distinct correlation noted between intensity of (yellow to orange) coloration of subiculum and intensity of violaceous reaction in KOH, also in specimens of yellow subiculum lacking perithecia. Violaceous spots turning bright yellow, and granules partly condensing to golden-yellow clumps between subiculum hyphae after addition of cotton blue/lactic acid (Fig. 4l).
Perithecial subiculum after reconstitution in water bright orange 5A–B5–8, perithecia larger, with little yellow subiculum in between; perithecia orange, sphaeroid with papillate to conical ostioles, after addition of 3% KOH subiculum immediately red (Fig. 4n) to pink, pigment soluble, pink to violaceous on white paper; spreading and darkening, perithecia and subiculum becoming indistinguishable.
In section generally masses of minute, hyaline to yellowish granules (Fig. 4j) seen. Ostioles (Fig. 4o) (38–)47–60(−65) μm (n = 30) long, projecting to (10–)16–27(−30) μm (n = 30), (23–)29–48(−70) μm (n = 30) diam at apex, conical, periphysate, of a palisade of narrow, cylindrical, hyaline cells ca. 1.5–3.0(−3.5) μm wide, convergent at apex, apex more or less flat, ostioles sometimes surrounded by free ends of subicular hyphae. Perithecia (Fig. 4p) (130–)165–218(−230) μm high, (125–)147–200(−225) μm (n = 30) diam, height including ostiole (150–)188–240(−250) μm (n = 30), sphaeroid to pyriform, gradually merging into ostioles. Peridium (Fig. 4q, r) (13–)16–21(−25) (n = 60) wide at base and sides, of hyaline, refractive, elongated, thick-walled, compressed cells inside, and minute more isodiametric cells outside, partly covered by hyphae, therefore hyphal to textura epidermoidea in face view. Subiculum hyphae (1.5–)2.0–4.0(−6.5) μm (n = 163) wide, hyaline, branched, walls to ca. 1 μm thick. Asci (Fig. 4s–u) (59–)70–86(−98) × (3.0–)3.7–4.3(−5.0) μm, including a stipe (2–)6–20(−33) μm (n = 190) long, cylindrical, fasciculate, apex truncate, thickened to 1.0–1.5 μm, with a minute pore, flat ring seen below thickening when young, base thickened, croziers present. Ascospores hyaline, verruculose, cells dimorphic, but often showing little difference, with 1 large to several minute guttules in each cell, distal cell (2.7–)3.0–3.7(−4.3) × (2.4–)2.7–3.2(−4.0) μm, l/w (0.9–)1.0–1.3(−1.6) (n = 267), (sub)globose to ellipsoidal (to nearly wedge-shaped), proximal cell (3.0–)3.5–4.3(−5.7) × (2.0–)2.4–3.0(−3.5) μm, l/w (1–)1.3–1.6(−2) (n = 267), oblong to plump wedge-shaped (to subglobose).
Cultures and anamorph
On CMD growth slow, colony radius less than 20 mm at 25 C after 1 wk, plate covered entirely by mycelium after >3 wk; mycelium dense, colony (Fig. 5p) hyaline, more or less circular, not zonate, colorless, surface becoming white and downy from long, narrow aerial hyphae; no odor, no chlamydospores, no pigment noted, sometimes yellow spots appearing late (after ca. 3 wk). Conidiation noted after ca. 3 d, effuse, of solitary plus fasciculate phialides on aerial hyphae to ca. 1.5 mm long and often numerous, short Gliocladium conidiophores (Fig. 5r) to 100–180 μm long with (often minute) heads to ca. 80 μm diam; sometimes larger (Fig. 5q). Gliocladium conidiophores to ca. 6 μm wide at base, gradually attenuated upward, simple, branched at 3 apical levels, frequently only 1 branch at lowest branching point, several at other branching points; terminal branches 2.0–2.5 μm wide, metulae ca. 3–4 μm wide, phialides solitary to densely packed and numerous in compact heads. Phialides (7–)8–13(−16) × (1.5–)1.7–2.2(−2.5) μm, (1.0–)1.2–1.7(−2.2) μm wide at base, l/w (3.2–)4.4–6.9(−9) (n = 60), lageniform to subulate. Conidia (Fig. 5v) (2.7–)3.0–4.2(−7.0) × 1.5–2.5(−3.2) μm, l/w (1.2–)1.5–2.3(−4.3) (n = 100), hyaline, smooth, oblong to (sub)allantoid, few ellipsoidal or subglobose, with 1 to several guttules, scar indistinct.
On PDA growth slow, typically colony radius less than 20 mm after 1 wk, plate covered by mycelium after 4 or more wk; colony circular, dense, surface becoming white, zonate or not, of a dense white mat of aerial hyphae with thick strands, margin hyaline, sometimes with central radial streaks due to extremely thick strands; no pigment formed, reverse at most pale yellowish; no odor noted. Conidiation effuse, scant or abundant, dependent on age of examined isolate. Conidiophores erect, originating more or less at right angles on surface hyphae, or on aerial hyphae, but phialides also solitary on aerial hyphae. Conidiophores (Fig. 5s–u) mostly to ca. 150(−220) μm long including phialides, with 1–3 apical branching points on a straight main axis, base to ca. 6–7 μm wide, tapering upward to ca. 3–4 μm below lowest branching point, typically asymmetric and of 1 branch 2–3 μm wide, parallel to main axis, further branching symmetric, with fasciculate branches; terminal branches typically 1-celled, 15–20 μm long and 1.5–2.0 μm wide; each branch with a whorl of phialides on slightly thickened ends, 2.5–3.5 μm wide. Phialides (Fig. 5s, u) solitary, often divergent in small whorls of ca. 3–6, or numerous, dense and parallel in large whorls. Conidial heads minute to large, wet, mucous, deliquescent, to 50–80 μm or 250–300 μm diam, depending on the isolate. Phialides (6–)10–21(−32) × (1.5–)1.8–2.2(−2.7) μm, (1.0–)1.3–1.8(−2.2) μm wide at base, l/w (3.6–)4.6–11(−20.2) (n = 100), subulate, straight or basally strongly curved, length apparently dependent on their number per whorl (shorter with increasing number). Conidia (Fig. 5w) (2.7–)3.0–6.2(−12.5) × (1.5–)2.0–2.5(−3.0) μm, l/w 1.4–2.8(−4.8) (n = 93), hyaline, smooth, mostly oblong to (sub)allantoid, variable fractions narrowly ellipsoidal (to subglobose), with several minute guttules, often a group close to each end; with a marked tendency to smaller size and ellipsoidal to subglobose shape when formed on dense whorls of phialides.
Anamorph on natural substrate (including lectotype) (Fig. 5m–o): present in most specimens on the margin of the teleomorph, around perithecia or in patches separated from the teleomorph, also on the upper side of basidiomata. Conidiophores to ca. 160 μm long, 5.0–6.5 μm wide at base, 4–4.5 below first branching; terverticillate, branches 2.0–2.5 μm wide, terminating in dense whorls of numerous parallel phialides. Conidial heads colorless to white (rarely yellow to orange), 30–60 μm diam. Phialides (5–)8–13(−16) × (1.5–)2.0–2.5(−3.0) μm, (1.0–)1.2–1.8(−2.2) μm wide at base, l/w (3–)3.6–5.6(−7.3) (n = 35), narrowly lageniform to subulate, straight or basally curved. Conidia (2.5–)2.8–4.0(−11) × (1.2–)1.5–2.2(−3) μm, l/w (1.3–)1.6–2.2(−3) (n = 93), hyaline, smooth, mostly oblong to allantoid.
Habitat
on basidiomata of Oligoporus spp. and Tyromyces spp., in Europe chiefly on O. tephroleucus and O. balsameus, in North America chiefly on T. chioneus, possibly also on other polypores.
Known distribution
Europe, North America and Japan (see Doi and Yamatoya 1989)
Lectotype chosen here: USA. New Jersey: Newfield, on a resupinate polypore, 18 Oct 1880, Harkness, det. J.B. Ellis (NY).
Epitype, designated here to establish the connection of teleomorph, anamorph, culture and gene sequences: USA. New York: Rockland County, woods along Stony Brook, east of Sloatsburg, Harriman State Park, on polypore, 4 Aug 1967, E. Yarrow and S. Stein, det. C.T. Rogerson C.T.R. 67-51 (NY, living culture CBS 299.78).
Other specimens examined
AUSTRIA. Oberösterreich: Kleines Kesselbachtal, W Wesenufer, MTB 7548/2, on Oligoporus sp., holomorph (perithecia scant), 2 Oct 1993, H. Voglmayr. DENMARK. Sjaelland: Sollerod Kirkeskov, on hymenium of Oligoporus tephroleucus on Fagus sylvatica, holomorph, 27 Oct 2005, A. Jørgensen, comm. T. Læssøe (WU 28451, culture CBS 121552 = C.P.K. 2432). CANADA. Prince Edward Island: on Tyromyces chioneus, teleomorph, J. Macoun, date unknown, confirmed by J.B. Ellis (NY, examined for North American Flora). ESTONIA. Jõgevamaa County, Alam-Pedja Nature Reserve, Altnurga, on Oligoporus sp. on Alnus glutinosa, anamorph, 08 Oct 1997, I. Parmasto (TAA 169075, culture TFC 97-129). FRANCE. Ariège: Rimont, on unidentifiable polypore/bark of Quercus sp., 14 Dec 2002, J. Fournier 02242. GERMANY. Baden Württemberg: Tübingen, Schönbuch, Eisenbachhain, on Oligoporus cf. balsameus on Fagus sylvatica, teleomorph, 03 Oct 2006, L. Beenken, det. W. Gams/J.A. Stalpers, W.J. 3053 (WU 28452, culture CBS 120648 = C.P.K. 2860). –, on the hymenium of Oligoporus cf. balsameus on Betula pendula, holomorph, 03 Oct 2006, P. Karasch, det. W. Gams/J.A. Stalpers, W.J. 3054 (WU 28453, culture CBS 120647 = C.P.K. 2861). UNITED KINGDOM. Hertfordshire: Welwyn Garden City, Sherrardspark Wood, on Oligoporus cf. tephroleucus on Betula pendula, holomorph, teleomorph immature, 12.09.2007, W. Jaklitsch, K. Robinson and H. Voglmayr, W.J. 3157 (WU 28454). USA. Connecticut: West Haven, on polypore, exceptionally strongly colored (reddish-brown) teleomorph, West R., Nov. 1888, det. Ellis, (NFC 197, BPI 631554). NSew Hampshire: Cheshire County, Campus Franklin Pierce College, Rindge, on Tyromyces chioneus (as Polyporus albellus), holomorph, 19 Aug 1987, A. Northrup, Northeast Foray Group, det G.J. Samuels (NY; culture G.J.S. 89-83). New Jersey: Essex County, 2 miles east of Lewis, on Tyromyces chioneus (as Polyporus albellus), anamorph and few perithecia, 8 Sep 1967, C.T. Rogerson and S.J. Smith (NY). – Gloucester County, Newfield, on a resupinate polypore stated as Trametes nivosa, teleomorph, 18 Oct 1883, J.B. Ellis (NY). – Newfield, on old polypore, possibly Tyromyces, holomorph, Oct. 1885, J.B. Ellis (herb W.G. Farlow 221.67 (ex herb. Ellis)). – Newfield, on polypore, in 2 parts (part 1: teleomorph; part 2: identity unclear); det. Ellis, 4 May 1890 (NY). New York: – locality unknown, R. Lowen, 1999 (TAA 170370; culture TFC 99-238). Twin Valleys Camp, Plattsburg State College, near Wadhams, on Tyromyces chioneus, mainly anamorph plus white to yellowish subiculum and few perithecia, 9 Sep 1967, C.T. Rogerson, S.C. Canham and S.J. Smith (NY). Saratoga County, Gansevoort, on Tyromyces chioneus, teleomorph, Jul 1897, C.H. Peck, holotype of Hypocrea aurantiaca Peck (NY). – Schenectady County, Featherstonhaugh Lake State Park, vic. Mariaville, on Tyromyces chioneus, only anamorph, mycelium with yellow to orange color, 22 Oct 1969, C.T. Rogerson, S.J. Smith and J. Haines, C.T.R. 69-224 (NY).– Suffolk County, woods north of Manorville, on Tyromyces chioneus; anamorph and mycelium, 5 Oct 1967, C.T. Rogerson, S.J. Smith and E.M. Reilly (NY). – Oneigo County, Cleaveland, Widrig road, 2 Oct 1999, K. Põldmaa (TAA 170286; culture TFC 99-209). Wisconsin: Sauk County, Baxter’s Hollow Nature Conservancy, on T. chioneus on Betula papyrifera, 22 Sep 1990, T. Volk (BPI 1107149; culture G.J.S. 90-27).
Comments
While P. farinosa has been misinterpreted during its long history, P. pallida has been recognized unequivocally since its establishment. This species originally was described from the USA as Hypocrea pallida. In the protolog, published Jun 1886, J.B. Ellis cited two specimens, collected in Oct 1880 and Oct 1886. The first is the oldest mentioned and the specimen in best agreement with the description in the protolog (i.e. pallid). It therefore is selected as lectotype. The date of the second specimen is obviously wrong and probably addresses the specimen collected in Oct 1885, preserved in FH.
Generally “stromata” of P. pallida are more conspicuous than those of P. farinosa due to the brightly colored perithecia in the former. The second, most prominent distinguishing trait is the violaceous KOH reaction of the yellow pigment excreted by hyphae of P. pallida, staining the host basidiomata yellow. The subiculum is generally better developed in P. pallida, while perithecia of P. farinosa often emerge directly from the pores of the host. In P. farinosa also the occurrence of solitary perithecia is not uncommon and pronounced in specimens on old and torn host basidiomata, and then often lacking an anamorph, while this is practically unknown in P. pallida.
The lectotype of Hypocrea pallida is characterized by a pallid effuse fungus with well developed white subiculum with gregarious to crowded, bright orange perithecia, collapsed from top or sides, immersed in it. Ostioles invisible or papillate and concolorous with perithecium. Peridium hyphal to cellular in face view, slightly yellowish in KOH. Subiculum thin, translucent, white, farinose to arachnoid; hyphae with some thickenings; largest part KOH−, only few small yellowish KOH+ violaceous spots present. Numerous hyaline to yellowish-brown granules seen in mounts.
While Doi and Yamatoya (1989) observed perithecia in some isolates of P. pallida, this was not observed for those characterized in this study. The only exception is TFC 99-209 that produced perithecia on MEA (particularly on the Oxoid product). These were yellowish-orange with equivalent strong KOH reaction as in nature. Only the placement of perithecia in the subiculum is more scattered.
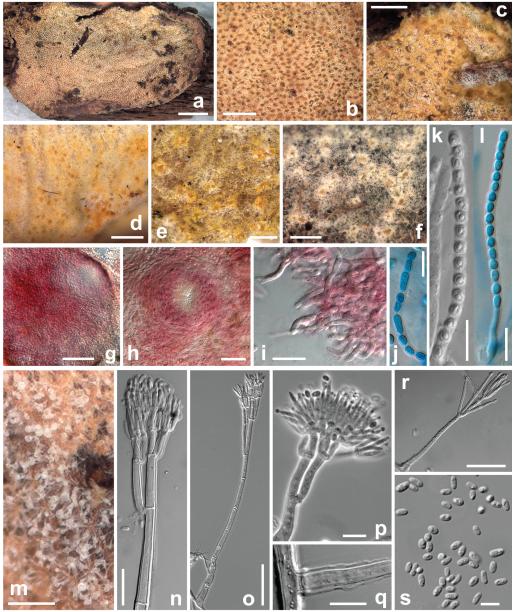
Protocrea illinoënsis K. Põldmaa & Samuels, sp. nov. Figs. 6a–l
Fig. 6.
Protocrea illinoënsis. a. Habit on the hymenophore of Skeletocutis nivea. b–f. Perithecia in subiculum (c. covered by white mass of extruded ascospores). g, h. Perithecia in 3% KOH (g. side view, h. ostiolar area). i. Free ends of subiculum hyphae on perithecial surface. j–l. Asci with ascospores (k. in KOH; j, l. in cotton blue/lactic acid). m. Conidiophores with conidial heads. n, p. top of conidiophore. o, r. conidiophore. q. base of conidiophore covered by sheath. s. conidia. a–n. On natural substrate. o–s. On CMD. a–d, g–k, m, n. TAA 169608; e, f, k. BPI 749349; o–s. TFC 96-98. Bars: a = 3 mm; b, d = 1 mm; c, e, f, m = 0.5 mm; g, o = 50 μm; h, n, r = 20 μm; i–l, p, q = 10 μm; s = 5 μm.
MycoBank MB 512132
Anamorph: Gliocladium sp. Figs. 6m–s
Differt a Protocrea farinosa in mutatione purpurea coloris KOH ope provocata, a P. farinosa et P. pallida in incremento celeriore in agaris CMD et MEA et in conidiophoribus longioribus, ad 370 μm. Holotypus TAA 169608.
Perithecial subiculum ca. 20 × 10 mm, ca. 0.2–0.3 mm high, effused across the host’s hymenophore and partly also the reflexed pileus with perithecia densely aggregated and forming a continuous layer with farinose surface or scattered to gregarious in a scarce and thin subiculum; occasionally solitary, free and naked perithecia at the margin. Subiculum arachnoid to cottony, whitish to pale (orange-)yellow; hyphae hyaline, 1.5–3.5 μm wide, loosely arranged, with numerous free ends at the surface, restricted to a thin layer around densely aggregated perithecia or widely effused bearing scattered perithecia. Perithecia immersed except the very top to semi-immersed, loosely attached to subicular hyphae or situated directly on host’s pore surface, generally easily detachable. Perithecia globose, often appearing flat from above, collapsed discoidal when dry, sometimes laterally compressed when old, 170–230 μm high, 150–230 μm wide in crush mounts, amber to ochraceous, or (orange-)yellow. Papilla short and conical, concolorous with perithecial body, 30–55 μm high, ostioles of narrow cylindrical vertical elements, ca. 1–3 μm wide, with convergent periphyses inside the papilla. In patches with (orange-)yellow subiculum and perithecia the latter becoming purple in 3% KOH solution, no color change seen in other parts. Microscopic reaction to 3% KOH strong, bright purple to violaceous formed in various areas of the subiculum and apical parts of perithecia, mostly dissolved outside the cells but appearing also inside the cells on the surface of papilla.
Peridium ca. 15 μm thick at base and sides, directly merging with ostiolum in upper part, hyaline, innermost layer composed of ellipsoidal thin-walled cells but principal layer of tightly packed, thin, thick-walled cells with walls 1.0–1.8 μm thick, outermost layer composed of loosely interwoven, thin-walled hyphae. Asci cylindrical, 68–80 × 2.5–4.0 μm, including a stipe 5–8 μm long, apex thickened to ca. 1.0–1.5 μm. Ascospores hyaline, finely spinulose, cells dimorphic, but often with little difference in shape and size, distal cell mostly subglobose, some oblong to wedge-shaped, (2.5–)2.8–3.3(−3.8) × (2.0–)2.4–3.0(−3.3) μm, l/w (0.9–)1.0–1.3(−1.4) (n = 90), proximal cell mostly oblong, ellipsoidal to wedge-shaped, some subglobose (2.5–)3.0–3.5(−4.5) × (1.7–)2.0–2.7(−3.0) μm, l/w (1.1–)1.2–1.6(−2.2) (n = 90).
Anamorph on natural substrate
Conidiophores scattered on host’s hymenophore, simple, straight, 90–150(−230) μm long and 4–7 μm wide at base, 2.5–4 μm below the first branching, thick-walled, wall smooth, with 3–5 septa, bearing a small conidial head of ca. 20–35 μm diam, bi- to terverticillate, up to 5 metulae formed from one supporting branch, phialides held in dense penicilli, ca. 10 × 2 μm, cylindrical or strongly curved upward. Conidia ellipsoidal to oblong, equilateral, rarely suballantoid, (2.5–)2.7–3.6(−4.5) × (1.2–)1.5–2.0(−2.5) μm, l/w (1.3–)1.6–2.3(−3.1) (n = 100), hyaline, smooth.
Cultures and anamorph
On CMD: growth slow (G.J.S. 94-54) to moderately fast (TFC 96-98) with colony radius ca. 30 or 50 mm after 1 wk at 25 C, respectively. Colonies hyaline, margin even, aerial mycelium absent to scarce; reverse pale yellow (TFC 96-98) or yellowish peach (G.J.S. 94-54) after ca. 2 wk, pigmentation in the center and/or in the margin; surface becoming slightly farinose and whitish by conidiation; no chlamydospores noted, odor sweetish after ca. 1 mo.
Conidiation colorless, verticillate and penicillate conidiophores arising from aerial and submerged hyphae. Gliocladium conidiophores with a well differentiated stipe arising mostly from submerged hyphae arranged singly or in ropes of 10–15 μm diam, conidiophores loosely to densely disposed, of a stipe with 1 apical head 30–100(−150) μm diam, discrete, erect, mononematous, 100–370 μm long (including penicillus), branched at top with 2–3(−4) levels of supporting branches, the uppermost bearing the phialides; typically asymmetrically branched on the lowest level with one lateral branch, symmetrically at 1–2(−3) points above, with 3–4 branches at the intermediate and up to 6 branches at the uppermost level, dense whorls of phialides and conidia of each conidiophore joined into one head; heads globose, wet, mucous, colorless to milky white in the stereomicroscope, variable in size, often joined with others into larger heads. Stipe thick-walled (to 0.8 μm), mostly covered with an undulating sheath, 1.0–1.8 μm thick; frequently septate, with up to 8 septa, (40–)80–210 μm long, 4–8 μm wide at the base (mostly wider than the hyphae from which they arise), gradually attenuated upward, 2–4 μm below first branching level, lowest branches (15–)25–35(−60) long, 2.5–4.5 μm wide, in narrow angles to nearly parallel; metulae 11–22 × 1.5–2.5 μm long and 3.0–3.5 μm wide, forming numerous, densely packed phialides. Phialides mostly subulate to almost cylindrical, symmetrical or inaequilateral and curved on sides of whorls, (5–)8–14(−17) × (1.0–)1.2–1.8(−2.2) μm, 0.5–1.0 μm wide at tip, l/w (3.2–)4.8–10.7(−14.6) (n = 35), held by 4–6 in parallel. Conidia mostly homogeneous in size and shape, ellipsoidal to oblong, rarely suballantoid, mostly equilateral, sometimes one side flattened, (2.3–)3.0–4.0(−5.0) × (1.3–)1.5–2.0(−2.5) μm, l/w (1.3–)1.7–2.4(−2.9) (n = 125), hyaline, smooth, with 1 to several guttules, base undifferentiated.
On PDA growth slow, with colony radius 30–36 mm in 1 wk; aerial mycelium absent to scanty, forming strands, surface wrinkled in the center, partly farinose; reverse pale to orange-yellow in 1 wk, turning ocher to pale peach or orange-brownish during following weeks.
Conidiation moderate to abundant, loosely disposed or dense in varying areas, simple, erect conidiophores arising from submerged mycelium; Gliocladium conidiophores less developed than on CMD, with shorter stipes mostly up to 100 μm long and ca. 4 μm wide at base, and smaller conidial heads; verticillium-like conidiophores abundant. Conidia oblong, cylindrical to ellipsoidal, 2.3–5.7(−14.0) × (1.5–)1.8–2.5(−2.7) μm, l/w 1.3–2.7(−6.6) (n = 60), hyaline, smooth, scar indistinct or projecting and truncate at base.
Etymology
refers to the locality of the known specimens.
Habitat
on the hymenophore of Skeletocutis nivea and a similar unidentifiable resupinate polypore.
Known distribution
North America (USA, Illinois)
Holotype
USA. Illinois: Ogle County, White Pines Forest State Park, Sleepy Hollow Trail, on Skeletocutis nivea, 28 Sep 1996, K. Põldmaa (TAA 169608, isotype BPI 746696, culture TFC 96-98).
Other specimens examined
USA. Illinois: Union County, vic. Carbondale, Giant City State Park, on a decayed effused polypore (Skeletocutis sp.?), 19 Sep 1994, G. J. Samuels (BPI 749349, culture G.J.S. 94-54 = CBS 121670).
Notes
Protocrea illinoënsis shares teleomorph characters with P. farinosa as well as P. pallida. Like P. farinosa it grows on Skeletocutis nivea. Colors observed in the teleomorph of P. illinoënsis occur also in the other two Protocrea species. While the orange color of some perithecia in both collections is more yellowish and not as bright as in P. pallida, the KOH reaction (Figs. 6g–i) is as strong as in that species. In the larger piece of the holotype and in the isotype of P. illinoënsis there are amber to ochraceous perithecia formed in a dense layer (Figs. 6a–c), while on another piece of the holotype orange-yellow perithecia are scattered in a well developed subiculum (Fig. 6d). In the other specimen yellowish perithecia, some with an orange tinge, are solitary or in small groups in a scarce arachnoid subiculum (Figs. 6e–f). In both collections some asci contain continuous, slipper-shaped ascospores, reminiscent of those of Sphaerostilbella.
The anamorphs and colonies in culture offer good characters for the delimitation of P. illinoënsis. Conidiophores are distinctly longer than in P. farinosa and P. pallida, especially in TFC 96-98, extending 180–370 μm. Frequently an undulating sheath covering the wall of conidiophores either at their bases or along the whole stipe up to the metulae can be seen (Fig. 6q). In TFC 96-98 it is noteworthy that the metulae and their supporting branches are thick-walled. An additional short conidiophore sometimes is seen at the base of the stipe of well developed conidiophores.
In culture P. illinoënsis can be distinguished also by its growth rate on CMD and MEA, which exceeds that of P. farinosa and P. pallida. Characters of colonies on CMD reveal differences between the two isolates. In addition the more slowly growing colonies of G.J.S. 94-54 acquire a tinge of peach in time compared to the initial pale yellow. In the faster growing TFC 96-98 very pale yellow appears only after 3 wks. A sweetish odor was noted in cultures of both strains, while no distinct odor has been noted in cultures of P. farinosa and P. pallida.
Hypocrea decipiens Jaklitsch, K. Põldmaa & Samuels, sp. nov. Figs. 3n–q
MycoBank MB 512133
= ‘Hypocrea farinosa Berk. & Broome’ sensu Overton et al, Stud Mycol 56:59. 2006.
[non Hypocrea farinosa Berk. & Broome, Ann. Mag. Nat. Hist. Ser. 2, 7:186. 1851.]
Anamorph
Trichoderma sp., acremonium/verticillium-like (see Overton et al 2006b)
Stromata alba ad luteo-brunnea, tenua, effusa, prosenchymatosa supra peritheciis et pseudoparenchymatosa sub peritheciis. Ascosporae hyalinae, spinulosae; pars distalis globosa ad ellipsoidea, (3.0–)3.3–3.7(−3.7) × 3.0–3.2(−3.5) μm; pars proxima oblonga ad cuneiformis, (3.2–)3.5–4.5(−5.5) × (2.2–)2.3–2.7(−3.0) μm. Anamorphosis Verticillio similis, conidia subglobosa ad ellipsoidea, 4–7 × 2.5–3.5 μm, hyalina, glabra.
Stromata (Fig. 3n, o; holotype, dry) thinly effuse, 5–43 × 2–17 mm, 0.1–0.4 mm (n = 9) thick, indeterminate, whitish to pale orange or light brown, ca. 5C–D4–5(−5B3), partly more lively brown 6C–D5–6, with a dull orange- to reddish-brown spot 8C–D5–8 (apparently KOH treated); broadly attached including margin; margin hyphal, whitish to yellowish, stroma surface also whitish between perithecia. Stroma surface smooth to more or less farinose, uneven, partly rugose, depending on substrate; only ostioles projecting for the most part, perithecial outlines translucent. Stroma surface of hyphae (3.0–)3.5–6.0(−7.5) μm (n = 30) wide; subperithecial tissue compact, whitish-yellowish, a textura angularis of hyaline, thin-walled angular to globose cells (5–)6–12(−15) × (3–)5–9(−10) μm (n = 30), mixed with some wide hyaline hyphae. Perithecia brownish, numerous, crowded, slightly projecting, some free at the margin, globose, not collapsed except for few old perithecia; visible part (35–)45–155(−205) μm (n = 30) diam in face view when dry. Ostioles (16–)22–38(−47)μm (n = 30) diam in face view when dry, prominent, papillate to conical, concolorous with or lighter than perithecium, some surrounded by a white fringe of hyphae at the apex. Younger stroma parts lighter, with perithecia in larger distances. Spore deposits fine, white. Stroma turning orange-brown to dark brown in 3% KOH, with stromal hyphae and cells remaining unchanged; peridium turning bright orange (Fig. 3o), bright yellow after subsequent treatment with cotton blue/lactic acid. Asci (Fig. 3p, q) (57–)65–73(−76) × (3.0–)3.5–4.5 μm (n = 31), including a stipe (1–)2–6(−8) μm (n = 31) long, fasciculate on long ascogenous hyphae, cylindrical, with 16 more or less uniseriate part ascospores, apex thickened to ca. 1 μm, base thickened or not, no croziers seen. Ascospores hyaline, spinulose, cells dimorphic, distal cell (3.0–)3.3–3.7(−3.7) × 3.0–3.2(−3.5) μm, l/w (1.0–)1.1–1.2(−1.3) (n = 30), (sub)globose to ellipsoidal, proximal cell (3.2–)3.5–4.5(−5.5) × (2.2–)2.3–2.7(−3.0) μm, l/w (1.1–)1.4–2.0(−2.3) (n = 31), oblong, wedge-shaped (to subglobose). See Overton et al (2006b) for the description of the anamorph.
Etymology
decipiens = deceiving, due to earlier misinterpretations.
Known distribution
France, ?Japan, USA.
Habitat
bark, aphyllophoraceous fungi (Stereum, Lentinula cultures, Phellinus gilvus)
Holotype
FRANCE. Pyrenées atlantiques: Foret Domaniale d’Oloron, on Quercus sp., soc. effete stromatic pyrenomycete, 30 Aug 1997, F. Candoussau 513 (BPI 747356; culture G.J.S. 97-207 = CBS 121307).
Other specimens examined
USA. Maryland: unidentified mushroom farm, locality not known, on wood and grain inoculated with Lentinula edodes, 1989, comm. D. Farr (BPI 802598, culture G.J.S. 89-139 = CBS 131208). – Prince George County, Greenbelt, Greenbelt Forest, on bark, fall 1991, S.A. Rehner (BPI 1112870, culture G.J.S. 91-101). Ohio: Warren County, Fort Ancient along Little Miami River, on Phellinus gilvus, 17 Nov 1996, K. Põldmaa and G.J. Samuels (TAA 169648, BPI 744529; culture G.J.S. 96-281).
DISCUSSION
In this work we combined the study of type and many recently collected specimens with molecular phylogenetic analyses to demonstrate that the concept of Protocrea proposed by Doi (1972, 1978) and followed by Overton et al (2006b) was based on misinterpretation of the type specimen of P. farinosa. Further we demonstrate, contrary to the results of Overton et al (2006b), that P. farinosa is not a species of Hypocrea. According to the concept of Protocrea outlined in this study species of Protocrea have Hypocrea-like ascospores, perithecia formed in a subiculum, anamorphs typical of Gliocladium s. str., and they occur on aphyllophoraceous fungi with a polyporous hymenophore. We selected a replacement epitype specimen for P. farinosa, the type species of the genus. This replaces the erroneous designation of Overton et al (2006b). The species previously included in Protocrea are excluded as species of Arachnocrea or Hypocrea. Analyses of morphological and molecular data however distinguish at least six species in the genus including P. pallida, which has occupied an enigmatic position since 1994 (Rehner and Samuels 1994, Põldmaa et al 1999, Põldmaa 2000).
Our study did not involve material collected outside Europe and North America. It is most likely that morphologically similar collections from other regions of the world belong to genus Protocrea, representing either the described or new species. Therefore the current treatment does not pretend to be the complete account of the genus.
Protocrea pallida (as Hypocrea pallida) was treated in detail by Doi and Yamatoya (1989), who distinguished it and related species from Protocrea by a two-layered perithecial wall. The inner wall was reported to consist of indistinct thin-walled cells that become roseous in 3% KOH. In fact both P. farinosa and P. pallida exhibit the same structure of the peridium and also a rosy tint can be seen on the inner wall of both species in KOH, but this is distinct from the bright violaceous discoloration in P. pallida.
Considering the same morphology, geographical distribution and occurrence on the same host, we agree with Barr et al (1986) and Doi and Yamatoya (1989) that H. aurantiaca is P. pallida with a slightly stronger developed yellow to orange pigmentation of the subiculum.
Hypocrea ampulliformis Yoshim. Doi & Yamatoya (Doi and Yamatoya 1989) was described as a close ally of H. pallida, occurring on Trichaptum biforme (Fr.) Ryvarden (syn. Hirschioporus elongatus (Berk.) Teng). It differs from P. farinosa and P. pallida by ampulliform perithecia and distinctly cellular perithecial walls. The anamorph is easily distinguishable from those of the Protocrea species treated in this study. The conidiophore stipe is wider (mostly >10 μm at base), clearly verrucose throughout its length, bearing large white heads of broadly ellipsoidal to subglobose conidia. Preliminary molecular data reveal true Hypocrea species to be its closest relatives. This species will be treated in more detail in a future publication.
The verticillium-like anamorphs exclude Protocrea latissima Mercuri & Ranalli and P. seminuda Yoshim. Doi from Protocrea; thus they are not treated here.
Based on sequences of the 28S rDNA, Rehner and Samuels (1994) detected that H. pallida does not belong in Hypocrea while Põldmaa et al (1999) and Põldmaa (2000), using the same gene, determined an uncertain position for it among species of Hypomyces. Even though constraining the monophyly of P. pallida with species of Sphaerostilbella yielded MP trees with better likelihood values than for those in which P. pallida was forced into one clade with Hypocrea species, the observed differences were statistically insignificant (Põldmaa et al 1999). The failure of LSU rDNA data to distinguish phylogenetic relationships among the larger groups involved was observed even when involving extended sets of species of the Hypocreaceae (Põldmaa and Samuels 2004).
Molecular phylogenetic analyses with rpb2 and LSU rDNA data place Protocrea within the Hypocreaceae and distinct from the main genera of the family Hypocrea, Hypomyces and Sphaerostilbella. However, because there is no support for the “backbone” of the family, we are not able to confirm the closest relatives of Protocrea. Shared morphology suggests affinities with Arachnocrea, Hypomyces and Sphaerostilbella, where perithecia are also formed in a subiculum. The disarticulating ascospores however do not differ in any way from those typical of Hypocrea species. The anamorph on the other hand is almost indistinguishable from the type species of Gliocladium, G. penicillioides Corda, which is the asexual stage of Sphaerostilbella aureonitens (Tul.) Seifert, Samuels & W. Gams (Seifert 1985). Although anamorphs of other Hypocrealean genera having Gliocladium-like, stipitate, penicillately branched conidiophores have been placed in Gliocladium, their divergent morphology readily separates them from Gliocladium s. str. These include most notably Clonostachys rosea (Link : Fr.) Schroers et al (Gliocladium roseum (Link) Thom), the anamorph of Bionectria ochroleuca (Schwein.) Schroers & Samuels (Schroers et al 1999), and several other species of Clonostachys, Trichoderma virens (J.H. Mill. et al) Arx (=Gliocladium virens J.H. Mill. et al), the anamorph of Hypocrea virens Chaverri et al (Chaverri et al 2001), and the anamorph of Roumegueriella rufula (Berk. & Broome) Malloch & Cain (Yaguchi et al 1997, Rossman et al 1999). While these and other hypocrealean anamorphs are easily distinguished from true Gliocladium, we do not see any morphological separation between the anamorphs of Protocrea and Sphaerostilbella. Their independent development in closely related but phylogenetically distinct groups seems much less likely than the loss of this morphotype during evolution. At the same time it has to be considered that both in Protocrea as circumscribed here and Sphaerostilbella, verticillium-like conidiophores are common in culture and in the latter genus synnematous and verticillium-like anamorphs occur in nature for all the species except S. aureonitens. Moreover verticillium-like conidiophores, bearing conidia in clear green drops and termed “synanamorphs” by Chaverri et al (2003) and which form in addition to typical Trichoderma conidiophores, are common in Trichoderma, also a member of the Hypocreaceae. Such plasticity seems to add evidence for considering these anamorphs as plesiomorphic in the family.
Results of molecular analysis presented herein do not reveal close relationships of Protocrea to any of the previously mentioned genera. Instead we suggest that Protocrea represents an earlier diverged lineage within the Hypocreaceae, presumably sharing a common ancestor with the other, highly divergent groups corresponding to more exclusive genera based on morphological characteristics. This hypothesis could serve as a starting point for more detailed studies on the phylogeny of hypocreaceous ascomycetes in future.
ACKNOWLEDGMENTS
We thank the curators of BPI, FH, K and NY for the loan of the type and many other specimens of P. farinosa, P. pallida and others. We thank Thomas Læssøe, Walter Gams and Hermann Voglmayr for providing fresh specimens and Erast Parmasto for the identification of hosts in many of the American and Estonian collections. We thank Gams also for support in nomenclatural issues. The financial support by the Austrian Science Fund (FWF Project P19143-B17) to WMJ and by the Estonian Science Foundation (grant No. 6939) to KP is gratefully acknowledged.
Contributor Information
Walter M. Jaklitsch, Faculty Centre for Systematic Botany, University of Vienna, Rennweg 14, A-1030 Vienna, Austria.
Kadri Põldmaa, Natural History Museum, University of Tartu, Vanemuise 46, EE-51014, Tartu, Estonia.
Gary J. Samuels, United States Department of Agriculture, Agricultural Research Service, Systematic Mycology and Microbiology Laboratory, Room 304, B-011A, BARC-W, Beltsville, Maryland 20705
LITERATURE CITED
- Barr ME, Rogerson CT, Smith SJ, Haines JH. An annotated catalog of the Pyrenomycetes described by Charles H. Peck. New York State Mus Bull. 1986;459:1–74. [Google Scholar]
- Chaverri P, Castlebury LA, Overton BE, Samuels GJ. Hypocrea/Trichoderma species with conidiophore elongations and green conidia. Mycologia. 2003;95:1100–1140. [PubMed] [Google Scholar]
- Chaverri P, Samuels GJ, Stewart EL. Hypocrea virens sp. nov., the teleomorph of Trichoderma virens. Mycologia. 2001;93:1113–1124. [Google Scholar]
- Doi Y. Revision of the Hypocreales with cultural observations IV. The genus Hypocrea and its allies in Japan. (2) Enumeration of the species. Bull Nat Sci Mus (Tokyo) 1972;15:649–751. [Google Scholar]
- Doi Y. Revision of Hypocreales with cultural observations XI. Additional notes on Hypocrea and its allies in Japan (1) Bull Nat Sci Mus Ser B. 1978;4:19–26. [Google Scholar]
- Doi Y, Yamatoya K. Hypocrea pallida and its allies (Hypocreaceae) Mem New York Bot Gard. 1989;49:233–242. [Google Scholar]
- Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. Hypocrea crystalligena sp. nov., a common European species with a white-spored Trichoderma anamorph. Mycologia. 2006;98:499–513. doi: 10.3852/mycologia.98.3.499. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nuc Acid Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. Taschenlexikon der Farben. Muster-Schmidt Verlag; Zürich-Göttingen: 1981. [Google Scholar]
- Liu YL, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Mercuri OA, Ranalli ME. Estudio systemático y biológico de Hypocreales (Ascomycetes) de Argentina I. Protocrea latissima sp. nov. estudios de cultivo. Physis (Buenos Aires) 1977;35:303–317. 1976. [Google Scholar]
- Moravec Z. Arachnocrea, un genre nouveau de la famille des Nectriaceae. Bull Soc mycol Fr. 1956;72:160–166. [Google Scholar]
- Nicholas KB, Nicholas HB, Jr, Deerfield DW., II Gene-Doc: analysis and visualization of genetic variation. EMBNET News. 1997;4:1–4. Program distributed by the authors. [Google Scholar]
- Nylander JAA. MrModeltest v2. Evolutionary Biology Centre, Uppsala University; 2004. Program distributed by the author. [Google Scholar]
- Overton BE, Stewart EL, Geiser DM, Jaklitsch WM. Systematics of Hypocrea citrina and related taxa. Stud Mycol. 2006a;56:1–38. doi: 10.3114/sim.2006.56.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton BE, Stewart EL, Geiser DM. Taxonomy and phylogenetic relationships of nine species of Hypocrea with anamorphs assignable to Trichoderma section Hypocreanum. Stud Mycol. 2006b;56:39–65. doi: 10.3114/sim.2006.56.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petch T. Notes on British Hypocreaceae III. J Bot. 1937;75:217–230. [Google Scholar]
- Petch T. British Hypocreales. Trans Br Mycol Soc. 1938;21:243–305. [Google Scholar]
- Põldmaa K. The genus Hypomyces and allied fungicolous fungi in Estonia I. Species growing on aphyllophoralean basidiomycetes. Folia Cryptog Estonica. 1999;34:15–31. [Google Scholar]
- Põldmaa K. Generic delimitation of the fungicolous Hypocreaceae. Stud Mycol. 2000;45:83–94. [Google Scholar]
- Põldmaa K, Larsson E, Kõljalg U. Phylogenetic relationships in Hypomyces and allied genera, with emphasis on species growing on wood-decaying homobasidiomycetes. Can J Bot. 1999;77:1756–1768. [Google Scholar]
- Põldmaa K, Samuels GJ. Fungicolous Hypocreaceae (Ascomycota: Hypocreales) from Khao Yai National Park, Thailand. Sydowia. 2004;56:79–130. [Google Scholar]
- Rehner SA, Samuels GJ. Taxonomy and phylogeny of Gliocladium analyzed by large subunit rDNA sequences. Mycol Res. 1994;98:625–634. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes) Stud Mycol. 1999;42:1–248. [Google Scholar]
- Samuels GJ, Dodd S, Lu B-S, Petrini O, Schroers H-J, Druzhinina IS. The Trichoderma koningii aggregate species. Stud Mycol. 2006;56:67–133. doi: 10.3114/sim.2006.56.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroers H-J, Samuels GJ, Seifert KA, Gams W. Classification of the mycoparasite Gliocladium roseum in Clonostachys as C. rosea, its relationship to Bionectriaochroleuca, and notes on other Gliocladium-like fungi. Mycologia. 1999;91:365–385. [Google Scholar]
- Seifert KA. A monograph of Stilbella and some allied hyphomycetes. Stud Mycol. 1985;27:1–235. [Google Scholar]
- Sung G-H, Hywel-Jones NL, Sung J-M, Luangsa-ard JJ, Shrestha B, Spatafora JW. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods), Version 4.0b10. Sinauer Associates; Sunderland, Massachusetts: 2002. [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nuc Acid Res. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- Yaguchi T, Someya A, Udagawa S-i. Isolation of Roumegueriella rufula as a new record from East Asia. Mycoscience. 1997;38:83–85. [Google Scholar]
- Yao Y-J, Pegler DN, Chase MW. Application of ITS (nrDNA) sequences in the phylogenetic study of Tyromyces s.l. Mycol Res. 1999;103:219–229. [Google Scholar]
- Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung G-H. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia. 2006;98:1076–1087. doi: 10.3852/mycologia.98.6.1076. [DOI] [PubMed] [Google Scholar]