Abstract
The emergence of autoreactivity that ultimately destroys insulin-producing β-cells and causes Type 1 diabetes (T1D) is a result of genetic susceptibility and environmental factors, such as viral infections. The ability to induce strong cellular immune responses and to cause inflammation in the target organ makes viral infections prime candidates for the initiation of islet autoreactivity. Indeed, certain viruses have been linked to the occurrence of T1D based on epidemiological, serological and histological findings; and several rodent studies clearly demonstrate that viral infections can trigger autoimmunity. However, viruses have also been shown to efficiently prevent autoimmunity, which underlines the beneficial aspects of exposure to microbial agents as suggested by the hygiene hypothesis. Here, we will try to untangle some aspects of the complex interplay between viruses and the immune system and we will recapitulate by what rationale certain viruses have been associated with T1D.
Keywords: autoimmunity, coxsackievirus, hygiene hypothesis, Type 1 diabetes, viral infections
The destruction of insulin-producing pancreatic β-cells by an autoreactive immune system is the hallmark of Type 1 diabetes (T1D) and the demise of pancreatic β-cell mass progressively impairs the body’s blood glucose control. The necessity to closely monitor blood glucose levels and inject insulin accordingly is the inherent consequence for affected patients. Although insulin therapy has shown marked improvements over the last few decades, disease progresses in the vast majority of diabetic patients and long-standing dysglycemia results in macroangiopathic, microangiopathic or neuropathic complications [1]. The incidence rates of T1D have been rising over the past few decades worldwide by an average of 3% per year [2] and it has recently been predicted that T1D incidence may double in children under the age of 5 years by the year 2020, especially in developed countries [3].
While T1D is a genetic disorder and several genetic variations can undoubtedly be associated with disease occurrence, environmental factors that are largely unknown are thought to ultimately trigger diabetogenesis in susceptible individuals (reviewed in [4]). This concept evolved for several reasons. First, to date no genetic pattern has been described that leads to total disease penetration [5]. Second, the concordance rate in monozygotic twins is well below 50% [6,7]. Finally, disease occurs even in those individuals that carry protective genetic variations [5]. Environmental triggers that turn genetic susceptibility into overt diabetes have drawn much attention, since a detailed knowledge of these factors may be key to preventing the disease in the future.
Even though several other factors may contribute to T1D pathogenesis, there is compelling evidence that viruses are major players in both disease initiation and in disease protection. In this article, we will summarize which viruses have been associated with T1D and recapitulate some aspects of the complex interplay between viruses and the immune system that result in the protection against, or triggering of, T1D.
Humans versus rodent models
The limited availability of relevant human samples is a major obstacle in T1D research. Since the pancreas is very difficult to access, it cannot be routinely biopsied in order to study the immunological or virological determinants of the disease in the target organ. Moreover, a large number of patients with recent onset of the disease are children, which limits the amount and frequency of blood draws for scientific studies. These barriers heavily impair the opportunities for investigators to unravel the early events in diabetogenesis in humans. In an effort to partly overcome this obstacle, the Network for Pancreatic Organ Donors with Diabetes has been initiated, which enables investigators to access tissues of deceased patients with T1D [101]. However, much of our knowledge on pathogenesis is derived from animal models. There is a rich variety of mainly rodent models to study various aspects of diabetes pathogenesis, but also to explore new therapeutic interventions. Nonobese diabetic (NOD) mice and biobreeding rats spontaneously develop autoimmune diabetes. Several aspects of their disease development closely mimic the disease in humans, such as certain susceptibility genes and the repertoire of autoantigens targeted by the immune system [8–10]. Rat insulin reporter-glycoprotein (RIP-GP) or RIP-nucleoprotein (NP) mice express immunodominant epitopes from the lymphocytic choriomeningitis virus (LCMV) on their pancreatic β cells under the control of the RIP and, following viral infection, the primed and activated antiviral T cells attack and destroy the β cells expressing the viral antigen [11,12]. Other models that have been employed to study the influence of viral infections on diabetogenesis carry T-cell receptor transgenes that are specific for MHC class I or class II-restricted autoantigenic epitopes, such as the BDC2.5 mice [13].
Evidence for an involvement of viruses in T1D pathogenesis
Several observations in humans and animal models have contributed to the hypothesis that viral infections could trigger the autoreactivity that ultimately results in β-cell destruction. It could be shown that several viruses, such as certain coxsackieviruses, Rubella virus and mumps virus, are able to directly infect and lyse insulin-producing β-cells [14–16]. Thus, one mechanism by which viruses might induce T1D is through direct cytopathic effects, resulting in β-cell death. However, this may not be the mechanism by which viruses commonly initiate diabetogenesis. While fulminant cases of diabetes that can be induced through viruses that directly infect and lyse β cells have been reported in humans [17], they are rather uncommon. Moreover, it does not necessarily explain the emergence of autoreactivity, a crucial player in T1D pathogenesis.
The evidence for an involvement of viruses in triggering islet autoreactivity is of a different nature. Besides several in vivo studies in mice that will be discussed later in more detail, there is epidemiological, serological and also histological evidence in humans supporting this hypothesis. A critical part of the host’s immune response to invading viruses is the secretion of interferons that initiate inflammation [18]. One mechanism by which interferons trigger inflammation in the pancreas is the upregulation of MHC class I molecules on β cells, thereby unmasking them for immune attack [19,20]. Indeed, histological analysis of pancreatic tissue from recently diagnosed but deceased diabetic patients repeatedly revealed the upregulation of MHC class I molecules in the islets of Langerhans that is accompanied by lymphocytic infiltrations in those islets with remaining β-cell mass [21,22]. The critical requirement for MHC class I upregulation in β-cell destruction has been shown in rodent studies where activated CD8+ effector T cells only attacked and destroyed those cells in vitro that have been unmasked by elevated MHC class I levels [20]. Even though the degree of lymphocytic infiltrations is considerably lower in humans compared with NOD mice or RIP-LCMV mice and we do not know precisely at what point insulitis arises in humans, the coexistence of high expression levels of MHC class I molecules and insulitis, especially compared with nondiabetic controls, suggests that the sequence of virus-induced islet inflammation is also of importance in humans.
While the composition of the islet infiltrates is suggestive of the presence of viruses, other groups were able to directly detect viral particles on pancreatic tissue or even isolate live virus from a pancreas derived from a newly diagnosed but deceased patient [23,24]. In addition, a recent study added evidence for a virus–diabetes link by identifying four protective genetic variations in the IFIH1 gene, a helicase enzyme also known as melanoma-associated differentiation 5 (MDA5), which is responsible for type I interferon production in response to a viral infection [25]. Each genetic variant impaired the function of the protein product MDA5 and individuals carrying these genetic variations had a significantly lower risk of developing autoimmune diabetes. Interestingly, MDA5 senses viruses from the picornavirus family, which includes enteroviruses such as coxsackieviruses, the most common group of viruses reported to be associated with T1D. Mechanistically, enteroviral infections could activate MDA5 and induce secretion of type I interferons, which would lead to upregulation of MHC class I on β cells, thereby enhancing recognition of β cells by autoreactive CD8+ cytotoxic T lymphocytes. In summary, the described findings strongly support the hypothesis that viral infections contribute to T1D pathogenesis. However, these associations do not provide a mechanistic explanation as to how viruses can initiate autoimmunity.
Molecular mimicry
The emergence of cross-reactive T-cell clones could be one such mechanism and this phenomenon has been termed molecular mimicry. The classic example for an association of a microbial pathogen with an autoimmune disease comes from β-hemolytic streptococci, which are associated with acute rheumatic fever (ARF). Initially, patients infected with Streptococcus pyogenes develop impetigo and pharyngitis. If untreated, disease can progress to ARF, including arthritis of the joints and rheumatic heart disease due to cross-reactive immunity [26]. A study in rats could show that inoculation of recombinant M protein from streptococci can activate CD4+ T cells that cross-react with cardiac myosin, and myosin-immunized rats developed valvular lesions similar to those found in ARF [27]. In T1D, the evidence for an involvement of molecular mimicry in pathogenesis is less clear-cut. While there is a remarkable structural similarity between a group B coxsackievirus (CVB) epitope (P2-C 35–43) and an epitope derived from the major autoantigen GAD65 (GAD65 258–266) in NOD mice and in humans [28], CVB infection had no effect on T-cell reactivity to the GAD65 peptide or on T1D incidence in mice [29] and CD4+ T-cell clones generated to the GAD65 258–266 epitope did not proliferate in response to the CVB P2-C 35–43 epitope in humans with T1D [30].
As mentioned previously, RIP-LCMV mice are a valuable mouse model for studying the involvement of viruses in diabetogenesis. Prior to infection with LCMV, RIP-LCMV mice do not display any signs of overt pathology, despite the transgenic expression of LCMV antigens, either NP or GP on pancreatic β cells [11,12,20]. Following LCMV infection, virus-specific T cells mediate viral clearance but also attack β cells, a so-called ‘artificial molecular mimicry’ and also a proof of principle. While this match of foreign and self antigen is artificially created and may not reflect the situation in humans, it shows that potentially autoreactive T-cell precursors can circulate in the body without attacking their target structure. Moreover, this model shows that a viral infection is able to induce an autoimmune disease by activating these precursors that ultimately turn auto-aggressive. However, a single amino acid change flanking a cytotoxic T-lymphocyte epitope was found to interfere with the development of T1D, suggesting that complete homology between viral and β-cell antigens is required [31]. Moreover, infection with a variant of LCMV expressing a lower-avidity GP CD8+ epitope significantly reduced T1D incidence in this model. Thus, only molecular epitope identity, but not mimicry (cross-reactivity), was sufficient to trigger diabetes in an animal model, raising questions as to under which circumstances molecular mimicry might contribute to autoimmunity [32]. Interestingly, infection with Pichinde virus (PV) results in cross-reactive immunity of a PV epitope and a subdominant LCMV epitope and PV infection is able to augment pre-existing autoimmunity in RIP-LCMV mice. However, PV infection alone does not generate a sufficient autoreactive T-cell response that is able to initiate disease in LCMV-naive RIP-LCMV mice [33]. These observations argue for a role of molecular mimicry in enhancing or accelerating ongoing autoimmune processes rather than causing initiation of autoimmunity.
Bystander activation & antigenic spreading
Another mechanism that may be triggered by viral infections of the pancreas and contribute to T1D pathogenesis is the unspecific activation of unrelated T-cell specificities due to ongoing tissue inflammation. As part of the anti-viral immune response, the local release of inflammatory mediators such as nitric oxide can result in the bystander destruction of the neighboring, yet uninfected, cells [34,35]. Moreover, the release of self-antigens in the context of inflammation can lead to the presentation of those antigens by antigen-presenting cells (APCs) and consequently priming or activation of other autoreactive immune cell clones (epitope or antigenic spreading). Indeed, different mouse models nicely show that virally induced tissue inflammation can result in the development of autoreactivity to unrelated self-proteins. RIP-LCMV mice develop autoantibodies to insulin and GAD in response to islet inflammation induced by LCMV infection [36]. In addition, insulin-specific immunomodulation is capable of preventing diabetes in this system, clearly showing the importance of bystander activation in the destruction of β cells [37]. Another autoimmune model where antigenic spreading is crucial to the emergence of autoreactivity is the Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. Disease-susceptible mice are unable to clear an intracranial TMEV-infection, which results in chronic viremia and CNS inflammation. In this milieu, autoreactive T-cell clones emerge that target the self-proteolipid myelin, which is also targeted in human multiple sclerosis [38].
In an effort to discriminate between molecular mimicry and bystander activation, Horwitz et al. infected wild-type NOD mice and NOD.BDC2.5 mice with a coxsackievirus B4 strain (CVB4) [29]. BDC2.5 mice carry a T-cell receptor transgene specific for an MHC class II-restricted autoantigen and spontaneously develop insulitis but have a much lower incidence of diabetes compared with wild-type NOD mice. CVB4 infection at 6–8 weeks of age induced severe pancreatitis, predominantly of the exocrine tissue, and interestingly, resulted in rapid diabetes onset in BDC2.5 mice but not in wild-type NOD mice [29]. The observation that wild-type NOD mice did not develop diabetes upon CVB4 infection suggests that the presence of autoreactive T cells, in this case the BDC2.5 clone, is an important prerequisite for bystander activation to contribute to disease development and that molecular mimicry does not significantly influence diabetogenesis in this system since both mouse strains had the same genetic background and were infected with the same virus. Moreover, the degree of pre-existing insulitis was higher in BDC2.5 mice, suggesting that islet inflammation at the time of infection may be another harmful precondition.
Evidence for an involvement of viruses in T1D protection
The hygiene hypothesis suggests that exposure to microbial agents may be beneficial for the development of a balanced immune system and its ability to maintain self tolerance [39]. Initially, it had been proposed to explain the increase of allergic diseases in industrialized countries [40] but it seems to be valid for several other immune disorders, such as T1D, multiple sclerosis and also Crohn’s disease [41]. While the incidence of various infectious diseases have decreased over the last few decades due to elevated hygiene standards and the widespread use of antibiotics and vaccination programs, the occurrence of autoimmune disorders has increased rapidly [41]. It has proven to be very difficult to dissect which of the aforementioned changes in living conditions accounts for this increase in immune disorders. The Environmental Determinants of Diabetes in the Young study [102] is currently underway and will hopefully help to characterize such environmental factors that might contribute to disease protection or penetration [42]. One aspect that is repeatedly discussed to support the importance of the hygiene hypothesis is the strong influence of various pathogens on the diabetes incidence in NOD mice [8]. Not only have infections with several pathogens such as LCMV, enteroviruses, helminths or Salmonella been shown to significantly decrease diabetes incidence [43–46], but the maintenance of high diabetes incidence rates in this mouse strain strongly requires specific pathogen-free housing conditions [103]. Studies in NOD mice also revealed the mechanistic aspects of how viruses may mediate protection from diabetes. Upon infection with LCMV or CVB, two distinct mechanisms were found to reduce the incidence or delay the onset of T1D [46]. It could be shown that the expansion of autoreactive CD8+ T cells expressing programmed cell death-1 was inhibited by lymphoid cells that transiently upregulated programmed cell death-1 ligand 1. Moreover, viral infection resulted in elevated levels of TGF-β and an increase of CD4+CD25+ regulatory T cells producing this cytokine and resulted in the maintenance of long-term tolerance [46,47]. Recent data suggest that the protection from T1D through viruses and other microbes may be dependent on signaling through Toll-like receptors (TLRs), which will be discussed in a separate section.
Enteroviruses, other viruses & T1D
Although a clear association with a particular virus, or viral infections in general, and T1D could not be established in humans, there is ever-increasing evidence from epidemiological and experimental studies that strongly suggest this association exists, especially for human enteroviruses (HEVs). HEVs frequently circulate in the population, however, most infections remain clinically asymptomatic. Within the large family of enteroviruses, CVB have been identified to trigger T1D [48]. Based on studies in mice, we can draw a picture on how CVB could modulate autoimmune diabetes incidence. Studies performed by Tracy et al. suggest that the degree of pre-existing insulitis, the dose and the replication rate of the inoculated virus seem to be the key variables [48–50]. In NOD mice, the degree of islet inflammation increases with age, and infection with various CVB strains, which differed in terms of pathogenicity, islet-tropism and replication rates, significantly decreased diabetes incidence in young NOD mice with no or mild insulitis. Interestingly, the strongest protection was conferred by rapidly replicating strains. By contrast, infection of older, prediabetic NOD mice with this rapidly replicating strain accelerated T1D onset compared with uninfected control animals, whereas infection with a slowly replicating strain decelerated diabetogenesis [49]. These observations clearly suggest that pre-existing insulitis seems to be a crucial requirement for rapidly replicating viruses to promote T1D. Moreover, the importance of the inoculation dose became apparent by the observation that lower doses of the highly replicating strain resulted in decreased T1D incidence compared with infection with high doses. Similarly, higher doses of the slowly replicating strain resulted in acceleration of disease [50]. Importantly, viral titers in the pancreas correlated with the inoculation dose [48].
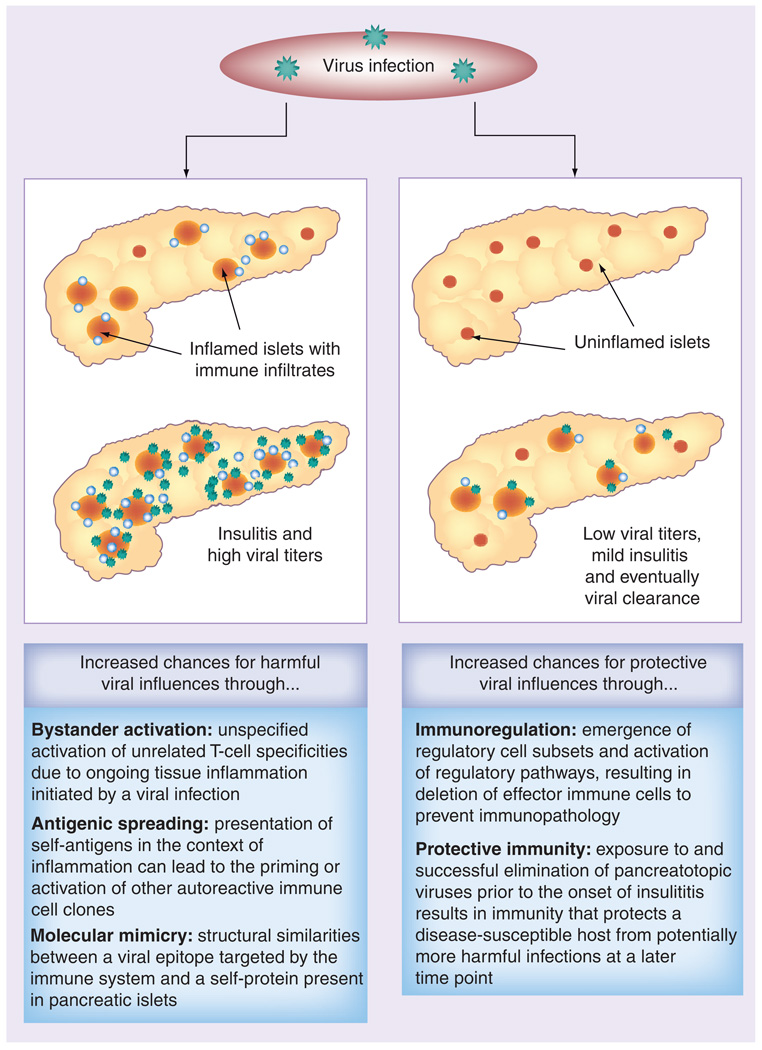
Thus, the observation that inflammation in the host’s pancreas may codetermine whether HEV accelerates or decelerates diabetogenesis suggests that viral infections, even with the same virus, can have directly opposing outcomes in shaping the immune response to self-antigens (Figure 1). It also teaches us that the hygiene hypothesis is not necessarily in contrast to the hypothesis that HEV can trigger T1D. Finland has one of the highest T1D incidences world-wide and, while T1D diabetes incidence has been increasing over the last decades, exposure to enteroviruses has been dropping [51]. One could hypothesize that, based on the reduced exposure to HEV in industrialized countries, fewer individuals have acquired protective immunity in their early childhood and are not protected from T1D by virally triggered immunoregulatory mechanisms such as programmed cell death 1 ligand 1 upregulation on lymphoid cells and increasing numbers of circulating regulatory T cells (Tregs), as has been shown to occur in NOD mice [46]. Consequently, when host-driven islet inflammation starts to occur in genetically predisposed individuals, they are more susceptible to symptomatic HEV infection that may initiate overt autoreactivity. Indeed, numerous reports suggested an association between CVB and T1D in humans. Viral RNA has been detected in serum samples and peripheral blood mononuclear cells of pediatric patients with recent-onset diabetes, whereas control samples were largely negative [52,53] and signs of persistent enterovirus infection were found in upper intestinal biopsy samples from diabetic patients [54]. The appearance of T1D autoantibodies could be correlated with seasonal CVB infections [55,56]. Moreover, enteroviruses could be detected and even isolated from pancreata of deceased recent-onset diabetes patients [23,24,57,58]. However, in contrast to rodent studies, viruses were found almost exclusively within the islets of Langerhans [21,23,50,59,60]. It has to be mentioned that in 2004, a systematic review on 26 case–control studies between 1966 and 2002 did not support a causal relationship between CVB and T1D pathogenesis [61]. Some reports suggested an association of other viruses, such as the mumps virus [62] and the rubella virus [63], with T1D. Since vaccination programs in developed countries have widely eliminated infections with those viruses and T1D incidence has increased, it is unlikely that these viruses commonly contribute to T1D pathogenesis. The same holds true for other reports associating childhood vaccination programs with T1D [64]. Among other studies, in 2004 a study was published that included almost 740,000 children that received the recommended childhood vaccines and did not reveal any association between the development of autoimmune diabetes and any particular vaccine [65].
Figure 1. Schematic model of how viral infections with pancreatotropic viruses could have opposing outcomes depending on various factors.
Pre-existing islet inflammation at the time of infection with a rapidly replicating virus, in the absence of protective immunity, could increase the chances of promoting or accelerating Type 1 diabetes onset through mechanisms such as bystander activation, molecular mimicry or antigenic spreading. By contrast, viral infection in the absence of insulitis is more likely to result in protection from Type 1 diabetes through upregulation of immunoregulatory mechanisms and acquisition of protective immunity that prevents infection at later time points when host-driven insulitis could be present.
Viruses & TLRs in T1D
As part of the innate immune system, TLRs recognize defined patterns that are commonly shared by groups of pathogens [66]. TLR ligation is also an important player in the initiation and shaping of adaptive immune responses to various antigens, and several studies suggest an important role for TLRs in T1D (reviewed in [67]). While this role is obviously not restricted to the action of viruses, we will focus on some reports that suggest that the emergence of autoreactivity can be prevented but also boosted by virally mediated engagement of different TLRs. It has been observed for viruses, TLR stimulation has been shown to substantially accelerate disease development, while other studies report that engagement of TLRs is involved in protection from T1D [68,69].
Indeed, treatment with the TLR-3 stimulus poly I:C has been shown to prevent diabetes in the disease-prone Biobreeding rat model when administered at low doses [68], whereas higher doses of poly I:C accelerated diabetes in a dose-dependent manner [69].
T cells with a regulatory phenotype can express TLR-2, TLR-4, TLR-5, TLR-7 and TLR-8 [70,71] and several studies suggest that TLR signaling, especially through TLR-2, is a crucial player in Treg biology [70]. TLR-2-deficient mice have fewer Tregs and administration of TLR-2 ligands results in increased Treg numbers in mice and Treg proliferation in vitro and in vivo [71]. Our recent data suggest that protection from diabetes through infection with LCMV is dependent on the emergence of Tregs and TLR-2 involvement, since TLR-2 ligation increased Treg numbers in NOD mice and those mice that received a TLR-2 antagonist were not protected from disease following LCMV infection [Filippi C et al., Manuscript in Preparation].
These data are in contrast to other reports showing that TLR-2-dependent interaction of apoptotic β cells and APCs resulted in increased priming of diabetogenic T cells and the finding that diabetes incidence is significantly lower in TLR-2-deficient mice on a NOD background compared with wild-type NOD mice [72]. The observation that TLR-2 and TLR-4 mRNA is significantly upregulated in monocytes derived from patients with T1D suggests an involvement of TLR-2 signaling in the autoreactive immune response [73].
Other models in which TLR ligation has been shown to promote islet autore-activity include the virally induced diabetes models RIP-LCMV and the diabetes-resistant Biobreeding rats. Approximately 30% of the latter develop diabetes when infected with the Kilham rat virus and TLR-3, -4, -6, -7, -8 and -9 ligation, in addition to the Kilham rat virus infection, signif icantly increased diabetes incidence [74]. In the RIP-LCMV mouse model, LCMV infection results in β-cell destruction, whereas injection of the peptide that is expressed on the β cells alone fails to induce autoreactivity. However, in conjunction with TLR activation, peptide injection induced diabetes even in the absence of the virus [75].
Taken together, these data suggest that TLR signaling is one tool that is employed by viruses to either protect from diabetes or promote disease development.
Expert commentary & five-year view
The association between viruses and T1D is complex; besides genetics and other environmental influences, viruses may be only one factor that contributes to the development of islet auto-reactivity. In humans, our insights are rather limited into what the contributions of viruses to T1D development are. The main reasons for this lack of evidence are: the inherent difficulties in accessing tissue samples from patients with recent-onset disease; the possibility that a triggering viral infection may occur weeks or month before hyperglycemia appears; the heterogeneity of viruses that have been associated with T1D; and the fact that most infections with these viruses are clinically silent.
Encouragingly, there is increasing information from epidemiological observations and studies on pancreata from deceased T1D patients that is of great value for the T1D research community. With the initiation of the Network for Pancreatic Organ Donors with Diabetes, we will be able to learn a lot more about the mechanisms that contribute to pathogenesis in humans. Until now, the majority of knowledge has been derived from studies in mice. These studies suggest that several prerequisites have to be met for a specific virus, such as, an enterovirus, to trigger autoimmunity. These include: pre-existing insulitis of the host; infection with high doses of a rapidly replicating strain; and the absence of protective immunity to the specific virus strain. If this hypothesis proves true, diabetes development could be prevented in the presence of protective immunity to these virus strains. If not acquired through early childhood exposure due to our hygiene standards, we should seek ways to establish a protective vaccination that provides immunity to the most aggressive or rapidly replicating strains.
Another hypothesis on how viruses can initiate autoimmunity is the fertile field hypothesis [76]. It proposes that viral infection of the pancreas provides a transient, localized fertile field where autoreactive T cells can easily emerge. Bystander activation and molecular mimicry represent two mechanistic models by which those self-aggressive T cells might develop. Interestingly, however, molecular mimicry and bystander activation seem to be incapable of triggering autoimmune disease onset independently [30,77]. While the fertile field hypothesis proposes that the viral infection may be the initial trigger that initiates islet inflammation and autoreactive T cells emerge in this proinflammatory milieu, the CVB studies in NOD mice suggest that this islet inflammation is a prerequirement in order for viruses to trigger autoimmunity. Although these two scenarios in essence disagree on what is ‘the chicken’ and what is ‘the egg’, insulitis or viral infection, they do not necessarily exclude each other. It is well conceivable that pre-existing host-driven islet inflammation is a requirement for a virus to prepare the fertile field in which full-blown autoimmunity can develop (Figure 1). Moreover, although not specifically addressed in experimental studies, it is probable that under certain circumstances two (or even more) consecutive infections may be required; the first infection prepares the fertile field through initiation of islet inflammation and in this milieu the second infection induces autoreactivity and finally β-cell destruction.
These theories address mechanistic aspects on how viruses are capable of inducing T1D but there are limited and even conflicting data on which viruses can be held responsible. While several reports discovered significantly more traces of recent or persistent enterovirus infections in diabetic patients compared with controls, the frequency with which enteroviruses circulate in the population and the aforementioned systematic review by Green et al. on the case–control studies somewhat put these findings in perspective [61]. However, this does not necessarily negate the role of enteroviruses in triggering T1D. We have to keep in mind that T1D is a multifactorial disease and studies analyzing viral involvement in T1D pathogenesis need to acknowledge the possibility that a viral infection in a genetically predisposed individual may have fundamentally different consequences compared with the same infection in nonsusceptible individuals. In other words, we should not necessarily expect more viral infections in recent-onset patients compared with control populations. It is probably the genetic susceptibility (and also the host-driven insulitis) that makes the difference. In this respect, we eagerly await the results of the The Environmental Determinants of Diabetes in the Young study [42], where we can compare such environmental influences within a group of genetically susceptible individuals.
Acknowledgments
Tobias Boettler was supported by a grant from the Deutsche Forschungsgemeinschaft, Bonn, Germany. Matthias von Herrath is supported by NIH/NAID grant P01 AI058105-05.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr. Clin. North Am. 2005;52(6):1553–1578. doi: 10.1016/j.pcl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet. 2000;355(9207):873–876. [PubMed] [Google Scholar]
- 3.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood Type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–2020: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in Type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler AG, Nepom GT. Prediction and pathogenesis in Type 1 diabetes. Immunity. 2010;32(4):468–478. doi: 10.1016/j.immuni.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of Type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes. 2003;52(4):1052–1055. doi: 10.2337/diabetes.52.4.1052. [DOI] [PubMed] [Google Scholar]
- 7.Redondo MJ, Rewers M, Yu L, et al. Genetic determination of islet cell autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of patients with Type 1 diabetes: prospective twin study. Br. Med. J. 1999;318(7185):698–702. doi: 10.1136/bmj.318.7185.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson MA, Leiter EH. The NOD mouse model of Type 1 diabetes: as good as it gets? Nat. Med. 1999;5(6):601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 10.Wallis RH, Wang K, Marandi L, et al. Type 1 diabetes in the BB rat: a polygenic disease. Diabetes. 2009;58(4):1007–1017. doi: 10.2337/db08-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. • Describes the engineering of transgenic rat insulin promoter–lymphocytic choriomeningitis virus mice.
- 12. Ohashi PS, Oehen S, Buerki K, et al. Ablation of ‘tolerance’ and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. • Describes the engineering of transgenic rat insulin promoter–lymphocytic choriomeningitis virus mice.
- 13.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74(6):1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg-Fellner F, Witt ME, Fedun B, et al. Diabetes mellitus and autoimmunity in patients with the congenital rubella syndrome. Rev. Infect. Dis. 1985;7 Suppl. 1:S170–S176. doi: 10.1093/clinids/7.supplement_1.s170. [DOI] [PubMed] [Google Scholar]
- 15.Notkins AL. On the track of viruses. Nature. 1984;311(5983):209–210. doi: 10.1038/311209b0. [DOI] [PubMed] [Google Scholar]
- 16.Jenson AB, Rosenberg HS, Notkins AL. Pancreatic islet-cell damage in children with fatal viral infections. Lancet. 1980;2(8190):354–358. [PubMed] [Google Scholar]
- 17.Shimada A, Maruyama T. Encephalomyocarditis-virus-induced diabetes model resembles ‘fulminant’ Type 1 diabetes in humans. Diabetologia. 2004;47(10):1854–1855. doi: 10.1007/s00125-004-1538-9. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7(2):131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 19.Thomas HE, Parker JL, Schreiber RD, Kay TW. IFN-γ action on pancreatic β cells causes class I MHC upregulation but not diabetes. J. Clin. Invest. 1998;102(6):1249–1257. doi: 10.1172/JCI2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Herrath MG, Dockter J, Oldstone MB. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1(3):231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 21.Coppieters KT, von Herrath MG. Histopathology of Type 1 diabetes: old paradigms and new insights. Rev. Diabet. Stud. 2009;6(2):85–96. doi: 10.1900/RDS.2009.6.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N. Engl. J. Med. 1985;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 23.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of β cells and natural killer cell insulitis in recent-onset Type 1 diabetic patients. Proc. Natl Acad. Sci. USA. 2007;104(12):5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 1979;300(21):1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 25. Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against Type 1 diabetes. Science. 2009;324(5925):387–389. doi: 10.1126/science.1167728. • The melanoma-associated differentiation 5 receptor is responsible for type I interferon production in response to viral infections and mutations in the gene encoding this receptor protect against Type 1 diabetes (T1D).
- 26.Guilherme L, Cunha-Neto E, Coelho V, et al. Human heart-infiltrating T-cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteins. Circulation. 1995;92(3):415–420. doi: 10.1161/01.cir.92.3.415. [DOI] [PubMed] [Google Scholar]
- 27.Quinn A, Kosanke S, Fischetti VA, Factor SM, Cunningham MW. Induction of autoimmune valvular heart disease by recombinant streptococcal M protein. Infect. Immun. 2001;69(6):4072–4078. doi: 10.1128/IAI.69.6.4072-4078.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, Maclaren NK. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J. Clin. Invest. 1994;94(5):2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 1998;4(7):781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 30.Schloot NC, Willemen SJ, Duinkerken G, Drijfhout JW, de Vries RR, Roep BO. Molecular mimicry in Type 1 diabetes mellitus revisited: T-cell clones to GAD65 peptides with sequence homology to Coxsackie or proinsulin peptides do not crossreact with homologous counterpart. Hum. Immunol. 2001;62(4):299–309. doi: 10.1016/s0198-8859(01)00223-3. [DOI] [PubMed] [Google Scholar]
- 31.Sevilla N, Homann D, von Herrath M, et al. Virus-induced diabetes in a transgenic model: role of cross-reacting viruses and quantitation of effector T cells needed to cause disease. J. Virol. 2000;74(7):3284–3292. doi: 10.1128/jvi.74.7.3284-3292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronski MA, Boulter JM, Moskophidis D, et al. TCR affinity and negative regulation limit autoimmunity. Nat. Med. 2004;10(11):1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 33.Christen U, Edelmann KH, McGavern DB, et al. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J. Clin. Invest. 2004;114(9):1290–1298. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duke RC. Self recognition by T cells. I. Bystander killing of target cells bearing syngeneic MHC antigens. J. Exp. Med. 1989;170(1):59–71. doi: 10.1084/jem.170.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stassi G, De Maria R, Trucco G, et al. Nitric oxide primes pancreatic β cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J. Exp. Med. 1997;186(8):1193–1200. doi: 10.1084/jem.186.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holz A, Dyrberg T, Hagopian W, Homann D, von Herrath M, Oldstone MB. Neither B lymphocytes nor antibodies directed against self antigens of the islets of Langerhans are required for development of virus-induced autoimmune diabetes. J. Immunol. 2000;165(10):5945–5953. doi: 10.4049/jimmunol.165.10.5945. [DOI] [PubMed] [Google Scholar]
- 37.Coon B, An LL, Whitton JL, von Herrath MG. DNA immunization to prevent autoimmune diabetes. J. Clin. Invest. 1999;104(2):189–194. doi: 10.1172/JCI7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller SD, Vanderlugt CL, Begolka WS, et al. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 1997;3(10):1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 39.von Mutius E. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: farm lifestyles and the hygiene hypothesis. Clin. Exp. Immunol. 2010;160(1):130–135. doi: 10.1111/j.1365-2249.2010.04138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strachan DP. Hay fever, hygiene and household size. Br. Med. J. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347(12):911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 42.TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr. Diabetes. 2007;8(5):286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 43.Oldstone MB. Prevention of Type I diabetes in nonobese diabetic mice by virus infection. Science. 1988;239(4839):500–502. doi: 10.1126/science.3277269. [DOI] [PubMed] [Google Scholar]
- 44.Cooke A, Tonks P, Jones FM, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21(4):169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 45.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune Type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 2007;75(1):397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Filippi CM, Estes EA, Oldham JE, von Herrath MG. Immunoregulatory mechanisms triggered by viral infections protect from Type 1 diabetes in mice. J. Clin. Invest. 2009;119(6):1515–1523. doi: 10.1172/JCI38503. • Provides a mechanistic explanation of how viruses confer protection from T1D.
- 47.Richer MJ, Straka N, Fang D, Shanina I, Horwitz MS. Regulatory T-cells protect from Type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-β. Diabetes. 2008;57(5):1302–1311. doi: 10.2337/db07-1460. [DOI] [PubMed] [Google Scholar]
- 48.Tracy S, Drescher KM, Jackson JD, Kim K, Kono K. Enteroviruses, Type 1 diabetes and hygiene: a complex relationship. Rev. Med. Virol. 2010;20(2):106–116. doi: 10.1002/rmv.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tracy S, Drescher KM, Chapman NM, et al. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 2002;76(23):12097–12111. doi: 10.1128/JVI.76.23.12097-12111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kanno T, Kim K, Kono K, Drescher KM, Chapman NM, Tracy S. Group B coxsackievirus diabetogenic phenotype correlates with replication efficiency. J. Virol. 2006;80(11):5637–5643. doi: 10.1128/JVI.02361-05. • Describes the importance of pre-existing insulitis in the host, inoculation dose and viral loads in the target organ in the triggering of T1D by coxsackieviruses.
- 51.Viskari H, Ludvigsson J, Uibo R, et al. Relationship between the incidence of Type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia. 2005;48(7):1280–1287. doi: 10.1007/s00125-005-1780-9. [DOI] [PubMed] [Google Scholar]
- 52.Schulte BM, Bakkers J, Lanke KH, et al. Detection of enterovirus RNA in peripheral blood mononuclear cells of Type 1 diabetic patients beyond the stage of acute infection. Viral. Immunol. 2010;23(1):99–104. doi: 10.1089/vim.2009.0072. [DOI] [PubMed] [Google Scholar]
- 53.Clements GB, Galbraith DN, Taylor KW. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346(8969):221–223. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 54.Oikarinen M, Tauriainen S, Honkanen T, et al. Detection of enteroviruses in the intestine of Type 1 diabetic patients. Clin. Exp. Immunol. 2008;151(1):71–75. doi: 10.1111/j.1365-2249.2007.03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lonnrot M, Korpela K, Knip M, et al. Enterovirus infection as a risk factor for β-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000;49(8):1314–1318. doi: 10.2337/diabetes.49.8.1314. [DOI] [PubMed] [Google Scholar]
- 56.Lonnrot M, Salminen K, Knip M, et al. Enterovirus RNA in serum is a risk factor for β-cell autoimmunity and clinical Type 1 diabetes: a prospective study. Childhood Diabetes in Finland (DiMe) Study Group. J. Med. Virol. 2000;61(2):214–220. [PubMed] [Google Scholar]
- 57. Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human Type 1 diabetes. Diabetologia. 2009;52(6):1143–1151. doi: 10.1007/s00125-009-1276-0. • Analysis performed on pancreata recovered postmortem from T1D patients demonstrated the presence of enteroviral group B coxsackievirus vp1 protein.
- 58.Rasilainen S, Ylipaasto P, Roivainen M, et al. Mechanisms of βcell death during restricted and unrestricted enterovirus infection. J. Med. Virol. 2004;72(3):451–461. doi: 10.1002/jmv.20003. [DOI] [PubMed] [Google Scholar]
- 59.Ylipaasto P, Klingel K, Lindberg AM, et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet βcells. Diabetologia. 2004;47(2):225–239. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 60.Drescher KM, Kono K, Bopegamage S, Carson SD, Tracy S. Coxsackievirus B3 infection and Type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology. 2004;329(2):381–394. doi: 10.1016/j.virol.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 61.Green J, Casabonne D, Newton R. Coxsackie B virus serology and Type 1 diabetes mellitus: a systematic review of published case-control studies. Diabet. Med. 2004;21(6):507–514. doi: 10.1111/j.1464-5491.2004.01182.x. [DOI] [PubMed] [Google Scholar]
- 62.Hyoty H, Leinikki P, Reunanen A, et al. Mumps infections in the etiology of Type 1 (insulin-dependent) diabetes. Diabetes Res. 1988;9(3):111–116. [PubMed] [Google Scholar]
- 63.Forrest JM, Menser MA, Burgess JA. High frequency of diabetes mellitus in young adults with congenital rubella. Lancet. 1971;2(7720):332–334. doi: 10.1016/s0140-6736(71)90057-2. [DOI] [PubMed] [Google Scholar]
- 64.Classen JB, Classen DC. Immunization in the first month of life may explain decline in incidence of IDDM in The Netherlands. Autoimmunity. 1999;31(1):43–45. doi: 10.3109/08916939908993858. [DOI] [PubMed] [Google Scholar]
- 65.Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Childhood vaccination and Type 1 diabetes. N. Engl. J. Med. 2004;350(14):1398–1404. doi: 10.1056/NEJMoa032665. [DOI] [PubMed] [Google Scholar]
- 66.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Filippi CM, von Herrath MG. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: viruses, autoimmunity and immunoregulation. Clin. Exp. Immunol. 2010;160(1):113–119. doi: 10.1111/j.1365-2249.2010.04128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sobel DO, Goyal D, Ahvazi B, et al. Low dose poly I:C prevents diabetes in the diabetes prone BB rat. J. Autoimmun. 1998;11(4):343–352. doi: 10.1006/jaut.1998.0203. [DOI] [PubMed] [Google Scholar]
- 69.Sobel DO, Newsome J, Ewel CH, et al. Poly I:C induces development of diabetes mellitus in BB rat. Diabetes. 1992;41(4):515–520. doi: 10.2337/diab.41.4.515. [DOI] [PubMed] [Google Scholar]
- 70.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 2003;197(4):403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutmuller RP, den Brok MH, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 2006;116(2):485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim HS, Han MS, Chung KW, et al. Toll-like receptor 2 senses β-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27(2):321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with Type 1 diabetes: further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2008;93(2):578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zipris D, Lien E, Xie JX, Greiner DL, Mordes JP, Rossini AA. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J. Immunol. 2005;174(1):131–142. doi: 10.4049/jimmunol.174.1.131. [DOI] [PubMed] [Google Scholar]
- 75.Lang KS, Recher M, Junt T, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 2005;11(2):138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 76.von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile? Nat. Rev. Microbiol. 2003;1(2):151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- 77.Marttila J, Juhela S, Vaarala O, et al. Responses of coxsackievirus B4-specific T-cell lines to 2C protein-characterization of epitopes with special reference to the GAD65 homology region. Virology. 2001;284(1):131–141. doi: 10.1006/viro.2001.0917. [DOI] [PubMed] [Google Scholar]
Websites
- 101.nPOD, Network for Pancreatic Organ Donors with Diabetes. www.jdrfnpod.org.
- 102.TEDDY, The Environmental Determinants of Diabetes in the Young. http://teddy.epi.usf.edu.
- 103.The Jackson Laboratory, Incidence study. http://type1diabetes.jax.org/gqc_ incidence_studies.html.