This work examines alterations of epigenetic marks in the phytochrome A locus in response to light and finds that rapid changes in histone methylation and acetylation accompany phyA repression by light.
Abstract
Recent genome-wide surveys showed that acetylation of H3K9 and H3K27 is correlated with gene activation during deetiolation of Arabidopsis thaliana seedlings, but less is known regarding changes in the histone status of repressed genes. Phytochrome A (phyA) is the major photoreceptor of deetiolation, and phyA expression is reversibly repressed by light. We found that in adult Arabidopsis plants, phyA activation in darkness was accompanied by a significant enrichment in the phyA transcription and translation start sites of not only H3K9/14ac and H3K27ac but also H3K4me3, and there was also moderate enrichment of H4K5ac, H4K8ac, H4K12ac, and H4K16ac. Conversely, when phyA expression was repressed by light, H3K27me3 was enriched with a corresponding decline in H3K27ac; moreover, demethylation of H3K4me3 and deacetylation of H3K9/14 were also seen. These histone modifications, which were focused around the phyA transcription/translation start sites, were detected within 1 h of deetiolation. Mutant analysis showed that HDA19/HD1 mediated deacetylation of H3K9/14 and uncovered possible histone crosstalk between H3K9/14ac and H3K4me3. Neither small RNA pathways nor the circadian clock affected H3 modification status of the phyA locus, and DNA methylation was unchanged by light. The presence of activating and repressive histone marks suggests a mechanism for the rapid and reversible regulation of phyA by dark and light.
INTRODUCTION
The nucleosomal organization of chromatin has been known to be an important element in the transcriptional regulation of eukaryotic genes. Core histone tails in nucleosomes can be reversibly modified through multiple posttranslational tags, including acetylation, methylation, ubiquitination or sumoylation of Lys residues, methylation of Arg residues, and phosphorylation of Ser and Thr residues (Strahl and Allis, 2000; Kouzarides, 2007). The impact of these modifications on the activation or repression of plant gene expression is being actively investigated on a genomic basis and for various signaling pathways (Charron et al., 2009; Roudier et al., 2009; Zhang et al., 2009; Zhou, 2009; Liu et al., 2010; Zhou et al., 2010).
After the first identification of VERNALIZATION2 encoding a homolog of one of the Polycomb group proteins (Gendall et al., 2001) and VERNALIZATION INSENSITIVE3 encoding a plant homeodomain protein (Sung and Amasino, 2004), chromatin modification has emerged as a critical regulatory mechanism in Arabidopsis thaliana vernalization. The molecular connection between vernalization and flowering prompted extensive studies on the chromatin status of the FLOWERING LOCUS C (FLC) locus (Kim et al., 2009). Activation of FLC expression prior to vernalization is associated with various activating histone marks such as histone H3 and H4 acetylation (He et al., 2003; Peng et al., 2006), H3K4 methylation (He et al., 2004), H3K36 methylation (Zhao et al., 2005), H2B monoubiquitination (Cao et al., 2008), and H2A.Z deposition (Deal et al., 2007), whereas vernalization-mediated FLC repression is associated with two repressive histone marks, H3K9 and H3K27 methylation (Bastow et al., 2004; Sung and Amasino, 2004).
Histone H3 modifications marked with both K4me3 (active) and K27me3 (repressive) are referred to as “bivalent domains” in embryonic stem cells (Bernstein et al., 2006), and such domains have been proposed to poise genes for rapid activation during differentiation or specific developmental stages. Both FLC and FT carry bivalent marks, H3K4me3 and H3K27me3, suggesting the role of H3K27me3 as a strong repression marker of the Polycomb complex (Finnegan and Dennis, 2008; Jiang et al., 2008).
Light (L) is among the most important environmental factors that impact plant development. When germinated in darkness (D), seedlings are etiolated, having elongated hypocotyls, closed cotyledons, and undifferentiated plastids. Upon exposure to L, seedlings undergo photomorphogenesis, during which hypocotyl elongation is inhibited, etioplasts differentiate into chloroplasts, chlorophyll is synthesized, and the cotyledons become expanded. These L-dependent morphological changes are underpinned primarily by changes in gene expression (Jiao et al., 2007), and it has been estimated that L alters the expression of ~20% of genes in the Arabidopsis genome (Jiao et al., 2005). The genome-wide reprogramming of gene expression during photomorphogensis suggests regulation at the chromatin level, including changes in histone modifications. Indeed, several reports have appeared on histone modifications of photosynthetic genes mediated by L. Chua et al. (2001, 2003) found that shoot-specific and L-induced accumulation of pea (Pisum sativum) PetE transcripts is associated with histone H3 and H4 acetylation in the PetE enhancer/promoter region. Genetic studies of Arabidopsis mutants deficient in histone acetyltransferase (TAF1/HAF2 and GCN5) and histone deacetylase (HDA19/HD1) provided evidence for the importance of histone acetylation/deacetylation in the expression of a number of photosynthetic genes (Bertrand et al., 2005; Benhamed et al., 2006). Subsequent genome-scale screening of target promoters bound by GCN5 identified early L-responsive genes similar to those targeted by HY5 (Lee et al., 2007; Benhamed et al., 2008). Recently, Charron et al. (2009) surveyed genome-wide distributions of four histone modifications (H3K9ac, H3K9me3, H3K27ac, and H3K27me3) during Arabidopsis seedling deetiolation and found acetylation of H3K9 but not H3K27 of the HY5 and HYH loci upon L activation.
In contrast to genes that are induced by L, there is also a group of genes that are repressed by L. A notable example is phytochrome A (phyA), which encodes the major photoreceptor mediating far-red (FR) signaling. PhyA accumulates in D as the inactive, stable Pr form, but upon illumination it is converted to the active Pfr form (Sharrock and Clack, 2002), which is rapidly degraded by the COP1 E3 ligase (Seo et al., 2004). Along with phyA depletion in the L, phyA transcript levels are also strongly repressed by both FR and red (R) light (Cantón and Quail, 1999), suggesting L-mediated transcriptional regulation through both phyA and phyB photoreceptors. The negative regulation of phyA by L has been known for more than two decades; therefore, possible changes in phyA chromatin status during the D/L transition are of great interest.
Here, we confirmed that phyA transcript was repressed by L in a reversible manner and found that changes in transcript levels were correlated with alterations in specific histone marks. In the dark, acetylation of histones H3 (K9/14 and K27) and H4 (K5, K8, K12, and K16) and methylation of histone H3 (K4me3) were detected at the active phyA locus, whereas upon L treatment, increased H3K27me3 mark was associated with the repressed gene. The activating H3K4me3 mark and the repressive H3K27me3 mark were enriched around phyA transcription/translation start sites. The presence of these opposing marks enables rapid activation and inactivation of phyA in response to changing L conditions. Deacetylation of H3K9/14ac in the L was mediated by HISTONE DEACETYLASE19, and there is a coordinated behavior between H3K9/14ac and H3K4me3 suggesting crosstalk between these two marks. Finally, we found that that L-mediated phyA repression is regulated by chromatin remodeling without DNA methylation and without any participation of small RNA pathways.
RESULTS
PhyA Regulation in Adult Plants
We first used 2-week-old Arabidopsis plants to study global chromatin changes associated with D/L transitions. In general, there was an increase in histone H3 tail modifications in L compared with D, and notable changes are found with K9/K14ac, K4me2, K4me3, K27me3, and K36me3 (see Supplemental Figure 1 online). Time-course experiments during seedling deetiolation showed that the changes in H3 modification were rapid, as they can be detected even after only 1 h of L exposure; moreover, enrichments of both activating marks (e.g., K9/14ac and K4me3) and repressive marks (K27me3) were seen. These modifications reflect the sum of all histone H3 changes associated with both upregulation and downregulation of gene expression upon L treatment.
To investigate alterations in histone modifications specifically associated with phyA expression, we used 2-week-old plants grown under the same conditions. At the end of the L period (L1), plants were kept in D for 24 h (D1), transferred to L for 24 h (L2), and then returned to D for another 24 h (D2). Figure 1A shows that phyA transcript levels were clearly repressed by L in a reversible manner, confirming previous results (Cantón and Quail, 1999). The difference in transcript levels between D and L was approximately five to seven times.
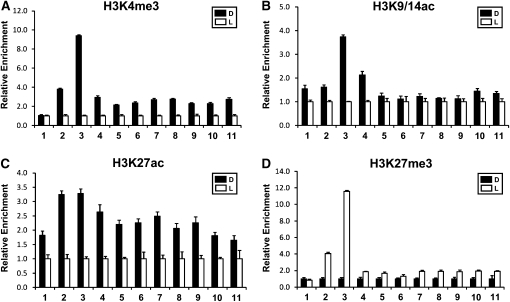
Figure 1.
Changes of Histone Modifications on phyA in the D/L Transition.
(A) phyA expression levels in the D/L transition. Two-week-old Arabidopsis plants grown under long-day conditions (16 h of L/8 h of D) were harvested at the end of the L period (L1) and followed by D/L treatment for 24-h intervals (D1, L2, and D2). PhyA expression levels were determined by quantitative RT-PCR with a gene-specific primer set.
(B) Schematic diagram of phyA gene structure. Promoter (P), exons (e1, e2, e3, and e4), and 5′ UTR (5′U) and 3′ UTR (3′U) are represented by black, gray and white boxes, respectively. The amplification sites for ChIP analyses are indicated as numbers (1–11) with black bars below the map. ATG is the translation start site.
(C) RNA Pol II binding affinity on the phyA locus (regions 3, 7, and 11) during the D/L transition. The relative enrichment of RNA Pol II binding affinity was determined using anti-Pol II antibody with region-specific primers.
(D) and (E) Relative levels of histone H3 modifications (K4me2, K4me3, K9/14ac, K9me2, K9me3, K27ac, K27me3, and K36me3) at region 3 (D) and region 7 (E) in the D/L transition.
(F) Relative levels of histone H3 acetylation (K9ac and K14ac) in the D/L transition.
(G) Relative levels of histone H4 acetylation (K5ac, K8ac, K12ac, and K16ac) in the D/L transition.
Error bars indicate sd (n = 3).
Arabidopsis encodes only a single phyA gene of ~4 to 5 kb (Sharrock and Quail, 1989). We designed primers to interrogate 11 different regions of this phyA genomic locus from the promoter to the 3′ untranslated region (UTR; Figure 1B). Using polymerase II (Pol II)–specific antibodies, we detected specific enrichment of Pol II with region 3 in D as compared with L samples. Note that region 3 covers the genomic region just upstream of the ATG start codon. No Pol II enrichment was detected in regions 7 and 11, which correspond to the 3′ end of exon1 and the 3′ UTR, respectively. These results suggest that the reversible increase in phyA transcript levels in D is likely associated with Pol II–mediated transcription.
To investigate changes in chromatin status accompanying L repression and D activation of phyA, we performed chromatin immunoprecipitation (ChIP) using a battery of antibodies specific for modifications on H3 and H4. We focused on histone H3 changes in region 3 using regions 7 and 11 as negative controls. Figure 1D shows that D activation of phyA was accompanied by an enrichment (2.0- to 4.0-fold) of H3K4me3 and H3K9/14ac in region 3. This is in contrast to the general global increase of H3K4me3 and H3K9/14ac in L (see Supplemental Figure 1 online). The use of specific antibodies revealed increased levels of both H3K9ac and H3K14ac (Figure 1F). There was also a clear enrichment of H3K27ac and a corresponding depletion of H3K27me3 in D as compared with L samples. No significant and reproducible changes were detected with H3K4me2, H3K9me2, H3K9me3, and H3K36me3 levels. Small changes (<1.4-fold) in H3 marks in response to D and L were also seen in regions 7 and 11, but these were not considered to be significant (Figure 1E; see Supplemental Figure 2 online). We also examined possible H4 changes in region 3 and found only moderate enrichment (1.5- to 2.0-fold) of K5ac, K8ac, K12ac, and K16ac marks in D as compared with L (Figure 1G).
We compared changes of four key histone H3 marks (K4me3, K9/14ac, K27ac, and K27me3) over the 11 regions of the phyA locus between D and L samples. Figure 2 shows that D activation of phyA was clearly associated with an enrichment of H3K4me3 (four- to ninefold) in regions 2 and 3 and H3K9/14ac (two- to fourfold) in regions 3 and 4. Note that region 2 includes the phyA promoter, whereas region 3 spans the ATG translation start site. A strong enrichment of H3K4me3 (fourfold) but not H3K9/14ac (1.5-fold) was also found in region 2, which includes the phyA promoter region. The enrichment of the H3K27ac mark (1.8- to 3.3-fold) was detected throughout all phyA regions but with a maximum in regions 2 and 3 (3.3-fold). By contrast, there was a 12-fold enrichment of H3K27me3 mark in region 3 and a fourfold enrichment of the same mark in region 2 upon silencing of phyA by L, indicating the importance of this repressive mark in phyA downregulation.
Figure 2.
Enrichment of H3K4me3, H3K9/14ac, H3K27ac, and H3K27me3 at the phyA Locus.
Relative levels of H3K4me3 (A), H3K9/14ac (B), H3K27ac (C), and H3K27me3 (D) at the phyA locus. Two-week-old Arabidopsis plants were grown under long-day conditions (16 h of L/8 h of D). Plants at the end of a L period were incubated in the D for 24 h (D) and then treated with white light for 24 h (L). The numbers on the x axis indicate the positions of the PCR-amplified sites described in Figure 1B. Error bars indicate sd (n = 3).
PhyA Regulation during Deetiolation
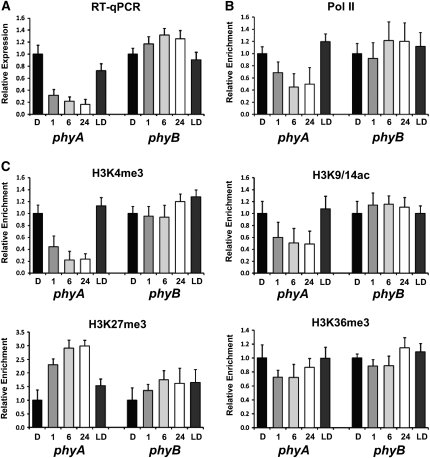
Experiments with adult plants showed that the most prominent changes in the chromatin status of phyA during the D/L transition occurred around region 3 and, to a lesser extent, in region 2. As an important photoreceptor for seedling deetiolation, phyA transcript level is high in etiolated seedlings but rapidly declines upon exposure to L. We investigated whether chromatin changes would correlate with rapid phyA repression by L during seedling deetiolation. Figure 3A confirms a previous report (Cantón and Quail, 1999) that phyA transcripts accumulated in etiolated seedlings, but phyA was rapidly down-regulated upon irradiation with white light. However, this decrease in transcript level was reversed when seedlings were returned to D for 24 h (Figure 3A). By contrast, transcript levels of phyB, which was used as a negative control gene, were slightly increased in L as compared with D. The downregulation of phyA was accompanied by a decrease in Pol II association with region 3, suggesting reduced transcription (Figure 3B). The L-mediated repression of phyA was accompanied by a decrease in H3K4me3- and H3K9/14ac activating marks but an increase in H3K27me3 repressive mark (Figure 3C). These chromatin changes were readily detected after one hour of deetiolation, and they reached a steady state after 6 h of L. The changes were reversed when seedlings were returned to D for 24 h. No significant changes in these histone marks were seen in phyB, whose transcript levels only increased moderately in L. We have also checked Pol II enrichment and histone modifications in two different regions (regions 7 and 11 shown in Figure 1B) on the phyA locus. The enrichment of Pol II and histone modifications in region 7 showed similar patterns to those of region 3, but the fold change was less than 1.5 (see Supplemental Figures 3A and 3B online). No Pol II enrichment and changes of histone modifications were detected in region 11 (see Supplemental Figures 3A and 3C online).
Figure 3.
Changes of Histone Modifications on phyA during Deetiolation.
(A) Relative phyA and phyB expression levels during deetiolation. Four-day-old etiolated Arabidopsis seedlings (D) were treated with white light (100 μmol/m2/s) as indicated (1, 6, and 24 h) and transferred to D (LD) for 24 h. Expression levels were determined by quantitative RT-PCR with gene-specific primer sets.
(B) Relative RNA Pol II binding affinity on phyA and phyB during deetiolation. The relative enrichment of RNA polymerase binding affinity at region 3 of the phyA locus was determined using anti-Pol II antibody.
(C) Relative levels of histone H3 modifications (K4me3, K9/14ac, K27me3, and K36me3) on phyA and phyB during deetiolation. The relative enrichment of histone H3 modifications at region 3 of the phyA locus was determined by ChIP assays using specific antibodies. Amplification sites of phyB for ChIP assay are similar to those of phyA.
Error bars indicate sd (n = 3).
PhyA Regulation during D/L Diurnal Cycles
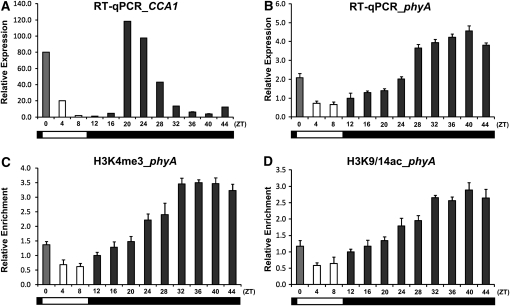
We also investigated phyA chromatin changes in 2-week-old plants grown under short-day conditions (8 h of L/16 h of D). At the end of a photoperiod, plants were kept in continuous D for another 24 h and samples were collected at 4-h intervals. Control experiments showed that transcript levels of CCA1 decreased during the subjective L, as expected for this circadian regulated gene (Figure 4A). PhyA transcript levels were higher (~2.5-fold) in the D compared with the L period (Figure 4B). However, under prolonged D, phyA transcript levels continued to increase during the subjective L period. At the end of the second D period, phyA transcript levels were four to five times that at the beginning of the L period. These results clearly indicate that in contrast to CCA1, phyA is regulated by D/L only but not by the circadian clock. The increase in phyA transcript levels in D was accompanied by a three- to fourfold enrichment of H3K4me3 and H3K9/14ac marks in region 3 (Figures 4C and 4D), but no significant changes of these modifications were seen in regions 7 and 11 (see Supplemental Figures 4A and 4B online).
Figure 4.
Changes of Histone Modifications on phyA during the Diurnal Cycle.
(A) and (B) Relative CCA1 (A) and phyA (B) expression levels during the diurnal cycle. Two-week-old Arabidopsis plants were grown under short-day conditions (8 h of L/16 h of D), transferred to D for 24 h, and sampled at 4-h intervals. Expression levels were determined by quantitative RT-PCR with gene-specific primer sets.
(C) and (D) Relative levels of histone H3 modifications, K4me3 (C) and K9/14ac (D), of phyA during the diurnal cycle. The relative enrichment of histone H3 modifications on region 3 of the phyA locus was determined by ChIP assays using specific antibodies.
Error bars indicate sd (n = 3).
PhyB Signals phyA Chromatin Changes
The rapid repression of phyA expression has been reported to be regulated by both FR and R (Cantón and Quail, 1999). Figure 5A shows that phyA repression by R was blocked in the phyB-9 mutant, consistent with the major role of the phyB photoreceptor in R responses. ChIP experiments showed that the continued high phyA expression in phyB-9 in R was accompanied by a relative increase of H3K4me3, H3K9/14ac, and H3K27ac activating marks in the phyA region 3 in the phyB-9 mutant as compared with the wild-type ecotype Columbia (Col-0; Figure 5B). Moderate increases (less than 1.5-fold) of these modifications were found in the phyA region 7 of the phyB-9 mutant, but there were no differences in the phyA region 11 between the wild type and the phyB-9 mutant (see Supplemental Figures 5A and 5B online).
Figure 5.
Enrichment of H3K4me3, H3K9/14ac, and H3K27ac at the phyA locus in the phyB Mutant Exposed to R.
(A) Relative phyA expression level in Col-0 and phyB mutant under FR or R. Four-day-old etiolated Arabidopsis seedlings were treated with FR (5 μmol/m2/s) or R (20 μmol/m2/s) as indicated (3, 9, and 24 h). Expression levels were determined by quantitative RT-PCR with a phyA-specific primer set.
(B) Relative levels of active histone H3 modifications (K4me3, K9/14ac, and K27ac) of phyA (region 3) in Col-0 and the phyB mutant after R (20 μmol/m2/s) treatment for 24 h.
Error bars indicate sd (n = 3).
[See online article for color version of this figure.]
Deacetylation of H3K9/14 on the phyA Locus Depends on HDA19/HD1
The observation that phyA repression is accompanied by a decrease in acetylated H3K9/14 levels suggests that L mediates deacetylation of K9 and K14 on histone H3 tails. The Arabidopsis genome encodes a number of histone acetylases and deacetylases. At least three such enzymes are related to L responses, since mutants deficient in GCN5 and TAF1 have been shown to be hyposensitive to L whereas a mutant lacking in HDA19/HD1 is hypersensitive (Bertrand et al., 2005; Benhamed et al., 2006).
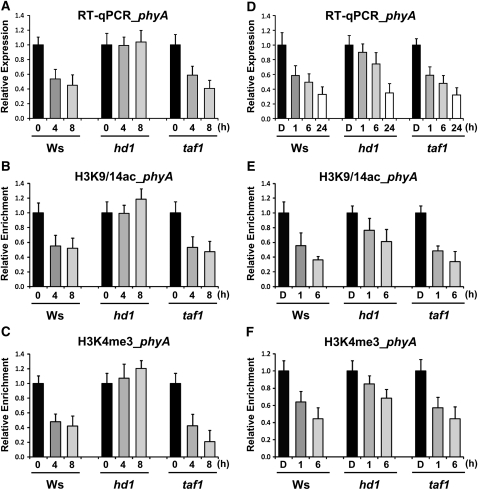
To investigate possible functions of these genes in regulating phyA expression, we compared phyA transcript levels of adult plants of hda19/hd1 and taf1 with those of the wild-type (Wassilewskija [Ws]) control. Figure 6A shows that L-dependent repression of phyA expression in taf1 was similar to that in the wild-type (Ws) control but blocked in hda19/hd1. Consistent with this result, H3K9/14 acetylation was sustained at the D level in hda19/hd1 even after 8 h of L exposure (Figure 6B), although in the wild type (Ws) and taf1, the H3K9/14 acetylation level declined to ~50% of the D level after the same L regimen. Further analysis showed that L-mediated demethylation of H3K4me3 occurred in taf1 similar to the wild-type (Ws) control (Figure 6C), but surprisingly, the demethylation of H3K4me3 was blocked in hda19/hd1 as well, which lacks HISTONE DEACETYLASE19. Our result shows that HDA19/HD1 but not TAF1 regulates phyA expression through deacetylation of H3K9/14. This finding confirms a previous report of an enrichment of H3K9ac associated with two photosynthetic genes in the hda19/hd1 mutant compared with the wild type (Benhamed et al., 2006). No significant changes in H3 marks in regions 7 and 11 on the phyA locus were seen (see Supplemental Figures 6A and 6B online).
Figure 6.
Changes of Histone Modifications on phyA in hda19/hd1 and taf1 Compared with the Wild Type (Ws).
(A) Relative phyA expression level in Ws, hda19/hd1, and taf1 under the D/L transition. Two-week-old Arabidopsis plants were grown under short-day conditions (8 h of L/16 h of D), harvested at the end of the D period (0), and sampled at 4-h intervals (4 and 8) under L conditions. The expression level of phyA was determined by quantitative RT-PCR.
(B) and (C) Relative levels of histone H3 modifications, K9/14ac (B) and K4me3 (C), of phyA (region 3) in Ws, hda19/hd1, and taf1 under the D/L transition.
(D) Relative phyA expression level in Ws, hda19/hd1, and taf1 during deetiolation. Four-day-old etiolated Arabidopsis seedlings (D) were treated with white light as indicated (1, 6, and 24 h). The expression level of phyA was determined by quantitative RT-PCR.
(E) and (F) Relative levels of histone H3 modifications, K9/14ac (E) and K4me3 (F), of phyA (region 3) in Ws, hda19/hd1, and taf1 during deetiolation.
Error bars indicate sd (n = 3).
To see if HDA19/HD1 plays a similar role in phyA repression in young seedlings, we also investigated the responses of hd1 and taf1 during deetiolation, and essentially similar results were obtained. The kinetics of L-mediated phyA repression in taf1 and the wild type (Ws) during deetiolation were similar, and after 6 h of L phyA transcript levels decreased to ~50% of the D level (Figure 6D). Similar time-course analysis shows that the L-dependent decline in phyA expression was significantly delayed although not totally arrested in hda19/hd1. After 6 h in L, phyA transcript level was ~70% of the D value. These differences in phyA expression in L between hd1 and the wild type (Ws) and taf1 were paralleled by differences in H3K9/14ac and H3K4me3 levels (Figures 6E and 6F). The less severe effect of the hda19/hd1 mutation on phyA repression during deetiolation may be explained if there is functional redundancy contributed by other histone deacetylases at this stage of plant development.
Dynamic Chromatin Changes Not Linked to Changes in DNA Methylation Status
We investigated possible linkage between D/L-mediated changes in phyA chromatin modifications and small RNA pathways. Supplemental Figure 7A online shows that a number of mutants (rdr2, dcl3-1, ddc, nrpd1a-2, and nrpd1b-1) deficient in small RNA pathway components were not significantly different from the wild-type control with respect to their D/L regulation of phyA. H3K4me3 changes accompanying D activation of phyA were clearly detected in dcl3-1 and nrpd1a-2 as in the wild-type control (see Supplemental Figure 7B online).
Chawla et al. (2007) previously reported transcriptional silencing of the endogenous phyA gene in transgenic plants carrying a transgenic fragment of phyA. In these plants, they observed methylation of certain phyA exonic regions, suggesting a correlation between phyA repression and DNA methylation. Therefore, we investigated whether the repression of phyA by L was accompanied by any DNA methylation. We performed sequencing of bisulfite-treated DNA collected from D and L samples and examined possible alterations in DNA methylation in six different regions of phyA (Figure 7; see Supplemental Figures 8 and 9 online). No significant changes in DNA methylation were detected between D and L samples in which phyA transcript levels were altered by six- to sevenfold (Figures 1A, D1 and L2, and 7; see Supplemental Figures 8 and 9 online). From these results, we conclude that reversible DNA methylation does not play a role in changes of phyA transcript levels during the D/L transition.
Figure 7.
Comparison of DNA Methylation Status of the phyA Locus in the D/L Transition.
(A) Schematic diagram of partial phyA gene structure showing the promoter (P), 5′ UTR (5′U), and exon 1 (e1). Black bars below the map represent regions analyzed by bisulfite sequencing. Each fragment was amplified using bisulfite-modified genomic DNA and inserted into pCR2.1-TOPO vector. P1 and P1, Promoter regions; U1 and U2, 5′ UTRs; E1 and E2, exon 1 regions.
(B) Analysis of average cytosine methylation (CG, CHG, and CHH) in the D/L transition. Sequences of 10 to 25 clones for each region were analyzed, and the results are shown in Supplemental Figure 9 online. H = A, T, or C.
DISCUSSION
PhyA plays an important role in early seedling development by mediating photomorphogenesis when germinated seedlings just emerge from the soil surface. Highly expressed in etiolated seedlings, this photoreceptor is rapidly downregulated by L both at the transcript (Cantón and Quail, 1999) and the protein levels (Seo et al., 2004). Although the L-mediated repression of phyA has been known for more than a decade, little is known regarding the molecular mechanisms of this repression. Here, we have focused on investigating chromatin changes on the phyA locus for three reasons: (1) phyA is the major photoreceptor for deetiolation; (2) its expression is repressed rather than activated by L; and (3) the phyA locus is longer than 5 kb, which allows more detailed investigations of changes in histone modifications along different genomic regions.
We show that the reversible activation and repression of phyA expression by D and L, respectively, is accompanied by reversible changes in H3 and H4 modifications of the phyA locus, particularly in regions surrounding the translation and transcription start sites. Because these histone modifications were seen when phyA transcript levels were altered under three different sets of conditions (16 h of L/8 h of D, exposure of etiolated seedlings to continuous L, and 8 h of L/16 h of D) irrespective of the growth stage of the plants, they must be related to the effects of L regulation on phyA expression. In contrast to previous work (Charron et al., 2009), we performed time-course studies to obtain information on the dynamics of expression changes and correlated these with changes in histone modifications.
Activating Histone Marks
Four histone modifications, H3K4me3, H3K9ac, H3K14ac, and K3K27ac, were found to accompany phyA activation in D. Comparison of D and L samples showed that these marks were enriched by at least three- to ninefold. In addition, moderate enrichment (1.5- to 2.0-fold) of H4K5ac, H4K8ac, H4K12ac, and H4K16ac was also seen. H3K4me3, H3K9ac, and H3K14ac have been linked to active gene transcription in yeast and mammalian cells (Li et al., 2007) and more recently with activation of plant genes as well (Pfluger and Wagner, 2007; Kim et al., 2010).
With respective to L-responsive genes, activation of HY5 and HYH during deetiolation is accompanied by a significant increase in H3K9ac (Charron et al., 2009), which is distributed over the entire transcribed region. By contrast, there is no detectable difference in H3K27ac levels associated with these two genes in D and L, and possible changes in H3K4me3 were not investigated. Notwithstanding the opposing mode of regulation, D activation of phyA was marked by an increase of not only H3K9ac but also H3K27ac. In addition, we observed an enrichment of H3K4me3 (10 fold), which was higher than that (three- to fourfold) found with H3K9/14ac and H3K27ac. Based on the relative enrichment, we suggest that H3K4me3 may play a more important role in phyA activation than the other two histone marks.
Charron et al. (2009) have raised a possible fundamental difference between yeast/mammalian and plant genes regarding the location of the activating H3K9ac mark. In yeast and mammals, H3K9ac is enriched around the gene promoter region, whereas in plants, H3K9ac and H3K27ac are found around the translation rather than transcription start site of active genes (Kim et al., 2008; Charron et al., 2009; Wang et al., 2009). Our results here are consistent with this finding, since not only H3K9/14ac but also the activating H3K4me3 mark are more enriched at the genomic region surrounding the translation start site (region 3), although significant changes were observed around the transcriptional start site as well (region 2). The case of H3K27ac deserves special comment. In contrast to the other activating marks, there was a general increase of H3K27ac along the entire transcribed region of active phyA, with a moderate peak surrounding the transcription and translation start sites. This result suggests that H3K27ac may be important for transcriptional elongation as well as transcriptional initiation.
At this moment, it is not clear whether all four activating histone marks (H3K4me3, H3K9/14ac, and H3K27ac) are needed incrementally for full phyA transcription or if they function redundantly in some combinatorial fashion. In mammalian cells, phosphorylation of H3S10 affects acetylation of H3K9 and H3K14 in an opposite manner and inhibiting acetylation at H3K14 blocks trimethylation of H3K4 (Latham and Dent, 2007; Lee et al., 2010). Whether such crosstalk among histone modifications operates at the phyA locus is an interesting regulatory issue for future investigation.
Other than H3 modifications, we have also observed changes in H4 acetylation at K5, K8, K12, and K16, which were rather modest (1.5- to 2.0-fold) compared with those obtained with H3 modifications. Further experiments are needed to assess the contributions of these H4 modifications to phyA regulation by D/L.
Repressive Histone Marks
A simple model for reversible D/L regulation of phyA chromatin would be to assume erasure of activating histone marks along with L-mediated inactivation of phyA. Although deacetylation of H3K9/14 and H3K27 was indeed seen, we also found that phyA repression was an active process reinforced by a big increase (12-fold) of the H3K27me3 repressive mark. Genome-wide analyses of Arabidopsis have established the importance of H3K27me3 as a major mechanism for silencing of a large number of genes (Turck et al., 2007; Oh et al., 2008; Zhang et al., 2008, Charron et al., 2009). With respect to L-responsive genes, Charron et al. (2009) noted that photosynthetic genes regulated by HY5 and HYH are targeted by acetylation of H3K9 and H3K27 but not by H3K9me3 or H3K27me3. On the other hand, they also found that two genes involved in GA metabolism, GA2ox7 and GA3ox2, that are induced during deetiolation displayed reduced H3K27me3 marks in L. These results suggest the importance of H3K27me3 in restraining GA2ox7 and GA3ox2 expression in D, similar to its role in silencing phyA expression in L.
It is significant that the decrease in H3K9ac was not accompanied by an increase in the repressive H3K9me3 mark known to be associated with 40% of Arabidopsis genes (Turck et al., 2007). This is perhaps not surprising, as H3K9me3 is associated mostly with heterochromatin, whose silencing depends on small RNA pathways and associated DNA methylation. Here, we present clear evidence that neither small RNA pathways nor DNA methylation is involved in phyA repression.
The presence of both activating and repressive histone marks on phyA suggests a mechanism to ensure rapid turnoff in the L during deetiolation and transcriptional reactivation when plants are returned to D.
Relationship to Histone-Modifying Enzymes
The importance of H3K4me3, H3K9/14ac, H3K27ac, and H3K27me3 in D/L regulation of the phyA locus implicates enzymes that catalyze reversible acetylation/deacetylation and methylation/demethylation in this process. One working hypothesis is to assume that in D-appropriate histone acetyltransferases, methyltransferases, and demethylases are recruited to the phyA locus to demethylate H3K27me3, to acetylate H3K9/14 and H3K27, and to methylate H3K4. In L, similar enzymes are recruited to deacetylate H3K9/14ac and H3K27ac, demethylate H3K4me3, and methylate H3K27.
Since chromatin modifications associated with phyA repression are blocked in the phyB-9 mutant in red light, these changes must be mediated by the phyB signaling pathway, although the precise mechanism is unknown at present.
The Arabidopsis genome encodes a large number of histone acetyltransferases/deacetylases and methyltransferases/demethylases; however, the functions of individual enzymes might be hard to determine because of possible functional redundancy. Nevertheless, among the acetylation/deacetylation enzymes, the roles of GCN5, TAF1/HAF2, and HDA19/HD1 in L response have been shown by genetic studies (Bertrand et al., 2005; Benhamed et al., 2006). Mutants deficient in GCN5 and TAF1 are hyposensitive to L with respect to hypocotyl elongation, whereas the reverse is true of the hda19/hd1 mutant. A simple and straightforward interpretation of these results is that GCN5 and TAF1 are positive regulators whereas HDA19/HD1 is a negative regulator of L responses. For example, GCN5 and/or TAF1/HAF2 might acetylate H3K9/14 in D to activate phyA transcription, whereas HDA19/HD1 might deacetylate H3K9/l4ac in L to repress phyA expression. Indeed, we found that hda19/hd1 is unable to down-regulate phyA expression in L. Consistent with the higher phyA expression in hda19/hd1 in L, mutant seedlings display L hypersensitivity compared with wild-type seedlings (Benhamed et al., 2006).
Our analysis of histone modifications in hda19/hd1 uncovered coordinated behavior between the two activating marks, H3K9/14ac and H3K4me3. The mutant hda19/hd1 is impaired in deacetylation of H3K9/14 in L, which can be attributed to a deficiency of HISTONE DEACETYLASE19. However, to our surprise, this mutant is also unable to demethylate H3K4me3, whose levels in L are about the same as those in D. This result suggests that deacetylation of H3K9/14ac is required for H3K4me3 demethylation. Whether demethylation of H3K4me3 is also needed for deacetylation of H3K9/14ac remains to be investigated with an appropriate mutant. Crosstalk between H3K4me3 and H3K9/14ac as well as between other histone marks has been reported previously in yeast and human cells (Latham and Dent, 2007; Lee et al., 2010).
Recent evidence shows that in Arabidopsis, the chromodomain of LHP1 binds to H3K27me3 of target genes to repress transcription (Turck et al., 2007; Exner et al., 2009). Since the phyA locus in L is enriched in H3K27me3, according to this scenario, its reactivation in D during D/L transitions must entail the removal of the effector LHP1 to unmask H3K27me3 so as to enable the demethylation of the latter followed by subsequent acetylation of H3K27. If this model is correct, we would expect lhp1 mutants to have less efficient repression and therefore display higher phyA expression levels in L compared with the wild type. Elucidating the possible role of LHP1 and other histone-modifying enzymes in phyA expression will lead to a better understanding of how changes in chromatin structure modify gene expression in response to D/L.
METHODS
Plant Materials and Growth Conditions
We used Arabidopsis thaliana wild type (Col-0 and Ws), phyB-9, hd1, taf1, rdr2, dcl3-1, ddc, nrpd1a-2, and nrpd1b-1 as plant materials (Reed et al., 1993; Xie et al., 2004; Pontier et al., 2005; Benhamed et al., 2006; Chan et al., 2006). All mutants were in the Col-0 background except hd1 and taf1 in the Ws background. Sterilized seeds were incubated on Murashige and Skoog medium supplemented with 1% Suc at 4°C for 4 d and transferred to long-day, short-day, or continuous D conditions. Seedlings were grown for 14 to 21 d in a growth chamber under long-day (16 h of L/8 h of D) or short-day (8 h of L/16 h of D) conditions from cool-white fluorescent light (100 μmol/m2/s). For deetiolation, sterilized seeds were germinated on Murashige and Skoog medium for 4 d in D after incubation for 4 d at 4°C and then transferred to continuous white light (100 μmol/m2/s), FR (5 μmol/m2/s), or R (20 μmol/m2/s) for the time periods described in the figure legends.
RNA Extraction and Quantitative RT-PCR
Total RNA isolated from Arabidopsis seedlings using Qiagen RNeasy plant mini kits were treated with RNase-free DNase (Qiagen) on column. After quantification of RNA, 1 μg of total RNA was used for cDNA synthesis using an oligo(dT) and SuperScript III RT kit (Invitrogen). The cDNA was quantified using SYBR Premix Ex Taq (TaKaRa) with gene-specific primers in a Bio-Rad CFX96 real-time system. ACTIN2 was used as an internal control. The primers used for real-time PCR are listed in Supplemental Table 1 online. Real-time quantitative PCR was repeated with two to five biological replicates, and each sample was assayed in triplicate by PCR. Error bars in each graph indicate sd of three technical repetitions. ACTIN2 was used as an internal control for normalization in each quantitative PCR experiment.
ChIP Assay
About 3 g of seedlings was used for ChIP assay. Chromatin preparation and immunoprecipitation were performed as described (Gendrel et al., 2005). Harvested seedlings were fixed in 1% formaldehyde for 10 min in a vacuum. Gly was added to a final concentration of 0.125 M, and the reaction was terminated by incubation for 5 min in a vacuum. Seedlings were rinsed three times with distilled water and frozen in liquid nitrogen. After isolation, chromatin was sheared to ~500-bp fragments by sonication (Diagenode Bioruptor UCD-200). Immunoprecipitation was performed by adding specific antibody in an extract and protein A or G agarose/salmon sperm DNA (Millipore). The antibodies used for ChIP assays were anti-H3K4me2 (Millipore; 07-030), anti-H3K4me3 (Active Motif; 39,159), anti-H3K9/14ac (Millipore; 06-599), anti-H3K9ac (Millipore; 07-352), anti-H3K14ac (Millipore; 07-353), anti-H3K9me2 (Active Motif; 39,239), anti-H3K9me3 (Active Motif; 39,161), anti-H3K27ac (Millipore; 07-360), anti-H3K27me3 (Millipore; 07-449), anti-H3K36me3 (Abcam; ab9050), anti-H4K5ac (Millipore; 07-327), anti-H4K8ac (Millipore; 07-328), anti-H4K12ac (Millipore; 07-595), anti-H4K16ac (Millipore; 07-329), and anti-RNA polymerase II (Santa Cruz Biotechnology; sc-33754). After washing, immune complexes were eluted from the protein A or G beads and reverse cross-linked by incubation for at least 6 h at 65°C. Samples were treated with proteinase K for 1 h at 65°C. DNA was extracted in a final volume of 80 μL using the QIAquick PCR purification kit (Qiagen). Chip assay was repeated with two to five biological replicates. One microliter of DNA was used for each real-time quantitative PCR with SYBR Premix Ex Taq (TaKaRa) in the CFX96 real-time system (Bio-Rad). Each sample was assayed in triplicate by PCR. Error bars in each graph indicate sd of three technical repetitions. We used ACTIN2 as internal control for H3K4me2, H3K4me3, H3K9/14ac, H3K9ac, H3K14ac, H3K27ac, H3K36me3, H4K5ac, H4K8ac, H4K12ac, H4K16ac, and anti-RNA polymerase II and Ta3 for H3K9me2, H3K9me3, and H3K27me3. Primers used for ChIP assays are listed in Supplemental Table 2 online.
Genomic DNA Isolation, Bisulfite Sequencing, and DNA Methylation Analysis
Methylation status of the phyA locus in the D/L transition was evaluated by bisulfite sequencing. Two-week-old Arabidopsis plants grown under long-day conditions (16 h of L/8 h of D) were used. Plants at the end of a L period were incubated in D for 24 h and then treated with white light (100 μmol/m2/s) for 24 h. Genomic DNA was isolated from ~1 g of plant materials treated with continuous D or L for 24 h using the DNeasy plant maxi kit (Qiagen) according to the manufacturer’s instructions. Two micrograms of genomic DNA was used for bisulfite treatment using the EpiTect bisulfite kit (Qiagen). Bisulfite DNA conversion was performed in a thermocycler according to the manufacturer’s protocol. For each PCR, 1 μL of bisulfite-treated genomic DNA was used with degenerate primers for amplification of the selected regions of the phyA locus. PCR products were inserted into pCR2.1-TOPO (Invitrogen) for blue/white selection. Fifteen to 30 white clones were selected for each sample and then sequenced with M13 forward or M13 reverse primer. The sequences were analyzed using the CyMATE program (http://www.gmi.oeaw.ac.at/en/cymate-index). The primers for sequencing of bisulfite-treated DNAs are listed in Supplemental Table 3 online.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: phyA (At1g09570), phyB (At2g18790), CCA1 (At2g46830), ACT2 (At5g09810), and Ta3 (At1g37110).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Immunoblot Analyses Showing Histone H3 Modifications during Deetiolation or the D/L Transition.
Supplemental Figure 2. Relative Levels of Histone H3 Modifications (K4me2, K4me3, K9/14ac, K9me2, K9me3, K27ac, K27me3, and K36me3) at Region 11 Shown in Figure 1B in the D/L Transition.
Supplemental Figure 3. Changes of Histone Modifications at Regions 7 (B) and 11 (C) of the phyA Locus during Deetiolation.
Supplemental Figure 4. Changes of Histone Modifications at Regions 7 (A) and 11 (B) of the phyA Locus during the Diurnal Cycle.
Supplemental Figure 5. Enrichment of H3K4me3, H3K9/14ac, and H3K27ac at Regions 7 (A) and 11 (B) of the phyA Locus in the phyB Mutant Exposed to R.
Supplemental Figure 6. Changes of Histone Modifications at Regions 7 (A) and 11 (B) of the phyA Locus in hda19/hd1 and taf1 Compared with the Wild Type (Ws).
Supplemental Figure 7. phyA Expression Levels in RNA–Directed DNA Methylation RdDM-Related Mutants.
Supplemental Figure 8. Cytosine Methylation in the Promoter, 5′ UTR, and Exonic Region of phyA in the D/L Transition.
Supplemental Figure 9. DNA Methylation Analyses of the phyA Locus in the D/L Transition Using Bisulfite Sequencing.
Supplemental Table 1. Primers for Real-Time Quantitative PCR.
Supplemental Table 2. Primers for ChIP Assays.
Supplemental Table 3. Degenerate Primers for Bisulfite Sequencing.
Acknowledgments
We thank Dao-Xiu Zhou for hd1 and taf1. We thank Sanghee Kim for setting up ChIP protocols in the laboratory and Rossana Henriques for discussion. C.J. was supported by the Korea Research Foundation funded by the Korean Government (Grant KRF-2008-357-F00008). This work was supported by National Institutes of Health (Grant GM 44640 to N.-H.C.).
References
- Bastow R., Mylne J.S., Lister C., Lippman Z., Martienssen R.A., Dean C. (2004). Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427: 164–167 [DOI] [PubMed] [Google Scholar]
- Benhamed M., Bertrand C., Servet C., Zhou D.X. (2006). Arabidopsis GCN5, HD1, and TAF1/HAF2 interact to regulate histone acetylation required for light-responsive gene expression. Plant Cell 18: 2893–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamed M., et al. (2008). Genome-scale Arabidopsis promoter array identifies targets of the histone acetyltransferase GCN5. Plant J. 56: 493–504 [DOI] [PubMed] [Google Scholar]
- Bernstein B.E., et al. (2006). A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125: 315–326 [DOI] [PubMed] [Google Scholar]
- Bertrand C., Benhamed M., Li Y.F., Ayadi M., Lemonnier G., Renou J.P., Delarue M., Zhou D.X. (2005). Arabidopsis HAF2 gene encoding TATA-binding protein (TBP)-associated factor TAF1, is required to integrate light signals to regulate gene expression and growth. J. Biol. Chem. 280: 1465–1473 [DOI] [PubMed] [Google Scholar]
- Cantón F.R., Quail P.H. (1999). Both phyA and phyB mediate light-imposed repression of PHYA gene expression in Arabidopsis. Plant Physiol. 121: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Dai Y., Cui S., Ma L. (2008). Histone H2B monoubiquitination in the chromatin of FLOWERING LOCUS C regulates flowering time in Arabidopsis. Plant Cell 20: 2586–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.W., Henderson I.R., Zhang X., Shah G., Chien J.S., Jacobsen S.E. (2006). RNAi, DRD1, and histone methylation actively target developmentally important non-CG DNA methylation in Arabidopsis. PLoS Genet. 2: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron J.B., He H., Elling A.A., Deng X.W. (2009). Dynamic landscapes of four histone modifications during deetiolation in Arabidopsis. Plant Cell 21: 3732–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla R., Nicholson S.J., Folta K.M., Srivastava V. (2007). Transgene-induced silencing of Arabidopsis phytochrome A gene via exonic methylation. Plant J. 52: 1105–1118 [DOI] [PubMed] [Google Scholar]
- Chua Y.L., Brown A.P., Gray J.C. (2001). Targeted histone acetylation and altered nuclease accessibility over short regions of the pea plastocyanin gene. Plant Cell 13: 599–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua Y.L., Watson L.A., Gray J.C. (2003). The transcriptional enhancer of the pea plastocyanin gene associates with the nuclear matrix and regulates gene expression through histone acetylation. Plant Cell 15: 1468–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal R.B., Topp C.N., McKinney E.C., Meagher R.B. (2007). Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner V., Aichinger E., Shu H., Wildhaber T., Alfarano P., Caflisch A., Gruissem W., Köhler C., Hennig L. (2009). The chromodomain of LIKE HETEROCHROMATIN PROTEIN 1 is essential for H3K27me3 binding and function during Arabidopsis development. PLoS ONE 4: e5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan E., Dennis E. (2008). Polycomb repression: It’s all in the balance. Plant Signal. Behav. 3: 412–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall A.R., Levy Y.Y., Wilson A., Dean C. (2001). The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107: 525–535 [DOI] [PubMed] [Google Scholar]
- Gendrel A.V., Lippman Z., Martienssen R., Colot V. (2005). Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2: 213–218 [DOI] [PubMed] [Google Scholar]
- He Y., Doyle M.R., Amasino R.M. (2004). PAF1-complex-mediated histone methylation of FLOWERING LOCUS C chromatin is required for the vernalization-responsive, winter-annual habit in Arabidopsis. Genes Dev. 18: 2774–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Michaels S.D., Amasino R.M. (2003). Regulation of flowering time by histone acetylation in Arabidopsis. Science 302: 1751–1754 [DOI] [PubMed] [Google Scholar]
- Jiang D., Wang Y., Wang Y., He Y. (2008). Repression of FLOWERING LOCUS C and FLOWERING LOCUS T by the Arabidopsis Polycomb repressive complex 2 components. PLoS ONE 3: e3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Lau O.S., Deng X.W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Jiao Y., Ma L., Strickland E., Deng X.W. (2005). Conservation and divergence of light-regulated genome expression patterns during seedling development in rice and Arabidopsis. Plant Cell 17: 3239–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Doyle M.R., Sung S., Amasino R.M. (2009). Vernalization: Winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 25: 277–299 [DOI] [PubMed] [Google Scholar]
- Kim J.M., To T.K., Ishida J., Morosawa T., Kawashima M., Matsui A., Toyoda T., Kimura H., Shinozaki K., Seki M. (2008). Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana. Plant Cell Physiol. 49: 1580–1588 [DOI] [PubMed] [Google Scholar]
- Kim J.M., To T.K., Nishioka T., Seki M. (2010). Chromatin regulation functions in plant abiotic stress responses. Plant Cell Environ. 33: 604–611 [DOI] [PubMed] [Google Scholar]
- Kouzarides T. (2007). Chromatin modifications and their function. Cell 128: 693–705 [DOI] [PubMed] [Google Scholar]
- Latham J.A., Dent S.Y. (2007). Cross-regulation of histone modifications. Nat. Struct. Mol. Biol. 14: 1017–1024 [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., Tongprasit W., Zhao H., Lee I., Deng X.W. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Smith E., Shilatifard A. (2010). The language of histone crosstalk. Cell 142: 682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Carey M., Workman J.L. (2007). The role of chromatin during transcription. Cell 128: 707–719 [DOI] [PubMed] [Google Scholar]
- Liu C., Lu F., Cui X., Cao X. (2010). Histone methylation in higher plants. Annu. Rev. Plant Biol. 61: 395–420 [DOI] [PubMed] [Google Scholar]
- Oh S., Park S., van Nocker S. (2008). Genic and global functions for Paf1C in chromatin modification and gene expression in Arabidopsis. PLoS Genet. 4: e1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Cui Y., Bi Y.M., Rothstein S.J. (2006). AtMBD9: A protein with a methyl-CpG-binding domain regulates flowering time and shoot branching in Arabidopsis. Plant J. 46: 282–296 [DOI] [PubMed] [Google Scholar]
- Pfluger J., Wagner D. (2007). Histone modifications and dynamic regulation of genome accessibility in plants. Curr. Opin. Plant Biol. 10: 645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier D., Yahubyan G., Vega D., Bulski A., Saez-Vasquez J., Hakimi M.A., Lerbs-Mache S., Colot V., Lagrange T. (2005). Reinforcement of silencing at transposons and highly repeated sequences requires the concerted action of two distinct RNA polymerases IV in Arabidopsis. Genes Dev. 19: 2030–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Nagpal P., Poole D.S., Furuya M., Chory J. (1993). Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier F., Teixeira F.K., Colot V. (2009). Chromatin indexing in Arabidopsis: An epigenomic tale of tails and more. Trends Genet. 25: 511–517 [DOI] [PubMed] [Google Scholar]
- Seo H.S., Watanabe E., Tokutomi S., Nagatani A., Chua N.H. (2004). Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 18: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R.A., Clack T. (2002). Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol. 130: 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R.A., Quail P.H. (1989). Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 3: 1745–1757 [DOI] [PubMed] [Google Scholar]
- Strahl B.D., Allis C.D. (2000). The language of covalent histone modifications. Nature 403: 41–45 [DOI] [PubMed] [Google Scholar]
- Sung S., Amasino R.M. (2004). Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427: 159–164 [DOI] [PubMed] [Google Scholar]
- Turck F., Roudier F., Farrona S., Martin-Magniette M.L., Guillaume E., Buisine N., Gagnot S., Martienssen R.A., Coupland G., Colot V. (2007). Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 3: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Elling A.A., Li X., Li N., Peng Z., He G., Sun H., Qi Y., Liu X.S., Deng X.W. (2009). Genome-wide and organ-specific landscapes of epigenetic modifications and their relationships to mRNA and small RNA transcriptomes in maize. Plant Cell 21: 1053–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Johansen L.K., Gustafson A.M., Kasschau K.D., Lellis A.D., Zilberman D., Jacobsen S.E., Carrington J.C. (2004). Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2: E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Rider S.D., Jr, Henderson J.T., Fountain M., Chuang K., Kandachar V., Simons A., Edenberg H.J., Romero-Severson J., Muir W.M., Ogas J. (2008). The CHD3 remodeler PICKLE promotes trimethylation of histone H3 lysine 27. J. Biol. Chem. 283: 22637–22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Bernatavichute Y.V., Cokus S., Pellegrini M., Jacobsen S.E. (2009). Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 10: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Yu Y., Meyer D., Wu C., Shen W.H. (2005). Prevention of early flowering by expression of FLOWERING LOCUS C requires methylation of histone H3 K36. Nat. Cell Biol. 7: 1256–1260 [DOI] [PubMed] [Google Scholar]
- Zhou D.X. (2009). Regulatory mechanism of histone epigenetic modifications in plants. Epigenetics 4: 15–18 [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang X., He K., Charron J.B., Elling A.A., Deng X.W. (2010). Genome-wide profiling of histone H3 lysine 9 acetylation and dimethylation in Arabidopsis reveals correlation between multiple histone marks and gene expression. Plant Mol. Biol. 72: 585–595 [DOI] [PubMed] [Google Scholar]