Abstract
Background
Inflammation and immune response have potential prognostic implications for breast cancer survivors. We examined how postdiagnosis diet quality is cross-sectionally related to biomarkers of inflammation and adipose-derived hormones among breast cancer survivors and determined whether physical activity or body size modified any observed associations.
Methods
Participants included 746 women diagnosed with stage 0-IIIA breast cancer. 30 months after diagnosis, women completed food frequency questionnaires. We scored diet quality with the Healthy Eating Index (HEI)-2005. Serum concentrations of C-reactive protein (CRP), serum amyloid A (SAA), leptin and adiponectin were measured in fasting 30-ml blood samples. Log biomarker values were regressed on quartiles of HEI-2005 scores in multivariate models, and beta scores were exponentiated and expressed as geometric means within quartiles of HEI-2005 scores.
Results
Women with better vs. poor quality postdiagnosis diets, as defined by higher HEI-2005 scores (Q4 vs. Q1), had lower concentrations of CRP (1.6mg/L vs. 2.5 mg/L), but no significant difference in concentrations of SAA, leptin or adiponectin. Among women not engaging in recreational physical activity after diagnosis, better diet quality was associated with lower CRP concentrations (2.5 mg/L vs. 5.0 mg/L), but no association was observed among women engaging in any recreational physical activity (1.4 mg/L vs. 1.6 mg/L) (p-heterogeneity=0.03).
Conclusions
Among breast cancer survivors, a better quality diet appears to be associated with lower levels of chronic inflammation.
Impact
Lower levels of chronic inflammation have been associated with improved survival after breast cancer.
Keywords: Diet, Inflammation, Breast Cancer Survivors
Introduction
In the United States (U.S), approximately 2.5 million women are thought to be living with a personal history of breast cancer and the many morbidities associated with life as a survivor (1). There is some evidence that a modifiable health habit that women can change, the quality of the diet, may be related to survival after breast cancer (2, 3). However, the mechanisms linking postdiagnosis diet to survival are not well-known.
Recent studies have highlighted the potential prognostic importance of inflammation and immune response for women with early stage breast cancer. Among these survivors, higher concentrations of C-reactive protein (CRP) and serum amyloid A (SAA), biomarkers of chronic inflammation, have been associated with poorer survival (4). Additionally, a higher concentration of adiponectin, a hormone involved in metabolism and inflammation (5–7) has been associated with improved survival (8).
These biomarkers that have been related to survival are of interest to study in relation to the dietary patterns women choose after a diagnosis of breast cancer, because certain dietary components and nutrients have anti-inflammatory properties (9), which may have synergistic or antagonistic effects on the biomarkers in context of total diet (10). Among obese women without cancer, overall diet quality has been associated with inflammation (11), whereas caloric restriction (12) and lower carbohydrate consumption (13) have been shown to be associated with favorable adipokine profiles. In animal studies, high-fat diets have resulted in diminished inflammatory response to adipose-derived hormones like leptin and adiponectin (14, 15).
Diet quality could be directly or indirectly related to inflammation and immune function through changes in body mass (11, 16, 17), being that higher body fat mass is associated with higher concentrations of CRP, SAA and leptin and lower concentrations of adiponectin (18, 19). We investigated these potential mechanistic pathways of inflammation biomarkers (CRP, SAA) and adipose-derived hormones (adiponectin, leptin) for postdiagnosis diet among women with early-stage breast cancer.
Materials and Methods
The Health, Eating, Activity, and Lifestyle (HEAL) study is a multi-ethnic prospective cohort study that has enrolled 1,183 breast cancer survivors women with their first primary breast cancer (0-IIIA) drawn from Surveillance, Epidemiology, and End Results (SEER) population-based cancer registries in New Mexico, Los Angeles County, and Western Washington. Survivors are being followed to determine whether lifestyle, hormones, and other exposures affect breast cancer prognosis. Details of the study have been published elsewhere (20–22).
In the HEAL Study, women completed extensive assessments, which were conducted at baseline, approximately 6-months after diagnosis, and at approximately 30-months after diagnosis. At the 30-month assessment, a blood draw was also taken.
944 participants were alive and completed the 30-month assessment. We excluded women who may have been receiving treatment for subsequent recurrences or new primaries at the time of their 30-month assessment (n=32), because active treatment may be associated with changes in diet. We retained women who had subsequent recurrences or new primaries well in advance of the 30-month assessment such that treatment would not affect exposure measurements (n=25). Sensitivity analyses confirmed that including these women did not change results. Of the remaining 912 women, we excluded women with incomplete data for diet (n=21), follow-up time (n=3), biomarkers (n=124), or BMI (n=18). Our final sample included 746 women. We obtained written informed consent from all study participants. The study was approved by the institutional review board at each participating center, in accord with assurances filed with and approved by the US Department of Health and Human Services.
Biomarker assessment
Inflammation biomarkers and adipose-derived hormones
A 30 mL fasting blood sample was collected from participants at the 30-month assessment. Blood was processed within 3 hours of collection and serum was stored in 1.8 mL tubes at −70° to −80° C until analysis.
CRP and SAA were measured by latex-enhanced nephelometry using high-sensitivity assays (23) on the Behring Nephelometer II analyzer (Dade Behring Diagnostics, Deerfield, IL) at the University of Washington Medical Center (Seattle, WA). Adiponectin was measured using a highly sensitive radioimmunoassay (RIA) (Linco Research, St. Charles, MI) at the Northwest Research Lipid Laboratories at the University of Washington. Leptin was measured using the Linco 125RIA kit at the Reproductive and Endocrine Research Laboratory at the University of Southern California for California participants and at the Aging and Genetic Epidemiology Program Laboratory at the University of New Mexico for New Mexico and Washington participants. The lowest detection limits for CRP, SAA, leptin and adiponectin assays were 0.2 mg/L, 0.7 mg/L, 0.5ng/mL, and 0.78 ng/mL. Inter-assay coefficients of variation were 5%–9% (CRP), 4%–8% (SAA), 5%–8% (leptin), and 19% (adiponectin).
Diet
To measure diet at the 30-month assessment, we used a 122-item self-administered food-frequency questionnaire developed and validated for the Women's Health Initiative (WHI) (24), adapted from the Health Habits and Lifestyle Questionnaire (25). The WHI-FFQ was designed to capture foods relevant for multi-ethnic and geographically diverse population groups and has been shown to produce reliable (rall nutrients=0.76) and comparable estimates to 8 days of dietary intake from 24-hour dietary recalls and 4-day food records (r= 0.37, 0.62, 0.41, 0.36, with energy, percent energy from fat, carbohydrate and protein) (24). New Mexico participants reported their usual dietary intake for the previous year, whereas participants at the other two centers reported usual intake for the previous month.
The nutrient database used to analyze the WHI-FFQ is derived from the Nutrition Data Systems for Research (NDS-R, version 2005, University of Minnesota, Minneapolis, MN) (26, 27). NDS-R provides necessary estimates for energy, saturated fat, and sodium, but does not link to the MyPyramid Equivalents Database (28). Thus, we established a customized link with the WHI-FFQ to calculate total fruit, whole fruit, total vegetables, dark green vegetables, orange vegetables, legumes, total grains, whole grains, milk, meat and beans, oils, solid fats, and added sugars. We also created variables for calories from solid fat, added sugar, alcohol, and calories from saturated fat.
We measured diet quality with the Healthy Eating Index-2005 (HEI-2005), (29–32) which uses an energy-adjusted density approach, and was jointly developed by the National Cancer Institute and U.S. Department of Agriculture to align with the U.S. Dietary Guidelines for Americans-2005 (33). Table 1 lists the 12 components and standards for scoring. For each participant, we scored each component and calculated a total score (100 possible points). We classified HEI-2005 scores into quartiles. The strength of HEI-2005 is its ability to distinguish those scoring well on virtually all of the components (Q4) vs. those scoring poorly on virtually all the components (Q1), and this is the comparison of our analysis. Scores in the middle quartiles (Q2–Q3) are more likely to reflect “mixed quality” diets, thus including individuals with somewhat similar total scores, but more widely varying component scores.
Table 1.
| Component | Maximum points | Standard for maximum score | Standard for minimum score of zero |
|---|---|---|---|
| Total Fruit (includes 100% juice) | 5 | ≥0.8 cup equiv. per 1,000 kcal | No Fruit |
| Whole Fruit (not juice) | 5 | ≥0.4 cup equiv. per 1,000 kcal | No Whole Fruit |
| Total Vegetables | 5 | ≥1.1 cup equiv. per 1,000 kcal | No Vegetables |
| Dark Green and Orange Vegetables and Legumes2 | 5 | ≥0.4 cup equiv. per 1,000 kcal | No Dark Green or Orange Vegetables or Legumes |
| Total Grains | 5 | ≥3.0 oz equiv. per 1,000 kcal | No Grains |
| Whole Grains | 5 | ≥1.5 oz equiv. per 1,000 kcal | No Whole Grains |
| Milk3 | 10 | ≥1.3 cup equiv. per 1,000 kcal | No Milk |
| Meat and Beans | 10 | ≥2.5 oz equiv. per 1,000 kcal | No Meat or Beans |
| Oils4 | 10 | ≥12 grams per 1,000 kcal | No Oil |
| Saturated Fat | 10 | ≤7% of energy5 | ≥15% of energy |
| Sodium | 10 | ≤0.7 gram per 1,000 kcal5 | ≥2.0 grams per 1,000 kcal |
| Calories from Solid Fats, Alcoholic beverages, and Added Sugars (SoFAAS) | 20 | ≤20% of energy | ≥50% of energy |
Intakes between the minimum and maximum levels are scored proportionately, except for Saturated Fat and Sodium (see 5)
Legumes counted as vegetables only after Meat and Beans standard is met
Includes all milk products, such as fluid milk, yogurt, and cheese, and soy beverages
Includes nonhydrogenated vegetable oils and oils in fish, nuts, and seeds.
Saturated Fat and Sodium get a score of 8 for the intake levels that reflect the 2005 Dietary Guidelines, <10% of calories from saturated fat and 1.1 grams of sodium/1,000 kcal, respectively
Anthropometry
Body mass index (BMI)
Height was measured postdiagnosis at the baseline assessment. For participants missing measured height, self-reported height at age 18 was used. At the 30-month assessment, trained staff measured weight to the nearest 0.1 kg, with women wearing light indoor clothing and no shoes. All measurements were performed twice and averaged for a final value. BMI was calculated as weight (kg)/ height (m2).
Recreational physical activity
We collected information on recreational aerobic physical activity using the Modifiable Activity Questionnaire developed by Kriska which has high validity and reliability (r= 0.73 with total energy expenditure assessed by doubly-labeled water, and r=0.92 for 3-week test-retest) (34). At the 30-month assessment, participants reported the type, duration, and frequency of aerobic recreational physical activities (e.g. brisk walking, biking, dancing, swimming, jogging, etc.) in the previous year. Activities were classified according to their corresponding metabolic equivalent of task value (MET) (35). For all activities with MET values ≥3, we summed the products of activity MET values and hours spent in each activity to arrive at the MET-hours/week spent in moderate/vigorous-intensity aerobic activity for each participant.
Similar to Irwin et al. (36), we classified recreational physical activity into three categories (inactive: 0; somewhat active: >0 to <9; active: ≥9 MET-hours/week), with 9 MET-hours/week approximately equal to 150 min/week of moderate-intensity physical activity, and meeting the general population guidelines (37). Given the benefit observed in HEAL for doing any postdiagnosis recreational physical activity (36), for stratified analyses, we dichotomized physical activity as none (0 MET-hours/week) vs. any (>0 MET-hours/week).
Additional risk factors
For participants' initial breast cancer diagnoses, disease stage and estrogen receptor status were obtained from Surveillance, Epidemiology, and End Results (SEER) cancer registry records and detailed information on treatment and surgical procedures were obtained from SEER registry, physician, and hospital records. At baseline, information was collected on recruitment site, date of birth, and race, and information on chronic conditions was abstracted from medical records. We calculated age at 30-month assessment using date of birth and 30-month assessment date. At the 30-month assessment, we collected information on tamoxifen use, use of non-steroidal anti-inflammatory drugs, smoking status, and physician-diagnosed Type II diabetes. We determined participants' menopausal status (pre-; post-; undetermined) at the 30-month assessment from medical records, hormone levels, and questionnaires. We considered each of these risk factors in model development.
Statistical Analyses
Differences in descriptive characteristics of women with better vs. poor quality diets were tested using likelihood ratio chi-square tests.
We examined the residuals of the analytes on theirs regressions and found that a logarithmic transformation yielded nearly homoscedastic variation about zero. The resulting normal quantile-quantile plots were nearly linear, confirming the suitability of the logarithmic transformation. Log biomarker values were regressed on quartiles of HEI-2005 scores in multivariate models, and beta scores were exponentiated and expressed as geometric means and 95% confidence intervals (CI) within quartiles of HEI-2005 scores.
We adjusted for factors changed the magnitude of beta values by at least 10%, improved model fit, and/or allowed comparison to the published literature. We presented a parsimonious model (Model 3) meeting these criteria that adjusts for age (continuous), total energy (kcal), BMI (continuous), race/ethnicity (non-Hispanic White, Hispanic, Black/African American, American Indian/Asian/Other), and MET-hours/week of recreational physical activity (continuous). For the adipose-derived hormones, we also presented a model with additional adjustments for stage, menopausal status, and smoking (Model 4), which were confounders in age and energy-adjusted models; however, adding these to the parsimonious model did not improve model fit. Using our biomarker specific models, we also investigated each HEI-2005 component separately, controlling for other components.
For any association observed, we investigated heterogeneity by postdiagnosis recreational physical activity and BMI by using likelihood ratio tests for both the interaction of diet quality with these factors (alpha=0.1) and the difference in model fit of full and reduced models. If an interaction was present, we stratified by the relevant factor and calculated a Cochran's Q heterogeneity statistic (38). All statistical tests were based on a priori hypotheses, and therefore there was no adjustment for multiple testing. All analyses were conducted with SAS (version 9.1.3, Cary, NC).
Results
Women with better vs. poor quality diets, defined as having higher HEI-2005 scores (Q4 vs. Q1), were older, more likely to be non-Hispanic White and postmenopausal, engaged in more postdiagnosis recreational physical activity, had lower BMIs, and were less likely to be current smokers (Table 2).
Table 2.
Characteristics of 746 Breast Cancer Survivors in the Health, Eating, Activity, Lifestyle Study by Quartiles of Healthy Eating Index (HEI)–2005 Scores
| Postdiagnosis Diet Quality | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| HEI-2005 Quartile 1 (35–57) “Poor” | HEI-2005 Quartile 2 (57–67) “Mixed” | HEI-2005 Quartile 3 (67–75) “Mixed” | HEI-2005 Quartile 4 (75–87) “Better” | ||||||
| No. | % | No. | % | No. | % | No. | % | p-value5 | |
| Number of participants | 186 | 187 | 187 | 186 | |||||
| Age1 | |||||||||
| Mean (SE) | 55.7 (0.7) | 58.1 (0.8) | 57.7 (0.7) | 60.1 (0.8) | <0.0001 | ||||
| Race/Ethnicity | <0.0001 | ||||||||
| White, non-Hispanic | 86 | 46 | 102 | 55 | 126 | 67 | 134 | 72 | |
| Hispanic | 29 | 16 | 24 | 13 | 17 | 9 | 1 | 6 | |
| Black, non-Hispanic | 67 | 36 | 55 | 29 | 39 | 21 | 35 | 19 | |
| American Indian, Asian, Other | 4 | 2 | 6 | 3 | 5 | 3 | 6 | 3 | |
| Recruitment Site | 0.001 | ||||||||
| Western Washington | 39 | 21 | 40 | 21 | 43 | 23 | 44 | 24 | |
| New Mexico | 80 | 43 | 93 | 50 | 105 | 56 | 107 | 58 | |
| Los Angeles County, California | 67 | 36 | 54 | 29 | 39 | 21 | 35 | 19 | |
| Menopausal Status1 | 0.01 | ||||||||
| Premenopausal | 65 | 35 | 62 | 33 | 63 | 34 | 54 | 29 | |
| Postmenopausal | 106 | 57 | 116 | 62 | 114 | 61 | 128 | 69 | |
| Unknown | 15 | 8 | 9 | 5 | 10 | 5 | 4 | 2 | |
| Treatment | 0.69 | ||||||||
| Surgery only | 65 | 35 | 59 | 32 | 54 | 29 | 58 | 31 | |
| + radiation | 71 | 38 | 68 | 36 | 74 | 40 | 72 | 39 | |
| +chemotherapy | 20 | 11 | 16 | 9 | 19 | 10 | 18 | 10 | |
| +radiation and chemotherapy | 30 | 16 | 44 | 24 | 40 | 21 | 38 | 20 | |
| Stage | |||||||||
| In situ | 50 | 27 | 41 | 22 | 46 | 25 | 39 | 21 | |
| Localized | 97 | 52 | 108 | 58 | 97 | 52 | 103 | 55 | |
| Regional | 39 | 21 | 38 | 20 | 44 | 24 | 44 | 24 | |
| Current Tamoxifen use | 0.14 | ||||||||
| No | 111 | 60 | 106 | 57 | 104 | 56 | 97 | 52 | |
| Yes | 75 | 40 | 81 | 43 | 83 | 44 | 89 | 48 | |
| Estrogen Receptor Status2 | 0.33 | ||||||||
| Positive | 94 | 51 | 105 | 56 | 106 | 57 | 113 | 61 | |
| Negative | 31 | 17 | 35 | 19 | 29 | 16 | 22 | 12 | |
| HEI-2005 score (100 points possible) | |||||||||
| Mean (SE) | 50.3 (0.4) | 62.6 (0.2) | 70.8 (0.2) | 79.0 (0.2) | <0.0001 | ||||
| Energy/day (kcal) | |||||||||
| Mean (SE) | 1723 (87) | 1514 (50) | 1396 (45) | 1256 (34) | <0.0001 | ||||
| Fruit (c eq/1000 kcal) | |||||||||
| Mean (SE) | 0.5 (0.03) | 0.9 (0.04) | 1.2 (0.06) | 1.7 (0.06) | <0.0001 | ||||
| Whole fruit (c eq/1000 kcal) | |||||||||
| Mean (SE) | 0.3 (0.02) | 0.6 (0.03) | 0.8 (0.05) | 1.2 (0.06) | <0.0001 | ||||
| Vegetables (c eq/1000 kcal)3 | |||||||||
| Mean (SE) | 0.8 (0.03) | 1.0 (0.05) | 1.1 (0.03) | 1.3 (0.04) | <0.0001 | ||||
| Dark green vegetables, orange vegetables, and legumes (c eq/1000kcal)3 | |||||||||
| Mean (SE) | 0.1 (0.01) | 0.2 (0.03) | 0.2 (0.01) | 0.3 (0.01) | <0.0001 | ||||
| Total grains (oz eq/1000 kcal) | |||||||||
| Mean (SE) | 2.5 (0.07) | 2.7 (0.07) | 2.8 (0.07) | 2.9 (0.06) | 0.0002 | ||||
| Whole grains (oz eq/1000 kcal) | |||||||||
| Mean (SE) | 0.4 (0.03) | 0.6 (0.03) | 0.7 (0.04) | 0.9 (0.04) | <0.0001 | ||||
| Meat & beans (oz eq/1000kcal)4 | |||||||||
| Mean (SE) | 3.0 (0.09) | 3.0 (0.08) | 3.0 (0.07) | 2.9 (0.07) | 0.38 | ||||
| Oils (g/1000 kcal) | |||||||||
| Mean (SE) | 9.6 (0.35) | 11.1 (0.36) | 11.5 (0.37) | 11.2 (0.40) | 0.003 | ||||
| Milk (c eq/1000 kcal) | |||||||||
| Mean (SE) | 0.7 (0.03) | 0.9 (0.04) | 0.9 (0.05) | 1.0 (0.05) | <0.0001 | ||||
| Sodium (g/1000 kcal) | |||||||||
| Mean (SE) | 1.5 (0.02) | 1.7 (0.02) | 1.6 (0.02) | 1.7 (0.02) | <0.0001 | ||||
| Percent calories from saturated fat | |||||||||
| Mean (SE) | 14.1 (0.24) | 12.4 (0.18) | 10.6 (0.16) | 8.6 (0.14) | <0.0001 | ||||
| Percent discretionary calories from solid fat, alcoholic beverages, and added sugars | |||||||||
| Mean (SE) | 40.5 (0.55) | 31.9 (0.40) | 27.1 (0.33) | 20.8 (0.33) | <0.0001 | ||||
| MET-hours/week of postdiagnosis recreational physical activity | |||||||||
| Mean (SE) | 8.5 (1.0) | 11.4 (1.1) | 13.4 (1.2) | 18.1 (1.8) | <0.0001 | ||||
| BMI1 | |||||||||
| Mean (SE) | 28.6 (0.5) | 28.9 (0.5) | 27.4 (0.4) | 26.6 (0.4) | 0.003 | ||||
| Current smoker1 | 37 | 20 | 22 | 12 | 21 | 11 | 13 | 7 | 0.003 |
| Current use of non-steroidal anti-inflammatory drugs1 | 78 | 42 | 56 | 30 | 71 | 38 | 78 | 42 | 1.00 |
At 30-month assessment
Confirmed positive/negative status for 535 participants
Includes legumes only after meat and beans standard has been met
Includes legumes only if meat and beans standard is otherwise not met
p-values are for likelihood ratio chi-square tests contrasting means (continuous variables) and percentages (categorical variables) for women with a better quality diet compared to those with a poor quality diet.
As shown in Table 3, women with better quality diets had significantly lower CRP concentrations (1.6 mg/L vs. 2.4 mg/L, p=0.004), but similar serum concentrations of SAA, leptin, or adiponectin. Controlling for BMI in models resulted attenuation of all diet quality-biomarker associations and a noticeable improvement in model fit, especially for CRP and leptin Addition of other confounders on top of models already including BMI did not change model fit.
Table 3.
Adjusted Geometric Means1 and 95% Confidence Intervals for Biomarkers of Inflammation and Adipose-derived Hormone Concentrations of 746 Breast Cancer Survivors by Quartiles of Healthy Eating Index (HEI)-2005 Scores
| Postdiagnosis Diet Quality | ||||||
|---|---|---|---|---|---|---|
| Model r22 | HEI-2005 Quartile 1 (35–57) “Poor” | HEI-2005 Quartile 2 (57–67) “Mixed” | HEI-2005 Quartile 3 (67–75) “Mixed” | HEI-2005 Quartile 4 (75 87) “Better” | p-value for Contrast `Better vs. Poor” | |
| N | 186 | 187 | 187 | 186 | ||
| C-reactive protein (mg/L) | ||||||
| Model 1 | 0.05 | 2.6 (2.1, 3.1) | 2.2 (1.9, 2.5) | 2.1 (1.8, 2.5) | 1.4 (1.2, 1.7) | <0.0001 |
| Model 2 | 0.30 | 2.4 (2.1, 2.8) | 2.0 (1.7, 2.3) | 2.2 (1.9, 2.6) | 1.6 (1.4, 1.9) | 0.0005 |
| Model 3 | 0.30 | 2.4 (1.9, 2.9) | 2.0 (1.6, 2.4) | 2.2 (1.8, 2.7) | 1.6 (1.4, 2.0) | 0.004 |
| Serum amyloid A (mg/L) | ||||||
| Model 1 | 0.07 | 6.6 (5.9, 7.4) | 5.5 (4.9, 6.2) | 6.2 (5.5, 6.9) | 5.8 (5.2, 6.6) | 0.17 |
| Model 2 | 0.15 | 6.5 (5.8 (7.2) | 5.2 (4.8, 5.9) | 6.3 (5.6, 7.0) | 6.1 (5.4, 6.8) | 0.48 |
| Model 3 | 0.15 | 6.5 (5.7, 7.5) | 5.4 (4.7, 6.2) | 6.4 (5.5, 7.3) | 6.3 (5.4, 7.2) | 0.63 |
| Leptin (ng/mL) | ||||||
| Model 1 | 0.01 | 21.4 (19.3, 23.8) | 19.5 (17.5, 21.6) | 18.3 (16.5, 20.4) | 17.2 (15.4, 19.1) | 0.004 |
| Model 2 | 0.58 | 20.5 (19.1, 21.9) | 17.8 (16.6, 19.0) | 19.1 (17.8, 20.4) | 19.0 (17.7, 20.3) | 0.13 |
| Model 3 | 0.59 | 19.7 (18.1, 21.4) | 17.4 (16.0, 18.9) | 18.9 (17.4, 20.6) | 19.0 (17.5, 20.7) | 0.63 |
| Model 4 | 0.60 | 19.8 (17.9, 21.9) | 17.2 (15.6, 19.1) | 18.8 (16.9, 20.8) | 18.7 (16.9, 20.8) | 0.36 |
| Adiponectin (μg/mL) | ||||||
| Model 1 | 0.05 | 12.8 (11.7, 14.0) | 13.5 (12.4, 14.8) | 14.0 (12.8, 15.3) | 14.1 (12.9, 15.5) | 0.15 |
| Model 2 | 0.11 | 13.0 (11.9, 14.2) | 14.0 (12.8, 15.2) | 13.8 (12.7, 15.1) | 13.7 (12.5, 15.0) | 0.43 |
| Model 3 | 0.19 | 12.7 (11.4, 14.1) | 13.3 (12.0, 14.8) | 12.7 (11.4, 14.2) | 12.6 (11.3, 14.1) | 0.94 |
| Model 4 | 0.19 | 13.0 (11.4, 14.7) | 13.6 (12.0, 15.4) | 13.0 (11.4, 14.8) | 12.9 (11.3, 14.7) | 0.94 |
Model 1: Adjusted for age (continuous), energy (kcals/day)
Model 2: Model 1 + body mass index (continuous)
Model 3: Model 1 + recreational moderate-vigorous physical activity (continuous), race/ethnicity (Non-Hispanic White; Hispanic; Black, African American; American Indian, Asian, other; Missing)
Model 4: Model 2 + stage (in situ, localized, regional), menopausal status (pre, post, undetermined), smoking (current, former, never, missing)
r2 for models only adjusting for age, energy, and BMI without HEI score were 0.29, 0.14, 0.58 and 0.11 for C-reactive protein, serum amyloid A, leptin and adiponectin, respectively
When we evaluated each HEI-2005 component individually, controlling for all other components, higher component scores for consumption of dark green and orange vegetables and legumes were significantly associated with lower CRP concentrations (data not shown). However, this component did not account for the full association observed for overall diet quality.
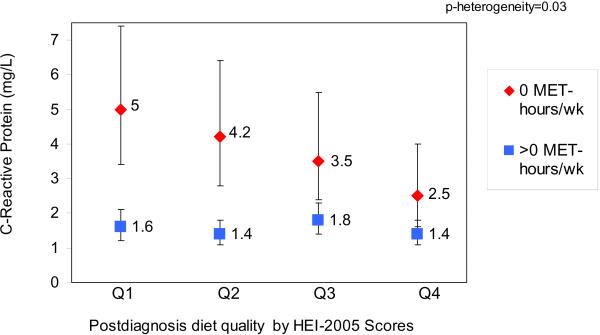
We found evidence of effect modification (p-heterogeneity=0.03) of the diet quality-CRP association by postdiagnosis recreational physical activity level (Table 4 and Figure 1). A better quality diet was associated with lower CRP concentrations among women engaging in no recreational physical activity (2.5 mg/L vs. 5.0 mg/L) but not among women who were engaging in any recreational physical activity (1.6 mg/L vs. 1.4 mg/L), whose concentrations of CRP were lower overall across quartiles of HEI-2005. We did not find evidence of effect modification of the diet-CRP relationship by BMI.
Table 4.
Adjusted Geometric Means and 95% Confidence Intervals1 for C-reactive Protein Concentrations of 746 Breast Cancer Survivors by Quartiles of Healthy Eating Index (HEI)-2005 Scores and Recreational Moderate/Vigorous Physical Activity
| Postdiagnosis Diet Quality | ||||||
|---|---|---|---|---|---|---|
| HEI-2005 Quartile 1 (35–57) “Poor” | HEI-2005 Quartile 2 (57–67) “Mixed” | HEI-2005 Quartile 3 (67–75) “Mixed” | HEI-2005 Quartile 4 (75–87) “Better” | p-value for Contrast `Better vs. Poor” | p-heterogeneity | |
| No recreational physical activity after diagnosis (0 MET-hours/week) | ||||||
| N | 89 | 71 | 62 | 54 | ||
| C-reactive protein (mg/L) | 5.0 (3.4, 7.4) | 4.2 (2.8, 6.4) | 3.5 (2.4, 5.5) | 2.5 (1.6, 4.0) | 0.001 | |
| 0.03 | ||||||
| Any recreational physical activity after diagnosis (>0 MET-hours/week) | ||||||
| N | 97 | 115 | 125 | 131 | ||
| C-reactive protein (mg/L) | 1.6 (1.2, 2.1) | 1.4 (1.1, 1.8) | 1.8 (1.4, 2.3) | 1.4 (1.1, 1.8) | 0.39 | |
Adjusted for age (continuous), energy (continuous), body mass index (continuous), race/ethnicity (Non-Hispanic White; Hispanic; Black, African American; American Indian, Asian, other; Missing)
Figure 1.
Adjusted Geometric Means of C-reactive Protein by Quartiles of Healthy Eating Index (HEI)-2005 Scores and Recreational Moderate/Vigorous Physical Activity
Discussion
This study fills an important gap in the literature by defining inflammation as a potential mechanism by which diet quality could affect survival, regardless of age, energy intake, body mass index, recreational physical activity, and race. This study complements our previous work in the HEAL study demonstrating a positive relationship between elevated CRP and mortality (4), independent of obesity.
We did not find evidence of associations between diet quality and SAA, leptin, or adiponectin. Although CRP and SAA are both biomarkers of inflammation, we found diet quality to be more strongly associated with CRP than SAA, which had also been reported our previous study of women without cancer (11). Given that both CRP and SAA were associated with mortality than CRP in our cohort (4), it is possible that, in contrast to CRP, SAA marks inflammation related to tumor progression or cardiovascular disease that is possibly less related to diet quality and less directly dependent on BMI. Future work is needed to investigate if our measure of diet quality is related to other important biomarkers of inflammation among survivors. If a better quality diet does influence adipose-derived hormone concentrations, is possible that larger contrasts of diet quality are needed to see the difference in these biomarkers.
Although relationships between diet quality and the biomarkers investigated were not explained by body size, controlling for BMI (Model 2) resulted in noticeable attenuation of relationships and increased the explanatory power of models greatly. Our results therefore suggest diet quality might also work indirectly to reduce inflammation through improving body size. Observational studies indicate that being in a normal weight range at the time of breast cancer diagnosis, as well as during and after treatment, is associated with improved prognosis (39).
In our study, the association between CRP and diet quality only held among women who were not engaging in recreational physical activity. It is possible that after a diagnosis of breast cancer, better diet quality may be anti-inflammatory, but relationships between diet quality and chronic inflammation are only evident when inflammation is high, as was the case among inactive survivors in our study. Because physical activity has been shown to reduce CRP concentrations among overweight and obese women (40), inactive women may show more room for the inflammation-lowering effects of the diet.
Few large studies of breast cancer survivors have been published, and this multiethnic cohort provides an important opportunity to investigate relationships between postdiagnosis diet, a modifiable factor, and biomarkers related to survival. Given that blood was collected after primary treatment was completed, the biomarkers measured in our study reflect ongoing host factors that may influence prognosis, as opposed to acute effects that may have been a result of the breast cancer treatments that participants received. We collected high-quality extensive data on clinical characteristics and treatment abstracted from physician and hospital records in addition to SEER cancer registry records, and objectively measured weight at the 30-month assessment. To assess diet, we used a valid and reliable dietary questionnaire designed for use by multiethnic postmenopausal women (37). Using the multidimensional HEI-2005, we were able to distinguish survivors with better vs. poor quality diets based on current dietary guidance. We also had a detailed postdiagnosis assessment of recreational physical activity (36).
In this cross-sectional analysis, we were unable to determine temporality. It also remains possible that underlying conditions of chronic inflammation or confounding by unmeasured factors could explain the results observed for CRP, given that we did not find associations with the other analytes. The self-report nature of our diet assessments may have resulted in exposure misclassification, though we would not expect this misclassification to be differential. There was a difference in response timeframe (last month vs. last year) for the FFQ by study site; however, it is reasonable to assume that women did not differentially make diet changes across sites during that time in the absence of an intervention. Our results are only generalizable to women who have completed treatment and survived at least 30 months after diagnoses of breast cancer. Last, we had insufficient statistical power to examine clinically important subpopulations of breast cancer survivors that may have poor prognosis (such as by race, ER-status, stage, and BMI).
Our study suggests that among inactive survivors, a better quality diet may be related to lower levels of chronic inflammation, which has been linked to improved survival. Future larger cohort studies of breast cancer survivors with sufficient follow-up and multiple measurements over time are needed to confirm our findings, investigate how inflammation may mediate the relationship between diet quality and improved survival, and understand potential heterogeneity of findings among clinically important subpopulations of patients.
Acknowledgments
We would like to thank Dr. Kathy B. Baumgartner, Charles L. Wiggins, HEAL study managers, Eric Meier, Todd Gibson, and the HEAL study participants.
Funding Support: This work was supported by National Cancer Institute Grants N01-CN-75036-20, NO1-CN-05228, NO1-PC-67010, T32 CA09661 and T32 CA105666. A portion of this work was conducted through the Clinical Research Center at the University of Washington and supported by the National Institutes of Health Grant M01-RR-00037 and the University of New Mexico Grant NCRR M01-RR-0997. Data collection for the Women's Contraceptive and Reproductive Experiences Study at the University of Southern California was supported by Contract No. N01-HD-3-3175 from the National Institute of Child Health and Human Development, and patient identification was supported in part by Contract 050Q-8709-S1528 from the California Department of Health Services.
References
- 1.American Cancer Society . Breast cancer facts and figures 2007–2008. Atlanta, GA: 2007. [Google Scholar]
- 2.Kroenke CH, Fung TT, Hu FB, Holmes MD. Dietary patterns and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:9295–303. doi: 10.1200/JCO.2005.02.0198. [DOI] [PubMed] [Google Scholar]
- 3.Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, Caan BJ. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J Clin Oncol. 2009;27:919–26. doi: 10.1200/JCO.2008.19.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce BL, Ballard-Barbash R, Bernstein L, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 7.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 8.Duggan C, Irwin ML, Xiao L, Henderson, et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J Clin Oncol. doi: 10.1200/JCO.2009.26.4473. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puglisi MJ, Fernandez ML. Modulation of C-reactive protein, tumor necrosis factor-alpha, and adiponectin by diet, exercise, and weight loss. J Nutr. 2008;138:2293–6. doi: 10.3945/jn.108.097188. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs DR, Jr., Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508S–513S. doi: 10.1093/ajcn/78.3.508S. [DOI] [PubMed] [Google Scholar]
- 11.Boynton A, Neuhouser ML, Wener MH, et al. Associations between healthy eating patterns and immune function or inflammation in overweight or obese postmenopausal women. Am J Clin Nutr. 2007;86:1445–55. doi: 10.1093/ajcn/86.5.1445. [DOI] [PubMed] [Google Scholar]
- 12.Lee I, Shin G, Choue RA. 12-week regimen of caloric restriction improves levels of adipokines and pro-inflammatory cytokines in Korean women with BMIs greater than 23 kg/m2. Inflamm Res. 2010;59(5):399–405. doi: 10.1007/s00011-009-0113-8. [DOI] [PubMed] [Google Scholar]
- 13.Ata SM, Vaishnav U, Puglisi M, et al. Macronutrient composition and increased physical activity modulate plasma adipokines and appetite hormones during a weight loss intervention. J Womens Health (Larchmt) 2010;19:139–45. doi: 10.1089/jwh.2009.1472. [DOI] [PubMed] [Google Scholar]
- 14.Mullen KL, Smith AC, Junkin KA, Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat-fed rats. Am J Physiol Endocrinol Metab. 2007;293:E83–90. doi: 10.1152/ajpendo.00545.2006. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg GR, Dyck DJ. Development of leptin resistance in rat soleus muscle in response to high-fat diets. Am J Physiol Endocrinol Metab. 2000;279:E1374–82. doi: 10.1152/ajpendo.2000.279.6.E1374. [DOI] [PubMed] [Google Scholar]
- 16.Pearson TA, Mensah GA, Alexander RW, Anderson, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 17.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-Reactive Protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 18.Schultz DR, Arnold PI. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 1990;20:129–47. doi: 10.1016/0049-0172(90)90055-k. [DOI] [PubMed] [Google Scholar]
- 19.Gnacińska M, Małgorzewicz S, Stojek M, Łysiak-Szydłowska W, Sworczak K. Role of adipokines in complications related to obesity. A review. Adv Med Sci. 2009;54:150–157. doi: 10.2478/v10039-009-0035-2. [DOI] [PubMed] [Google Scholar]
- 20.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–91. [PMC free article] [PubMed] [Google Scholar]
- 21.McTiernan A, Rajan KB, Tworoger SS, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–6. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wayne SJ, Baumgartner K, Baumgartner RN, Bernstein L, Bowen DJ, Ballard-Barbash R. Diet quality is directly associated with quality of life in breast cancer survivors. Breast Cancer Res Treat. 2006;96:227–232. doi: 10.1007/s10549-005-9018-6. [DOI] [PubMed] [Google Scholar]
- 23.Ledue TB, Weiner DL, Sipe JD, Poulin SE, Collins MF, Rifai N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A and mannose-binding protein in human serum. Ann Clin Biochem. 1998;35(Pt 6):745–53. doi: 10.1177/000456329803500607. [DOI] [PubMed] [Google Scholar]
- 24.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative Food Frequency Questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 25.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 26.Schakel SF, Buzzard IM, Gebhardt SE. Procedures for estimating nutrient values for food composition databases. J Food Compost Anal. 1997;10:102–114. [Google Scholar]
- 27.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–71. [PubMed] [Google Scholar]
- 28.Bowman SA, Friday JE, Moshfegh A. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004 [Internet] U.S. Department of Agriculture, Agricultural Research Service; Beltsville, MD: 2008. Available from: http://www.ars.usda.gov/ba/bhnrc/fsrg. [Google Scholar]
- 29.Guenther PM, Krebs-Smith SM, Reedy J, et al. Healthy Eating Index-2005. Center for Nutrition Policy and Promotion, United States Department of Agriculture; Beltsville, MD: 2008. [Google Scholar]
- 30.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB. Evaluation of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1854–64. doi: 10.1016/j.jada.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB, Basiotis PP. Development and evaluation of the Healthy Eating Index-2005: Technical Report. Center for Nutrition Policy and Promotion, U.S. Department of Agriculture; Beltsville, MD: 2007. [Google Scholar]
- 33.U.S. Department of Health and Human Services. U.S. Department of Agriculture . Dietary guidelines for Americans, 2005. U.S. Government Printing Office; Washington, DC: 2005. [Google Scholar]
- 34.Kriska A. Modifiable Activity Questionnaire. Med Sci Sports Exerc. 1997;29:73–78. [Google Scholar]
- 35.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 36.Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The Health, Eating, Activity, and Lifestyle study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Department of Health and Human Services . Physical activity guidelines for Americans: Be active, healthy, and happy. Washington, DC: 2008. [Google Scholar]
- 38.Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 39.Ballard-Barbash R, Hunsberger S, Alciati MH, et al. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101:630–43. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell PT, Campbell KL, Wener MH, et al. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med Sci Sports Exerc. 2009;41:1533–9. doi: 10.1249/MSS.0b013e31819c7feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guenther PM, Krebs-Smith SM, Reedy J, et al. Healthy Eating Index-2005 Fact Sheet. CNPP Fact Sheet No.1, December 2006. Slightly Revised. 2008 June; [Google Scholar]