Abstract
Mutations in the uncoupling protein 2 (Ucp2) gene are linked to type-2 diabetes. Here, a potential mechanism by which lack of UCP2 is cytoprotective in pancreatic β-cells was investigated. Nitric oxide (NO) production was elevated in Ucp2−/− islets. Proliferation (cyclin D2, Ccnd2) and anti-apoptosis (Tnfaip3) genes had increased expression in Ucp2−/− islets, whereas the mRNA of pro-apoptosis genes (Jun, Myc) was reduced. TNFAIP3 cellular localization was detected in both α- and β-cells of Ucp2−/− islets but in neither α- nor β-cells of UCP2+/+ islets, where it was detected in pancreatic polypeptide-expressing cells. TNFAIP3 distribution was not markedly altered 14 days after streptozotocin treatment. Basal apoptosis was attenuated in Ucp2−/− β-cells, while the nuclear factor κB (NF-κB) pathway was transactivated after islet isolation. Ucp2+/+ and Ucp2−/− islets were treated with cytokines for 24 h. Cytokines did not increase NF-κB transactivation or apoptosis in Ucp2−/− islets and TNFAIP3 was more strongly induced in Ucp2−/− islets. Inhibition of NO production strongly reduced NF-κB activation and apoptosis. These data show that null expression of Ucp2 induces transactivation of NF-κB in isolated islets, possibly due to NO-dependent up-regulation of inhibitor of κB kinase β activity. NF-κB transactivation appears to result in altered expression of genes that enhance a pro-survival phenotype basally and when β-cells are exposed to cytokines. TNFAIP3 is of particular interest because of its ability to regulate NF-κB signaling pathways.
Introduction
Uncoupling protein 2 (UCP2) belongs to a family of mitochondrial proteins characterized by an ability to transport ions, including protons and fatty acid anions (Esteves & Brand 2005); however, the specific physiological role of UCP2 is still unclear. In pancreatic β-cells, increased UCP2 expression is associated with suppression of glucose-stimulated insulin secretion (Chan et al. 1999, 2001) and mediates the negative effects of superoxide on insulin secretion (Krauss et al. 2003). In addition, the lack of UCP2 in mice protects them from the diabetogenic effects of a high-fat diet and also maintains glucose-stimulated insulin secretion (Joseph et al. 2002, 2004). Although baseline apoptosis is higher in Ucp2−/− mouse islets, and these islets produce higher levels of reactive oxygen species, no further increase in these parameters is observed after long-term high-fat diet treatment (Joseph et al. 2004). The mechanistic basis for protection from diabetes may be partially related to increased capacity for fatty acid oxidation (Joseph et al. 2004, Fatehi-Hassanabad & Chan 2007), but other mechanisms may also be operative.
It has been proposed that UCP2 modulates the inflammatory response and regulates the production of reactive oxygen species (Brookes 2005) and nitric oxide (NO) in cells (Bai et al. 2005). Depending on the amount produced, reactive oxygen species may stimulate or inhibit glucose-stimulated insulin secretion from β-cells (Pi et al. 2007), which may account for some inconsistencies in the literature regarding UCP2 function. Likewise, in the central nervous system, induction of UCP2 protects neurons from oxidative stress (Sullivan et al. 2004) but not all studies show this effect (de Bilbao et al. 2004, Prabhakaran et al. 2005). In our study of autonomic dysreflexia following spinal cord injury, no differences in cell damage were detected in Ucp2−/− versus Ucp2+/+ mice (Webb et al. 2006). In macrophages from Ucp2−/− mice, cytokine, NO and reactive oxygen species (ROS) production, as well as inducible NO synthase (iNOS) expression, were elevated (Bai et al. 2005). This proinflammatory state was attributed to increased activation of the transcription factor nuclear factor κB (NF-κB). It seems clear that under some conditions, UCP2 can provide protection from oxidative stress but such protection may depend on tissue-specific factors, such as the ability to induce other protective proteins, the overall effects on ATP production and the involvement of resident macrophages.
In β-cells, NF-κB is generally regarded as a mediator of inflammation, leading to induction of pro-apoptotic genes and cell death (Ho & Bray 1999, Ou et al. 2005, Eldor et al. 2006, Ortis et al. 2006). Acutely exposing β-cells to cytokines (Ortis et al. 2006) induces NF-κB nuclear translocation, whereas blocking NF-κB activation prevents β-cell damage (Eldor et al. 2006, Kwon et al. 2006). Reduction of the β-cell mass in type 2 diabetes is among the pathological effects caused by proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin 1β (IL1β) and interferon-γ (IFNγ; Eizirik & Mandrup-Poulsen 2001). In combination, IFNα and IL1β increase NO production and iNOS expression (Thomas et al. 2002). Application of the NO precursor nitroprusside is lethal to β-cells (Kroncke et al. 1991), while inhibition of iNOS with aminoguanidine is protective (Kuttler et al. 1987). An alternative role for NF-κB in regulation of β-cell function and survival has been revealed. Novel data from Hagerkvist et al. (2007) showed that the tyrosine kinase inhibitor imatinib mesylate protected β-cells from death in non-obese diabetic and streptozotocin (STZ)-induced diabetic mice. In vitro, imatinib reduced cytokine-stimulated apoptosis and this was associated with reduced association of inhibitor of κB (IκBα) with NF-κB subunit RelA. In another study, non-obese diabetic mice expressing a super-repressor of NF-κB activity had increased susceptibility to developing diabetes, which could be overcome by neutralizing TNF-α. These mice had reduced expression of anti-apoptotic proteins in β-cells (Kim et al. 2007).
Because we observed that lack of UCP2 protected β-cells from UCP2−/− mice from a high-fat diet-induced cell death (Joseph et al. 2004, 2005), we predicted that protection from other pro-apoptotic stimuli such as cytokines might also be afforded by lack of UCP2. Thus, the objective of this study was to examine the effects of cytokines on β-cells from Ucp2+/+ and Ucp2−/− mice, with emphasis on the role of NF-κB pathway. It was hypothesized that Ucp2−/− islets would have increased activation of the NF-κB pathway, similar to previous results reported in immune tissues (Bai et al. 2005).
Materials and methods
Materials
Cell culture medium (DMEM), antibiotic–antimycotic solution, calf and fetal bovine serum, Griess Reagent kit, TRIzol and Cloned AMV First-Strand cDNA Synthesis kit were purchased from Invitrogen. HEPES, BAY 11-7082, caffeic acid phenethyl ester (CAPE) and all cytokines were purchased from Sigma–Aldrich. Roche supplied BSA and the TUNEL assay kit. Nuclear extraction kits, NF-κB transactivation and IκBα phosphorylation ELISAs were purchased from Active Motif (Carlsbad, CA, USA). Antibodies to iNOS (BD Biosciences, Mississauga, Ontario, Canada), NF-κB (C-20), TNFAIP3 (H-100 and A-12), JUN (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin (Sigma–Aldrich) were also purchased. Insulin and somatostatin antibodies were purchased from Dako (Glostrup, Denmark), glucagon antibody from Abcam (Cambridge, UK) and pancreatic polypeptide from Thermo Fisher Scientific (Rockford, IL, USA).
Animals and pancreatic islet cultures
All procedures involving animals were approved by the respective university Animal Care Committees (Toronto and Prince Edward Island) and followed the guidelines of the Canadian Council on Animal Care. Littermate Ucp2+/+ and Ucp2−/− mice on a mixed C57Bl6/129 background bred from heterozygous mice (Zhang et al. 2001), age 16 weeks old and fed ad libitum were used for mouse islet experiments. Mice were anesthetised with pentobarbital (65 mg/ml, i.p.) and isolated pancreatic islets were obtained by collagenase digestion as described (Zhang et al. 2001). After digestion, the islets were purified by filtration (Salvalaggio et al. 2002), with minor modifications. Islets were cultured for 24 h in DMEM, 8·3 mmol/l glucose, supplemented with 10% calf serum, 1% antibiotic–antimycotic solution, 10 mmol/l HEPES with or without NF-κB inhibitors Bay 11-7082 (BAY, 10 μmol/l) or CAPE (10 μmol/l) or the iNOS inhibitor 1400W (10 μmol/l, Sigma) plus a combination of IL1β (50 ng/ml), IFNγ (50 ng/ml)±TNF-α (5 ng/ml). A density of 30 islets/well was incubated for each treatment. Mice made diabetic by STZ were treated according to Lee et al. (2009) and tissues were harvested 14 days later.
NF-κB RelA activity and IκBα phosphorylation assay
Isolated islets from Ucp2+/+ and Ucp2−/− mice were cultured as noted above for 24 h. Nuclear proteins were extracted according to the manufacturer's instructions (Active Motif). The supernatant (nuclear fraction) was kept at −80 °C until analysis. The active NF-κB contained in the nuclear extracts was measured by its DNA-binding activity on immobilized oligonucleotides encoding a specific consensus site using a NF-κB RelA transcription factor ELISA kit (Active Motif). Phosphorylated IκBα was measured in islet cellular extracts. Ucp2+/+ and Ucp2−/− islets were pretreated with BAY (10 μmol/l) or 1400W (10 μmol/l) for 2 h and then with a combination of cytokines, as described above. After 10 min incubation, islets were centrifuged and washed with PBS. Cellular extraction, protein determination and IκBα ELISA to measure phosphorylated IκBα were carried out according to ELISA kit instructions (Active Motif). Samples were measured by luminescence detection.
Apoptotic and necrotic cell determination
Determination of DNA strand breaks in islet cells was assessed by the TUNEL technique. After 24 h culture, islets were trypsinized (0·16 mg/ml trypsin) and the cells were fixed on precoated poly-l-lysine glass slides by cytospinning. Cells were sequentially stained for TUNEL and insulin, then the proportion of cells positive for TUNEL and insulin cells was quantified at 200× magnification. An average of 2000 cells/cell preparation was counted for each treatment condition and data were expressed as percentage of total β-cells. For necrosis measurements, control or treated mouse islets, or dispersed islet cells, plated on glass coverslips were incubated with propidium iodide (PI, 4 μmol/l) for 20 min at room temperature in darkness in buffer of the following composition (in mmol/l): 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 NaHCO3, 10 HEPES, pH 7·4. The coverslips were immediately washed in the same buffer, transferred to an open chamber, placed on the microscope stage and maintained at 36–37 °C using a Delta T4 Culture Dish Controller (Bioptechs, Butler, PA, USA). PI fluorescence was excited at 540 nm and emission measured with a 660 nm band pass filter, using a 550 nm beam splitter using an Olympus BX51W1 fluorescent microscope fitted with a 20×/0·95 water immersion objective and cooled CCD camera. For excitation, a xenon lamp-based DeltaRam high-speed monochromator from Photon Technology International (PTI, Lawrenceville, NJ, USA) was used. For control of the monochromator and videocamera, as well as for fluorescent imaging and collecting of data, the ImageMaster 3.0 software (PTI) was used. The data were expressed as percent of PI positive cells.
Nitrate/nitrite production
Nitrate/nitrite derived from NO produced in 24 h from 25 islets was measured by the Griess reaction.
Quantitative real time-PCR
Total RNA was extracted from 30 isolated islets by the TRIzol method (Invitrogen). cDNA was synthesized from 1 μg total RNA. Real-time-PCR was performed to measure changes in selected genes regulated by NF-κB or otherwise important to β-cell function. Primer sequences are listed in Table 1.
Table 1.
Primer sequences used for real-time PCR
| Gene (alternate name) | Forward | Reverse |
|---|---|---|
| Actb (Actin, beta) | 5′-CTGAATGGCCCAGGTCTGA-3′ | 5′-CCCTGGCTGCCTCAACAC-3′ |
| Ddit3 (Chop) | 5′-CAACAGAGGTCACACGCACAT-3′ | 5′-CCTGGGCCATAGAACTCTGACT-3′ |
| Myc | 5′-CGGTTCCTTCTGACAGAACTGA-3′ | 5′-CAGCCAAGGTTGTGAGGTTAGG-3′ |
| Ptgs2 (Cyclo-oxygenase-2) | 5′-CCTACTACAAGTGTTTCTTTTTTGCATT-3′ | 5′-TCACACCATAGTTCAGAGAGGTAATCA-3′ |
| Cpt1a | 5′-CAATGCAATTTTTTACTCCTTCCA-3′ | 5′-TTACCTCCTCCTTTGAACACTTACAG-3′ |
| Ccnd2 (cyclin D2) | 5′-TGACTGAACCATTTTGGATGTAAGAA-3′ | 5′-CCCGGACGCTCAGTCTTG-3′ |
| Gpx1 | 5′-GCGGCCCTGGCATTG-3′ | 5′-GGACCAGCGCCCATCTG-3′ |
| Ikbkb (IKKβ) | 5′-ACAGCCAGGAGATGGTACG-3′ | 5′-CGGACTTTGCTACAGGCGAT-3′ |
| Nos2 (inducible nitric oxide synthase (iNOS)) | 5′-CCTCCTTTGCCTCTCACTCT-3′ | 5′-CTTCAGTCAGGAGGTTGAGTTTTTC-3′ |
| Isl1 | 5′-GGAGATGACGGGCCTCAGT-3′ | 5′-CTGCGTTTCTTGTCCTTGCA-3′ |
| Jun (c-jun) | 5′-CCAGCAATGGGCACATCAC-3′ | 5′-TGCTCGTCGGTCACGTTCT-3′ |
| Mapk1 | 5′-TGCTGTGGAGTTGATGGTGTTAT-3′ | 5′-TTGAACAAGGCATATTTCTCATCAG-3′ |
| Pdx1 | 5′-TTTGAAGTCAGTCAGTTGCTCCTT-3′ | 5′-CCTTCAACCCCTCTCTTGCTATT-3′ |
| Sod2 (MnSOD) | 5′-CACATTAACGCGCAGATCATG-3′ | 5′-CCAGAGCCTCGTGGTACTTCTC-3′ |
| Sod3 | 5′-AGGTGGATGCTGCCGAGAT-3′ | 5′-TCCAGACTGAAATAGGCCTCAAG-3′ |
| Tnfaip3 (A20) | 5′-TGGTTCCAATTTTGCTCCTT-3′ | 5′-CGTTGATCAGGTGAGTCGTG-3′ |
| Ucp2 | 5′-CATCGCCTCCCCTGTTGAT-3′ | 5′-TGGCCCAAGGCAGAGTTC-3′ |
Western blotting
Groups of cultured islets were washed with 0·1 M PBS, then lyzed with Tris–EDTA–sucrose buffer (pH 7·4; containing 1% Triton X-100, 4 μg/ml aprotinin and 5 μg/ml bestatin), sonicated on ice for 5 min and separated by SDS-PAGE. Membranes were probed with specific primary antibodies and protein band intensities were normalized to β-actin.
Immunohistochemistry
Pancreas was harvested from mice just prior to islet isolation by taking a small piece of tissue from adjacent to the spleen and fixing in buffered formalin for 24 h. The tissues were dehydrated and embedded in paraffin using standard procedures and 5 μm sections cut and affixed to glass slides. Following dewaxing, sections were blocked using 5% normal serum complementary to the respective secondary antibody. Unmasking was done by immersing the slides in heated PBS (pH 7·2) for 30 min. The sections were then incubated with primary antibody overnight at 4 °C. Following stringent washing in PBS, appropriate secondary antibodies (conjugated to AlexaFluor dyes of 488 or 546 nm excitation wavelength) were applied for 1 h at room temperature. This process was repeated for blocking primary and secondary antibodies for dual-labeling experiments. Slides were coverslipped in SlowFade Gold containing DAPI (Invitrogen) in order to visualize the nuclei. Digital photomicrographs were obtained using a Zeiss fluorescence microscope and Axiovision 4.6 software.
Statistical analysis
All results are expressed as mean±s.e.m. Data were analyzed by using one or two-way ANOVA. Statistical analyses were done using Prism software (Graphpad, San Diego, CA. USA). P<0·05 was considered statistically significant.
Results
NO production is enhanced by UCP2 null expression
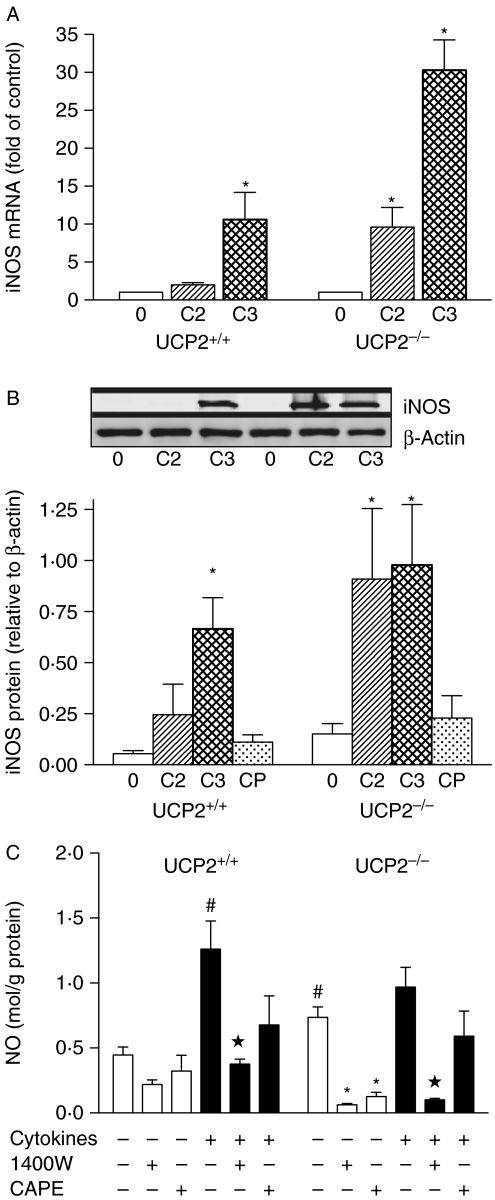
Increased NO and superoxide production has been reported in immune tissues of Ucp2−/− mice (Bai et al. 2005; Fig. 1). In pancreatic islets, basal Nos2 mRNA (Fig. 1A) and iNOS protein expression (Fig. 1B) were barely detectable relative to β-actin in both Ucp2+/+ and Ucp2−/− islets, but NO production, measured by Griess assay, was increased by 2-fold in Ucp2−/− versus Ucp2+/+ islets (P<0·05, Fig. 1C). Interestingly, the iNOS inhibitor 1400W at a concentration of 10 μmol/l more potently reduced basal NO production in Ucp2−/− than Ucp2+/+ islets (Fig. 1C), although a concentration of 50 μmol/l reduced NO to not-detectable levels in both islets (data not shown). Higher doses of 1400W non-specifically inhibit other NOS isoforms. Cytokine exposure of intact islets for 24 h increased iNOS mRNA (Fig. 1A) and protein (Fig. 1B). NO was increased significantly in Ucp2+/+ but not in Ucp2−/− islets when cytokines were present (P<0·05, Fig. 1C). In both cases, NO production was suppressed by 1400W but not by CAPE.
Figure 1.
Basal and cytokine-stimulated production of nitric oxide in islets. Expression of (A) iNOS (Nos2) mRNA and (B) iNOS protein in UCP2+/+ and UCP2−/− islets. 0=untreated control islets, C2=IL1β+IFNγ and C3=IL1β+IFNγ+TNF-α. Data were normalized to β-actin expression, as shown in the representative blot (inset). The effect of CAPE (CP) in the presence of three cytokines is also shown. N=3 or greater. (C) Nitric oxide (as nitrite normalized to total protein) was measured in supernatant from cultures of islets after 24 h in the absence or presence of iNOS inhibitor 1400W (10 μmol/l) or CAPE (10 μmol/l). N=4–8. *P<0·05 compared with respective basal group; star indicates P<0·05 compared with UCP2+/+ basal; #P<0·05 compared with respective cytokine-treated group.
No genotype-related differences in ROS formation were found under any experimental conditions (data not shown). Mn-superoxide dismutase, which may be induced by ROS, was quantified by western blotting and was similar between Ucp2−/− and Ucp2+/+ islets basally and after exposure to cytokines for 24 h. mRNA expression of Sod2, Sod3 and glutathione peroxidase-1 (Gpx1) was also not significantly different between genotypes (data not shown).
Effect of UCP2 null expression and cytokines on expression of selected genes
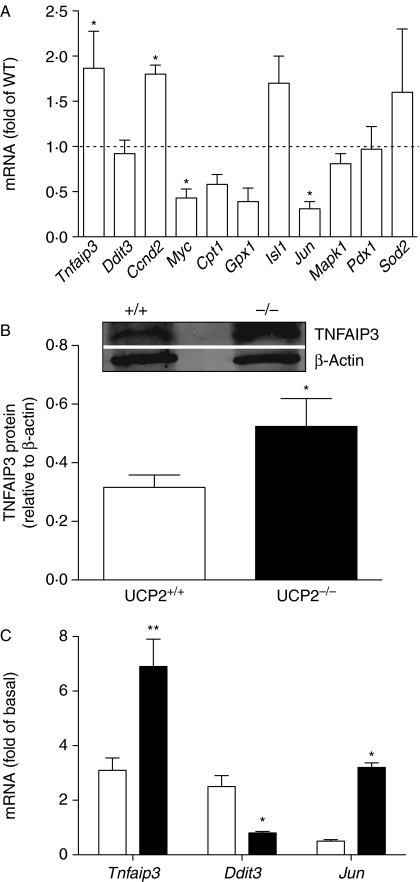
The adaptive response to cytokine exposure was compared between Ucp2−/− and Ucp2+/+ islets (Figs 2 and 3A). A significant increase in basal cyclin D2 (Ccnd2) and Tnfaip3 mRNA was detected in Ucp2−/− islets, whereas the mRNA expression of Myc and Jun was reduced (Fig. 2A). Basal protein expression of TNFAIP3 was also increased in Ucp2−/− islets (Fig. 2B). Immunohistochemistry also revealed strong staining of JUN in Ucp2+/+ relative to Ucp2−/− islets, and the majority positive cells also stained positively for insulin (Fig. 3A).
Figure 2.
Expression of selected genes regulated by NF-κB or important for β-cell differentiation and function. (A) Basal expression of selected genes. Data show mRNA levels in UCP2−/− islets relative to UCP2+/+ islets (dashed line). Carnitine palmitoyl transferase-1, Cpt1; chop, Ddit3; cyclin D2, Ccnd2; glutathione peroxidise-1, Gpx1; mitogen-activated protein kinase-1, Mapk1; pancreas duodenum homeobox-1, Pdx1; superoxide dismutase-2, Sod2. (B) Protein expression of TNFAIP3 in UCP2+/+ and UCP2−/− islets detected by immunoblotting. (C) Effects of cytokine treatment (IL1β+IFNγ+TNF-α) for 24 h on mRNA expression of TNFAIP3, Ddit3 (chop) and Jun expressed as fold of untreated controls in UCP2+/+ and UCP2−/− islets. *P<0·05 and **P<0·01 for n=3–4 separate preparations.
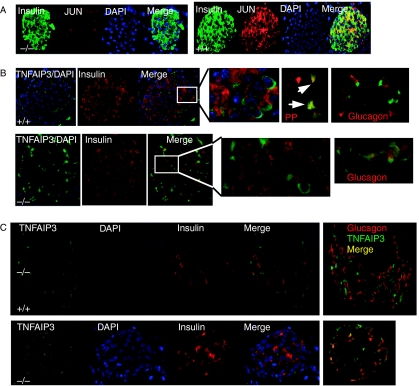
Figure 3.
(A) Protein expression of JUN in UCP2+/+ and UCP2−/− islets detected by immunohistochemistry. When images were merged, co-localization of insulin (green) and JUN (red) was detected as yellow in UCP2+/+ islets. The intensity of JUN staining also appeared more intense in the UCP2+/+ sections. (A) Representative photomicrographs showing localization of TNFAIP3 in islets by immunohistochemistry. TNFAIP3 (green) and insulin (red) were independently detected in islets from UCP2+/+ (top) and UCP2−/− (bottom) mice. Nuclei were visualized with DAPI. Merged images indicated that in UCP2+/+ islets, TNFAIP3 was detected mainly in the perinuclear region of non-β-cells (top, expanded from inset). There was co-labeling of TNFAIP3 with some pancreatic polypeptide-expressing cells (arrows) but not glucagon-labeled cells (far right). In UCP2−/− islets, TNFAIP3 was detected not only in the perinuclear regions of non-β-cells but also in the cytoplasm of cells co-staining for insulin (bottom, expanded from inset). In UCP2−/− islets, co-staining of TNFAIP3 and glucagon was detected (far right). (C) Effects of STZ treatment on TNFAIP3 immunostaining in islets. Tissues were obtained 14 days after STZ administration. In UCP2+/+ islets (top), TNFAIP3 staining was confined to non-β- and non-α-cells. Islets from UCP2−/− mice (bottom) expressed TNFAIP3 in most α-cells.
Expression of these genes was assessed after cytokine treatment of the islets (Fig. 2C). The mRNA for Tnfaip3, an important anti-apoptosis gene in β-cells, was induced ∼6-fold in Ucp2−/− islets (P<0·01) compared with 3-fold in Ucp2+/+ islets. The redox-sensitive transcription factor gene Jun was significantly increased in Ucp2−/− but not in Ucp2+/+ islets by 24 h cytokine treatment, whereas Ddit3 (chop), expressed in endoplasmic reticulum stress, was 3-fold higher in Ucp2+/+ islets after cytokine treatment (Fig. 2C). The expression of Myc was not altered by either cytokines in either genotype, while cytokines reduced expression of the rate-limiting gene of fat metabolism, Cpt1, in both (not shown). Lastly, expression of genes involved in cell growth and differentiation was measured. Overall, similar inhibition of expression of Ccnd2, Isl1, Mapk and Pdx1 was observed in both Ucp2+/+ and Ucp2−/− islets exposed to cytokines (not shown).
TNFAIP3 and NF-κB immunostaining: effects of STZ
TNFAIP3 is characterized as a stress-response gene and may have a critical role in protecting β-cells (Liuwantara et al. 2006; Figs 3B and C and 4). TNFAIP3 immunostaining was detected in both Ucp2+/+ and Ucp2−/− islets using H-100 polyclonal antibody. In Ucp2+/+ islets, TNFAIP3 was localized in the perinuclear region of cells on the periphery of islets and did not co-localize with insulin. However, in Ucp2−/− islets, perinuclear localization was detected in non-β-cells, while diffuse cytoplasmic staining was detected in β-cells (Fig. 3B). Whether TNFAIP3 co-localized with glucagon was investigated and found to differ by genotype. As shown in the micrographs at the far right of Fig. 3B, TNFAIP3 and glucagon were co-localized in Ucp2−/− but not in Ucp2+/+ islets. In Ucp2+/+ islets, the TNFAIP3 staining overlapped with a majority of pancreatic polypeptide-expressing cells. No TNFAIP3 staining was detected in somatostatin-expressing cells (not shown). A similar staining pattern of TNFAIP3 was found when a monoclonal antibody (A-12) was used.
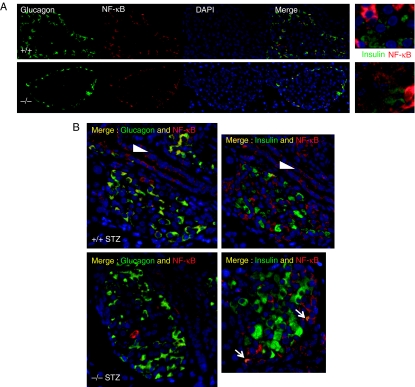
Figure 4.
Localization of NF-κB by immunohistochemistry. (A) Glucagon (green) and NF-κB (red) were independently detected in islets from UCP2+/+ (top) and UCP2−/− (bottom) mice. Nuclei were visualized with DAPI. Merged images indicated that in UCP2+/+ islets, NF-κB was detected in non-β-cells of both phenotypes. Less-intense staining was also detected in the islet core of UCP2−/− islets. Co-localization of insulin with NF-κB was detected in UCP2−/− but not in UCP2+/+ islets (far right panels). For these images, insulin staining was blunted equally in both photomicrographs using Image J (intensity range 43–446) and the maximum intensity of NF-κB increased equally (intensity range 0–140). (B) Effects of STZ treatment on NF-κB immunostaining in islets. Tissues were obtained 14 days after STZ administration. In UCP2+/+ islets (top), NF-κB staining was confined to non-β-cells that also stained for glucagon (left panel), whereas co-localization with insulin was not detected (right panel). Islets from UCP2−/− mice (bottom) expressed NF-κB in α-cells. A minority of β-cells also appeared to express NF-κB (arrows). In both genotypes, NF-κB was detected in duct cells (arrowheads).
STZ induces oxidative/nitrosative stress and kills β-cells. TNFAIP3 localization in pancreatic tissue from STZ-treated Ucp2−/− and Ucp2+/+ mice was compared 14 days after induction of diabetes. TNFAIP3 staining in Ucp2+/+ islets was not associated with either β- or α-cells (Fig. 3C, top panel), whereas in Ucp2−/− islets, some α-cells staining for glucagon also stained for TNFAIP3 (Fig. 3C, bottom panel).
NF-κB regulates Tnfaip3 transcription and TNFAIP3 acts to negatively regulate NF-κB transactivation in islets (Grey et al. 2003). We therefore investigated whether altered NF-κB expression or cellular localization could be detected in pancreatic sections. NF-κB immunostaining of pancreatic islets was more intense than of acinar cells and was similar in intensity in Ucp2−/− and Ucp2+/+ islets in fixed pancreatic tissue. NF-κB staining co-localized with α-cells in both Ucp2+/+ and Ucp2−/− islets (Fig. 4A and B). In islets from Ucp2−/− mice, NF-κB staining distinctly above background was also detected in non-α-cells. Because these cells were central to the islet, they appeared to be β-cells, although it was difficult to detect co-localization with insulin. Following STZ treatment, there appeared to be increased NF-κB staining intensity in non-β-cells and also in duct cells in islets from both genotypes (Fig. 4C and D). In all cases, the majority of staining was non-nuclear. A minority of β-cells also appeared to co-localize NF-κB in the Ucp2−/− islets.
Lack of UCP2 modifies basal NF-κB activation and apoptosis in isolated islets
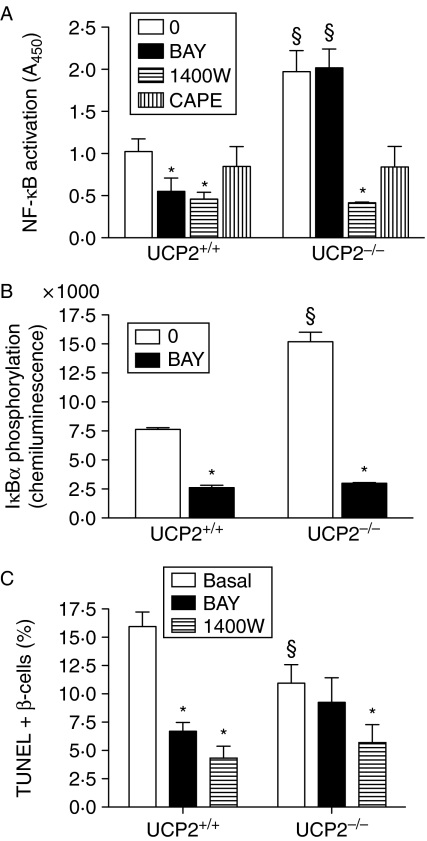
Because of the potential induction of NF-κB in islets from Ucp2−/− mice, further characterization was performed in isolated islets (Fig. 5). Basal NF-κB activity was significantly increased (P<0·05) by 2-fold in Ucp2−/− versus Ucp2+/+ islets after overnight culture (Fig. 5A). The compound BAY, which blocks phosphorylation of IκBα to prevent NF-κB nuclear translocation, inhibited NF-κB activation by >50% in Ucp2+/+ islets but had no effect in Ucp2−/− islets when applied for 2 h. However, blocking NO production with the iNOS inhibitor 1400W significantly inhibited basal NF-κB translocation in both genotypes. Likewise, CAPE, which interferes with the binding of the RelA subunit to DNA, reduced NF-κB activation in islets from both genotypes. The increase in basal NF-κB activity in Ucp2−/− islets could be attributed to 2-fold greater basal IκBα phosphorylation (Fig. 5B), downstream from increased basal IKKβ expression, as suggested from measurement of mRNA (Fig. 6C). IκBα phosphorylation was significantly inhibited by BAY within 2 h of application in Ucp2−/− and Ucp2+/+ islets, suggesting that, in a relatively acute timeframe, lack of effect of BAY on NF-κB activation is due to its sequestration in the nucleus.
Figure 5.
Characterization of basal NF-κB pathway and apoptosis in islets from UCP2+/+ and UCP2−/− mice. (A) Basal NF-κB (RelA subunit) activation (n=4) in control cells and those incubated with IKKβ inhibitor BAY 11-7082 (10 μM) or iNOS inhibitor 1400W (10 μM) for 2 h. Data were normalized to protein concentration. (B) Basal IκBα phosphorylation in the presence or absence of BAY (n=4). Effects were quantified by chemiluminescence and normalized to protein concentration. (C) Basal apoptosis, as a percentage of total β-cells, in UCP2+/+ and UCP2−/− islets, comparing the effects of BAY or 1400W with untreated controls. Cell apoptosis was assessed by TUNEL staining. Two thousand cells were counted on average at 200× magnification (n=4). *P<0·05, effect of inhibitor; §P<0·05, effect of genotype).
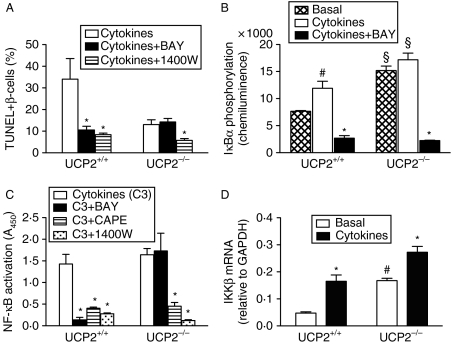
Figure 6.
Effects of 24 h exposure to cytokines on β-cell death and NF-κB pathway in islets from UCP2+/+ and UCP2−/− mice. In all cases, n=4. Basal=untreated control islets, cytokines=IL1β+IFNγ+TNF-α. (A) Cytokine-induced apoptosis, as percentage of total β-cells from UCP2+/+ and UCP2−/− islets after 24 h exposure. Effects of concurrent BAY 11-7082 (10 μM) are shown. The iNOS inhibitor 1400W (10 μM, 24 h) inhibited apoptosis induced by cytokines to 8·4±0·7% in UCP2+/+ and 5·8±0·9% in UCP2−/− islets (P<0·05 for both). (B) Effects of 24 h cytokines on NF-κB activation in UCP2+/+ and UCP2−/− islets in cytokine-treated cells and those incubated concurrently with inhibitors. The IKKβ inhibitor BAY strongly inhibited NF-κB activation in UCP2+/+ but not in UCP2−/− islets. The iNOS inhibitor 1400W inhibited NF-κB transactivation in both UCP2+/+ and UCP2−/− islets (P<0·05 for both). CAPE also inhibited NF-κB transactivation in both UCP2+/+ and UCP2−/− islets (P<0·05 for both). (C) Phosphorylation of IκBα basally or stimulated by cytokines in the presence and absence of BAY in UCP2+/+ and UCP2−/− islets. (D) Expression of IKKβ mRNA in control and cytokine-treated islets. *P<0·05, effect of cytokines; §P<0·05 for genotype effect; #P<0·05, effect of cytokines compared with basal).
To determine the effects of increased basal NF-κB activation on β-cell viability, we assessed apoptosis frequency by TUNEL, which was significantly higher in dispersed cells from Ucp2+/+ than Ucp2−/− islets (Fig. 5C). Ucp2+/+ islets had a significantly (P<0·05) reduced percentage of apoptotic cells when treated with BAY for 24 h but there was no effect in Ucp2−/− islets. Blocking NO production with 1400W reduced apoptosis by ∼75% in Ucp2+/+ islets. There was a 36% decrease in apoptosis in Ucp2−/− islets, from 11·0 to ∼7·0% (P<0·05).
Cytokine-mediated cell death has altered dependence upon NF-κB in Ucp2−/− islets
Culture for 24 h with a combination of three cytokines (50 ng/ml IL1β+50 ng/ml IFNγ+5 ng/ml TNF-α) increased apoptosis assessed by the TUNEL method in Ucp2+/+ but not in Ucp2−/− β-cells (Fig. 6A). In β-cells exposed to cytokines plus iNOS inhibitor 1400W, apoptosis was strongly inhibited in both genotypes (Ucp2+/+: 34·1±9·5% vs 8·4±0·7% and Ucp2−/−: 13·1±2·2 vs 5·8±0·9% in the absence and presence of 1400W, respectively, P<0·01). A significant reduction (P<0·01) in apoptotic cell number was observed in cytokine-exposed Ucp2+/+ but not in Ucp2−/− β-cells treated concurrently with BAY for 24 h (Fig. 6A). Necrosis (PI fluorescence) in both genotypes was increased equally by cytokines (data not shown).
NF-κB activity was stimulated by 2·5-fold by cytokines in Ucp2+/+ islets (P<0·05) and this was inhibited by >90% with 2 h BAY treatment (P<0·001). Conversely, neither cytokines nor BAY affected NF-κB activity in islets from Ucp2−/− mice (Fig. 6B). However, NF-κB activation was strongly inhibited by 1400W and CAPE in both genotypes. IκBα phosphorylation triggers translocation of RelA subunit of NF-κB to the nucleus. In Ucp2+/+ mice, cytokines increased IκBα phosphorylation, as expected, and this was significantly reduced by BAY. Ucp2−/− islets did not demonstrate any increase in IκBα phosphorylation in the presence of cytokines but BAY did reduce phosphorylation by 85% (Fig. 6C). By western blotting, there was no difference in total expression of either NF-κB or IκBα in Ucp2+/+ versus Ucp2−/− islets (data not shown). However, the mRNA expression of IKKβ, which phosphorylates IκBα, was elevated 2–3-fold in Ucp2−/− compared with Ucp2+/+ islets, both basally and after 24 h cytokine treatment (Fig. 6D).
Discussion
Null expression of UCP2 enhances β-cell adaptation to metabolic stress as shown by high-fat feeding studies in which glucose-stimulated insulin secretion remained robust and β-cell mass was expanded more than that in Ucp2+/+ mice (Joseph et al. 2002, 2004). These results are considered paradoxical by some authors in light of the hypothesis that UCP2 induction protects cells from oxidative stress (Pi et al. 2009). Indeed, this is demonstrated in stroke survival (Mehta & Li 2009). The purpose of the current study was to examine potential mechanisms contributing to β-cell protection in the absence of UCP2. We show that isolated Ucp2−/− islets have increased basal NO production and expression of Tnfaip3 (also called A20) and Ccnd2, two genes associated with cell proliferation, and reduced expression of Myc and Jun, two genes associated with apoptosis. The link between NO and the altered expression of these genes appears to be the transcription factor NF-κB. The resultant phenotype in isolated islets includes activation of NF-kB and induction of and/or altered cellular localization of TNFAIP3. These traits are linked to protection of β-cells from isolation- and cytokine-mediated apoptosis.
Basal production of NO was 2-fold higher in islets from Ucp2−/− than from Ucp2+/+ mice. Because basal NO synthesis of Ucp2−/− islets was inhibited by 1400W, enhanced activity of iNOS is suggested, whereas in Ucp2+/+ islets, basal production of NO was not inhibited by 1400W. Islets express neuronal NOS and endothelial NOS, both constitutively active isoforms (Spinas 1999) not sensitive to 1400W (Garvey et al. 1999), that are possibly the source of basal NO in Ucp2+/+ islets. These isoforms may also contribute to the fraction of basal NO not inhibited by 1400W in Ucp2−/− islets. Exposure to cytokines, which are known to induce iNOS, elicited similar increase in iNOS mRNA and protein in Ucp2+/+ and Ucp2−/− islets. The NO produced under cytokine stimulation was inhibited by 75 and 90% by 1400W in Ucp2+/+ and Ucp2−/− islets, respectively. These data suggest higher basal activity of iNOS in Ucp2−/− islets and is consistent with other studies showing elevated circulating NO, increased NO production by macrophages and increased iNOS expression in spleen (Bai et al. 2005). The physiological relevance of this relatively small increase in NO production remains unclear, but low concentrations of NO can act in signaling pathways, having been shown to promote insulin secretion via modulation of ATP-dependent K channels (Sunouchi et al. 2008).
NO is implicated in the regulation of gene transcription, working via NF-κB and the cAMP-response element binding protein, MYC (Contestabile 2008). A collection of pro-proliferative, pro-apoptotic, islet-specific and metabolic genes was screened for basal and cytokine-stimulated mRNA expression. NF-κB is known to respond to cytokines and is differentially regulated in immune tissues in Ucp2−/− mice (Bai et al. 2005); thus, some genes selected are regulated by NF-κB. Basally, four genes from this panel were differentially expressed in Ucp2−/− compared with Ucp2+/+ islets. Reduced expression of Myc and Jun is predicted to promote cell survival in Ucp2−/− islets. MYC is a transcription factor implicated in starvation-induced, caspase-mediated apoptosis in β-cells (Van de Casteele et al. 2003), and JUN, also a transcription factor, is involved in cytokine-mediated apoptosis (Ammendrup et al. 2000). In islets, Myc is regulated by NF-κB but Jun is induced by cytokines independent of NF-κB (Cardozo et al. 2001). Conversely, we observed induction of Ccnd2, which is essential for postnatal β-cell replication (Kushner et al. 2005) and could be associated with the enhanced proliferation of β-cells observed in islets from high-fat diet-fed mice (Joseph et al. 2002). Each of these observations is consistent with reduced frequency of apoptosis in Ucp2−/− β-cells observed after islet isolation.
The fourth gene of interest was Tnfaip3, which has been identified as a cardinal gene regulating anti-apoptosis in β-cells (Liuwantara et al. 2006) and the strong mRNA and protein induction observed is consistent with lower basal apoptosis in UCP2−/− islet cells. Changes in TNFAIP3 might occur as a result of islet isolation, which is known to induce apoptosis in a fraction of the β-cells (Thomas et al. 2009). We used immunohistochemistry to study in situ TNFAIP3 expression patterns in a less-stressed state, which revealed an altered pattern of expression in islets. In Ucp2+/+ islets, TNFAIP3 was most abundantly expressed in non-β-cells that stained for pancreatic polypeptide and was predominantly perinuclear in distribution. Conversely, TNFAIP3 was detected in both β- and non-β-cells in Ucp2−/− islets and was predominantly dispersed throughout the cytoplasm of β-cells while maintaining perinuclear localization in non-β-cells. Liuwantara et al. (2006) demonstrated localization of TNFAIP3 predominantly in the cytoplasm of β-cells but used cytokine-stimulated, dispersed FACS-purified cells for immunostaining, whereas the current results are from intact islets without exogenous cytokine stimulation. Predominantly perinuclear localization of TNFAIP3 was noted in a COS7 cell model (Li et al. 2008). Dispersal of TNFAIP3 throughout the cytoplasm in Ucp2−/− β-cells may have important functional consequences. Interestingly, islets from pancreatectomized rats exhibit strong, nearly 5-fold induction of Tnfaip3 mRNA and these rats are protected from cell death after STZ treatment (Laybutt et al. 2002), as are mice overexpressing TNFAIP3 in pancreas (Yu et al. 2004). Ucp2−/− mice are also partially resistant to STZ, maintaining higher circulating insulin concomitant with lower plasma glucose, although β-cell mass was not significantly protected (Lee et al. 2009). After STZ treatment, TNFAIP3 localization in Ucp2+/+ islets was maintained in both phenotypes and did not appear elevated relative to untreated islets. We speculate that co-localization of TNFAIP3 in α-cells of Ucp2−/− islets has important adaptive effects because Ucp2−/− α-cells are highly sensitive to cell death stimuli (Diao et al. 2008). Examination of islets in the early aftermath of STZ treatment (e.g. 3–7 days instead of 14 days) might reveal greater adaptive response and genotype differences.
Both iNOS and TNFAIP3 in islets are regulated by the transcription factor NF-κB (Cardozo et al. 2001, Laybutt et al. 2002). Although total NF-κB expression was similar to Ucp2+/+ islets (data not shown), translocation to the nucleus of RelA was significantly greater in isolated Ucp2−/− mouse islets. The mechanism appeared to be at the level of IKKβ expression, which was 3-fold higher in Ucp2−/− islets, and correlated with 2-fold higher IκBα phosphorylation. Normally, NF-κB RelA is retained in the cytoplasm by association with IκBα. IKKβ phosphorylation of IκBα allows it to enter the ubiquitin pathway for degradation, thereby permitting translocation of NF-κB RelA subunit to the nucleus (Magnani et al. 2000). Laybutt et al. (2002) also found increased NF-κB activity in their pancreatectomy model with induced TNFAIP3 expression and resistance to STZ. The current findings were also consistent with those in Ucp2−/− immune tissues, where IKKβ activity was enhanced and persistent NF-κB activation was noted (Bai et al. 2005). Curiously, while the IKKβ inhibitor BAY strongly reduced IκBα phosphorylation in Ucp2−/− islets, it had no effect on NF-κB nuclear translocation or apoptosis. One explanation is that once NF-κB is sequestered in the nucleus, it is immune to regulatory events occurring in the cytoplasm. This hypothesis is supported by the fact that CAPE, which inhibits NF-κB by blocking its binding to DNA, reduced basal and cytokine-stimulated NF-κB activation in Ucp2−/− islets as well as iNOS expression. However, further experiments to test this hypothesis more directly are required. In situ examination of NF-κB expression patterns by immunohistochemistry did not confirm increased translocation to the nucleus. Thus, like the changes seen in TNFAIP3, islet isolation may be the initial precipitating factor leading to increased RelA detected in the nucleus. Likewise, the lack of effect of STZ on the cellular distribution of NF-κB is possibly due to the extended sampling period of 14 days post-treatment.
In most studies of islets, NF-κB is associated with an increase in apoptosis but a growing amount of data (Laybutt et al. 2002, Hagerkvist et al. 2007, Kim et al. 2007), including the current report, suggest that longer-term or constitutive activation of NF-κB confers a pro-proliferative, anti-apoptotic phenotype in β-cells. The failure to detect a significant elevation in apoptosis in isolated Ucp2−/− islets may be due to strong induction of TNFAIP3 during islet isolation and suppression of Myc and Jun. Over-expression of TNFAIP3 in islets protected from NF-κB activation and cytokine-mediated apoptosis (Grey et al. 1999) and increased islet survival after transplantation (Grey et al. 2003). Cytokine-treated human islets also had increased expression of TNFAIP3 and other anti-apoptotic genes (Sarkar et al. 2009). NF-κB activation is also required for induction of other anti-apoptotic genes in mouse islets, including XIAP and c-FLIP, two inhibitors of caspases (Kim et al. 2007), which merits further investigation in our model. While concomitant up-regulation of TNFAIP3 and activation of NF-κB appear paradoxical, we concur with the suggestion of others (Laybutt et al. 2002) now generally accepted in multiple cell types (Hymowitz & Wertz 2010) that TNFAIP3 is working as a brake under these conditions and, possibly combined with increased expression of other anti-apoptotic genes, is protective. Of interest is the finding that TNFAIP3 is a substrate of IKKβ (Hutti et al. 2007). With an increase in IKKβ expression, as documented here, TNFAIP3 phosphorylation at serine 381 is predicted to be increased, which would increase its ability to inhibit the NF-κB pathway (Hutti et al. 2007). TNFAIP3 acts upstream of IKKβ to deubiquitinate substrates involved in NF-κB signaling, such as RIPK1 and TRAF6 (Hymowitz & Wertz 2010).
In conclusion, we find that isolated islets from Ucp2−/− mice display increased transactivation of NF-κB, which may contribute to enhanced expression of anti-apoptotic genes such as Tnfaip3 and pro-proliferative genes such as Ccnd2. This phenotype appears to be revealed by stressful events such as islet isolation because nuclear localization of NF-κB was not observed in intact pancreatic tissue. One possible mechanism is up-regulation of NO-dependent IKKβ activity. TNFAIP3 is of particular interest because of its ability to exert negative feedback on NF-κB signaling pathways to modulate apoptosis and other downstream events. Interestingly, TNFAIP3 had altered cellular and sub-cellular distribution in the islets from Ucp2−/− mice. This phenotype may contribute to the so-called paradoxical improved outcomes of Ucp2−/− mice to stressors such as cytokines, as shown here, or high-fat diet challenge (Joseph et al. 2002, 2004). Furthermore, the localization of TNFAIP3 in non-β-cells in situ has not previously been reported but may be of importance to islet function.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Canadian Diabetes Association (OG-1-09-2689-CC) and the Canadian Institutes for Health Research (MOP 43987).
References
- Ammendrup A, Maillard A, Nielsen K, Aabenhus Andersen N, Serup P, Dragsbaek Madsen O, Mandrup-Poulsen T, Bonny C. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic β-cells. Diabetes. 2000;49:1468–1476. doi: 10.2337/diabetes.49.9.1468. [DOI] [PubMed] [Google Scholar]
- Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW, et al. Persistent nuclear factor-κB activation in ucp2−/− mice leads to enhanced nitric oxide and inflammatory cytokine production. Journal of Biological Chemistry. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bilbao F, Arsenijevic D, Vallet P, Hjelle OP, Ottersen OP, Bouras C, Raffin Y, Abou K, Langhans W, Collins S, et al. Resistance to cerebral ischemic injury in UCP2 knockout mice: evidence for a role of UCP2 as a regulator of mitochondrial glutathione levels. Journal of Neurochemistry. 2004;89:1283–1292. doi: 10.1111/j.1471-4159.2004.02432.x. [DOI] [PubMed] [Google Scholar]
- Brookes PS. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radical Biology and Medicine. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Heimberg H, Heremans Y, Leeman R, Kutlu B, Kruhoffer M, Orntoft T, Eizirik DL. A comprehensive analysis of cytokine-induced and nuclear factor-κB-dependent genes in primary rat pancreatic β-cells. Journal of Biological Chemistry. 2001;276:48879–48886. doi: 10.1074/jbc.M108658200. [DOI] [PubMed] [Google Scholar]
- Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–1486. doi: 10.2337/diabetes.48.7.1482. [DOI] [PubMed] [Google Scholar]
- Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB. Increased uncoupling protein-2 levels in β-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302–1310. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Regulation of transcription factors by nitric oxide in neurons and in neural-derived tumor cells. Progress in Neurobiology. 2008;84:317–328. doi: 10.1016/j.pneurobio.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Diao J, Allister EM, Koshkin V, Lee SC, Bhattacharjee A, Tang C, Giacca A, Chan CB, Wheeler MB. UCP2 is highly expressed in pancreatic α-cells and influences secretion and survival. PNAS. 2008;105:12057–12062. doi: 10.1073/pnas.0710434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik DL, Mandrup-Poulsen T. A choice of death – the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C, et al. Conditional and specific NF-κB blockade protects pancreatic β cells from diabetogenic agents. PNAS. 2006;103:5072–5077. doi: 10.1073/pnas.0508166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves TC, Brand MD. The reactions catalysed by the mitochondrial uncoupling proteins UCP2 and UCP3. Biochimica et Biophysica Acta. 2005;1709:35–44. doi: 10.1016/j.bbabio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fatehi-Hassanabad Z, Chan CB. Expression of PPARα modifies fatty acid effects on insulin secretion in uncoupling protein-2 knockout mice. Nutrition and Metabolism. 2007;4:6. doi: 10.1186/1743-7075-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJR, Knowles RG. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. Journal of Biological Chemistry. 1999;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- Grey ST, Arvelo MB, Hasenkamp W, Bach FH, Ferran C. A20 inhibits cytokine-induced apoptosis and nuclear factor κB-dependent gene activation in islets. Journal of Experimental Medicine. 1999;190:1135–1146. doi: 10.1084/jem.190.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey ST, Longo C, Shukri T, Patel VI, Csizmadia E, Daniel S, Arvelo MB, Tchipashvili V, Ferran C. Genetic engineering of a suboptimal islet graft with A20 preserves β cell mass and function. Journal of Immunology. 2003;170:6250–6256. doi: 10.4049/jimmunol.170.12.6250. [DOI] [PubMed] [Google Scholar]
- Hagerkvist R, Sandler S, Mokhtari D, Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec): role of β-cell NF-kB activation and anti-apoptotic conditioning. FASEB Journal. 2007;21:618–628. doi: 10.1096/fj.06-6910com. [DOI] [PubMed] [Google Scholar]
- Ho E, Bray TM. Antioxidants, NFκB activation, and diabetogenesis. Proceedings of the Society for Experimental Biology and Medicine. 1999;222:205–213. doi: 10.1046/j.1525-1373.1999.d01-137.x. [DOI] [PubMed] [Google Scholar]
- Hutti JE, Turk BE, Asara JM, Ma A, Cantley LC, Abbott DW. IκB kinase β phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-κB pathway. Molecular and Cellular Biology. 2007;27:7451–7461. doi: 10.1128/MCB.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz SG, Wertz IE. A20: from ubiquitin editing to tumour suppression. Nature Medicine. 2010;10:332–340. doi: 10.1038/nrc2775. [DOI] [PubMed] [Google Scholar]
- Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51:3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- Joseph JW, Koshkin V, Saleh MC, Sivitz WI, Zhang CY, Lowell BB, Chan CB, Wheeler MB. Free fatty acid-induced β-cell defects are dependent on uncoupling protein 2 expression. Journal of Biological Chemistry. 2004;279:51049–51056. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- Joseph JW, Chan CB, Wheeler MB. UCP2 knockout mouse islets have lower consumption and faster oscillations of β cell oxygen. Canadian Journal of Diabetes. 2005;29:19–26. [Google Scholar]
- Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS. NF-κB prevents β cell death and autoimmune diabetes in NOD mice. PNAS. 2007;104:1913–1918. doi: 10.1073/pnas.0610690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang C-Y, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic β cell dysfunction. Journal of Clinical Investigation. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroncke KD, Funda J, Berschick B, Kolb H, Kolb-Bachofen V. Macrophage cytotoxicity towards isolated rat islet cells: neither lysis nor its protection by nicotinamide are β-cell specific. Diabetologia. 1991;34:232–238. doi: 10.1007/BF00405081. [DOI] [PubMed] [Google Scholar]
- Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF. Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Molecular and Cellular Biology. 2005;25:3752–3762. doi: 10.1128/MCB.25.9.3752-3762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttler B, Schmidt S, Kloting I, Stark O. In vitro and in vivo behaviour of rat islets of Langerhans treated with MHC antisera and complement. Experimental and Clinical Endocrinology. 1987;89:283–289. doi: 10.1055/s-0029-1210651. [DOI] [PubMed] [Google Scholar]
- Kwon KB, Kim EK, Jeong ES, Lee YH, Lee YR, Park JW, Ryu DG, Park BH. Cortex cinnamomi extract prevents streptozotocin- and cytokine-induced β-cell damage by inhibiting NF-kB. World Journal of Gastroenterology. 2006;12:4331–4337. doi: 10.3748/wjg.v12.i27.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybutt DR, Kaneto H, Hasenkamp W, Grey S, Jonas J-C, Sgroi DC, Groff A, Ferran C, Bonner-Weir S, Sharma A, et al. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to β-cell survival during chronic hyperglycemia. Diabetes. 2002;51:413–423. doi: 10.2337/diabetes.51.2.413. [DOI] [PubMed] [Google Scholar]
- Lee SC, Robson-Doucette CA, Wheeler MB. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. Journal of Endocrinology. 2009;203:33–43. doi: 10.1677/JOE-09-0117. [DOI] [PubMed] [Google Scholar]
- Li L, Hailey DW, Soetanydo N, Li W, Lippincott-Schwartz J, Shu H-B, Ye Y. Localization of A20 to a lysosome-associated compartment and its role in NFkB signaling. Biochimica et Biophysica Acta. 2008;1783:1140–1149. doi: 10.1016/j.bbamcr.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuwantara D, Elliot M, Smith MW, Yam AO, Walters SN, Marino E, McShea A, Grey ST. Nuclear factor-kB regulates β-cell death. A critical role for A20 in β-cell protection. Diabetes. 2006;55:2491–2501. doi: 10.2337/db06-0142. [DOI] [PubMed] [Google Scholar]
- Magnani M, Crinelli R, Bianchi M, Antonelli A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB) Current Drug Targets. 2000;1:387–399. doi: 10.2174/1389450003349056. [DOI] [PubMed] [Google Scholar]
- Mehta SL, Li PA. Neuroprotective role of mitochondrial uncoupling protein 2 in cerebral stroke. Journal of Cerebral Blood Flow and Metabolism. 2009;29:1069–1078. doi: 10.1038/jcbfm.2009.4. [DOI] [PubMed] [Google Scholar]
- Ortis F, Cardozo AK, Crispim D, Storling J, Mandrup-Poulsen T, Eizirik DL. Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-κB activation. Molecular Endocrinology. 2006;20:1867–1879. doi: 10.1210/me.2005-0268. [DOI] [PubMed] [Google Scholar]
- Ou D, Wang X, Metzger DL, Robbins M, Huang J, Jobin C, Chantler JK, James RFL, Pozzilli P, Tingle AJ. Regulation of TNF-related apoptosis-inducing ligand-mediated death-signal pathway in human β cells by Fas-associated death domain and nuclear factor κB. Human Immunology. 2005;66:799–809. doi: 10.1016/j.humimm.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;150:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, Daniel KW, Liu D, Lyght O, Edelstein D, Brownlee M, Corkey BE, Collins S. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic β-cell function. Endocrinology. 2009;150:3040–3048. doi: 10.1210/en.2008-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K, Li L, Mills EM, Borowitz JL, Isom GE. Up-regulation of uncoupling protein 2 by cyanide is linked with cytotoxicity in mesencephalic cells. Journal of Pharmacology and Experimental Therapeutics. 2005;314:1338–1345. doi: 10.1124/jpet.105.088625. [DOI] [PubMed] [Google Scholar]
- Salvalaggio PR, Deng S, Ariyan CE, Millet I, Zawalich WS, Basadonna GP, Rothstein DM. Islet filtration: a simple and rapid new purification procedure that avoids ficoll and improves islet mass and function. Transplantation. 2002;74:877–879. doi: 10.1097/00007890-200209270-00023. [DOI] [PubMed] [Google Scholar]
- Sarkar SA, Kutlu B, Velmurugan K, Kizaka-Kondoh S, Lee CE, Wong R, Valentine A, Davidson HW, Hutton JC, Pugazhenthi S. Cytokine-mediated induction of anti-apoptotic genes that are lined to nuclear factor-κB (NF-κB) signalling in human islets and in a mouse β cell line. Diabetologia. 2009;52:1092–1101. doi: 10.1007/s00125-009-1331-x. [DOI] [PubMed] [Google Scholar]
- Spinas GA. The dual role of nitric oxide in islet β-cells. News in Physiological Sciences. 1999;14:49–54. doi: 10.1152/physiologyonline.1999.14.2.49. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Springer JE, Hall ED, Scheff SW. Mitochondrial uncoupling as a therapeutic target following neuronal injury. Journal of Bioenergetics and Biomembranes. 2004;36:353–356. doi: 10.1023/B:JOBB.0000041767.30992.19. [DOI] [PubMed] [Google Scholar]
- Sunouchi T, Suzuki K, Nakayama K, Ishikawa T. Dual effect of nitric oxide on ATP-sensitive K+ channels in rat pancreatic β cells. Pflügers Archiv. 2008;456:573–579. doi: 10.1007/s00424-008-0463-z. [DOI] [PubMed] [Google Scholar]
- Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus γ-interferon-induced pancreatic β-cell dysfunction is mediated by β-cell nitric oxide production. Diabetes. 2002;51:311–316. doi: 10.2337/diabetes.51.2.311. [DOI] [PubMed] [Google Scholar]
- Thomas HE, McKenzie MD, Angstetra E, Campbell PD, Kay TW. β-Cell apoptosis in diabetes. Apoptosis. 2009;14:1389–1404. doi: 10.1007/s10495-009-0339-5. [DOI] [PubMed] [Google Scholar]
- Van de Casteele M, Kefas BA, Cai Y, Heimberg H, Scott DK, Henquin JC, Pipeleers D, Jonas JC. Prolonged culture in low glucose induces apoptosis of rat pancreatic β-cells through induction of c-myc. Biochemical and Biophysical Research Communications. 2003;312:937–944. doi: 10.1016/j.bbrc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Webb AA, Chan CB, Brown A, Saleh TM. Estrogen reduces the severity of autonomic dysfunction in spinal cord-injured male mice. Behavioural Brain Research. 2006;171:338–349. doi: 10.1016/j.bbr.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Yu LY, Lin B, Zhang ZL, Guo LH. Direct transfer of A20 gene into pancreas protected mice from streptozotocin-induced diabetes. Acta Pharmacologica Sinica. 2004;25:721–726. [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, β cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/S0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]