Introduction
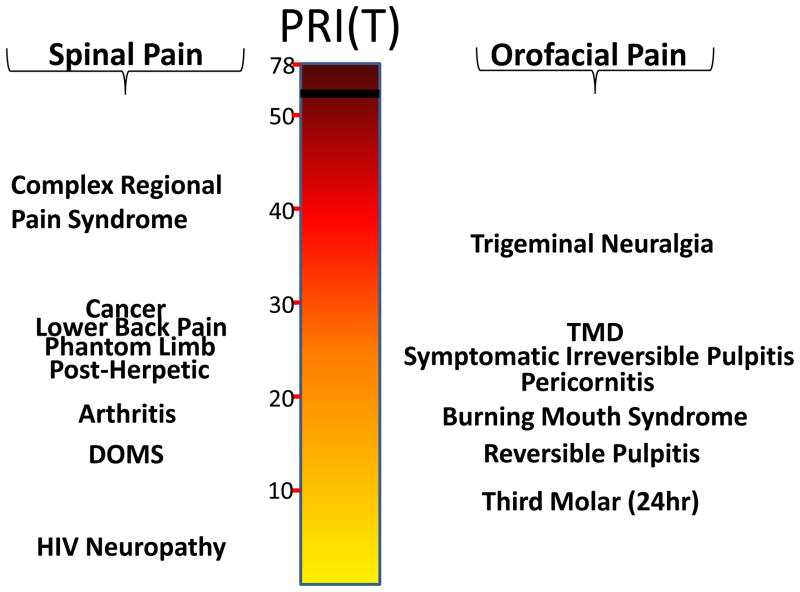
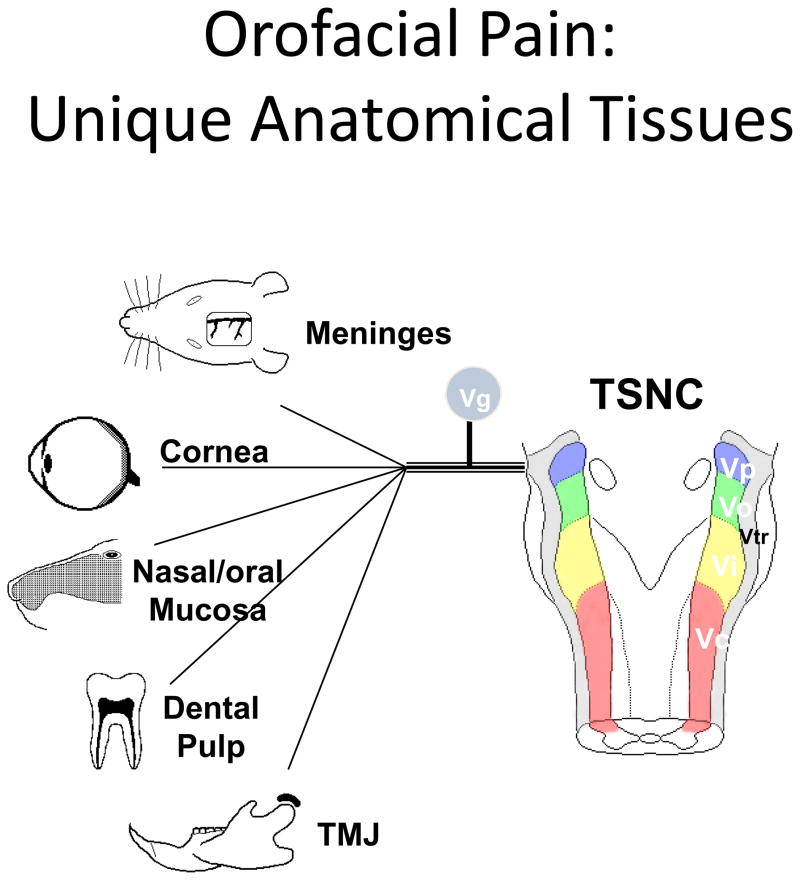
Pain in the oral and craniofacial system represents a major medical and social problem. Indeed a U.S. Surgeon General’s report on orofacial health concludes that, “…oral health means much more than healthy teeth. It means being free of chronic oral-facial pain conditions…” (1). Community-based surveys indicate that many subjects commonly report pain in the orofacial region, with estimates of > 39 million, or 22% of Americans older than 18 years of age, in the United States alone (2). Other population-based surveys conducted in the United Kingdom (3, 4), Germany (5)or regional pain care centers in the U.S. (6) report similar occurrence rates (7). Importantly, chronic widespread body pain, patient sex and age and psychosocial factors appear to serve as risk factors for chronic orofacial pain (8–12). In addition to its high degree of prevalence, the reported intensities of various orofacial pain conditions are similar to that observed with many spinal pain disorders (Fig 1). Moreover, orofacial pain is derived from many unique target tissues such as the meninges, cornea, tooth pulp, oral/nasal mucosa, and temporomandibular joint (Fig 2) and thus has several unique physiologic characteristics compared with the spinal nociceptive system (13). Given these considerations, it is not surprising that accurate diagnosis and effective management of orofacial pain conditions represents a significant health care problem.
Figure 1.
Comparison of pain intensity among spinal and orofacial pain disorders using the McGill Total Rank Pain Index (PRI(T)). The PRI(T) is an ordinal scale consisting of the sum of the ranks of words in each of the 20 sub-categories on the MPQ and ranges from 0–78. Data taken from (90, 95, 150–158).
Fig 2.
Unique target tissues innervated by the trigeminal sensory system. Taken from Bereiter et al., 2008, with permission
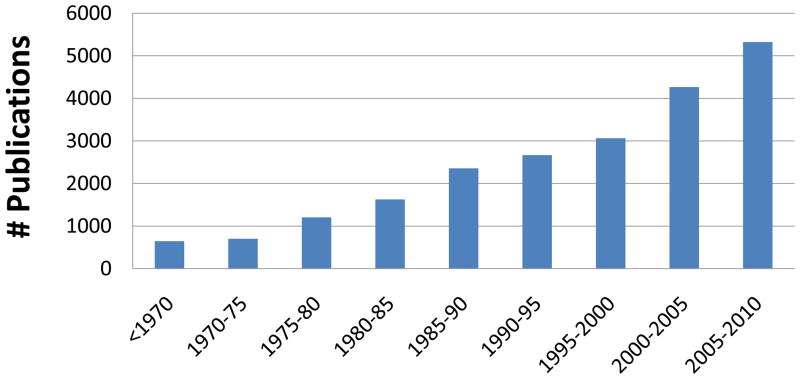
Publications in the field of orofacial pain demonstrate a steady increase over the last several decades (Fig 3). This is a complex literature; a recent bibliometric analysis of orofacial pain papers published in 2004–5 indicated that 975 papers on orofacial pain were published in 275 journals from authors representing 54 countries (14). Thus, orofacial pain disorders represent a complex constellation of conditions with an equally diverse literature base. Accordingly, this review will focus on a summary of major research foci on orofacial pain without attempting to provide a comprehensive review of the entire literature.
Fig 3.
Rates of papers published on orofacial pain. Data was acquired from a PubMed search (August 2010) using the search criteria of: (orofacial or trigeminal or temporomandibular or dental or tooth) and (pain or headache or hyperalgesia or allodynia or nociceptor or nociceptive).
Physiologic Studies on Trigeminal Pain
Several reviews are available that document the historical development of physiologic research on the trigeminal nociceptive system (15–19). More recent studies have characterized differences in electrophysiological (20), anatomical (21) or pharmacological (22, 23) properties of trigeminal afferents innervating distinct target tissues. Collectively, many of these studies provide support for the hypothesis that target tissue interactions with trigeminal neuron terminals, via either soluble factors such as neurotrophins (24), or by integrin binding to extracellular matrix molecules (25, 26), regulate the expression or trafficking of neuronal proteins including ion channels and receptors (27, 28) or second messenger signaling pathways (25). Thus, the presence of unique target tissues innervated by trigeminal afferent fibers likely contributes to differences in the responsiveness of these neurons. A recent review characterizes differences between the trigeminal and spinal afferent systems under basal conditions (13). Table 1 illustrates differences between the trigeminal and spinal systems after various forms of injury. Collectively, these studies indicate that the trigeminal system has many unique features that may contribute to distinct response patterns to tissue injury.
Table I.
Comparison of the Trigeminal and Spinal Afferent Systems After Injury
| Marker | Injury Model | Comparison | Authors |
|---|---|---|---|
| Galanin | Axotomy | TG ~ DRG for upregulation | (Arvidsson et al. 1994; Zhang et al. 1996) |
| NPY | Axotomy | TG ~ DRG for upregulation | (Arvidsson et al. 1994; Zhang et al. 1996) |
| Sympathetic fiber sprouting into ganglion and basket formation | Axotomy or CCI | TG: No DRG: Yes | (Bongenhielm et al. 1999; Benoliel et al. 2001) |
| Sympathetic fiber sprouting into ganglion | NGF infusion icv X 14d | DRG > TG | (Nauta et al. 1999) |
| SNS/PN3 = NaV1.xx | Axotomy | TG: Downregulation followed by normalization DRG: Persistent downregulation |
(Bongenhielm et al. 2000) |
| Ankyrin(G) | Axotomy | TG: Persistent downregulation | (Bongenhielm et al. 2000) |
| Ectopic firing of afferents | Axotomy | TG < DRG | (Tal et al. 1992) |
| Augmented excitability | Axotomy | TG ~ DRG | (Tal and Devor 1992; Zhang et al. 2002; Cherkas et al. 2004) |
| Frequency and rhythmicity of sponataneous discharges | Tight ligation of infraorbital vs sciatic nerves | DRG had significantly greater spontaneous discharge rate than TG neurons for both myelinated and unmyelinated fibers. DRG afferents had rhythmic discharge rates (not seen with TG) |
(Tal and Devor 1992) |
| Satellite glial cells | Axotomy | TG ~ DRG for upregulation of GFPA, proliferation | (Woodham et al. 1989; Stephenson et al. 1995; Cherkas et al. 2004) |
| NOS | Axotomy | TG ~ DRG for upregulation | (Hokfelt et al. 1994) |
| P2X3 & ATF-3 expression | Partial axotomy | TG ~ DRG | (Tsuzuki et al. 2001) |
| GM3 ganglioside. | Knockout GM2/GD2 and the GD3 synthase gene | Facial wounding > Rest of the body With peripheral nerve degeneration | (Inoue et al. 2002) |
| Peripheral chromatolysis | LiCl | TG ~ DRG | (Levine et al. 2004) |
| Sensory neuropathy with neuronal degeneration | Sjogren’s Syndrome | TG ~ DRG | (Malinow et al. 1986) |
| Infectivity of contralateral ganglia | Herpes simplex virus-1 (HSV) infection | 70% of TG contralateral to side of HSV injection produced infections after inoculation, whereas only 10% of contralateral DRG produced infections. | (Thackray et al. 1996) |
| HSV polypeptide ICP4 (VP175) expression in ganglia | Herpes simplex virus-1 (HSV) infection | TG ~ DRG | (Pepose et al. 1986) |
| Viral replication and degradation of host cells, mRNA | Herpes simplex virus-1 (HSV) infection | TG ~ DRG with wildtype HSV more virulent in both ganglia than HSV mutants lacking virion host shutoff (vhs) protein | (Smith et al. 2002) |
| Infectivity of ganglia | Simian varicella virus (SVV) | TG ~ DRG | (White et al. 2001) |
| Substance P in ganglia | streptozotocin-diabetes | TG had 26% reduction (p<0.01), but DRG = 11% non- significant reduction | (Robinson et al. 1987) |
| Substance P in ganglia | mf rat (mutilated foot; an autosomal recessive sensory neuropathy with reduced pain responsiveness | DRG < TG | (Scaravilli 1983) |
| Caspase-3 mediated neuronal apoptosis | Knockout of Rb (retinoblastoma tumor suppressor protein) | TG ~ DRG for protection from apoptosis in double knockout of Rb and caspase-3 compared to single Rb knockout | (Simpson et al. 2001) |
| Number of neurons in ganglia | TRKa knockout | TG ~ DRG for extensive neuronal loss | (Smeyne et al. 1994) |
| Reactivation of virus | HSV mutant with gamma34.5 gene deletion | TG > resistant to reactivation than DRG | (Spivack et al. 1995) |
| Wide-spread numbness and pain 4–12d after antibiotic treatment | Acute sensory neuronopathy syndrome in humans | TG ~ DRG | (Sterman et al. 1980) |
| Pain | Trigeminal neuralgia in humans | TG: Yes (max/mand divisions > ophthalmic) DRG: No equivalent |
(Jannetta 1980; Sweet 1984; Wilkins 1985; Goya et al. 1990; Hamlyn 1997; Hamlyn 1997; Tacconi et al. 2000) |
| Spontaneous behavior | Formalin | OVX females exhibited significantly greater increase informalin hyperalgesia after orofacial injection (upper lip) compared to hindpaw injection. Result is consistent with hypothesis of a difference in sex steroid regulation of nociception between TG and DRG systems | (Pajot et al. 2003) |
The hypothesis of peripheral regulation of neuronal phenotype has been expanded by the recognition that estradiol selectively alters gene transcription in trigeminal neurons, with increased expression of neuropeptides, such as prolactin, that are capable of sensitizing neuronal responses to capsaicin or noxious heat (29). Additional studies have demonstrated that trigeminal peptidergic neurons undergo morphological changes (“sprouting”) in response to injury-induced inflammation of in target tissues (19). In contrast, there is a lack of sympathetic fiber sprouting in trigeminal ganglion cells, unlike the well recognized occurrence in the spinal system (30–32). Thus, an emerging body of evidence reveals the dynamic and specific responsiveness of the trigeminal system to either injury of its various target tissues or to the presence of certain gonadal steroids.
Other studies have employed cultured trigeminal ganglia (TG) to evaluate cellular mechanisms of neuronal activation. For example, cannabinoids activate a calcineurin pathway leading to the rapid dephosphorylation and desensitization of TRPV1, thereby contributing to an ionotropic mechanism for peripheral cannabinoid antinociception (33–36). Moreover, accumulating evidence indicates a functional cross-desensitization between TRPV1 and TRPA1 on trigeminal neurons, possibly via formation of a heteromer (33, 37–39). Additional studies have used cultured TG to demonstrate that opioid receptors are expressed on sensory neurons, but are not coupled to inhibitory signaling pathways under basal experimental conditions. Instead, pretreatment with arachidonic acid or with agonists to receptors coupled to Gαq signaling pathways (eg., bradykinin, trypsin) is required to induce the rapid development of a functional competence for opioid receptor signaling to Gαi pathways leading to inhibition of neuronal activities (25, 40–43). These cellular findings are consistent with the observation that opioids have little efficacy for peripheral antinociception under basal conditions, but rapidly gain functional competence following injection of inflammatory mediators (44) or the development of inflammation.
Recent studies have employed cultured trigeminal neurons to identify endogenous TRPV1 agonists (45, 46). Heating of isolated superfused peripheral tissue to a noxious temperature range leads to the release of oxidized linoleic acid metabolites (OLAMs), including 9- and 13-hydroxyoctadecadienoic acid (HODE). The administration of synthetic 9- and 13-HODE (and their oxoODE metabolites) selectively activates TRPV1, leading to inward currents, increased accumulation of intracellular calcium, and triggering exocytosis of neuropeptides from TG neurons and thermal allodynia. These effects are blocked by TRPV1 antagonists and are only observed in trigeminal neurons from wildtype mice but not TRPV1 knockouts (46). Moreover, the intracellular delivery of compounds that block OLAM formation (eg., nordihydroguaiaretic acid) or a combination of anti-9- and anti-13-HODE antibodies both significantly inhibit heat-evoked activation of trigeminal neurons. Collectively, these findings strongly implicate the OLAMs as a family of endogenous TRPV1 agonists. Interestingly, the pronounced effect of TRPV1 antagonists for blocking heat hyperalgesia in inflammation as well as mechanical allodynia (after intrathecal administration) has led to the hypothesis that an endogenous TRPV1 system might be activated under conditions of tissue injury. In support of this hypothesis, the administration of anti-OLAM antibodies produce a peripherally-mediated thermal antinociception and a centrally-mediated blockade of mechanical allodynia in the complete Freund’s model of inflammation (45, 46). Thus, the OLAM system appears to contribute to acute heat detection by TRPV1 and to regulate more persistent conditions such as inflammatory pain.
Future research directions may include preclinical studies focusing on mechanisms underlying differences between trigeminal and spinal pain conditions, mechanisms of sex-dependent differences in pain transduction and processing, and on the biological basis and pharmacological regulation of acute and chronic orofacial pain conditions. Many of these studies would be promoted by the development of standardized preclinical pain models and assessment methods.
In addition to research on the biological mechanisms of nociceptive transmission, numerous clinical studies have described strong psychosocial/disability components to orofacial pain. Indeed, some diagnostic classification schemes differentiate the dimension of tissue contributions from psychosocial/disability factors (47) contributing to orofacial pain disorders. These studies demonstrate that the orofacial pain patient is confronted with a complex, multidimensional disorder that is best managed with appropriate treatment for all underlying factors (10, 48–53).
Studies on Trigeminal Inflammatory Disorders
Many translational studies have evaluated acute inflammatory injury to the trigeminal system. The dental impaction pain model has been developed as a standard clinical method for evaluating many analgesic drugs (54–56). Other investigators have used this model of acute inflammatory pain to evaluate preemptive anesthesia (57, 58), activation of endogenous opioid analgesic systems (59–62), local release of inflammatory mediators as collected by implanted microdialysis probes (63–66), other physiologic mechanisms (67), or the association of genetic polymorphisms with post-operative pain (68–70). This clinical model has several notable advantages including participation of relatively healthy subjects not taking concurrent drugs, standardized surgical procedures leading to reduced variance and relatively large numbers of potential participants. Collectively, these studies on patients undergoing surgical dental extractions have contributed greatly to evaluation of analgesics, anesthetics and anxiolytics as well as basic biological research on human subjects.
Other studies have focused on chronic inflammation of the oral and craniofacial region. Clinical studies on irreversible pulpitis in teeth (“toothache”) have demonstrated that this condition of bacterial-induced inflammation/necrosis is associated with significant changes in expression of ion channels (71–76), receptors (77) and neuropeptides (19, 78). Moreover, inflammation of a single tooth in patients appears sufficient to trigger central sensitization (79–82). Animal studies on inflammation in the trigeminal region have demonstrated target-site dependent differences in sensitization/activation (83–85)as well as sex-dependent differences in neuronal activities (86–89).
Future research directions on trigeminal inflammatory disorders may include preclinical and translational clinical studies focusing on mechanisms underlying the development and maintenance of inflammatory hyperalgesia/allodynia. Importantly, the clinical condition of pulpitis results in a very restricted pain locus (often within a tissue volume of <200 uL), intense pain reports (90) and dynamic neuronal and immunoplasticity. Thus, the pulpitis pain model is important not only from the perspective of high prevalence (2) and health care disparity (91), but also serves as a useful model for translational research (92).
Studies on Trigeminal Neuropathic Disorders
The orofacial region has unique neuropathic pain disorders not seen in the spinal system, including trigeminal neuralgia and glossopharyngeal neuralgia (93). Numerous clinical reports document these and other orofacial neuropathic or neuritic/neuralgic pain conditions and their responsiveness to surgical or pharmacological treatments (93–99). Several etiologic factors appear to contribute to the development of neuropathic pain disorders. Proposed mechanisms include injury/compression to the trigeminal nerve, inflammatory insult (possibly including glial contributions), or infection with herpes virus (93, 100–105). However, not all injuries to the trigeminal nerve lead to neuropathic pain disorders; indeed, the incidence of neuropathic pain after injury to orofacial structures is relatively low after dental treatment (106–108), facial trauma (100), orthognathic surgery (109), tooth extraction (110–113), or placement of dental implants (114). This apparent resistance of the trigeminal system for development of neuropathic conditions is an interesting clinical observation that should prompt preclinical research comparing trigeminal to spinal afferent systems for susceptibility to neuropathic pain disorders. It is interesting that the trigeminal system appears programmed for periodic loss of innervated structures during post-natal development, with the shedding of 20 deciduous teeth per person, with minimal development of neuropathic pain conditions.
Several risk factors for trigeminal neuralgia have been found including multiple sclerosis (115, 116) and hypertension (117). Additional studies have reported changes in the expression of ion channels (eg., NaV1.3, 1.7, 1.8, TRPA1, etc) in surgical biopsies collected from patients suffering from neuropathic orofacial pain (118, 119). Animal models of trigeminal neuropathic pain have been developed and include chronic constriction injury of the infraorbital nerve as well as transaction of the inferior alveolar nerve (101, 103). Interestingly, both preclinical and clinical studies have implicated constriction of peripheral nerves as an etiologic mechanism for inducing neuropathic pain via alteration in primary afferent functions (120), although certain cortical changes have been reported as well (121). This has led to the development of clinical surgical decompression procedures to treat patients with trigeminal neuralgia (98, 122). Several preclinical studies have implicated ion channels, endothelin receptors as well as glial mechanisms in contributing to the development of these models of neuropathic pain conditions (104, 105, 120, 123, 124).
Studies on Chronic Trigeminal Myofascial and Joint Pain
The diagnosis and management of many chronic orofacial pain conditions has been greatly hampered by confusion in determining etiologies from the temporomandibular joint versus myofascial sources. This has led to clinical studies difficult to interpret and diagnostic classifications that did not have a strong biological basis due to the lack of differentiation between joint and muscle contributions to the patient’s pain condition. Clinical studies on myofascial pain or temporomandibular dysfunction (TMD) were considerably improved by the development of the Research Diagnostic Criteria (47, 49), which highlighted the need for developing standardized diagnostic methods and definitions. Considerable evidence has been published demonstrating that patient sex/gender and exposure to sex steroids serve as risk factors for developing chronic orofacial pain conditions (50, 125–129). However, this is not observed in all studies, and other risk factors such as chronic widespread body pain, a prior history of physical abuse or health anxiety have also been reported to be associated with the development of chronic orofacial pain disorders (8, 11, 47, 50, 127, 130). The reasons why some but not all studies detect sex/gender as a significant risk factor for orofacial pain disorders is not clear, but may be due to differences in patient populations, case definitions or experimental approaches. Related preclinical studies have demonstrated that trigeminal neurons express estrogen receptors and undergo dramatic changes in gene expression (29, 131, 132) or firing rates (84) following exposure to estradiol.
Other clinical studies have focused on synovial fluid levels of inflammatory mediators to test for other possible biological mechanisms (133, 134) or have evaluated the role of peripheral glutamate receptors in triggering myofascial pain (135, 136). A very interesting approach is the application of genetics to patients with TMD. A haplotype of the COMT gene in patients is associated with reduced responsiveness to experimental pain and to reduced risk for TMD (137). Moreover, a mechanistic hypothesis for the protective effect of this haplotype has been advanced (138) and TMD patients with this COMT haplotype respond with increased analgesia from drugs such as propranolol (139).
Studies on Other Orofacial Pain Conditions
Many other orofacial pain disorders have been also evaluated. The trigeminal autonomic cephalgias (TAC) include cluster headache, paroxysmal hemicrania and unilateral neuralgiform headaches (140). This collection of pain disorders is characterized by unilateral head pain in association with autonomic features such as tearing and conjunctival involvement and considerable research has shed light on pain referral patterns, and issues related to proper diagnosis and treatment (141–145). Most cases of TAC reflect primary headaches, although rare cases may be associated with pituitary tumors (140). Pain is a major aspect of oral cancer (146)and often represents the initial symptom that prompts patients to seek health providers. Pain due to oral cancer may be due to soluble factors released from tumor cells, a localized inflammatory response to the tumor or even nerve entrapment. Several recent studies have implicated the endothelin system and proteases (eg., PAR-2 receptor activation) in mediating mechanical allodynia experimental models of oral cancer pain (147, 148). Burning mouth syndrome is a rare disorder, commonly characterized by spontaneous burning pain and mechanical allodynia. Although idiopathic, it has many features of neuropathic pain and has been reported to be associated with altered peripheral expression of voltage gated sodium channels (72).
Discussion
Orofacial pain disorders comprise a major and expensive component of health care and collectively have a high prevalence rate, a large range in pain intensity with a commensurate, often devasatating impact on quality of life (1). Although there are many common aspects of pain transduction and processing between the trigeminal and spinal systems, there are numerous examples of unique features in the peripheral and central components of the trigeminal pain system. Accordingly, ongoing basic and clinical research focused on acute and chronic orofacial pain conditions is required to understand the unique features of this pain system and to develop and evaluate better ways to treat patients with orofacial pain.
A major barrier for improved patient care and translational research is the lack of validated diagnostic criteria. Although efforts have been made for classifying TMD patients with the RDC TMD, headache patients with the International Headache Society criteria and orofacial pain with the American Academy of Orofacial Pain standards, clinical research indicates that each of these three methods are incomplete for comprehensive diagnosis of orofacial pain patients (142). Thus, further research is critically required to establish comprehensive, sensitive and specific diagnostic classification scheme for all orofacial pain patients. This would provide a critical contribution to practitioners and foster the development of a powerful dataset for clinical research. In addition, recent studies have incorporated quality of life indices, which provide important additional information on clinical outcomes (149).
Taken together, orofacial pain conditions represent a highly prevalent spectrum of pain disorders with pain intensities similar to those observed with many chronic spinal pain conditions. However, the unique anatomical, biochemical and associated psychosocial components provide compelling evidence for specific research focused on orofacial pain disorders.
Acknowledgments
This work was supported in part by R01 NS72890, R01 NS58655, R01 DA19585, and NCRR U54RR02438.
Footnotes
The author denies any conflicts of Interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Services UDoHaH; U.S. Department of Health and Human Services NIoDaC, Research NIoH. Oral Health in America: A Report of the Surgeon General. Rockville, MD: 2000. [Google Scholar]
- 2.Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1994;124:115–21. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane TV, Blinkhorn AS, Davies RM, Ryan P, Worthington HV, Macfarlane GJ. Orofacial pain: just another chronic pain? Results from a population-based survey. Pain. 2002;99:453–8. doi: 10.1016/S0304-3959(02)00181-1. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane TV, Blinkhorn AS, Craven R, Zakrzewska JM, Atkin P, Escudier MP, Rooney CA, Aggarwal V, Macfarlane GJ. Can one predict the likely specific orofacial pain syndrome from a self-completed questionnaire? Pain. 2004;111:270–7. doi: 10.1016/j.pain.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 5.John MT, LeResche L, Koepsell TD, Hujoel P, Miglioretti DL, Micheelis W. Oral health-related quality of life in Germany. Eur J Oral Sci. 2003;111:483–91. doi: 10.1111/j.0909-8836.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin SF, Huggins KH, LeResche L, Von Korff M, Howard J, Truelove E, Sommers E. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc. 1990;120:273–81. doi: 10.14219/jada.archive.1990.0043. [DOI] [PubMed] [Google Scholar]
- 7.Pau AK, Croucher R, Marcenes W. Prevalence estimates and associated factors for dental pain: a review. Oral Health Prev Dent. 2003;1:209–20. [PubMed] [Google Scholar]
- 8.Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain--results of the North Cheshire oro-facial pain prospective population study. Pain. 2010;149:354–9. doi: 10.1016/j.pain.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal VR, Macfarlane TV, Macfarlane GJ. Why is pain more common amongst people living in areas of low socio-economic status? A population-based cross-sectional study. Br Dent J. 2003;194:383–7. doi: 10.1038/sj.bdj.4810004. discussion 0. [DOI] [PubMed] [Google Scholar]
- 11.John MT, Miglioretti DL, LeResche L, Von Korff M, Critchlow CW. Widespread pain as a risk factor for dysfunctional temporomandibular disorder pain. Pain. 2003;102:257–63. doi: 10.1016/S0304-3959(02)00404-9. [DOI] [PubMed] [Google Scholar]
- 12.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004;5:317–28. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Bereiter DA, Hargreaves KM, Hu JW. Trigeminal mechanisms of nociception: Peripheral and Brainstem organization. In: Bushnell MCBA, editor. The Senses, A Comprehensive Reference. San Diego: Academic Press; 2008. pp. 435–60. [Google Scholar]
- 14.Robert C, Wilson CS, Donnadieu S, Gaudy JF, Arreto CD. Bibliometric analysis of the scientific literature on pain research: a 2006 study. Pain. 2008;138:250–4. doi: 10.1016/j.pain.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- 16.Matthews B. The mechanisms of pain from dentine and pulp. Br Dent J. 1976;140:57–60. doi: 10.1038/sj.bdj.4803691. [DOI] [PubMed] [Google Scholar]
- 17.Sessle BJ, Hu JW. Mechanisms of pain arising from articular tissues. Can J Physiol Pharmacol. 1991;69:617–26. doi: 10.1139/y91-092. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev. 1993;5:159–77. [PubMed] [Google Scholar]
- 19.Byers MR, Narhi MV. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 1999;10:4–39. doi: 10.1177/10454411990100010101. [DOI] [PubMed] [Google Scholar]
- 20.Harriott AM, Gold MS. Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. Journal of Neurophysiology. 2009;101:3126–34. doi: 10.1152/jn.91339.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain. 2005;117:280–91. doi: 10.1016/j.pain.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–8. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- 23.Harriott AM, Gold MS. Serotonin type 1D receptors (5HTR) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia. 2008;28:933–44. doi: 10.1111/j.1468-2982.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. Journal of Dental Research. 2007;86:550–5. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- 25.Berg KA, Zardeneta G, Hargreaves KM, Clarke WP, Milam SB. Integrins regulate opioid receptor signaling in trigeminal ganglion neurons. Neuroscience. 2007;144:889–97. doi: 10.1016/j.neuroscience.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bereiter DA, Cioffi JL, Bereiter DF, Zardeneta G, Milam SB. Local blockade of integrins in the temporomandibular joint region reduces Fos-positive neurons in trigeminal subnucleus caudalis of female rats produced by jaw movement. Pain. 2006;125:65–73. doi: 10.1016/j.pain.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone. 2010;46:306–13. doi: 10.1016/j.bone.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price TJ, Helesic G, Parghi D, Hargreaves KM, Flores CM. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience. 2003;120:155–62. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. Journal of Neuroscience. 2006;26:8126–36. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bongenhielm U, Boissonade FM, Westermark A, Robinson PP, Fried K. Sympathetic nerve sprouting fails to occur in the trigeminal ganglion after peripheral nerve injury in the rat. Pain. 1999;82:283–8. doi: 10.1016/S0304-3959(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 31.Benoliel R, Eliav E, Tal M. No sympathetic nerve sprouting in rat trigeminal ganglion following painful and non-painful infraorbital nerve neuropathy. Neurosci Lett. 2001;297:151–4. doi: 10.1016/s0304-3940(00)01681-5. [DOI] [PubMed] [Google Scholar]
- 32.Fried K, Bongenhielm U, Boissonade FM, Robinson PP. Nerve injury-induced pain in the trigeminal system. Neuroscientist. 2001;7:155–65. doi: 10.1177/107385840100700210. [DOI] [PubMed] [Google Scholar]
- 33.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. Journal of Physiology. 2007;583:175–93. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akopian AN, Ruparel NB, Jeske NA, Patwardhan A, Hargreaves KM. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends in Pharmacological Sciences. 2009;30:79–84. doi: 10.1016/j.tips.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11393–8. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. Journal of Biological Chemistry. 2006;281:32879–90. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. European Journal of Neuroscience. 2009;29:1568–78. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135:271–9. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. Journal of Neuroscience. 2008;28:1064–75. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehrenbacher JC, Sun XX, Locke EE, Henry MA, Hargreaves KM. Capsaicin-evoked iCGRP release from human dental pulp: a model system for the study of peripheral neuropeptide secretion in normal healthy tissue. Pain. 2009;144:253–61. doi: 10.1016/j.pain.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berg KA, Patwardhan AM, Sanchez TA, Silva YM, Hargreaves KM, Clarke WP. Rapid modulation of micro-opioid receptor signaling in primary sensory neurons. Journal of Pharmacology & Experimental Therapeutics. 2007;321:839–47. doi: 10.1124/jpet.106.116681. [DOI] [PubMed] [Google Scholar]
- 42.Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. Journal of Neuroscience. 2005;25:8825–32. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, Hargreaves KM. PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain. 2006;125:114–24. doi: 10.1016/j.pain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. Peripheral delta opioid receptors require priming for functional competence in vivo. European Journal of Pharmacology. 2009;602:283–7. doi: 10.1016/j.ejphar.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18820–4. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. Journal of Clinical Investigation. 2010;120:1617–26. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 48.Diatchenko L, Anderson AD, Slade GD, Fillingim RB, Shabalina SA, Higgins TJ, Sama S, Belfer I, Goldman D, Max MB, Weir BS, Maixner W. American Journal of Medical Genetics. 141B. 2006. Three major haplotypes of the beta2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder; pp. 449–62. Part B, Neuropsychiatric Genetics: the Official Publication of the International Society of Psychiatric Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dworkin SF, Sherman J, Mancl L, Ohrbach R, LeResche L, Truelove E. Reliability, validity, and clinical utility of the research diagnostic criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain. 2002;16:207–20. [PubMed] [Google Scholar]
- 50.Fillingim RB, Maixner W, Sigurdsson A, Kincaid S. Sexual and physical abuse history in subjects with temporomandibular disorders: relationship to clinical variables, pain sensitivity, and psychologic factors. Journal of Orofacial Pain. 1997;11:48–57. [PubMed] [Google Scholar]
- 51.Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, Siddiqi MQ, Maixner W. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. Pain. 2009;141:215–21. doi: 10.1016/j.pain.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroner-Herwig B. Chronic pain syndromes and their treatment by psychological interventions. Current Opinion in Psychiatry. 2009;22:200–4. doi: 10.1097/YCO.0b013e3283252d5a. [DOI] [PubMed] [Google Scholar]
- 53.Slade GD, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim RB, Belfer I, Max MB, Goldman D, Maixner W. Influence of psychological factors on risk of temporomandibular disorders. Journal of Dental Research. 2007;86:1120–5. doi: 10.1177/154405910708601119. [DOI] [PubMed] [Google Scholar]
- 54.Cooper SA, Desjardins PJ. The value of the dental impaction pain model in drug development. Methods in Molecular Biology. 2010;617:175–90. doi: 10.1007/978-1-60327-323-7_15. [DOI] [PubMed] [Google Scholar]
- 55.Mehlisch DR, Aspley S, Daniels SE, Southerden KA, Christensen KS. A single-tablet fixed-dose combination of racemic ibuprofen/paracetamol in the management of moderate to severe postoperative dental pain in adult and adolescent patients: a multicenter, two-stage, randomized, double-blind, parallel-group, placebo-controlled, factorial study. Clinical Therapeutics. 2010;32:1033–49. doi: 10.1016/j.clinthera.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database of Systematic Reviews. 2009:CD001548. doi: 10.1002/14651858.CD001548.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon SM, Dionne RA, Brahim J, Jabir F, Dubner R. Blockade of peripheral neuronal barrage reduces postoperative pain. Pain. 1997;70:209–15. doi: 10.1016/s0304-3959(96)03315-5. [DOI] [PubMed] [Google Scholar]
- 58.Gordon SM, Brahim JS, Dubner R, McCullagh LM, Sang C, Dionne RA. Attenuation of pain in a randomized trial by suppression of peripheral nociceptive activity in the immediate postoperative period. Anesthesia & Analgesia. 2010;95:1351–7. doi: 10.1097/00000539-200211000-00047. [DOI] [PubMed] [Google Scholar]
- 59.Hargreaves KM, Dionne RA, Mueller GP, Goldstein DS, Dubner R. Naloxone, fentanyl, and diazepam modify plasma beta-endorphin levels during surgery. Clin Pharmacol Ther. 1986;40:165–71. doi: 10.1038/clpt.1986.159. [DOI] [PubMed] [Google Scholar]
- 60.Levine JD, Gordon NC, Jones RT, Fields HL. The narcotic antagonist naloxone enhances clinical pain. Nature. 1978;272:826–7. doi: 10.1038/272826a0. [DOI] [PubMed] [Google Scholar]
- 61.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2:654–7. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 62.Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature. 1983;306:264–5. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- 63.Gordon SM, Brahim JS, Rowan J, Kent A, Dionne RA. Peripheral prostanoid levels and nonsteroidal anti-inflammatory drug analgesia: replicate clinical trials in a tissue injury model. Clinical Pharmacology & Therapeutics. 2002;72:175–83. doi: 10.1067/mcp.2002.126501. [DOI] [PubMed] [Google Scholar]
- 64.Hargreaves KM, Costello A. Glucocorticoids suppress levels of immunoreactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther. 1990;48:168–78. doi: 10.1038/clpt.1990.132. [DOI] [PubMed] [Google Scholar]
- 65.Swift JQ, Garry MG, Roszkowski MT, Hargreaves KM. Effect of flurbiprofen on tissue levels of immunoreactive bradykinin and acute postoperative pain. J Oral Maxillofac Surg. 1993;51:112–6. doi: 10.1016/s0278-2391(10)80002-3. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 66.Dionne RA, Gordon SM, Rowan J, Kent A, Brahim JS. Dexamethasone suppresses peripheral prostanoid levels without analgesia in a clinical model of acute inflammation. J Oral Maxillofac Surg. 2003;61:997–1003. doi: 10.1016/s0278-2391(03)00310-0. [DOI] [PubMed] [Google Scholar]
- 67.Hamza M, Wang X-M, Adam A, Brahim JS, Rowan JS, Carmona GN, Dionne RA. Kinin B1 receptors contributes to acute pain following minor surgery in humans. Molecular Pain. 2010;6:12. doi: 10.1186/1744-8069-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee Y-S, Kim H, Wu T-X, Wang X-M, Dionne RA. Genetically mediated interindividual variation in analgesic responses to cyclooxygenase inhibitory drugs. Clinical Pharmacology & Therapeutics. 2006;79:407–18. doi: 10.1016/j.clpt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Kim H, Lee H, Rowan J, Brahim J, Dionne RA. Genetic polymorphisms in monoamine neurotransmitter systems show only weak association with acute post-surgical pain in humans. Mol Pain. 2006;2:24. doi: 10.1186/1744-8069-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim H, Ramsay E, Lee H, Wahl S, Dionne RA. Genome-wide association study of acute post-surgical pain in humans. Pharmacogenomics. 2009;10:171–9. doi: 10.2217/14622416.10.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC. 17-Beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beneng K, Renton T, Yilmaz Z, Yiangou Y, Anand P. Sodium channel Na v 1.7 immunoreactivity in painful human dental pulp and burning mouth syndrome. BMC Neuroscience. 2010;11:71. doi: 10.1186/1471-2202-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warren CA, Mok L, Gordon S, Fouad AF, Gold MS. Quantification of neural protein in extirpated tooth pulp. Journal of Endodontics. 2008;34:7–10. doi: 10.1016/j.joen.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alvarado LT, Perry GM, Hargreaves KM, Henry MA. TRPM8 Axonal expression is decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia. J Endod. 2007;33:1167–71. doi: 10.1016/j.joen.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo S, Perry GM, Levinson SR, Henry MA. Pulpitis increases the proportion of atypical nodes of Ranvier in human dental pulp axons without a change in Na v 1.6 sodium channel expression. Neuroscience. 2010;169:1881–7. doi: 10.1016/j.neuroscience.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 76.Henry MA, Luo S, Foley BD, Rzasa RS, Johnson LR, Levinson SR. Sodium channel expression and localization at demyelinated sites in painful human dental pulp. Journal of Pain. 2009;10:750–8. doi: 10.1016/j.jpain.2009.01.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li YQ, Li H, Wei J, Qu L, Wu LA. Expression changes of K+-Cl- co-transporter 2 and Na+-K+-Cl- co-transporter1 in mouse trigeminal subnucleus caudalis following pulpal inflammation. 2010. [DOI] [PubMed] [Google Scholar]
- 78.Bowles WR, Withrow JC, Lepinski AM, Hargreaves KM. Tissue levels of immunoreactive substance P are increased in patients with irreversible pulpitis. Journal of Endodontics. 2003;29:265–7. doi: 10.1097/00004770-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 79.Falace DA, Reid K, Rayens MK. The influence of deep (odontogenic) pain intensity, quality, and duration on the incidence and characteristics of referred orofacial pain. J Orofac Pain. 1996;10:232–9. [PubMed] [Google Scholar]
- 80.Sigurdsson A, Maixner W. Effects of experimental and clinical noxious counterirritants on pain perception. Pain. 1994;57:265–75. doi: 10.1016/0304-3959(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 81.Owatz CB, Khan AA, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. The incidence of mechanical allodynia in patients with irreversible pulpitis. Journal of Endodontics. 2007;33:552–6. doi: 10.1016/j.joen.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 82.Khan AA, Owatz CB, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. Measurement of mechanical allodynia and local anesthetic efficacy in patients with irreversible pulpitis and acute periradicular periodontitis. Journal of Endodontics. 2007;33:796–9. doi: 10.1016/j.joen.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 83.Harriott AM, Dessem D, Gold MS. Inflammation increases the excitability of masseter muscle afferents. Neuroscience. 2006;141:433–42. doi: 10.1016/j.neuroscience.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 84.Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. Journal of Neurophysiology. 2005;93:1585–97. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaughn AH, Gold MS. Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. Journal of Neuroscience. 2010;30:7878–88. doi: 10.1523/JNEUROSCI.6053-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tashiro A, Okamoto K, Bereiter DA. Chronic inflammation and estradiol interact through MAPK activation to affect TMJ nociceptive processing by trigeminal caudalis neurons. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tashiro A, Okamoto K, Bereiter DA. Chronic inflammation and estradiol interact through MAPK activation to affect TMJ nociceptive processing by trigeminal caudalis neurons. Neuroscience. 2009;164:1813–20. doi: 10.1016/j.neuroscience.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Okamoto K, Bereiter DF, Thompson R, Tashiro A, Bereiter DA. Estradiol replacement modifies c-fos expression at the spinomedullary junction evoked by temporomandibular joint stimulation in ovariectomized female rats. Neuroscience. 2008;156:729–36. doi: 10.1016/j.neuroscience.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bereiter DA, Benetti AP. Amino acid release at the spinomedullary junction after inflammation of the TMJ region in male and female rats. Pain. 2006;126:175–83. doi: 10.1016/j.pain.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 90.Grushka M, Sessle BJ. Applicability of the McGill Pain Questionnaire to the differentiation of ‘toothache’ pain. Pain. 1984;19:49–57. doi: 10.1016/0304-3959(84)90064-2. [DOI] [PubMed] [Google Scholar]
- 91.Vargas CM, Macek MD, Marcus SE. Sociodemographic correlates of tooth pain among adults: United states, 1989. Pain. 2000;85:87–92. doi: 10.1016/s0304-3959(99)00250-x. [DOI] [PubMed] [Google Scholar]
- 92.Billinton A. Human dental pulp as a source of native functional nociceptor pharmacology. Pain. 2009;144:227. doi: 10.1016/j.pain.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 93.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurology. 2010;9:807–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 94.Oh IH, Choi SK, Park BJ, Kim TS, Rhee BA, Lim YJ. The Treatment Outcome of Elderly Patients with Idiopathic Trigeminal Neuralgia: Micro-Vascular Decompression versus Gamma Knife Radiosurgery. Journal of Korean Neurosurgical Society. 2008;44:199–204. doi: 10.3340/jkns.2008.44.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zakrzewska JM, Jassim S, Bulman JS. A prospective, longitudinal study on patients with trigeminal neuralgia who underwent radiofrequency thermocoagulation of the Gasserian ganglion. Pain. 1999;79:51–8. doi: 10.1016/S0304-3959(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 96.Cheshire WP., Jr . Defining the role for gabapentin in the treatment of trigeminal neuralgia: a retrospective study. 2010. [DOI] [PubMed] [Google Scholar]
- 97.Park S-S, Lee M-K, Kim J-W, Jung J-Y, Kim I-S, Ghang C-G. Percutaneous balloon compression of trigeminal ganglion for the treatment of idiopathic trigeminal neuralgia: experience in 50 patients. Journal of Korean Neurosurgical Society. 2008;43:186–9. doi: 10.3340/jkns.2008.43.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tarricone R, Aguzzi G, Musi F, Fariselli L, Casasco A. Cost-effectiveness analysis for trigeminal neuralgia: Cyberknife vs microvascular decompression. Neuropsychiatric Disease & Treatment. 2008;4:647–52. doi: 10.2147/ndt.s2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller JP, Acar F, Burchiel KJ. Classification of trigeminal neuralgia: clinical, therapeutic, and prognostic implications in a series of 144 patients undergoing microvascular decompression. 2010. [DOI] [PubMed] [Google Scholar]
- 100.Benoliel R, Birenboim R, Regev E, Eliav E. Neurosensory changes in the infraorbital nerve following zygomatic fractures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:657–65. doi: 10.1016/j.tripleo.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 101.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci. 1994;14:2708–23. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oh IH, Choi SK, Park BJ, Kim TS, Rhee BA, Lim YJ. The Treatment Outcome of Elderly Patients with Idiopathic Trigeminal Neuralgia: Micro-Vascular Decompression versus Gamma Knife Radiosurgery. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahn DK, Lim EJ, Kim BC, Yang GY, Lee MK, Ju JS, Han SR, Bae YC. Compression of the trigeminal ganglion produces prolonged nociceptive behavior in rats. European Journal of Pain: Ejp. 2009;13:568–75. doi: 10.1016/j.ejpain.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang C-Y, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. Journal of Neuroscience. 2009;29:11161–71. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chichorro JG, Zampronio AR, Cabrini DA, Franco CRC, Rae GA. Mechanisms operated by endothelin ETA and ETB receptors in the trigeminal ganglion contribute to orofacial thermal hyperalgesia induced by infraorbital nerve constriction in rats. Neuropeptides. 2009;43:133–42. doi: 10.1016/j.npep.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 106.Campbell RL, Parks KW, Dodds RN. Chronic facial pain associated with endodontic therapy. Oral Surg Oral Med Oral Pathol. 1990;69:287–90. doi: 10.1016/0030-4220(90)90288-4. [DOI] [PubMed] [Google Scholar]
- 107.Lobb WK, Zakariasen KL, McGrath PJ. Endodontic treatment outcomes: do patients perceive problems? J Am Dent Assoc. 1996;127:597–600. doi: 10.14219/jada.archive.1996.0271. [DOI] [PubMed] [Google Scholar]
- 108.Polycarpou N, Ng YL, Canavan D, Moles DR, Gulabivala K. Prevalence of persistent pain after endodontic treatment and factors affecting its occurrence in cases with complete radiographic healing. Int Endod J. 2005;38:169–78. doi: 10.1111/j.1365-2591.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 109.Cheung LK, Lo J. The long-term clinical morbidity of mandibular step osteotomy. Int J Adult Orthodon Orthognath Surg. 2002;17:283–90. [PubMed] [Google Scholar]
- 110.Carmichael FA, McGowan DA. Incidence of nerve damage following third molar removal: a West of Scotland Oral Surgery Research Group study. Br J Oral Maxillofac Surg. 1992;30:78–82. doi: 10.1016/0266-4356(92)90074-s. [DOI] [PubMed] [Google Scholar]
- 111.Robinson PP, Smith KG. Lingual nerve damage during lower third molar removal: a comparison of two surgical methods. Br Dent J. 1996;180:456–61. doi: 10.1038/sj.bdj.4809126. [DOI] [PubMed] [Google Scholar]
- 112.Valmaseda-Castellon E, Berini-Aytes L, Gay-Escoda C. Lingual nerve damage after third lower molar surgical extraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:567–73. doi: 10.1067/moe.2000.110034. [DOI] [PubMed] [Google Scholar]
- 113.Berge TI. Incidence of chronic neuropathic pain subsequent to surgical removal of impacted third molars. Acta Odontol Scand. 2002;60:108–12. doi: 10.1080/000163502753509518. [DOI] [PubMed] [Google Scholar]
- 114.Gregg JM. Neuropathic complications of mandibular implant surgery: review and case presentations. Ann R Australas Coll Dent Surg. 2000;15:176–80. [PubMed] [Google Scholar]
- 115.Putzki N, Pfriem A, Limmroth V, Yaldizli O, Tettenborn B, Diener HC, Katsarava Z. Prevalence of migraine, tension-type headache and trigeminal neuralgia in multiple sclerosis. European Journal of Neurology. 2009;16:262–7. doi: 10.1111/j.1468-1331.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- 116.Cruccu G, Biasiotta A, Di Rezze S, Fiorelli M, Galeotti F, Innocenti P, Mameli S, Millefiorini E, Truini A. Trigeminal neuralgia and pain related to multiple sclerosis. Pain. 2009;143:186–91. doi: 10.1016/j.pain.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 117.Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 1990;27:89–95. doi: 10.1002/ana.410270114. [DOI] [PubMed] [Google Scholar]
- 118.Morgan CR, Bird EV, Robinson PP, Boissonade FM. TRPA1 expression in human lingual nerve neuromas in patients with and without symptoms of dysaesthesia. 2010. [DOI] [PubMed] [Google Scholar]
- 119.Siqueira SR, Alves B, Malpartida HM, Teixeira MJ, Siqueira JT. Abnormal expression of voltage-gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. 2010. [DOI] [PubMed] [Google Scholar]
- 120.Harriott AM, Gold MS. Contribution of primary afferent channels to neuropathic pain. Current Pain & Headache Reports. 2009;13:197–207. doi: 10.1007/s11916-009-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blatow M, Nennig E, Sarpaczki E, Reinhardt J, Schlieter M, Herweh C, Rasche D, Tronnier VM, Sartor K, Stippich C. Altered somatosensory processing in trigeminal neuralgia. Human Brain Mapping. 2009;30:3495–508. doi: 10.1002/hbm.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tanaka T, Sakamoto E, Shiiba S, Oda M, Kito S, Wakasugi-Sato N, Matsumoto-Takeda S, Imamura Y, Nakanishi O, Morimoto Y. Relationship between the curative effects of carbamazepine administration and the neurovascular compression volume of the trigeminal nerve measured using magnetic resonance cisternography. 2010. [DOI] [PubMed] [Google Scholar]
- 123.Chichorro JG, Zampronio AR, Cabrini DA, Franco CR, Rae GA. Mechanisms operated by endothelin ETA and ETB receptors in the trigeminal ganglion contribute to orofacial thermal hyperalgesia induced by infraorbital nerve constriction in rats. 2010. [DOI] [PubMed] [Google Scholar]
- 124.Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, Gold MS, Porreca F, Strichartz GR. The role of sodium channels in chronic inflammatory and neuropathic pain. Journal of Pain. 2006;7:S1–29. doi: 10.1016/j.jpain.2006.01.444. [DOI] [PubMed] [Google Scholar]
- 125.Goncalves DAdG, Dal Fabbro AL, Campos JADB, Bigal ME, Speciali JG. Symptoms of temporomandibular disorders in the population: an epidemiological study. Journal of Orofacial Pain. 2010;24:270–8. [PubMed] [Google Scholar]
- 126.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–61. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 127.LeResche L, Mancl LA, Drangsholt MT, Huang G, Von Korff M. Predictors of onset of facial pain and temporomandibular disorders in early adolescence. Pain. 2007;129:269–78. doi: 10.1016/j.pain.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.LeResche L, Mancl LA, Drangsholt MT, Saunders K, Korff MV. Relationship of pain and symptoms to pubertal development in adolescents. Pain. 2005;118:201–9. doi: 10.1016/j.pain.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 129.LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69:153–60. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- 130.Lim PF, Smith S, Bhalang K, Slade GD, Maixner W. Development of temporomandibular disorders is associated with greater bodily pain experience. Clinical Journal of Pain. 2010;26:116–20. doi: 10.1097/AJP.0b013e3181c507ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Archives of Oral Biology. 2005;50:971–9. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 132.Amandusson A, Blomqvist A. Estrogen receptor-alpha expression in nociceptive-responsive neurons in the medullary dorsal horn of the female rat. European Journal of Pain: Ejp. 2010;14:245–8. doi: 10.1016/j.ejpain.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 133.Bouloux GF. Temporomandibular joint pain and synovial fluid analysis: a review of the literature. Journal of Oral & Maxillofacial Surgery. 2009;67:2497–504. doi: 10.1016/j.joms.2009.04.103. [DOI] [PubMed] [Google Scholar]
- 134.Stohler CS, Kowalski CJ. Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain. 1999;79:165–73. doi: 10.1016/s0304-3959(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 135.Arendt-Nielsen L, Svensson P, Sessle BJ, Cairns BE, Wang K. Interactions between glutamate and capsaicin in inducing muscle pain and sensitization in humans. European Journal of Pain: Ejp. 2008;12:661–70. doi: 10.1016/j.ejpain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alstergren P, Ernberg M, Nilsson M, Hajati AK, Sessle BJ, Kopp S. Glutamate-induced temporomandibular joint pain in healthy individuals is partially mediated by peripheral NMDA receptors. 2010. [PubMed] [Google Scholar]
- 137.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Human Molecular Genetics. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 138.Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–3. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 139.Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenetics & Genomics. 2010;20:239–48. doi: 10.1097/FPC.0b013e328337f9ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cittadini E, Matharu MS. Symptomatic trigeminal autonomic cephalalgias. 2010. [Review] [57 refs] [DOI] [PubMed] [Google Scholar]
- 141.Dodick DW. Migraine with isolated facial pain: a diagnostic challenge. Cephalalgia. 2007;27:1199–200. doi: 10.1111/j.1468-2982.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 142.Benoliel R, Birman N, Eliav E, Sharav Y. The International Classification of Headache Disorders: accurate diagnosis of orofacial pain? Cephalalgia. 2008;28:752–62. doi: 10.1111/j.1468-2982.2008.01586.x. [DOI] [PubMed] [Google Scholar]
- 143.Hussain A, Stiles MA, Oshinsky ML. Pain remapping in migraine: a novel characteristic following trigeminal nerve injury. Headache. 2010;50:669–71. doi: 10.1111/j.1526-4610.2009.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eross E, Dodick D, Eross M. The Sinus, Allergy and Migraine Study (SAMS) Headache. 2007;47:213–24. doi: 10.1111/j.1526-4610.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 145.Gaul C, Sandor PS, Galli U, Palla S, Ettlin DA. Orofacial migraine. Cephalalgia. 2007;27:950–2. doi: 10.1111/j.1468-2982.2007.01349.x. [DOI] [PubMed] [Google Scholar]
- 146.Epstein JB, Hong C, Logan RM, Barasch A, Gordon SM, Oberlee-Edwards L, McGuire D, Napenas JJ, Elting LS, Spijkervet FKL, Brennan MT. A systematic review of orofacial pain in patients receiving cancer therapy. Supportive Care in Cancer. 2010;18:1023–31. doi: 10.1007/s00520-010-0897-7. [DOI] [PubMed] [Google Scholar]
- 147.Dolan JC, Lam DK, Achdjian SH, Schmidt BL. The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. Journal of Neuroscience Methods. 2010;187:207–15. doi: 10.1016/j.jneumeth.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Quang PN, Schmidt BL. Endothelin-A receptor antagonism attenuates carcinoma-induced pain through opioids in mice. J Pain. 2010;11:663–71. doi: 10.1016/j.jpain.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ni Riordain R, Moloney E, O’Sullivan K, McCreary C. Burning mouth syndrome and oral health-related quality of life: is there a change over time? Oral Dis. 2010;16:643–7. doi: 10.1111/j.1601-0825.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- 150.Youle M, Osio M. A double-blind, parallel-group, placebo-controlled, multicentre study of acetyl L-carnitine in the symptomatic treatment of antiretroviral toxic neuropathy in patients with HIV-1 infection. HIV Med. 2007;8:241–50. doi: 10.1111/j.1468-1293.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 151.Barlas P, Craig JA, Robinson J, Walsh DM, Baxter GD, Allen JM. Managing delayed-onset muscle soreness: lack of effect of selected oral systemic analgesics. Arch Phys Med Rehabil. 2000;81:966–72. doi: 10.1053/apmr.2000.6277. [DOI] [PubMed] [Google Scholar]
- 152.Graham C, Bond SS, Gerkovich MM, Cook MR. Use of the McGill pain questionnaire in the assessment of cancer pain: replicability and consistency. Pain. 1980;8:377–87. doi: 10.1016/0304-3959(80)90081-0. [DOI] [PubMed] [Google Scholar]
- 153.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 154.Melzack R, Jeans ME, Stratford JG, Monks RC. Ice massage and transcutaneous electrical stimulation: comparison of treatment for low-back pain. Pain. 1980;9:209–17. doi: 10.1016/0304-3959(80)90008-1. [DOI] [PubMed] [Google Scholar]
- 155.Tahmoush AJ. Causalgia: redefinition as a clinical pain syndrome. Pain. 1981;10:187–97. doi: 10.1016/0304-3959(81)90194-9. [DOI] [PubMed] [Google Scholar]
- 156.Campbell WI, Kendrick RW. Pre-emptive analgesia using local anaesthesia: a study in bilaterally symmetrical surgery. Br J Anaesth. 1997;79:657–9. doi: 10.1093/bja/79.5.657. [DOI] [PubMed] [Google Scholar]
- 157.Seymour RA, Charlton JE, Phillips ME. An evaluation of dental pain using visual analogue scales and the Mcgill Pain Questionnaire. J Oral Maxillofac Surg. 1983;41:643–8. doi: 10.1016/0278-2391(83)90017-4. [DOI] [PubMed] [Google Scholar]
- 158.Vickers ER, Cousins MJ, Woodhouse A. Pain description and severity of chronic orofacial pain conditions. Aust Dent J. 1998;43:403–9. doi: 10.1111/j.1834-7819.1998.tb00200.x. [DOI] [PubMed] [Google Scholar]