Abstract
Superior vena cava (SVC) syndrome is a rare but serious complication after pacemaker implantation. This report describes three cases of SVC syndrome treated with venoplasty and venous stenting, with an average follow-up of 30.7 (±3.1) months. These cases illustrate that the definitive diagnosis, and the extent and location of venous obstruction, can only be determined by venography.
Keywords: Superior vena cava syndrome, Venous stenting, Pacemaker
Introduction
Venous thrombosis or stenosis after implantation of transvenous pacemaker leads occurs frequently, although this occlusion is usually asymptomatic. The reported incidence is 30–64% [1, 2]. The incidence of pacemaker-induced superior vena cava (SVC) syndrome, however, is rare, ranging from 1 in 40,000 to 1 in 250 patients [3–5]. The mechanical stress associated with pacemaker wires may lead to vessel wall inflammation, thrombus formation, and ultimately to venous obstruction and occlusion. This usually occurs early after implantation, but can even occur after many years. Predisposing factors for the development of SVC syndrome are thrombophilia, the use of hormone therapy, infection, the presence of a temporary wire before implantation, and the presence of multiple, active, or retained pacing leads [1–7]. Venous obstruction is usually asymptomatic, because slow progression allows time for a collateral circulation to develop [1, 6]. However, the absence of an adequate collateral circulation may result in invalidating symptoms. The most common symptoms are an inability to bend over without flushing and headache, exercise-induced flushing, and, in the worst-case scenario, a typical superior vena cava syndrome develops [3]. Multiple treatment options are available including percutaneous transluminal angioplasty, implantation of metallic stents, thrombolysis, mechanical thrombectomy, and venous grafting. A combination of therapies is often required [1, 2, 7–15]. We present three cases of SVC syndrome successfully treated with venoplasty and stenting.
Case Reports
Patient A was a 56-year-old woman who developed sick sinus syndrome which was treated by implantation of a DDDR pacemaker. Eight years later, she was hospitalised with symptoms that included neck pain, dysphagia, and facial flushing during exercise. On examination, she showed engorged neck veins. Ultrasound revealed normal flow through the jugular and subclavian veins on both sides. Computed tomography of the chest and neck did not suggest any abnormality of the venous system; there was no lymphadenopathy. Dental and ear–nose–throat examination was normal. Given the discordance between the clinical and imaging findings, she was discharged with planned outpatient follow-up. Two months later, she was readmitted with multiple rib fractures after a collapse. Bilateral upper limb venography showed obstruction of the superior vena cava (Fig. 1). Given the invalidating symptoms, a decision was made to remove the pacemaker leads and to attempt recanalisation of the SVC with venoplasty and stenting. Recanalisation was attempted using a combined approach from the left arm and right leg. After predilatation, a self-expanding stent (Boston Scientific, Wallstent, 16 × 60 mm) was placed in the SVC. Angiography post-placement showed reduced flow from the left arm with angiographic appearances of dissection and thrombus. After placement of an additional self-expanding stent (Abbott Vascular, Absolute, 10 × 40 mm) normal flow from the left and right arm was restored (Fig 2). A new DDDR pacemaker was implanted a few days later. Despite resolution of the symptoms related to SVC occlusion, she complained of persistent pain on the left side of the chest during left arm movements, most probably caused by the jammed stent between the clavicle and the first left rib. This could be resolved by resection of the first left rib. Thereafter, the patient was symptom-free with no signs of recurrence 30 months after stenting.
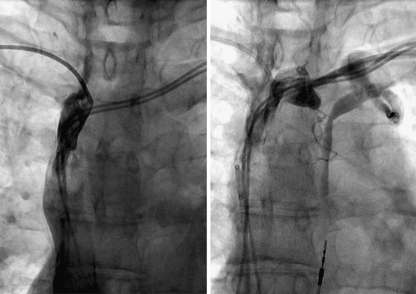
Fig. 1.
Venogram of right subclavian vein (left panel) and left subclavian vein (right panel) in patient A, showing extensive thrombosis in the anonyma veins, with occlusion of the SVC
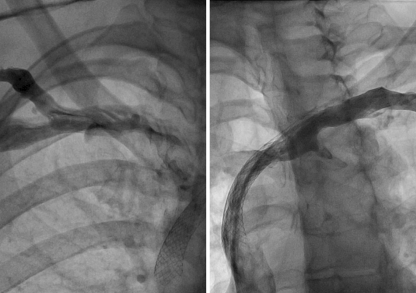
Fig. 2.
Selective injection from right (left panel) and left subclavian vein (right panel) after dilatation and stenting of the same patient as Fig. 1
Patient B was a 61-year-old woman who had a VVI pacemaker implanted for sick sinus syndrome at the age of 33 years. Fifteen years later, a low-grade infection of the pacemaker lead occurred. Laser lead extraction was complicated by a rupture of the superior vena cava, which was closed by a pericardial patch during an emergency thoracotomy. A new pacemaker was inserted below the rectus abdominal muscle with an epicardial atrial lead. Swelling of the upper thorax and head necessitated a re-thoracotomy the next day. A Goretex prosthesis (W.L. Gore & Associates, Inc. Medical Products Division, PO Box 2400, Flagstaff, Arizona, USA) was implanted between the innominate vein and the right atrium. Nine years later, she was admitted with swelling of the head, headache, and nausea. Computed tomography showed extensive venous collaterals, but blockade of the venous downstream could not be determined from this study. A venogram from the right jugular vein and right femoral vein revealed occlusion of the Goretex shunt (Fig. 3 left panel). Recanalisation of the prosthesis was attempted using a combined approach from the right jugular vein and right leg (Fig. 3 right panel). Repeated attempts to cross the occlusion with dedicated peripheral hydrophilic wires via guiding catheters placed distal and proximal to the occlusion were unsuccessful. Using over-the-wire balloons and dedicated guide wires for chronically occluded coronary vessels, a small channel was created that allowed both guiding catheters to be juxtaposed within the occlusion. An exchange length coronary wire was passed from the arm and recovered from the groin. Subsequently, the guiding catheters were retracted and the channel was enlarged by inflating progressively larger non-compliant coronary balloons (Quantum, Boston Scientific). The guide catheters were then readvanced until they were juxtaposed and a stiff exchange length Amplatz wire could be advanced from the arm to the groin. Subsequently, the guiding catheters were then removed and a self-expanding stent (Boston Scientific, Wallstent, 16 × 60 mm) was placed within the graft, followed by additional dilatations with a 10.0 and 12.0 balloon (Fig. 4). This patient remained asymptomatic 28 months after stenting.
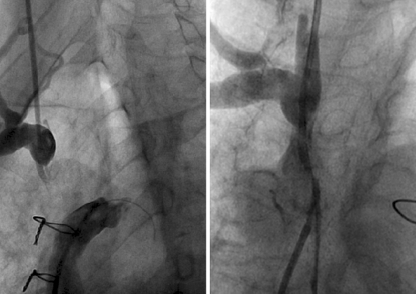
Fig. 3.
Angiogram in right anterior oblique position (left panel) of patient B showing discontinuity between right innominate vein and vena cava superior. Detail of the innominate vein in anteroposterior view is shown in the right panel
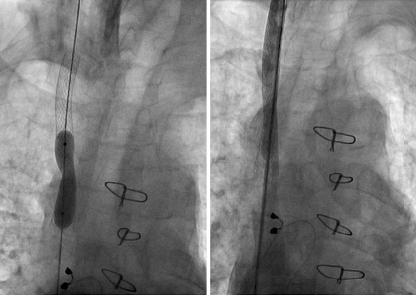
Fig. 4.
Balloon dilatation of the same patient as Fig. 3, after placement of a stent (left panel) with the final angiographic result shown in the right panel
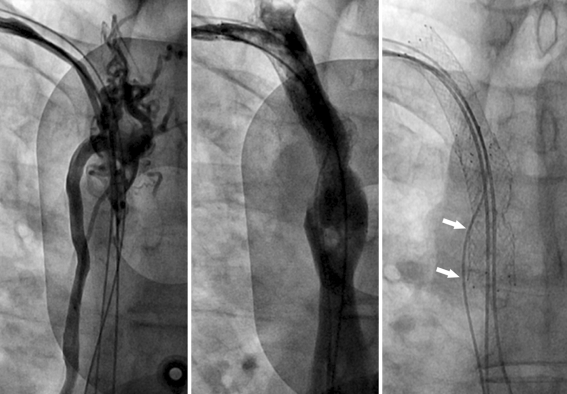
Patient C was a 64-year-old man with severe heart failure due to a dilated cardiomyopathy, who had a biventricular pacemaker implanted 4 years before. Three years later, he developed progressive dyspnoea during exercise and fatigue due to dislocation of the left ventricular lead. Repositioning of the lead was unsuccessful due to thrombosis of the left brachiocephalic and subclavian vein. A new biventricular pacemaker system was implanted from the right subclavian vein. Several months later, he developed clinical signs of SVC syndrome. A computed tomography scan was inconclusive and the diagnosis could only be confirmed by bilateral upper limb venography. The atrial and ventricular leads were removed; however, a Medtronic Starfix lead implanted in the coronary sinus had to be left in situ, because the fixation lobes could not be retracted. Recanalisation was attempted using a combined approach from the right subclavian and right femoral vein. The SVC and brachiocephalic vein were dilated and stented using a Cordis SMART, nitinol self-expanding stent resulting in a good runoff and a reduction in collateral flow (Fig. 5). New atrial and ventricular leads were implanted. The original coronary sinus lead was re-used and functioned properly although the lead was trapped between the stent and the vessel wall. Five months later, he developed swelling of the thorax, neck and head and gained 6 kg in weight. Both stents were occluded and the proximal end of the stent in the SVC was dislocated. After several balloon inflations employing 6.0 and 10.0 mm balloons, a Wallstent (16 × 60 mm) was inserted on this occasion from the right internal jugular vein to the superior vena cava re-establishing venous drainage from the head. A second overlapping Wallstent (16 × 60 mm) was positioned from the right atrium into the superior vena cava. Additional dilatations with 12.0 and 14.0 balloons obtained a good angiographic result. The patient remained asymptomatic at 34 months follow-up.
Fig. 5.
Left panel shows angiogram from right subclavian vein, illustrating occlusion of the vena cava superior and collateral circulation through the azygos vein. Middle panel shows the angiographic result after angioplasty and stenting of the vena cava superior. Radiographic appearance of both stents and the position of the newly implanted atrial and ventricular lead are shown in the right panel. The coronary sinus was re-used but functioned properly although the lead was trapped between the stent and the vessel wall (arrows) as illustrated here
Discussion
We present three cases with pacemaker-mediated SVC syndrome successfully treated with venous stenting. SVC syndrome is a rare but serious and debilitating complication after transvenous pacemaker implantation. The diagnosis of SVC syndrome is based on clinical signs that could only be confirmed by venography in all three patients. The diagnosis could not be completed by computed tomography in any of the patients. Venography, however, provided excellent characterisation of the venous anatomy and the site and extent of venous obstruction, necessary for decision-making for the therapeutic strategy. Notwithstanding the limitations of computed tomography in delineating venous anatomy, it is an essential examination to exclude external compression as a cause of SVC syndrome and to characterise the nature of the extrinsic compression [6, 16, 17].
Because of its rarity, and in view of the variations in anatomical obstruction associated with the SVC syndrome, there is no unique and unequivocal strategy. Most patients are unresponsive to anticoagulation alone which appears to be effective only in the mildest cases. However, life-long anticoagulation, after definitive endovascular therapy, is important to reduce the incidence of re-occlusion and may play a role in maintaining collateral circulation [2]. Many authors report successful treatment of SVC syndrome by venoplasty with or without stenting. In our three patients, successful treatment with venoplasty and venous stenting could be accomplished. The procedure is time-consuming, may entail a high volume of contrast and a high radiation dose, and is prone to acute and sub-acute complications. In the first patient, a first rib resection was necessary to relieve symptoms due to the need to place a self-expanding stent between the left clavicle and first rib. The third patient had a recurrent SVC syndrome after 5 months due to stent dislocation. He was successfully treated was additional stenting.
The nature of adequate adjuvant antithrombotic therapy remains difficult and is unanswered in the literature. We decided to treat all three patients with clopidogrel for 6 months in conjunction with long-term anticoagulation.
Follow-up, with respect to patency, in the literature is limited to 50 months [5, 14]. So far, none of our three patients has shown signs of recurrence (at 28–34 months). Further study and longer follow-up are necessary to determine long-term patency of venous stenting as a treatment for SVC syndrome.
In conclusion, venoplasty and venous stenting can be successfully performed in patients with SVC syndrome. Short- and long-term complications may be encountered, necessitating repeated intervention. However, this has to be accepted due to the lack of adequate therapeutic alternatives. Ultimately, if endovascular procedures fail, surgery is the last resort, requiring thoracotomy, which can be associated with significant morbidity [5].
Contributor Information
B. Klop, Email: boudewijn.klop@gmail.com
M. G. Scheffer, Email: mgscheffer@hotmail.com
E. McFadden, Email: Eugene.McFadden@hse.ie
F. Bracke, Email: f.bracke@me.com
B. van Gelder, Email: l.vangelder@onsnet.nu
References
- 1.Lindsay HSJ, Chennells PM, Perrins EJ. Successful treatment by balloon venoplasty and stent insertion of obstruction of the superior vena cava by an endocardial pacemaker lead. Br Heart J. 1994;71:363–5. doi: 10.1136/hrt.71.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rozmus G, Daubert JP, Huang DT, Rosero S, Hall B, Francis C. Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J Interv Card Electrophysiol. 2005;13:9–19. doi: 10.1007/s10840-005-1140-1. [DOI] [PubMed] [Google Scholar]
- 3.Melzer C, Lembcke A, Ziemer S, Eddicks S, Witte J, Baumann G, et al. Pacemaker-induced superior vena cava syndrome: clinical evaluation of long term follow-up. Pacing Clin Electrophysiol. 2006;29:1346–51. doi: 10.1111/j.1540-8159.2006.00546.x. [DOI] [PubMed] [Google Scholar]
- 4.Goudevenos JA, Reid PG, Adams PC, Holden MP, Williams DO. Pacemaker induced superior vena cava syndrome: report of four cases and review of the literature. Pacing Clin Electrophysiol. 1989;12:1890–5. doi: 10.1111/j.1540-8159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 5.Riley RF, Petersen SE, Ferguson JD, Bashir Y. Managing superior vena cava syndrome as a complication of pacemaker implantation: a pooled analysis of clinical practice. Pacing Clin Electrophysiol. 2010;33:420–5. doi: 10.1111/j.1540-8159.2009.02613.x. [DOI] [PubMed] [Google Scholar]
- 6.Park HW, Kim W, Cho JG, Kang JC. Multiple pacing lead lead-induced superior vena cava syndrome: succesful treatment by balloon angioplasty. J Cardiovasc Electrophysiol. 2005;16:221–3. doi: 10.1046/j.1540-8167.2005.40511.x. [DOI] [PubMed] [Google Scholar]
- 7.Bracke F, Meijer A, Gelder B. Venous occlusion of the access vein in patients referred for lead extraction: influence of patients and lead characteristics. Pacing Clin Electrophysiol. 2003;26:1649–52. doi: 10.1046/j.1460-9592.2003.t01-1-00247.x. [DOI] [PubMed] [Google Scholar]
- 8.Qanadli SD, El Hajjam ME, Mignon F, Kerviler E, Rocha P, Barré O, et al. Subacute and chronic benign superior vena cava obstructions: endovascular treatment with self-expanding metallic stents. AJR Am J Roentgenol. 1999;173:159–64. doi: 10.2214/ajr.173.1.10397119. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal E, Shakeel AQ, Tynan M, Bucknall CA. Percutaneous pacemaker lead extraction and stent implantation for superior vena cava occlusion due to pacemaker leads. Am J Cardiol. 1996;77:670–2. doi: 10.1016/S0002-9149(97)89333-2. [DOI] [PubMed] [Google Scholar]
- 10.Teo N, Sabharwal T, Rowland E, Curry P, Adam A. Treatment of superior vena cava obstruction secondary to pacemaker wires with balloon venoplasty and insertion of metallic stents. Eur Heart J. 2002;23:1465–70. doi: 10.1053/euhj.2002.3260. [DOI] [PubMed] [Google Scholar]
- 11.Tan CW, Vijitbenjaronk P, Khuri B. Superior vena cava syndrome due to permanent transvenous pacemaker electrodes: successful treatment with combined thrombolysis and angioplasty. Angiology. 2000;51:963–9. doi: 10.1177/000331970005101110. [DOI] [PubMed] [Google Scholar]
- 12.Chan AW, Bhatt DL, Wilkoff BL, Roffi M, Mukherje D, Gray BH, et al. Percutaneous treatment for pacemaker-associated superior vena cava syndrome. Pacing Clin Electrophysiol. 2002;25:1628–33. doi: 10.1046/j.1460-9592.2002.01628.x. [DOI] [PubMed] [Google Scholar]
- 13.Kastner RJ, Fisher WG, Blacky AR, Bacon ME. Pacemaker-induced superior vena cava syndrome with successful treatment by balloon venoplasty. Am J Cardiol. 1996;77:789–90. doi: 10.1016/S0002-9149(97)89222-3. [DOI] [PubMed] [Google Scholar]
- 14.Gilard M, Pérennes A, Mansourati J, Etienne Y, Fatemi M, Blanc JJ, Boschat J. Stent implantation for the treatment of superior vena cava syndrome related to pacemaker leads. Europace. 2002;4:155–8. doi: 10.1053/eupc.2002.0230. [DOI] [PubMed] [Google Scholar]
- 15.Garlitsky AC, Swingle JD, Aizer A, Holmes DS, Bernstein NE, Chinitz LA. Percutaneous treatment of the superior vena cava syndrome via an excimer laser sheath in a patient with a single chamber atrial pacemaker. J Interv Card Electrophysiol. 2006;16:203–6. doi: 10.1007/s10840-006-9041-5. [DOI] [PubMed] [Google Scholar]
- 16.Conte FA, Orzel JA. Superior vena cava syndrome and bilateral subclavian vein thrombosis CT and radionuclide venography correlation. Clin Nucl Med. 1986;11:698–700. doi: 10.1097/00003072-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Qanadli SD, El Hajjam M, Bruckert F, Judet O, Barré O, Chagnon S, Lacombe P. Helical CT phlebography of superior vena cava: diagnosis and evaluation of venous obstruction. AJR Am J Roentgenol. 1999;172:1327–33. doi: 10.2214/ajr.172.5.10227511. [DOI] [PubMed] [Google Scholar]