Abstract
Objectives
There is no consensus among physicians as to whether or not pectus excavatum can produce symptoms sometimes even severe enough to justify a surgical procedure. The aim of this study was to assess the prevalence and severity of complaints and symptoms of senior patients with a pectus excavatum and to evaluate the results of surgical correction of the chest deformation.
Design
This is a prospective clinical study, case series.
Participants
The participants are 42 senior patients with a pectus excavatum and somatic complaints.
Methods
Cardiological screening included medical history taking, physical examination, electrocardiography, transthoracic echocardiography and treadmill cardiography. Complaints, symptoms and test results were arranged in a clinical score. Indication for a therapeutic surgical correction by a modified Ravitch operation was a high clinical score in combination with radiological evidence of cardiac compression on CT or MRI.
Results
The clinical picture of the 42 senior patients with a pectus excavatum showed complaints of fatigue and low exercise tolerance, shortness of breath, palpitations, inspiratory obstruction and sometimes chest discomfort or pain. The serious and sometimes invalidating complaints of 19 patients (45%) had started only in their fourth or fifth decade of life and were labelled in 12 patients (63%) as ‘Unexplained cardiovascular complaints’. To date, 11 patients have undergone surgical procedures. Symptoms were reduced substantially or had disappeared completely. All patients indicated that their health-related quality of life was significantly improved.
Conclusion
Recognising the clinical picture of SPES is relevant because surgical reconstruction of the chest can provide substantial relief of symptoms.
Keywords: Pectus excavatum, Unexplained cardiovascular complaints, Ravitch, Fatigue, Low exercise tolerance, Shortness of breath, Palpitations, Inspiratory obstruction
Introduction
Twenty years ago most family physicians, internists and paediatricians regarded pectus excavatum principally as a cosmetic problem, and it was discussed as such in standard texts [1]. A relation between pectus excavatum and clinical symptoms was traditionally not recognised by most surgeons. Even nowadays, there is no consensus among physicians as to whether or not the deformity can produce symptoms, sometimes even severe enough to justify a surgical procedure [2]. In the past 20 years, a steadily increasing flow of reports has appeared from surgical groups, describing a relationship between symptoms and this malformation. After surgical intervention, most patients were relieved of their complaints [3–7]. A large follow-up study, where a relationship was supposed between pectus excavatum and pulmonary function before and after operation, was negative; but in this study, symptoms were not included [8]. Only a few authors give information on results of cardiovascular diagnostic tests relative to pectus excavatum, suggesting a causal relationship [4, 9, 10]. Apart from two case reports, one of which was from our group, we did not find any publications about cardiovascular complaints in 50-plus patients (seniors) with a PE [11–13]. In this case series, we will report the results of a prospective study of senior patients with a symptomatic pectus excavatum.
Pectus excavatum is a congenital deformation of the chest, presenting as a funnel-shaped impression in the median frontal chest between the fourth and the seventh rib. Asymmetric presentation occurs, and the extent of deformation may vary from slight to serious. Exact numbers about prevalence are difficult to obtain, as the deformation is often not noted or—when noted—is not mentioned in medical reports. Pectus excavatum is found in Europe in at least one in every 1000 births, mainly in men (70–85%) and often within the same families (35–45%). A correlation is mentioned between pectus excavatum and relatively rare syndromes as Ehlers Danlos, Marfan syndrome, or Poland syndrome [3, 5, 7, 14]. Mitral valve prolapse occurs in 15% of patients with pectus excavatum [3, 9]. In severe forms of pectus excavatum, the inward deformation of the sternum creates an impression on the right atrium or ventricle. This can cause cardiac compression [3, 9, 15]. By deviation of the heart to the left, cardiac compression can be avoided. This is the case in younger patients who still have flexible chests. This flexibility decreases as age advances and the chest wall gets stiffer. By this time, side deviation is no longer possible and cardiac compression increases. Thus, seniors with a pectus excavatum that was asymptomatic during their youth can gradually develop a symptomatic pectus excavatum.
Clinical Picture of Symptomatic Pectus Excavatum in Seniors
Complaints and symptoms encountered in children and adults with a symptomatic pectus excavatum are fatigue, reduced exercise tolerance, shortness of breath, palpitations, mitral valve prolapse and chest pain. When young and in good health, these complaints occur during high-level exercises and are often not mentioned or taken seriously for that reason [3–7]. Gradually and in slow progression, senior patients develop exactly the same clinical picture at lower levels of exercise [11–13]. Certainly, at advanced age, ischaemic heart disease and COPD could fit into this clinical picture and therefore should be excluded. Our first surgical procedure for a patient with symptomatic pectus excavatum in seniors (SPES) took place after he had been turned away often by other colleagues [11, 12].
Methods
This prospective and descriptive study includes all patients above 50 years of age (seniors) with a pectus excavatum and somatic complaints, who consulted us as a cardiologist or as a surgeon. Medical data regarding these patients, as recorded in written or computerised medical files, were dynamically collected in a structured MS Access-2002 database. This database was used for analysis and generation of reports. Cardiological screening included medical history-taking, physical examination, electrocardiography, transthoracic echocardiography and treadmill cardiography. All results were scored in the so-called SPES score and a Pectus Evaluation Index score (PEI score). To assess to what extent patients suffered from symptoms related to SPES, the signs, symptoms and possible test results were arranged in a SPES score from 1 to 10 (maximum) by giving a severity score to each finding (Table 1). The SPES score was 0 in case of absence of a pectus excavatum; no specific symptoms or pre-existing disorders, such as COPD and cardiac ischaemia, could be responsible for the symptoms. A routine chest radiograph in two directions and a CT scan or MRI of the thorax were analysed by a radiologist. The results of the visual inspection of the funnel chest by the surgeon and the radiological analysis were represented in the PEI score. We applied an ordinal scale from 0 (flat or no pectus excavatum) to 5 (deep or wedge formed excavation). Next to this, we calculated the Haller index, which is derived by dividing the transverse chest diameter by the anteroposterior diameter [16, 17]. Patients with a SPES index > 5, a PEI score > 3 and signs of cardiac compression were eligible for surgery. After careful instruction, they were asked by the surgeon to decide if they wanted to undergo a possibly curative correction of their pectus excavatum.
Table 1.
SPES score calculation
| Clinical finding | Score | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Dyspnoea | Minor | Moderate | Severe |
| Palpitations | Minor | Severe | |
| Fatigue, low exercise tolerance | Minor/moderate | Severe | |
| Chest pain | Yes | ||
| Postural symptoms | Possible | Clearly present | |
| ECG/Holter recording: (supra)ventricular arrhythmia; erratic conduction; partial AV block | Clearly present | Severe | |
| Echocardiography: MV insufficiency and/or prolapse; TV insufficiency; enlarged RA | Minor | Clearly present | |
| Treadmill ECG | Stopped due to exhaustion | ||
| Spirometry: inspiratory obstruction | Moderate | Clearly present | |
MV mitral valve, TV tricuspid valve, RA right atrium
Surgical correction basically followed the four steps described by Ravitch in 1949 using the modifications proposed by Robiseck [15, 18]. After sub-perichondral resection of the deformed costal cartilages and detachment of the xiphoid process, transverse sternotomy is performed at the upper level of the deformed sternum, which is then lifted up 4–5 cm. The corrected sternal position is secured by one or two cerclages and two strips of synthetic mesh (Marlex), spread behind the sternum, and secured tautly to the lateral osseous costal borders. The pectoralis muscles are then united presternally. Follow-up checks by the surgeon usually took place after 1, 3, 12 and 24 months. If possible, a cardiological follow-up was also done in our clinic. Clinical and cosmetic improvements were registered in the medical file of the patients. Before and after the surgical correction medical photographs were taken to document the cosmetic improvement. Subjective health-related quality of life improvement was measured by asking the patients the simple straight question: ‘How was your quality of life before and after the operation expressed as a number on a scale of 1 (minimal) to 10 (maximal)’? Patients were instructed beforehand about quality of life in relation to functional capacity, exercise capacity and emotional well-being.
Results
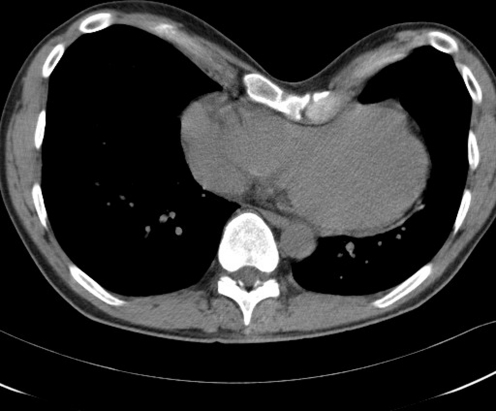
In the past 3 years (2006–2009), we examined 138 patients with a pectus excavatum who consulted us for cosmetic and somatic complaints. The 96 patients younger than 49 years came mainly for cosmetic reasons, but 27% (n = 26) appeared to have mild to considerable somatic complaints. Their data will be published separately. One nearly 49-year-old patient was regarded as a senior patient because of his matching case history and the minimal distance from the arbitrary age limit. One patient had a recurrence of all his previous complaints after a failed Ravitch correction elsewhere. The Kirschner wires, used for fixation, had to be removed after 3 months and his chest wall collapsed again. Two pairs of patients were brother and sister. Nearly all 42 senior patients (n = 38) were referred to our department after Dutch newspapers drew attention to a case history published by our group. They reported that symptoms such as shortness of breath and tiredness in elderly people could be related to an already long-existing funnel chest. The clinical picture of the 42 senior patients showed complaints of fatigue and low exercise tolerance, shortness of breath, palpitations, inspiratory obstruction and sometimes chest discomfort or pain. Nearly 45% of the 42 patients (n = 19) had an SPES score > 5 and a PEI score > 3 and were diagnosed as suffering from SPES. They had a long history of a slow but steady progression of their complaints and a declining quality of life. The complaints, serious and sometimes invalidating, had only started in their fourth or fifth decade of life and in 63% of the patients (n = 12) were labelled as ‘unexplained cardiovascular complaints’. In 16 of the 19 SPES patients, a postural component was present: complaints increased by bowing to the front and decreased in overstretching the back together with pulling both arms backwards. In 11 patients, the ECG recording showed moderate or serious conduction disturbances or arrhythmia. Abnormalities such as mild mitral valve insufficiency, mitral valve prolapse, or a slightly enlarged right atrium were seen in the transthoracic echogram of nine patients. Specific signs of heart compression, clearly visible on the CT scan (Figs. 1, 2) or MRI of the thorax, were also present in the echogram of two patients (Table 2). The treadmill ECG did not present any new specific information in any of the patients. Normal results were obtained in the pulmonary function tests performed on indication in five patients. The anaesthetist measured the central venous pressure invasively in two patients the day before the operation. Central venous pressure increased by 5 cm when the patients changed from a sitting position to a forward bowing position and normalised again in sitting position. In another patient, right cardiac catheterisation had been performed earlier and the pressure in the right atrium appeared to be substantially raised (13 mmHg).
Fig. 1.
Transversal CT of the chest
Fig. 2.
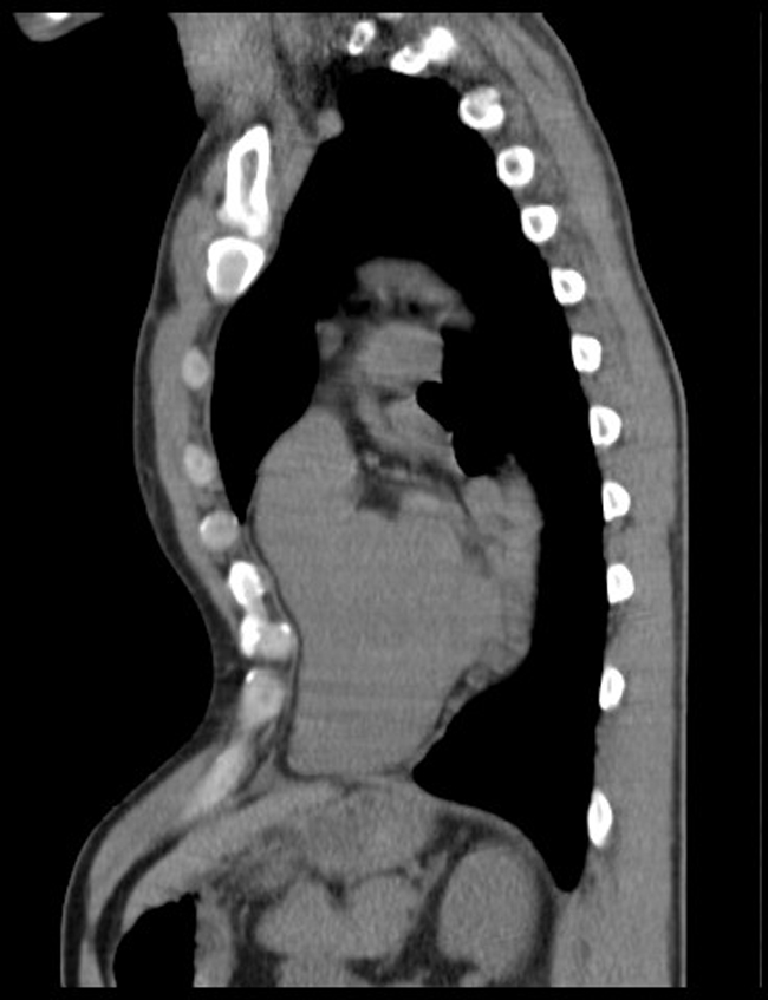
Sagittal CT of the chest
Table 2.
Complaints and symptoms in 19 SPES patients: frequency of distribution
| Complaints/symptoms | Frequency of distribution of SPES scores 0–3 | |||
|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | |
| n= | n= | n= | n= | |
| Dyspnoea | 0 | 2 | 12 | 5 |
| Palpitations | 5 | 7 | 7 | |
| Fatigue | 5 | 10 | 4 | |
| Chest pain | 13 | 6 | ||
| Postural symptoms | 3 | 4 | 12 | |
| Abnormal ECG/Holter | 8 | 4 | 7 | |
| Abnormal echography | 10 | 6 | 3 | |
See Table 1 for explanation of the scores and the empty fields
A therapeutic surgical correction by a modified Ravitch operation was advised to all SPES patients with signs of cardiac compression (68%; n = 13). To date, 11 patients have had surgical procedures. Their clinical data are represented in Table 3.
Table 3.
Preoperative data of 11 operated SPES patients
| Preoperative clinical picture represented by the calculated SPES index | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at operation (years) | PEI score | HALLER index | Dyspnoea | Palpitations/tachycardia | Fatigue; diminished endurance | Pain | Postural component present | ECG abnormal | Echo abnormal | SPES score |
| 69 | 5 | 2.5 | 2 | 2 | 1 | 0 | 2 | 2 | 0 | 9 |
| 55 | 5 | 6.8 | 1 | 2 | 0 | 0 | 1 | 2 | 1 | 7 |
| 57 | 4 | 3.2 | 2 | 1 | 1 | 0 | 2 | 0 | 2 | 8 |
| 55 | 5 | 3.2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 11 |
| 64 | 4 | 2.7 | 2 | 2 | 1 | 0 | 2 | 1 | 0 | 8 |
| 60 | 5 | 2.9 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 6 |
| 64 | 5 | 4.5 | 3 | 1 | 2 | 1 | 2 | 2 | 0 | 11 |
| 58 | 4 | 2.3 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 6 |
| 54 | 5 | 3.9 | 3 | 0 | 2 | 1 | 2 | 0 | 1 | 9 |
| 58 | 5 | 3.7 | 2 | 1 | 0 | 0 | 1 | 0 | 2 | 6 |
| 48 | 5 | 4.9 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 6 |
The clinical and cosmetic result of the surgical correction of the pectus excavatum, as judged by the surgeon or cardiologist at postoperative follow-up, was good to excellent in all cases (Table 4, Figs. 3, 4). Symptoms such as dyspnoea, fatigue and palpitations were substantially reduced or had disappeared completely. Mild chest pain was a complication of the surgical intervention mentioned by most patients. All patients regarded their health-related quality of life as substantially improved, with an average of 2.7 points on a scale of 1–10.
Table 4.
Postoperative data of 11 operated SPES patients
| Result of the operation on a 10-point scale | ||||
|---|---|---|---|---|
| As recorded in the medical file | Health-related quality of life | |||
| Months after surgery | Clinical | Cosmetic | Before operation | At last postoperative follow-up |
| 24 | 8 | 8 | 3 | 8 |
| 9 | 7 | 6 | 5 | 7 |
| 3 | 8 | 8 | 3 | 8 |
| 10 | 7 | 8 | 5 | 6 |
| 5 | 9 | 7 | 5 | 8 |
| 27 | 7 | 8 | 5 | 7 |
| 4 | 8 | 7 | 4 | 7 |
| 10 | 7 | 8 | 4 | 7 |
| 5 | 8 | 9 | 5 | 7 |
| 5 | 7 | 8 | 6 | 8 |
| 11 | 7 | 8 | 6 | 8 |
1 = bad, 10 = excellent
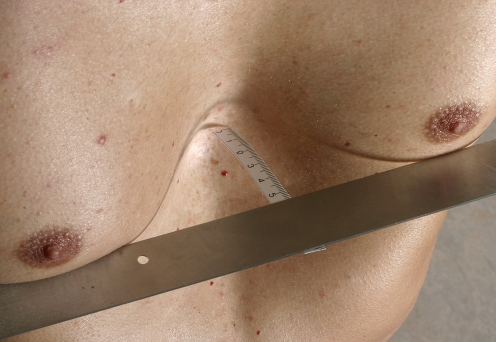
Fig. 3.
A 49-year-old man before surgical correction of pectus excavatum
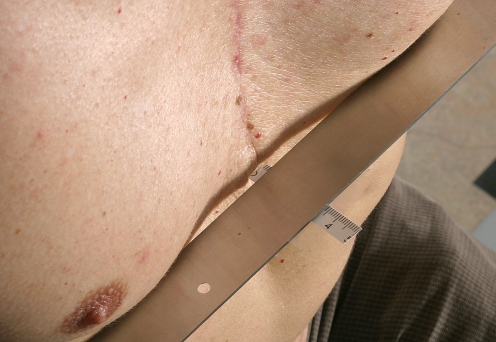
Fig. 4.
A 49-year-old man after surgical correction of pectus excavatum
Discussion and Conclusions
The SPES and PEI scores developed previously by our group were useful tools for selecting patients for operative correction. Only selected patients with signs of cardiac compression were considered eligible for operation. Chest radiography should be used as a primary modality for preoperative imaging of pectus excavatum. However, in most elderly patients, additional information from a CT scan or MRI will be required to measure the PA chest diameter and to discover if cardiac compression is present. In our patients, the calculated Haller index varied too much and manifested itself as an unreliable diagnostic or indicative tool. The treadmill ECG was considered normal in all the postoperative patients and has no place in a future diagnostic protocol. The former reluctance of clinicians to perform a surgical correction of a pectus excavatum can partly be explained by the fact that surgical repair of pectus excavatum does not significantly improve pulmonary function. Malek and colleagues suggest that these findings may be a result of testing pulmonary function under conditions in which pectus excavatum does not manifest itself [8]. In our opinion, pulmonary function testing should only be done on indication, to exclude expiratory pulmonary obstruction. The clinical picture of the patient, as judged by the surgeon or cardiologist in follow-up examinations, and the improvement of the quality of life, as experienced by the patient, were our indicators for the postoperative relief of symptoms.
Due to the abnormal anatomical shape of the chest in pectus excavatum, information obtained through the apical view of a transthoracic echogram is unreliable. In a recently published prospective study of 17 patients, Thorsten Kruger et al. assessed end-diastolic right ventricular (RV) dimensions and left ventricular (LV) ejection fraction by use of intraoperative transoesophageal echocardiography before and after surgical correction of pectus excavatum in adults. Surgical correction of pectus excavatum according to the Ravitch–Shamberger technique resulted in a significant increase in end-diastolic RV dimensions and a significantly increased LV ejection fraction [10].
Our data show a clear relation between a pectus excavatum in senior patients and complaints such as shortness of breath, palpitations, chronic fatigue and symptoms as ventricular ectopic beats. A postural component, aggravating the complaints and symptoms, was often present. ECG recordings showed moderate or serious conduction disturbances and arrhythmia. Heart failure and ischaemic heart disease can present a similar clinical picture and should be excluded. Cardiac compression by the inward deformation of the sternum and the presence of a postural component are indicative for diagnosing SPES.
The clinical picture we encountered in our patients with a pectus excavatum suggests a cardiovascular problem. In fact, 12 of the 19 patients with SPES were diagnosed previously as having unexplained cardiovascular complaints.
Diagnosing SPES is relevant because surgical reconstruction of the chest can provide complete relief of symptoms.
References
- 1.Crump HW. Pectus excavatum—pathophysiology, clinical presentation, surgical repair. Am Fam Physician. 1992;46:173–9. [PubMed] [Google Scholar]
- 2.Koumbourlis AC. Pectus excavatum. Pathophysiology and clinical characteristics. Paediatr Respir Rev. 2009;10:3–6. doi: 10.1016/j.prrv.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Fonkalsrud EW. Open pectus excavatum repairs. Changing trends, lessons learned. One surgeon’s experience. World J Surg. 2009;33:180–90. doi: 10.1007/s00268-008-9793-4. [DOI] [PubMed] [Google Scholar]
- 4.Jaroszewski DE, Fonkalsrud EW. Repair of pectus chest deformities in 320 adult patients: 21 year experience. Ann Thorac Surg. 2007;84:429–33. doi: 10.1016/j.athoracsur.2007.03.077. [DOI] [PubMed] [Google Scholar]
- 5.Goretsky MJ, Kelly RE, Croitoru D, Nuss D. Chest wall anomalies. Pectus excavatum and pectus carinatum. Adolesc Med. 2004;15(3):455–71. doi: 10.1016/j.admecli.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Mansour KA, Thourani VH, Odessey EA, Durham MM, Miller JI. Thirty-year experience with repair of pectus deformities in adults. Ann Thorac Surg. 2003;76:391–5. doi: 10.1016/S0003-4975(03)00441-7. [DOI] [PubMed] [Google Scholar]
- 7.Kelly RE, Shamberger RC, Mellins RB, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function. Facilitated by internet-based data collection. J Am Coll Surg. 2007;205:205–216. doi: 10.1016/j.jamcollsurg.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Malek MH, Berger DE, Marelich WD, Coburn JW, Beck TW, Housh TJ. Pulmonary function following surgical repair of pectus excavatum. A metaanalysis. Eur J Cardiothorac Surg. 2006;30(4):637–643. doi: 10.1016/j.ejcts.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Malek MH, Berger DE, Housh TJ, Marelich WD, Coburn JW, Beck TW. Cardiovascular function following surgical repair of pectus excavatum. A metaanalysis. Chest. 2006;130:506–5. doi: 10.1378/chest.130.2.506. [DOI] [PubMed] [Google Scholar]
- 10.Krueger T, Chassot PG, Christodoulou M, Cheng C, Ris HB, Magnusson L. Cardiac function assessed by transesophageal echocardiography during pectus excavatum repair. Ann Thorac Surg. 2010;89:240–4. doi: 10.1016/j.athoracsur.2009.06.126. [DOI] [PubMed] [Google Scholar]
- 11.Guldemond FI, Höppener PF, Kragten JA, Leeuwen YD, Siebenga J. Cardiale klachten door een pectus excavatum bij een 55-plusser. Ned Tijdschr Geneeskd. 2008;152:337–41. [PubMed] [Google Scholar]
- 12.Ron W, Frank G, Paul H, Hans K, van Leeuwen Y. Pectus excavatum, not always as harmless as it seems. BMJ Case Reports. 2009 doi: 10.1136/bcr.10.2009.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaroszewski D, Steidley E, Galindo A, Francisco Arabia A. Treating heart failure and dyspnoea in a 78-year-old man with surgical correction of pectus excavatum. Ann Thorac Surg. 2009;88:1008–10. doi: 10.1016/j.athoracsur.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 14.Creswick HA, Stacey W, Kelly RE, et al. Family study of the inheritance of pectus excavatum. J Pediatr Surg. 2006;41:1699–03. doi: 10.1016/j.jpedsurg.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 15.Robicsek F, Watts LT, Fokin AA. Surgical repair of pectus excavatum and carinatum. Semin Thorac Cardiovasc Surg. 2009;21:64–75. doi: 10.1053/j.semtcvs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Haller JA, Jr, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg. 1987;22:904–6. doi: 10.1016/S0022-3468(87)80585-7. [DOI] [PubMed] [Google Scholar]
- 17.Daunt SW, Cohen JH, Miller SF. Age related normal ranges for the Haller index in children. Pediatr Radiol. 2004;34:326–30. doi: 10.1007/s00247-003-1116-1. [DOI] [PubMed] [Google Scholar]
- 18.Karagounis VA, Wasnick J, Gold JP. An innovative single-stage repair of severe asymmetric pectus excavatum defects using substernal mesh bands. Ann Thorac Surg. 2004;78:19–21. doi: 10.1016/j.athoracsur.2004.02.044. [DOI] [PubMed] [Google Scholar]