Abstract
We present a case of iatrogenic left main coronary artery dissection, successfully treated by prompt bail-out stenting, and provide a brief discussion on its occurrence and treatment, as well as the immediate and long-term outcome of percutaneous coronary intervention, including our own single-centre experience, for this potentially catastrophic complication.
Keywords: Left main coronary artery, Dissection, Percutaneous intervention
Introduction
Iatrogenic left main coronary artery (LMCA) dissection is one of the complications most dreaded by the interventional cardiologist. Although rare, with a reported incidence of less than 0.1% [1], its occurrence can have devastating consequences if not promptly treated with immediate revascularisation. LMCA dissection often leads to abrupt vessel closure and cessation of blood flow towards a large portion of myocardium resulting in acute pump failure and haemodynamic collapse. Before 1993, when the first successful percutaneous bail-out LMCA stenting was performed, urgent coronary artery bypass surgery (CABG) was the treatment of choice. However, many patients died before even making it to the operation theatre. Hence immediate percutaneous coronary intervention (PCI) seems to be an appropriate and feasible alternative if performed by an experienced interventionalist, as demonstrated by the next case.
Case
A 74-year old woman with evidence of silent anterior myocardial ischaemia on non-invasive stress testing was referred for coronary angiography, which showed single-vessel disease with a significant lesion in the mid-part of the left anterior descending coronary artery (LAD) (Fig. 1, Panels a + b). Because the lesion had a heavily calcified appearance, the patient was scheduled for elective PCI with a plan to perform rotational atherectomy for plaque debulking prior to stent deployment. A right femoral approach was chosen with insertion of a 6 F introducer sheath and an Extra Back-Up 3.75 guiding catheter (Medtronic®) was used. Engagement of the LMCA proved rather difficult due to its superior location but was successfully established after several attempts. The pressure curve remained normal throughout without damping or ventricularisation. However, upon first injection of contrast dye, an antegrade dissection was visualised ‘as it happened’, extending from the proximal to the distal LMCA (Fig. 2, Panels c + d), which abruptly halted coronary flow, almost immediately resulting in cardiogenic shock. Treatment with vasopressors, inotropes and artificial ventilation was promptly initiated and an intra-aortic balloon pump (IABP) was inserted via the contralateral groin. Nevertheless, the haemodynamic condition of the patient remained so precarious that we thought she would not survive any further delay while awaiting emergent surgery. Therefore we opted for percutaneous treatment. We were able to carefully negotiate a wire in the true lumen of the LMCA and a bare metal stent (Vision 3.5 × 28 mm, Abbott Vascular®) was subsequently placed from the ostial LMCA towards the LAD (Fig. 3, Panel e), re-establishing TIMI 3 flow in both the LAD and the circumflex artery (Fig. 3, panel f). The angiographic result was further optimised using non-compliant balloon post-dilatations (Sprinter NC 3.75 × 15 mm, Medtronic®), after which the patient was transferred to the intensive care unit for further monitoring. Vasopressive and inotropic agents could quickly be titrated down and stopped, and the IABP was removed the next day. Extubation had to be delayed due to aspiration pneumonia, but after 10 days the patient was discharged home in a good overall condition. Pre-discharge echocardiography revealed a normal systolic left ventricular function, and maximum troponin-I elevation during the entire hospital course was 0.49 μg/l (upper limit of normal: 0.13 μg/l).
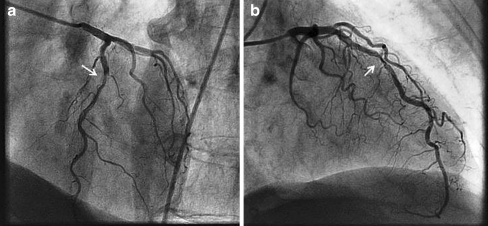
Fig. 1.
Coronary angiogram in left anterior oblique cranial (panel a) and right anterior oblique cranial (panel b) view showing a severe, highly calcified lesion (arrow) in the middle part of the left anterior descending artery
Fig. 2.
Iatrogenic dissection (arrow) upon dye injection extending from the proximal (panel c) to distal (panel d) left main coronary artery
Fig. 3.
Deployment of a 3.5 × 28 mm bare metal stent from the ostial left main coronary artery towards the proximal left anterior descending artery (panel e), sealing the dissection and re-establishing TIMI 3 flow (panel f)
Discussion
Iatrogenic LMCA dissection results from mechanical injury to the arterial wall during catheter manipulation or passage or deployment of an interventional device. Extensive catheter manipulation, catheter type (e.g. Amplatz catheter, small Judkins catheter resulting in deep LMCA intubation), stiffer and less manageable guide wires (e.g. pressure wire), unusual LMCA anatomy or location, operator experience, and presence of LMCA atherosclerosis have all been associated with an increased risk of dissection [2, 3].
In our case, the introduction of the catheter in the LMCA was more difficult than expected due to its high location. Although no damping or ventricularisation of the pressure curve was seen, the tip of the catheter was ‘scraping’ against the wall of the LMCA, thereby probably causing the dissection when injecting dye. Hence, the importance of careful positioning of the catheter in co-axial alignment with the artery before any vigorous contrast injection cannot be stressed enough, especially when confronted with an already calcified LMCA.
Once dissection occurs, the clinical picture differs, depending on the remaining antegrade coronary flow, and can range from an asymptomatic patient with preserved TIMI 3 flow, to a patient in refractory cardiogenic shock whose LMCA is ‘amputated’. Nevertheless, even in case of initial TIMI 3 flow and haemodynamic stability, rapid deterioration may occur shortly after because of abrupt flow compromise due to progressive dissection or superimposed thrombus formation, which is why it seems imperative to always envisage a ‘revascularisation plan’ by PCI or CABG.
A literature review by Cheng et al. [4] (Table 1) of 36 patients who underwent stenting of an iatrogenic LMCA dissection showed a favourable immediate outcome with achievement of procedural, angiographic success in 32 patients (88.9%). Four patients needed emergent CABG of whom 2 eventually died. This strategy, consisting of a primary attempt to stent with back-up CABG when needed, thus resulted in an overall survival rate of 94.4%.
Table 1.
Literature review of iatrogenic LMCA dissection: review by Cheng [4], completed by cases from our own experience (n = 18) since 1998
| 1st author | Case number | Successful bail-out stenting | Emergent CABG | Survival |
|---|---|---|---|---|
| Garcia-Robles JA [6] | 1 | 0 | 1 | 1 |
| Hennessy TG [7] | 1 | 1 | 0 | 1 |
| Cameron J [8] | 6 | 6 | 0 | 6 |
| Al-Saif SM [9] | 1 | 1 | 0 | 1 |
| Nageh T [10] | 1 | 1 | 0 | 1 |
| Jain D [11] | 1 | 1 | 0 | 1 |
| Mulvihill NT [12] | 1 | 1 | 0 | 1 |
| Awadalla H [13] | 1 | 0 | 1 | 1 |
| Lee SW [5] | 10 | 10 | 0 | 10 |
| Cheng Cl [4] | 13 | 11 | 2 | 11 |
| Onsea K | 18 | 18 | 0 | 16a |
| Total | 54 | 50 | 4 | 51 |
aOne patient died of pulmonary haemorrhage, the other of lung cancer
Long-term outcome of emergent LMCA bail-out stenting in a group of 13 patients [4] was similar to that of elective percutaneous treatment of an unprotected LMCA, with a binary restenosis rate of 30% of the LMCA segment (3 patients) at 5 ± 2 months follow-up. In another report, Lee and colleagues [5] reported no significant LMCA restenosis on 6-month follow-up angiogram of ten urgently treated patients, which was attributed to the minimal plaque burden in the iatrogenically dissected LMCA, leading to less in-stent neo-intimal proliferation.
In our own hospital—a tertiary reference centre—we have treated 18 patients for iatrogenic LMCA dissection since 1998. Immediate procedural success was 100% with a 1-year survival rate of 89% (16/18 patients). Of the two patients who died, one succumbed shortly after successful PCI due to uncontrollable lung bleeding, while the other patient died from lung cancer.
Follow-up angiogram at 6 months was performed in eight patients, one of whom showed significant restenosis at the LMCA stent necessitating subsequent CABG. Early stent thrombosis occurred in one patient 10 days after LMCA stenting, while being hospitalised for upper gastrointestinal bleeding interfering with normal dual antiplatelet therapy. Unfortunately emergent percutaneous treatment was unsuccessful in this patient, who subsequently underwent CABG, but eventually required a heart transplant because of persistent severe pump failure.
Conclusion
When confronted with an iatrogenic LMCA dissection, prompt bail-out stenting seems a reasonable and feasible first-choice option, with high immediate procedural success and acceptable long-term results. As always, prevention is better than cure and meticulous attention should be paid to catheter and device manipulation during each diagnostic or interventional procedure.
References
- 1.Awadalla H, Sabet S, Sebaie A, et al. Catheter-induced left main dissection incidence, predisposition and therapeutic strategies experience from two sides of the hemisphere. J Invasive Cardiol. 2005;17(4):233–6. [PubMed] [Google Scholar]
- 2.Kovac JD, Bono DP. Cardiac catheter complications related to left main stem disease. Int J Cardiol. 1999;69(3):299–303. doi: 10.1016/S0167-5273(98)00360-X. [DOI] [PubMed] [Google Scholar]
- 3.Slack JD, Pinkerton CA, VanTassel JW, et al. Left main coronary artery dissection during percutaneous transluminal coronary angioplasty. Cathet Cardiovasc Diagn. 1986;12(4):255–60. doi: 10.1002/ccd.1810120410. [DOI] [PubMed] [Google Scholar]
- 4.Cheng CI, Wu CJ, Hsieh YK, et al. Percutaneous coronary intervention for iatrogenic left main coronary artery dissection. Int J Cardiol. 2008;126(2):177–82. doi: 10.1016/j.ijcard.2007.03.125. [DOI] [PubMed] [Google Scholar]
- 5.Lee SW, Hong MK, Kim YH, et al. Bail-out stenting for left main coronary artery dissection during catheter-based procedure: acute and long-term results. Clin Cardiol. 2004;27(7):393–5. doi: 10.1002/clc.4960270705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Robles JA, Garcia E, Rico M, et al. Emergency coronary stenting for acute occlusive dissection of the left main coronary artery. Cathet Cardiovasc Diagn. 1993;30(3):227–9. doi: 10.1002/ccd.1810300310. [DOI] [PubMed] [Google Scholar]
- 7.Hennessy TG, McCann HA, Sugrue DD. Bailout Palmaz-Schatz stenting for iatrogenic dissection of the left main coronary artery. J Invasive Cardiol. 1996;8:450–2. [PubMed] [Google Scholar]
- 8.Cameron J, Aroney C, Bett J. Left main coronary artery dissection during coronary angioplasty or angiography treated by stent insertion without requirement for emergency bypass graft surgery. Aust N Z J Med. 2000;30(6):726–8. doi: 10.1111/j.1445-5994.2000.tb04370.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Saif SM, Liu MW, Al-Mubarak N, et al. Percutaneous treatment of catheter-induced dissection of the left main coronary artery and adjacent aortic wall: a case report. Catheter Cardiovasc Interv. 2000;49(1):86–9. doi: 10.1002/(SICI)1522-726X(200001)49:1<86::AID-CCD20>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Nageh T, Badawi R, Thomas MR. Left main coronary artery dissection detected by intravascular ultrasound following angiographically successful percutaneous coronary intervention. J Invasive Cardiol. 2004;16(5):279–80. [PubMed] [Google Scholar]
- 11.Jain D, Kurowski V, Katus HA, et al. Catheter-induced dissection of the left main coronary artery, the nemesis of an invasive cardiologist a case report and review of the literature. Z Kardiol. 2002;91(10):840–5. doi: 10.1007/s00392-002-0823-1. [DOI] [PubMed] [Google Scholar]
- 12.Mulvihill NT, Boccalatte M, Fajadet J, et al. Catheter-induced left main dissection: a treatment dilemma. Catheter Cardiovasc Interv. 2003;59(2):214–6. doi: 10.1002/ccd.10481. [DOI] [PubMed] [Google Scholar]
- 13.Awadalla H, Salloum JG, Smalling RW, et al. Catheter-induced dissection of the left main coronary artery with and without extension to the aortic root: a report of two cases and a review of the literature. J Interv Cardiol. 2004;17(4):253–7. doi: 10.1111/j.1540-8183.2004.00381.x. [DOI] [PubMed] [Google Scholar]