Abstract
Background
Coronary artery fistulas (CAFs) are infrequent anomalies, coincidentally detected during coronary angiography (CAG).
Aim
To elucidate the currently used diagnostic imaging modalities and applied therapeutic approaches.
Materials and Methods
Five Dutch patients were found to have CAFs. A total of 170 reviewed subjects were subdivided into two comparable groups of 85 each, treated with either percutaneous ‘therapeutic’ embolisation (PTE group) or surgical ligation (SL group).
Results
In our series, the fistulas were visualised with several diagnostic imaging tests using echocardiography, multidetector computed tomography, and CAG. Four fistulas were unilateral and one was bilateral; five originated from the left and one originated from the right coronary artery. Among the reviewed subjects, high success rates were found in both treatment groups (SL: 97% and PTE: 93%). Associated congenital or acquired cardiovascular disorders were frequently present in the SL group (23%). Bilateral fistulas were present in 11% of the SL group versus 1% of the PTE group. The fistula was ligated surgically in one and abolished percutaneously in another. Medical treatment including metoprolol was conducted in two, and watchful waiting follow-up was performed in one.
Conclusions
Several diagnostic imaging techniques are available for assessment of the anatomical and functional characteristics of CAFs.
Keywords: Coronary artery fistula, Positron emission tomography, Multidetector computed tomography, Coronary angiography, Surgical ligation, Percutaneous occlusion
Introduction
Treatment of congenital coronary artery fistulas (CAFs) is dependent on the presence of symptoms, the clinical significance of the fistula, the haemodynamic shunt magnitude and the morphological appearance and characteristics of the fistula visualised with different imaging techniques. In symptomatic adult patients with a large left-to-right shunt, a percutaneous approach for occlusion of the fistula can be accomplished by using detachable balloons, coils, microcoils, stent grafts and Amplatzer occluder devices [1–7].
Surgical ligation techniques include intracardiac and extracardiac closure combined with proximal and distal ligation of the fistula [8–12].
Diagnosis of the entire fistula (origin, pathway, and termination) is accomplished with multimodality invasive and non-invasive imaging techniques. A multidisciplinary therapeutic team (interventional radiologist, interventional cardiologist, and standby cardiac surgeon) might be needed for these patients [3].
The treatment strategies in the currently presented five patients were surgical ligation in one patient, percutaneous occlusion of the fistula using microcoils in one patient, and medical management and watchful waiting policy in three patients.
Comparison of percutaneous approaches and surgical ligation is outlined with a review of the literature.
Materials and Methods
From June 2007 to November 2009, five adult patients were diagnosed with congenital CAFs coincidentally detected during conventional coronary angiography (CAG). The patient data are summarised in Table 1. Patients were evaluated for palpitations and oppressive feeling in the chest (patient 1), progressive exertional dyspnoea (patient 2), episodes of oppressive chest pain radiating to the left shoulder that were nitroglycerin sensitive (patient 3), ST-elevation myocardial infarction with inferior localisation (patient 4) and acute coronary syndrome (patient 5). There were two males and three females with a mean age of 59.4 years (range 40–78). There was no evidence of associated congenital cardiovascular disorders. All patients underwent conventional CAG where the CAFs were detected.
Table 1.
Data of five patients with congenital CAFs
| Case/age/gender | Clinical presentation | Diagnostic workup | Fistula morphology | Management | Comments |
|---|---|---|---|---|---|
| 1–67/F | Chest pain | Echocardiography | Unilateral: LCx➔CS | PTE | Previous history: car accident as a teenager |
| Myocardial perfusion test | Origin: Multiple | Risk factors: positive family history and hyperlipidaemia | |||
| CMR | Pathway: Tortuous, | ||||
| MDCT | dilated | ECG: SR with complete LBBB | |||
| PET scan | Termination: Single | CC: Normal values with insignificant shunt | |||
| CC + CAG | Maintenance therapy: metoprolol and simvastatin | ||||
| 2–67/F | Dyspnoea on exertion | Echocardiography | Unilateral: LCx➔CS | SL | Previous history: Hypothyroidism |
| Myocardial perfusion test | Origin: Single | Risk factors: Hypertension, hyperlipidaemia and smoking | |||
| MDCT | Pathway: Tortuous, | ECG: SR with incomplete RBBB | |||
| CC + CAG | dilated | CC: Mild pulmonary hypertension with insignificant shunt | |||
| Termination: Single | Maintenance therapy: thyrax, emcor and pravastatin | ||||
| 3–40/M | Chest pain | Echocardiography | Unilateral: LCx➔CS | Refused intervention | Previous history: traumatic sternal and rib fracture |
| MDCT | Origin: Single | Watchful waiting | Risk factors: smoking | ||
| CAG | Pathway: Tortuous | ECG: SR with concave ST-segment up sloping | |||
| Termination: Single | CC: NA | ||||
| Maintenance therapy: None | |||||
| 4–78/F | Myocardial infarction | Echocardiography | Bilateral: | Medical | Previous history: COPD |
| MDCT | LAD + RCA➔PA | Risk factors: Hyperlipidaemia | |||
| CC + CAG | LAD: | ECG: SR with acute IMI | |||
| Origin: Multiple | CC: Normal values with insignificant shunt | ||||
| Pathway: Tortuous, dilated | Maintenance therapy: metoprolol, aspirin, clopidogrel and atorvastatin | ||||
| Termination: Single | |||||
| RCA: | |||||
| Origin: Multiple | |||||
| Pathway: Tortuous, dilated | |||||
| Termination: Single | |||||
| 5–45/M | Acute coronary syndrome | CAG | Unilateral: LAD➔PA | Medical | Previous history: none |
| Origin: Multiple | PCI of RCA | Risk factors: positive family history, DM, hypertension and smoking | |||
| Pathway: Tortuous, multiple | ECG: SR without shifting of the ST-segments | ||||
| Termination: Single | CC: NA | ||||
| Maintenance therapy: metoprolol, aspirin, clopidogrel and atorvastatin |
F female, M male, CMR cardiovascular magnetic resonance, MDCT multidetector computed tomography, PET positron emission tomography, CC cardiac catheterisation, CAG coronary angiography, RCA right coronary artery, Cx circumflex coronary artery, LAD left anterior descending coronary artery, CS coronary sinus, PA pulmonary artery, PCI percutaneous coronary intervention, PTE percutaneous transluminal ‘therapeutic’ embolisation, SL surgical ligation, SR sinus rhythm, LBBB left bundle branch block, RBBB right bundle branch block, IMI inferior myocardial infarction, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, NA not applicable
Significant atherosclerotic coronary artery disease (CAD) was defined as luminal stenosis of ≥ 75% in the epicardial coronary arteries or their main branches. Rest 12-lead ECG was performed in all patients. Transthoracic echocardiography (TTE) was performed in four patients. Four patients underwent multidetector computed tomography (MDCT). Myocardial perfusion tests were performed in two patients (patients 1 and 2). Cardiovascular magnetic resonance (CMR) and positron emission tomography (PET) were performed in one patient (patient 1).
Management
Three patients received conservative medical therapy for fistula management (patients 3, 4, and 5). Percutaneous therapeutic embolisation (PTE) was performed in one (patient 1), and surgical ligation (SL) was performed in another (patient 2).
Results
Selective CAG demonstrated a dilated and serpiginous circumflex coronary artery (Cx) terminating into the coronary sinus (CS) (patients 1, 2, and 3) (Fig. 1a, b and c), and the left anterior descending coronary artery (LAD) and the right coronary artery (RCA) were dilated and tortuous. From both arteries, multiple small and large fistulous vessels as well as vascular structures originated and terminated in the pulmonary artery (PA) (Fig. 2a, b). The morphological anatomy of the fistulous vessels from the LAD to the PA had the appearance of an ‘Asian dragon’ (Fig. 2c, d) (patient 4), and from the proximal segment of the LAD, a solitary fistula originated and connected to the PA (Fig. 3) (patient 5).
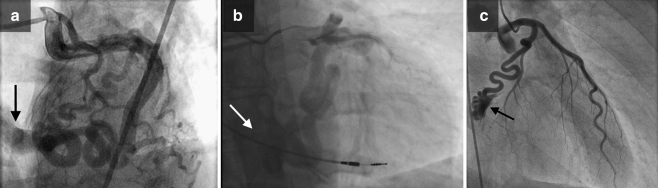
Fig. 1.
a Left anterior oblique (LAO) frame with cranial angulations shows CAFs from the Cx to the CS (arrow). b Right anterior oblique (RAO) frame with cranial angulations shows CAFs from Cx to CS (arrow); ventricular lead of the pacemaker is well appreciated. c RAO caudal angiographic view illustrates a fistula from the distal segment of the Cx terminating into the CS (arrow)
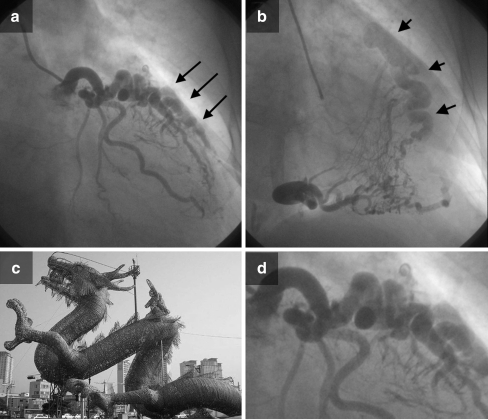
Fig. 2.
a RAO angiographic view shows an LAD-PA fistula (arrows). b RCA-PA fistula. Filling of the pulmonary artery occurs via a common channel (arrowheads). c and d ‘Asian dragon’: The fistula’s resemblance to an Asian dragon shape can be appreciated. The morphological anatomy of the fistulous vessels from the LAD to the PA shows the appearance of an ‘Asian dragon’ with a serpiginous vessel with multiple twists and turns terminating in the pulmonary artery via a dilated common channel
Fig. 3.
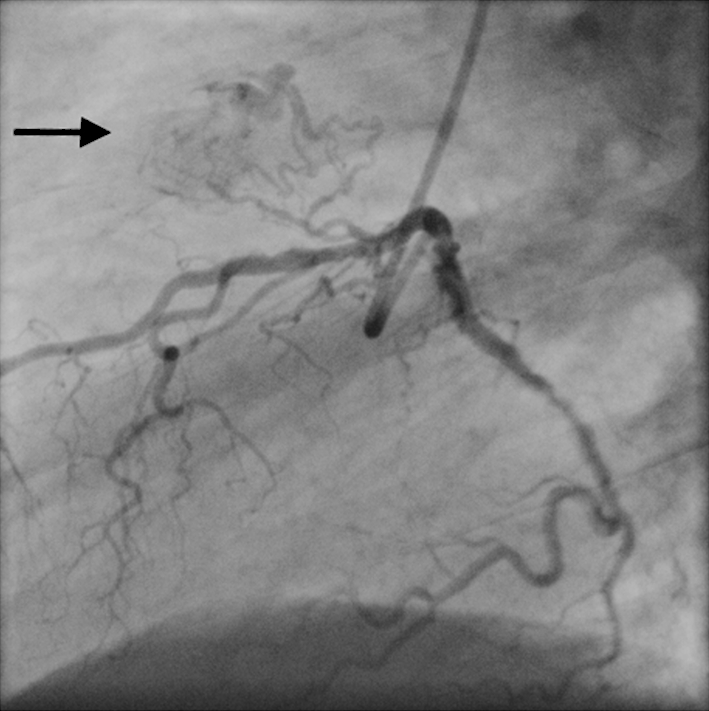
Left lateral angiographic view shows an LAD-PA fistula (arrow)
Three patients were free of atherosclerotic CAD (patients 1, 3, and 4). One patient had minimal luminal irregularities of the coronary arteries (patient 2), whereas another patient with an LCA-PA fistula had significant stenosis of the RCA.
All patients remained in sinus rhythm, and none of the patients developed atrial fibrillation. A complete left bundle branch block (patient 1) and an incomplete right bundle branch block (patient 2) were observed. The ECG was compatible with acute inferior wall myocardial infarction in one patient (patient 4). TTE was normal in patients 1 and 3, and mild left ventricular hypertrophy was detected in patients 2 and 4. The presence of a fistula was echocardiographically suspected and mild pulmonary hypertension was estimated in patient 2. Mild atrioventricular and semilunar valvular incompetence were found in patients 2 and 4.
Myocardial perfusion revealed an apico-anteroseptal defect without reversible ischaemia in one (patient 1) and dubious anteroseptal ischaemia in another (patient 2). Both had a left ventricular ejection fraction above 0.60.
CMR imaging failed to visualise the distal segment of the fistula and its termination into the CS (patient 1), but it showed a dilated proximal segment.
PET scanning revealed reversible myocardial ischaemia in the distal inferior segment of the left ventricle using adenosine stress and rest 13N-ammonia (Fig. 4a). The stress/rest ratio was 3.12 (LAD 2.89 = 33%, RCA 1.18 = 13.5%, and Cx 4.68 = 53.5%). Blood flow across the Cx (fistula-bearing vessel) (53.5%) was much (approximately fourfold) higher than the RCA (13.5%) and caused relative ischaemia of the inferior myocardial segment, which is supplied by the RCA.
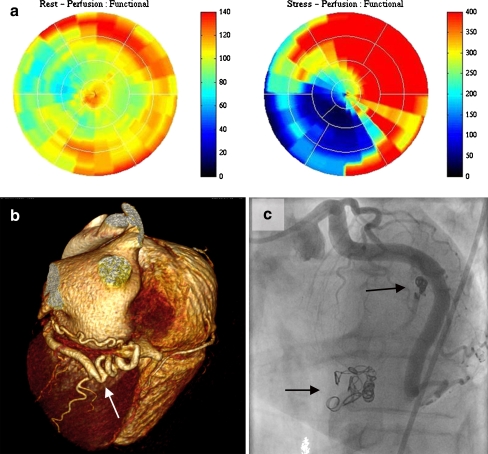
Fig. 4.
a Normal findings on the rest 13N-ammonia polar map (left panel) and a large absolute perfusion defect (dark blue) in the inferior wall on adenosine 13N-ammonia polar map (right panel). b Three-dimensional volume-rendered computed tomography coronary angiography demonstrates CAFs from Cx to CS (arrow). c After coiling of the fistula, abnormal flow was abolished (arrow)
On MDCT, the fistula was confirmed in four patients (patients 1, 2, 3, and 4). The Cx was dilated, tortuous and ended in the CS (patients 1, 2, and 3). Tortuous, serpiginous and dilated vascular structures were observed arising from both the RCA and LAD and terminating in the PA (patient 4). Dilatation of the coronary venous system was present in two patients (patients 2 and 4). The Cx was dilated (8 mm in diameter) with a very tortuous course (Fig. 4b).
Management
Three patients (patients 3, 4, and 5) received conservative medical management (CMM). Two were treated medically with a β-blocker (patients 3 and 5) and continued on watchful waiting (patient 4). PTE was performed in one (patient 1), and SL was performed in another (patient 2).
A PTE procedure was performed (patient 1) by an interventional cardiologist and an interventional radiologist for transcutaneous occlusion of the fistulas utilising multiple microcoils. The largest fistula was occluded using a thread coil of 12 mm × 20 cm, two of 5 mm × 8 cm and a VortX coil of 4 × 4 mm. The small fistula was obliterated by three VortX 4 × 4 mm coils. Immediately at the end of the procedure, CAG demonstrated complete occlusion of the large fistula and significant reduction of flow in the small fistulous vessel (Fig. 4c).
In patient 2, surgical ligation was performed based on the following findings: clinical data, size of the fistula, mild pulmonary hypertension and angiographic morphological aspects of the fistula (Fig. 1b). Postoperative recovery was uncomplicated.
One patient (patient 5) underwent a successful PCI procedure with the deployment of two bare metal stents (Prokinetic) across the stenotic segment of the RCA.
Review Subjects
A total of 170 subjects with congenital CAFs treated either percutaneously or surgically were collected from the literature. The cohort was subdivided into two comparable groups (Table 2). The first group included subjects treated with a percutaneous transluminal ‘therapeutic’ approach (PTE group, n = 85 patients) [1–7, 13–22], and the second group had subjects treated with surgical ligation techniques (SL group, n = 85 patients) [8–12,23–42].
Table 2.
Comparison between the percutaneous embolisation [1–7, 13–22] approach versus surgical ligation [8–12, 23–42]
| PTE group | SL group | |
|---|---|---|
| Number of patients | 85 | 85 |
| Origin of CAFs | ||
| LCA | 53 (62%) | 68 (80%) |
| RCA | 33 (38%) | 18 (20%) |
| Qp:Qs | 2.06:1.0 | 2.04:1.0 |
| Tortuousity | Frequently present | Prevalent |
| Aneurysm | Rare | Common |
| Unilateral fistula | (84/85) 99% | (76/85) 89% |
| Bilateral fistula | (1/85) 1% | (9/85) 11% |
| Diagnostic workup | Multimodality | Multimodality |
| Confirmation of complete occlusion | Multimodality | Multimodality |
| Associated congenital/acquired lesions | Infrequent | Frequent (23%) |
| Follow-up period | Mean 2.4 years (range 1 month–13 years) | Mean 2.2 years(range 3 months–9.6 years) |
CAFs coronary artery fistulas, LCA left coronary artery, PTE percutaneous transluminal ‘therapeutic’ embolisation, RCA right coronary artery, SL surgical ligation
In the PTE group, the mean Qp:Qs was 2.06:1.0. Confirmation of complete occlusion of the fistula was assessed post-procedurally by clinical examination and one or a combination of the following imaging techniques: phonocardiography, echocardiography, myocardial perfusion test, and CAG [16, 18–20, 22].
Complete closure was reached in 93% and near-complete closure in 4%. Urgent CABG was needed in 3% of the subjects (2 patients) due to myocardial infarction secondary to thrombotic occlusion of the fistula-related coronary artery in one patient and severe stent obstruction of the left main stem in the other patient [7, 14]. The materials used for closure of the fistula were as follows: detachable balloons (latex and silicone), stainless steel coils (Gianturco coils), platinum microcoils, polytetrafluoroethylene (PTEF)-covered graft stents, Amplatzer duct occluders, Amplatzer septal occluders, and floppy tips of guidewires [2–7, 14–21].
In the second group (SL group), the mean Qp:Qs was 2.04:1.0. Postoperative confirmation of complete occlusion of the fistula was achieved by clinical examination and one or more of the following imaging studies: echocardiography, MDCT, myocardial perfusion test, cardiac catheterisation, and CAG. Complete closure was reached in 97%, and near-complete closure was reached in 3% of the patients. Intracardiac and extracardiac closures were combined with proximal and distal ligation of the fistula [8–12]. Follow-up periods in both groups were 2.4 years (range 1 month–13 years) for the PTE group and 2.2 years (range 3 months–9.6 years) for the SL group.
Statistical Analysis
Values were expressed as means, averages, and percentages.
Discussion
CAFs are coincidentally detected, as demonstrated in all of the patients in the current series, during a conventional CAG performed for assessment of suspected coronary heart disease. However, conventional CAG has a two-dimensional imaging pattern that limits its ability to visualise the entire fistula. In the angiographic series by Vitarelli et al., termination of fistulas into the coronary sinus or right ventricle could not be delineated by conventional CAG in 19% of patients [43].
Although conventional CAG remains the gold standard for detection of congenital or acquired CAFs, Kacmaz et al. has suggested that 16-slice MDCT may serve as an alternative non-invasive diagnostic test to visualise coronary artery anomalies, especially CAFs coursing between two vascular territories (sensitivity 87%) [13].
Non-invasive coronary angiography using MDCT has recently been reported by Achenbach et al. [44]. and by Datta et al. [45]. MDCT has excellent spatial resolution [46, 47], which has improved tremendously due to the increase in the number of detector rows to 320 slices [48]. The diagnostic accuracy and sensitivity of MDCT in CAFs was determined by Kacmaz et al. in 2008 to be 58% for fistulas coursing between coronary arteries and cardiac chambers and 87% for fistulas coursing between two vascular structures with an overall sensitivity of 73% [13]. In this report, MDCT could not detect 27% of CAFs previously diagnosed with CAG, particularly CAFs originating from the RCA or LAD with outflow into the right atrium (RA), left ventricle (LV) or pulmonary artery (PA). In our patients, MDCT confirmed the diagnosis, and the origin; course and termination were clearly delineated.
Reports have been published regarding multimodality non-invasive and invasive diagnostic imaging of congenital [49] and acquired [50] CAFs in adults as well as exclusively non-invasive diagnostic tests [51].
With MDCT, the anatomy of the fistula and the coronary arteries and their relationship to other adjacent structures can be delineated. An additional advantage of MDCT is its ability to assess the venous system of the heart. Dilatation of the coronary venous system was clearly recognised in two patients in our current series.
In the presence of a large aneurysm, MDCT illustrated the circumflex-coronary sinus fistula but failed to delineate the RCA-RV fistula [52].
As was the case in one of our patients, CAFs could be suspected by TTE [43, 53]. However, TTE is inadequate to delineate the entire fistula (origin, pathway and drainage sites). In the series of Vitarelli et al., TTE was suggestive of CAFs in 27% of the patients, but transoesophageal echocardiography (TEE) adequately diagnosed the origin and termination of the fistulas in 100% of the patients. In 19% of these patients, conventional CAG was unable to identify the precise site of drainage [43]. With the application of three-dimensional transoesophageal [25] or contrast echocardiography [49, 54] visualisation of the drainage sites may be increased. TEE may act as a complementary non-invasive diagnostic imaging test to determine termination sites [43]. Two-dimensional echocardiography failed to define the anatomical and functional features of a fistula in a patient with a large aneurysm due to deformity of the surrounding structures [52].
Lehmkuhl et al. reported the use of fully non-invasive diagnostic methods (64-slice MDCT and CMR) to assess functional characteristics and to visualise the anatomic features of the fistula without a need for CAG [51]. The application of the 64-slice MDCT and the 16-slice MDCT have also primarily been used by Oncel and Kacmaz, respectively, to visualise CAFs [13, 55].
CMR has been applied to the identification congenital CAFs [56, 57]. CMR was able to detect CAFs correctly in 85% of patients with angiographically diagnosed fistulas, and full determination of origin and drainage site was accurately achieved in 93% [58]. As in our first patient, failure of CMR to detect the fistula has been described previously [27, 52].
Positron emission tomography scanning (13Nammonia-adenosine) in congenital CAFs (Cx-CS) can be of great value as a non-invasive diagnostic technique to assess the flow ratio of the different coronary arteries. As in the first patient, the amount of flow crossing the left Cx was increased up to fourfold that of the RCA and 1.6-fold of that of the LAD. ‘Steal phenomenon’ was observed as the ratio of regional distribution disturbance between both territories with increase in Cx flow by 20% (the difference between Cx 53.5% and RCA 13.5%). Based on these findings, angiographic characteristics and MDCT features, percutaneous transluminal ‘therapeutic’ embolisation was indicated.
To the best of our knowledge, this report is the first documented application of positron emission tomography (PET) 13N-adenosine scanning in congenital Cx-CS fistula. PET imaging offers the unique opportunity for absolute quantification of myocardial perfusion reserve. The advantages of PET techniques in various cardiovascular disorders have been described in previous studies. [59, 60]
Two of our patients presented with acute myocardial infarction (MI) and acute coronary syndrome; in one patient, MI was present ipsilateral to the shunt (patient 4), and in the other patient, it was located contralateral to the fistula (patient 5). In both, the presence of CAFs may have facilitated the acute coronary event. Responsible mechanisms may be either thromboembolic complications (in patient 4) due to turbulence of flow in the fistulous vessel and/or hypoperfusion of the stenotic coronary artery. This exaggerated ischaemic changes due to deviation of coronary blood flow into the fistulous vessel in the second patient (patient 5).
Percutaneous transluminal ‘therapeutic’ embolisation (PTE) has been advocated by many investigators as the current therapeutic strategy of choice for congenital CAFs in amenable morphological fistula anatomy [1, 20–22, 61]. A multidisciplinary approach was performed by Syed et al. [3]. The reported success rate was 87% in India [21], 93% in Russia, [20] 82% in the United States [19] and 97% in the United Kingdom [1]. In surgically treated patients in the series of Shuiyun et al., no residual shunt was found before hospital discharge [9]. The reported recurrence rate in the literature for SL is 25% [19] and 9–19% for PTE [19, 62].
In the SL group the origin from the LCA was more frequent (80% versus 62%), the tortuosity of the fistulous vessels was more prevalent, and aneurysmal formation was more common than in the PTE group. Furthermore, bilateral fistulas composed 11% of the SL group and 1% of the PTE group. Unilateral fistulas were comparable in the SL group and PTE groups, 89%, and 99%, respectively. Associated congenital or acquired cardiovascular disorders were frequently present in the SL group (23%).
Three patients of the current series were treated conservatively. Many reports have been published describing conservative medical management (medical and watchful waiting) in patients with congenital CAFs [63–66].
Therapeutic strategies in the recently reported series of Abdelmoneim et al. were CMM in 56.7% of patients, SL in 23.3% and PTE in 20% [67].
Conclusion
CAFs may have a complex anatomy; therefore, performing several diagnostic imaging techniques during pre-treatment workup is inevitable. A complementary multimodality approach to imaging can be used to reach an optimal informed management decision.
Determination of the optimal non-invasive test during follow-up and confirmation of complete closure of the fistula after surgical or non-surgical interventions remain to be solved, but both should be tailored to the patient’s needs and the available institution’s or country’s facilities.
Acknowledgement
The assistance of the following catheterisation laboratory technicians in alphabetical order is highly appreciated: Alkmaar, Amsterdam, Apeldoorn, Enschede, Hengelo and Zutphen. Special thanks goes to the staff of the Department of Radiodiagnostics, LUMC; the staff of the laboratory of Nuclear Medicine and Molecular Imaging, UMCG; and the staff of the Department of Cardiac Surgery of St. Antonius Hospital, Nieuwegein. The assistance of librarians Mrs A. Geerdink and Mr D. Maas of hospital ZGT is greatly acknowledged.
References
- 1.Qureshi SA, Tynan M. Catheter closure of coronary artery fistulas. J Interv Cardiol. 2001;14(3):299–307. doi: 10.1111/j.1540-8183.2001.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 2.Boccalandro F, Awadalla H, Smalling RW. Percutaneous transcatheter coil embolization of two coronary fistulas originating from the left main ostium and left anterior descending artery. Catheter Cardiovasc Interv. 2002;57(2):221–223. doi: 10.1002/ccd.10280. [DOI] [PubMed] [Google Scholar]
- 3.Syed MI, Kalweit WH, Shaikh A. Microcoil embolization for treatment of a right coronary arteriovenous fistula. J Interv Cardiol. 2003;16(4):347–350. doi: 10.1034/j.1600-6143.2003.08054.x. [DOI] [PubMed] [Google Scholar]
- 4.Atmaca Y, Altin T, Ozdol C, Pamir G, Caglar N, Oral D. Coronary-pulmonary artery fistula associated with right heart failure: successful closure of fistula with a graft stent. Angiology. 2002;53(5):613–616. doi: 10.1177/000331970205300519. [DOI] [PubMed] [Google Scholar]
- 5.Kassaian SE, Mahmoodian M, Salarifar M, Alidoosti M, Abbasi SH, Rasekh A. Stent-graft exclusion of multiple symptomatic coronary artery fistulae. Tex Heart Inst J. 2007;34(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- 6.Bonello L, Com O, Gaubert JY, Sbraggia P, Paganelli F. Covered stent for closure of symptomatic plexus-like coronary fistula. Int J Cardiol. 2006;109(3):408–410. doi: 10.1016/j.ijcard.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 7.Ascoop AK, Budts W. Percutaneous closure of a congenital coronary artery fistula complicated by an acute myocardial infarction. Acta Cardiol. 2004;59(1):67–69. doi: 10.2143/AC.59.1.2005161. [DOI] [PubMed] [Google Scholar]
- 8.Tirilomis T, Aleksic I, Busch T, Zenker D, Ruschewski W, Dalichau H. Congenital coronary artery fistulas in adults: surgical treatment and outcome. Int J Cardiol. 2005;98(1):57–59. doi: 10.1016/j.ijcard.2002.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Shuiyun W, Qingyu W, Shengshou H, et al. Surgical treatment of 52 patients with congeni coronary artery fistulas. Chin Med J. 2001;114:752–755. [PubMed] [Google Scholar]
- 10.Cherif A, Farhati A, Fajraoui M, et al. Coronary-pulmonary arterial fistula in the adult: report of 6 cases and review of the literature. Tunis Méd. 2003;81(8):595–599. [PubMed] [Google Scholar]
- 11.Katoh T, Zempo N, Minami Y, et al. Coronary arteriovenous fistulas with giant aneurysm: two case reports. Cardiovasc Surg. 1999;7(4):470–472. doi: 10.1016/S0967-2109(98)00102-1. [DOI] [PubMed] [Google Scholar]
- 12.Versaci F, Giudice C, Sperandio M, Simonetti G, Chiariello L. A case of coronary artery fistula visualized by 64-slice multidetector CT. Nat Clin Pract Cardiovasc Med. 2009;6(1):57–60. doi: 10.1038/ncpcardio1413. [DOI] [PubMed] [Google Scholar]
- 13.Kacmaz F, Ozbulbul NI, Alyan O, et al. Imaging of coronary artery anomalies: the role of multidetector computed tomography. Coron Artery Dis. 2008;19(3):203–209. doi: 10.1097/MCA.0b013e3282f528f1. [DOI] [PubMed] [Google Scholar]
- 14.Müller D, Wimmer-Greinecker G, Fichtelscherer S, Moritz A. Symptomatic coronary artery-pulmonary artery fistulae. Interact Cardiovasc Thorac Surg. 2004;20:192–193. doi: 10.1007/s12055-004-0085-9. [DOI] [Google Scholar]
- 15.Llera LS Diaz, Fournier Andray JA, Gomez MS, Mayol DA, Gonzalez GA, Perez Fernandez-Cortacero JA. Percutaneous occlusion with coils of coronary artery fistulas in adults. Rev Esp Cardiol. 2005;58(1):93–96. doi: 10.1157/13070512. [DOI] [PubMed] [Google Scholar]
- 16.Francoual M, Diebler C, Bouchet PF, Landau JF, Delamare J. Percutaneous occlusion by a detachable balloon of a fistula between the left coronary and the pulmonary artery. Arch Mal Coeur Vaiss. 1990;83(7):1003–1005. [PubMed] [Google Scholar]
- 17.Nguyen K, Myler RK, Hieshima G, Ashraf M, Stertzer SH. Treatment of coronary artery stenosis and coronary arteriovenous fistula by interventional cardiology techniques. Cathet Cardiovasc Diagn. 1989;18(4):240–243. doi: 10.1002/ccd.1810180410. [DOI] [PubMed] [Google Scholar]
- 18.Xie CH, Xia CS, Gong FQ, Zhou YB, Zhu WH. Interventional occlusion of congenital vascular malformations. World J Pediatr. 2009;5(4):296–299. doi: 10.1007/s12519-009-0056-8. [DOI] [PubMed] [Google Scholar]
- 19.Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE. Management of coronary artery fistulae. Patient selection and results of transcatheter closure. J Am Coll Cardiol. 2002;39(6):1026–1032. doi: 10.1016/S0735-1097(02)01742-4. [DOI] [PubMed] [Google Scholar]
- 20.Alekyan BG, Podzolkov VP, Cardenas CE. Transcatheter coil embolization of coronary artery fistula. Asian Cardiovasc Thorac Ann. 2002;10(1):47–52. doi: 10.1177/021849230201000112. [DOI] [PubMed] [Google Scholar]
- 21.Trehan V, Yusuf J, Mukhopadhyay S, et al. Transcatheter closure of coronary artery fistulas. Indian Heart J. 2004;56(2):132–139. [PubMed] [Google Scholar]
- 22.Collins N, Mehta R, Benson L, Horlick E. Percutaneous coronary artery fistula closure in adults: technical and procedural aspects. Catheter Cardiovasc Interv. 2007;69(6):872–880. doi: 10.1002/ccd.21085. [DOI] [PubMed] [Google Scholar]
- 23.Sato F, Koishizawa T. Stress/Rest (99 m)Tc-MIBI SPECT and 123I-BMIPP scintigraphy for indication of surgery with coronary artery to pulmonary artery fistula. Int Heart J. 2005;46(2):355–361. doi: 10.1536/ihj.46.355. [DOI] [PubMed] [Google Scholar]
- 24.Hajj-Chahine J, Haddad F, El-Rassi I, Jebara V. Surgical management of a circumflex aneurysm with fistula to the coronary sinus. Eur J Cardiothorac Surg. 2009;35(6):1086–1088. doi: 10.1016/j.ejcts.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Nekkanti R, Nanda NC, Angsingkar KG, Mukhtar O. Transesophageal three-dimensional echocardiographic assessment of left main coronary artery fistula. Echocardiography. 2001;18(4):305–308. doi: 10.1046/j.1540-8175.2001.00305.x. [DOI] [PubMed] [Google Scholar]
- 26.Ozeki S, Utsunomiya T, Kishi T, et al. Coronary arteriovenous fistula presenting as chronic pericardial effusion. Circ J. 2002;66(8):779–782. doi: 10.1253/circj.66.779. [DOI] [PubMed] [Google Scholar]
- 27.Olsen LA, Folke K, Kjaergard HK. Surgery of complex coronary arteriovenous fistula. Scand Cardiovasc J. 1997;31(3):169–171. doi: 10.3109/14017439709058089. [DOI] [PubMed] [Google Scholar]
- 28.Liu JC, Chan P, Chang TH, Chen RF. Off-pump surgery for multiple coronary artery fistulas with aneurysm. Ann Thorac Surg. 2006;81(2):729–732. doi: 10.1016/j.athoracsur.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Esmaeilzadeh M, Khaledifar A, Usefi A, Omrani GH. Right coronary artery-to-pulmonary artery fistula, the role of echocardiography. Iran Cardiovasc Res J. 2007;1:50–52. [Google Scholar]
- 30.Fujii H, Tsutsumi Y, Ohashi H, Kawai T, Lino K, Onaka M. Surgical treatment of multiple coronary artery fistulas with an associated small saccular aneurysm—a case report. J Card Surg. 2006;21(5):493–495. doi: 10.1111/j.1540-8191.2006.00308.x. [DOI] [PubMed] [Google Scholar]
- 31.Ryu JC, Choe YH, Park PW, Park JE, Chae H, Lee WR. Cardiac tamponade due to a rupture of the coronary arteriovenous aneurysm—a case report. J Korean Med Sci. 1997;12(2):143–145. doi: 10.3346/jkms.1997.12.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy Praschker BG, Rama A, Gandjbakhch I, Pavie A. Congenital bilateral coronary artery to pulmonary artery fistulas associated with left main trunk stenosis. Interact Cardiovasc Thorac Surg. 2008;7(2):360–361. doi: 10.1510/icvts.2007.171629. [DOI] [PubMed] [Google Scholar]
- 33.Dam DW, Noyez L, Skotnicki SH, Lacquet LK. Multiple fistulas between coronary and pulmonary arteries. Eur J Cardiothorac Surg. 1995;9(12):707–708. doi: 10.1016/S1010-7940(05)80130-7. [DOI] [PubMed] [Google Scholar]
- 34.Alkhulaifi AM, Horner SM, Pugsley WB, Swanton RH. Coronary artery fistulas presenting with bacterial endocarditis. Ann Thorac Surg. 1995;60(1):202–204. [PubMed] [Google Scholar]
- 35.Sun S, Li JY, Hu PY, Wu SJ. Starfish-assisted off-pump obliteration of massive coronary arteriovenous fistulae. Tex Heart Inst J. 2005;32(4):595–597. [PMC free article] [PubMed] [Google Scholar]
- 36.Shimamura Y, Yamaki F, Yamamoto H, Kouda T, Tsukagoshi M. Aneurysm in the pulmonary trunk associated with atrial septal defect, a left coronary artery fistula to the pulmonary trunk, and valvular pulmonary stenosis. Jpn J Thorac Cardiovasc Surg. 2000;48(5):329–333. doi: 10.1007/BF03218151. [DOI] [PubMed] [Google Scholar]
- 37.Hol PK, Geiran O, Andersen K, et al. Improvement of coronary artery fistula surgery by intraoperative imaging. Ann Thorac Surg. 2004;78(6):2193–2195. doi: 10.1016/j.athoracsur.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 38.Dimitrakakis G, Oppell U, Luckraz H, Groves P. Surgical repair of triple coronary-pulmonary artery fistulae with associated atrial septal defect and aortic valve regurgitation. Interact Cardiovasc Thorac Surg. 2008;7(5):933–934. doi: 10.1510/icvts.2008.181388. [DOI] [PubMed] [Google Scholar]
- 39.Sunder KR, Balakrishnan KG, Tharakan JA, et al. Coronary artery fistula in children and adults: a review of 25 cases with long-term observations. Int J Cardiol. 1997;58(1):47–53. doi: 10.1016/S0167-5273(96)02792-1. [DOI] [PubMed] [Google Scholar]
- 40.Midell AI, Bermudez GA, Replogle R. Surgical closure of left coronary artery-left ventricular fistula: the second case reported in the literature and a review of the five previously reported cases of coronary artery fistula terminating in the left ventricle. J Thorac Cardiovasc Surg. 1977;74(2):199–203. [PubMed] [Google Scholar]
- 41.Castedo E, Oteo JF, Burgos R, et al. Coronary artery fistula as a bypass of a left anterior descending coronary artery stenosis. Ann Thorac Surg. 1997;64(6):1813–1814. doi: 10.1016/S0003-4975(97)00932-6. [DOI] [PubMed] [Google Scholar]
- 42.Kamiya H, Yasuda T, Nagamine H, et al. Surgical treatment of congenital coronary artery fistulas: 27 years' experience and a review of the literature. J Card Surg. 2002;17(2):173–177. doi: 10.1111/j.1540-8191.2002.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 43.Vitarelli A, Curtis G, Conde Y, et al. Assessment of congenital coronary artery fistulas by transesophageal color Doppler echocardiography. Am J Med. 2002;113(2):127–133. doi: 10.1016/S0002-9343(02)01157-9. [DOI] [PubMed] [Google Scholar]
- 44.Achenbach S, Ulzheimer S, Baum U, et al. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation. 2000;102(23):2823–2828. doi: 10.1161/01.cir.102.23.2823. [DOI] [PubMed] [Google Scholar]
- 45.Datta J, White CS, Gilkeson RC, et al. Anomalous coronary arteries in adults: depiction at multi-detector row CT angiography. Radiology. 2005;235(3):812–818. doi: 10.1148/radiol.2353040314. [DOI] [PubMed] [Google Scholar]
- 46.Sato Y, Mitsui M, Takahashi H, et al. A giant left circumflex coronary artery–right atrium arteriovenous fistula detected by multislice spiral computed tomography. Heart Vessels. 2004;19(1):55–56. doi: 10.1007/s00380-003-0707-y. [DOI] [PubMed] [Google Scholar]
- 47.Tan KT, Chamberlain-Webber R, McGann G. Characterisation of coronary artery fistula by multi-slice computed tomography. Int J Cardiol. 2006;111(2):311–312. doi: 10.1016/j.ijcard.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 48.Weymann A, Lembcke A, Konertz WF. Right coronary artery to superior vena cava fistula: imaging with cardiac catheterization, 320-detector row computed tomography, magnetic resonance imaging, and transoesophageal echocardiography. Eur Heart J. 2009;30(17):2146. doi: 10.1093/eurheartj/ehp207. [DOI] [PubMed] [Google Scholar]
- 49.Kearney LG, Chan R, Srivastava PM. Multimodality imaging of circumflex artery fistula to coronary sinus with persistent left-sided superior vena cava. Eur Heart J. 2007;28(21):2652. doi: 10.1093/eurheartj/ehm201. [DOI] [PubMed] [Google Scholar]
- 50.Konings TC, Groenink M, Bouma BJ, Mulder BJ. Acquired left coronary artery fistula to right ventricular outflow tract. Neth Heart J. 2008;16(3):100–101. doi: 10.1007/BF03086126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehmkuhl L, Luecke C, Koesser A, et al. Right coronary artery fistula to the coronary sinus: Morphological and functional assessment by multidetector computed tomography and magnetic resonance flow measurement. Eur J Radiol Extra. 2009;70:e5–e9. doi: 10.1016/j.ejrex.2008.10.012. [DOI] [Google Scholar]
- 52.Shimaya K, Suzuki Y, Inoue Y. Right coronary artery aneurysm with associated arteriovenous fistula. Int J Cardiol. 1997;58(2):192–194. doi: 10.1016/S0167-5273(96)02869-0. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Li Z, Wang X. Assessment of Coronary Artery Fistula by Color Doppler Echocardiography. Echocardiography. 1998;15(1):67–72. doi: 10.1111/j.1540-8175.1998.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 54.Goswami NJ, Zabalgoitia M. Localization of a coronary artery fistula using contrast transesophageal echocardiography. J Am Soc Echocardiogr. 2002;15(8):839–840. doi: 10.1067/mje.2002.119912. [DOI] [PubMed] [Google Scholar]
- 55.Oncel D, Oncel G. Right coronary artery to left ventricle fistula–effective diagnosis with 64-MDCT. Int J Cardiovasc Imaging. 2007;23(2):287–291. doi: 10.1007/s10554-006-9136-1. [DOI] [PubMed] [Google Scholar]
- 56.Kubota S, Suzuki T, Murata K. Cine magnetic resonance imaging for diagnosis of right coronary arterial-ventricular fistula. Chest. 1991;100(3):735–737. doi: 10.1378/chest.100.3.735. [DOI] [PubMed] [Google Scholar]
- 57.Duerinckx AJ, Perloff JK, Currier JW. Arteriovenous fistulas of the circumflex and right coronary arteries with drainage into an aneurysmal coronary sinus. Circulation. 1999;99(21):2827–2828. doi: 10.1161/01.cir.99.21.2827. [DOI] [PubMed] [Google Scholar]
- 58.Said SA, Hofman MB, Beek AM, Werf T, Rossum AC. Feasibility of cardiovascular magnetic resonance of angiographically diagnosed congenital solitary coronary artery fistulas in adults. J Cardiovasc Magn Reson. 2007;9(3):575–583. doi: 10.1080/10976640601015433. [DOI] [PubMed] [Google Scholar]
- 59.Tio RA, Slart RH, Boer RA, et al. Reduced regional myocardial perfusion reserve is associated with impaired contractile performance in idiopathic dilated cardiomyopathy. Neth Heart J. 2009;17(12):470–474. doi: 10.1007/BF03086306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tio RA, Dabeshlim A, Siebelink HM, et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50(2):214–219. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 61.Reidy JF, Anjos RT, Qureshi SA, Baker EJ, Tynan MJ. Transcatheter embolization in the treatment of coronary artery fistulas. J Am Coll Cardiol. 1991;18(1):187–192. doi: 10.1016/S0735-1097(10)80239-6. [DOI] [PubMed] [Google Scholar]
- 62.Cheung DL, Au WK, Cheung HH, Chiu CS, Lee WT. Coronary artery fistulas: long-term results of surgical correction. Ann Thorac Surg. 2001;71(1):190–195. doi: 10.1016/S0003-4975(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 63.Ho YL, Chen WJ, Wu CC, Lee YT. Acute myocardial infarction in a case of myelofibrosis with patent coronary arteries and arteriovenous fistulae draining into the main pulmonary artery. Int J Cardiol. 1994;46(1):49–51. doi: 10.1016/0167-5273(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 64.Komatsu S, Hirayama A, Sato Y, Kodama K. Coronary-pulmonary fistula serving as a collateral source to the occluded coronary artery in a patient with myocardial infarction. Int J Cardiol. 2007;115(3):408–409. doi: 10.1016/j.ijcard.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 65.Millaire A, Goullard L, Groote P, Ducloux G. Congenital high flow coronary cameral fistula in an 81-year-old woman: management problems. Can J Cardiol. 1992;8(9):917–920. [PubMed] [Google Scholar]
- 66.Sercelik A, Mavi A, Ayalp R, Pestamalci T, Gumusburun E, Batiraliev T. Congenital coronary artery fistulas in Turkish patients undergoing diagnostic cardiac angiography. Int J Clin Pract. 2003;57(4):280–283. [PubMed] [Google Scholar]
- 67.Abdelmoneim SS, Mookadam F, Moustafa S, et al. Coronary artery fistula: single-center experience spanning 17 years. J Interv Cardiol. 2007;20(4):265–274. doi: 10.1111/j.1540-8183.2007.00267.x. [DOI] [PubMed] [Google Scholar]