Abstract
We previously reported that (-)-epigallocatechin-3-gallate (EGCG) and grape seed extract (GSE) at high concentration nearly blocked intestinal iron transport across the enterocyte. In this study, we aimed to determine whether small amounts of EGCG, GSE, and green tea extract (GT) are capable of inhibiting iron absorption, to examine if ascorbic acid counteracts the inhibitory action of polyphenols on iron absorption, and to explore the mechanisms of polyphenol-mediated apical iron uptake and basolateral iron release. An55Fe absorption study was conducted by adding various concentrations of EGCG, GSE, and GT using Caco-2 intestinal cells. Polyphenols were found to inhibit the transepithelial 55Fe transport in a dose-dependent manner. The addition of ascorbic acid offset the inhibitory effects of polyphenols on iron transport. Ascorbic acid modulated the transepithelial iron transport without changing the apical iron uptake and the expression of ferroportin-1 protein in the presence of EGCG. The polyphenol-mediated apical iron uptake was inhibited by membrane impermeable Fe2+ chelators (P < 0.001), but at a low temperature (4°C), the apical iron uptake was still higher than the control values at 37°C (P < 0.001). These results suggest that polyphenols enhance the apical iron uptake partially by reducing the conversion of ferric to ferrous ions and possibly by increasing the uptake of polyphenol-iron complexes via the energy-independent pathway. The present results indicate that the inhibitory effects of dietary polyphenols on iron absorption can be offset by ascorbic acid. Further studies are needed to confirm the current findings in vivo.
Introduction
Dietary polyphenolic compounds are known for their beneficial effects on health (1–4), mainly from their antioxidative properties. Their antioxidant activities have been shown to be dependent on the ability of their constituent phenolic compounds to scavenge free radicals and chelate metals such as iron (5, 6). Tea and red wine are thought to be important sources of these compounds. Tea, made from the leaves of the plant Camellia sinensis, is a popular beverage worldwide. The beneficial effects of green tea are attributed to its polyphenolic compounds, particularly the catechins. (-)-Epigallocatechin-3-gallate (EGCG),3 the most abundant green tea catechin, is the most bioactive disease-preventing polyphenol compound in green tea. In contrast, the phenolic compounds in grape seed extract (GSE) have 1 or more aromatic rings, which contain hydroxyl groups in different patterns. They vary in size from small simple molecules to huge polymerized complexes. However, the problem with some polyphenolic compounds is their effects on reducing iron absorption when included in the diet; multiple galloyl groups are responsible for inhibiting iron absorption from foods. Evidence suggests that polyphenolic compounds may be partly responsible for the low iron bioavailability in many vegetables (7, 8).
Dietary ferric ion in the lumen is thought to be reduced to ferrous ion by either ferrireductase (duodenal cytochrome b) activity on the cell surface (9–11) or exogenous dietary reducing agents, such as ascorbic acid (9). Iron is then transported across the apical membrane via the apical iron transporter, divalent metal transporter-1, into the enterocyte (12, 13). The newly acquired iron is then distributed to the basolateral surface or to iron-binding proteins (e.g. heme, nonheme iron-binding proteins, and ferritin). Finally, the newly obtained iron is transferred across the basolateral membrane of the enterocyte via the iron exporter, ferroportin-1 (14–16), and then it is oxidized to ferric ion by ferroxidase hephaestin (17) on the basolateral surface prior to release into the circulation.
The amounts of extracted polyphenols in green tea are variable and depend on many factors, including the brew conditions (18). The concentration of polyphenols in wine also varies. Red wine contains more polyphenolic compounds than white wine (19). We previously reported that selected dietary bioactive polyphenols, EGCG and GSE, nearly block the transepithelial iron transport by decreasing basolateral iron release in the enterocyte (20, 21) when they were added at a high level. However, previous studies did not, to our knowledge, evaluate the minimum dietary levels of polyphenols needed to inhibit iron transport across the enterocyte. Thus, the present study aimed to examine the relationship between the amount of polyphenolic compounds and the extent of inhibition on mucosal iron transport. Additionally, the effect of green tea extract (GT) on mucosal iron transport was also studied. The GT contains EGCG and other flavonoids, such as epicatechin gallate (ECG), epigallo catechin, epicatechin, and catechin. Ascorbic acid markedly enhances the absorption of dietary iron and strongly counteracts the inhibitory effects of dietary factors, such as phytate. Thus, another purpose of the present study was to examine the possibility of counteracting the inhibition of polyphenolic compounds on mucosal iron transport by adding nutritionally realistic amounts of ascorbic acid. Most dietary factors are known to affect intestinal iron absorption by modulating the apical iron uptake from the gastrointestinal lumen. However, previous results indicate that EGCG and GSE may inhibit iron absorption by preventing the exchange of Fe to the basolateral transfer mechanism. EGCG and GSE were shown to decrease the transepithelial iron transport across the enterocyte by decreasing the basolateral iron release and increasing the apical iron uptake in the enterocytes. This finding was unexpected, and the mechanism of EGCG and GSE on iron absorption remains to be elucidated. Thus, the 3rd objective of the current study was to explore the mechanism by which bioactive polyphenols enhance apical iron uptake and decrease basolateral iron release.
Materials and Methods
Reagents.
Tissue culture media, HBSS, glutamine, nonessential amino acids, and penicillin/streptomycin were purchased from Invitrogen. FBS was obtained from Hyclone. EGCG (TEAVIGO, purity > 95%), GSE, and GT were purchased from DSM Nutritional Products, Partoeno, and Pharmanex, respectively. The chemical characteristics and degree of polymerization for the GSE and GT used in these studies have been documented (22, 23). The 55Fe (as FeCl3) was purchased from PerkinElmer. Unless otherwise noted, all other reagents were purchased from Sigma Chemical, Fisher Scientific, or VWR.
Caco-2 cell culture.
The human Caco-2 cell line was purchased from American Type Culture Collection. Stock cultures were maintained as previously reported (20, 21). For the experiments, cells were cultured on microporous membrane inserts (4.9 cm2, BD Biosciences) that were coated with collagen and used after d 17 postconfluence for experiments as previously described (20, 21).
55Fe transport and uptake.
Transepithelial iron transfer from the apical compartment to the basolateral compartment and cellular iron accumulation were determined as previously detailed (9, 20). Briefly, cells were incubated at 37°C with 1.5 mL of 10 μmol/L 55Fe/56Fe-nitrilotriacetic acid (NTA)2 in an iron-uptake buffer containing the indicated bioactive compounds in the presence or absence of other testing compounds in the apical compartment and 2.5 mL of DMEM in the basolateral compartment. Approximately 7–10% of the added iron in the iron uptake buffer was radiolabeled iron (55Fe). All test solutions were freshly prepared before each experiment. Stock solutions of 10 mmol/L FeCl3⋅6H2O and 20 mmol/L NTA were prepared fresh in 1 mmol/L HCl (pH 3.0) and diluted 10-fold in sterile, deionized water. The diluted stock solutions were mixed with 55Fe3+Cl3 (specific radioactivity, 110–180 GBq/mmol; PerkinElmer) to provide 185 kBq/well for the uptake and transport studies. Once the 10 μmol/L 55Fe(NTA)2 solution was prepared, the indicated polyphenolic compounds were added and mixed and then ascorbic acid (or other testing compounds) was immediately added. The mixed test solutions were immediately placed on cells for the iron transport and uptake studies. The effect of polyphenolic compounds and ascorbic acid on the solubility of iron in iron uptake buffer was determined by measuring the radioactivity levels in vortexed aliquots before and after centrifugation at 10,600 × g for 10 min at 20°C. The effect that the order of adding the polyphenols and ascorbic acid to the iron uptake buffer has on the apical uptake and the transepithelial transport of Fe was also assessed by adding polyphenols before or after the ascorbic acid to the 55Fe solution. The integrity of tight junctions between cells was monitored by measuring transepithelial electric resistance and phenol red transport; any leaking cell monolayers were discarded. Cellular levels of 55Fe were measured as previously detailed (20). A green tea bag contains up to 20–200 mg EGCG. The contents of the extracted polyphenols in tea are dependent on the origin of tea, the manufacturer, and the brew conditions, which include water temperature, brew time, and tea:water ratio (18). Thus, a cup of green tea can provide various amounts of EGCG, ranging from ∼1–2 mg to a maximum of 200 mg. Most GSE supplements contain 100–500 mg GSE/capsule. However, red wine contains ∼132–366 mg/L of catechin equivalents and white wine contains 5–13 mg/L of catechin equivalents (19). Because the total gastric volume during meals can range from 1 to 2 L depending on the amounts of meal consumed (24), the concentrations of polyphenols (0.46–46 mg/L) used in the present study are within practical ranges. To study the temperature effect, uptake experiments were performed as described above at 4°C.
The effect of Fe2+ chelator on polyphenolic compound-mediated apical iron uptake.
To investigate the mechanism by which bioactive polyphenols enhance the apical nonheme iron uptake, 100 μmol/L of membrane-impermeable Fe2+ chelators, bathophenanthroline disulfonate (BPS) and ferrozine (FZ), were added in the apical compartment in the presence of 46 mg/L of EGCG and GSE. After 3 h of incubation, the apical iron uptake was assessed.
Western-blot analysis.
After the cells were incubated for 3 h with the various polyphenolic compounds in the presence or absence of ascorbic acid, protein samples were extracted and the expression of the ferroportin-1 protein was assessed as previously described (25).
Statistical analysis.
Values were expressed as means ± SEM. The experiments were repeated 3–6 times with 3–6 wells for each test variable. Data were analyzed using a 1-way or 2-way (treatment × time) ANOVA with Bonferroni’s multiple comparison tests post hoc for multiple comparisons using Prism 5.0 software (GraphPad). Data were log-transformed as necessary to attain homogeneity of variance and data are reported as nontransformed means. The REG (regression) procedure was used to perform simple linear regression analyses (Table 1). Differences were considered significant at P < 0.05.
TABLE 1.
Dose-dependent effects of EGCG, GSE, and GT on the rate of apical 55Fe transfer across the cell monolayer in fully differentiated Caco-2 cells1
| Treatments, mg/L | Rate of 55Fe transfer (AP to BL chamber), pmol/ (h ⋅ mg cellular protein) |
| + EGCG | |
| 0 | 9.56 ± 1.54a |
| 0.46 | 4.64 ± 0.83b |
| 4.6 | 0.77 ± 0.05c |
| 46 | 0.34 ± 0.01d |
| + GSE | |
| 0 | 17.1 ± 2.80a |
| 0.46 | 14.0 ± 2.75a |
| 4.6 | 12.3 ± 0.56a |
| 46 | 1.64 ± 0.13b |
| + GT | |
| 0 | 6.50 ± 0.18a |
| 0.46 | 6.35 ± 0.34a |
| 4.6 | 6.06 ± 0.32a |
| 46 | 1.04 ± 0.04b |
Values are means + SEM, = 6 or 3 (EGCG).
The rate of transepithelial 55Fe transport across the differentiated Caco-2 cell monolayers was calculated during 3-h incubation by linear regression analysis (+ EGCG, 2 > 0.97; + GSE, r2 > 0.97; + GT, r2 > 0.99). Values are means ± SEM, n = 6 or 3 (EGCG). Means within a treatment without a common letter differ, P < 0.05.
Results
Dose-dependent effects of polyphenolic compounds on the transepithelial iron transport.
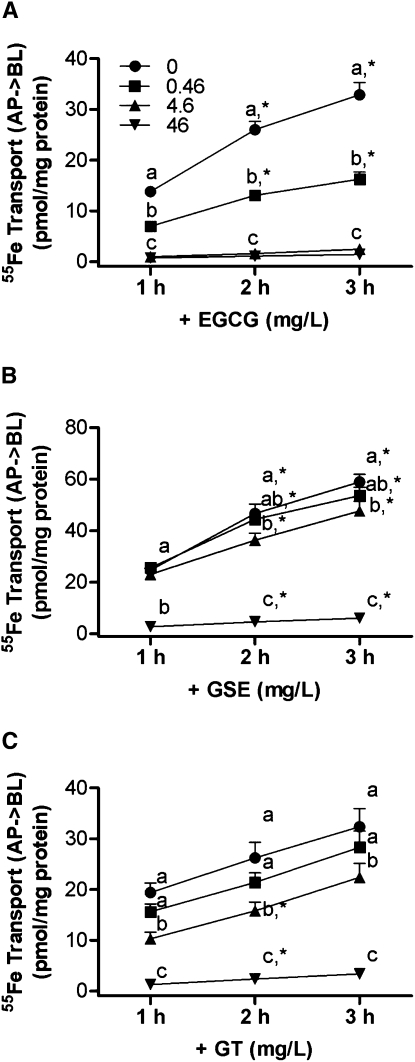
The solubility of Fe was not changed when polyphenols were added to the uptake buffer. The amount of 55Fe in the supernatant of the samples containing EGCG, GSE, and GT was the same as the amount in the control solution (data not shown). The quantity of 55Fe transferred from the apical to the basolateral compartment of the Caco-2 cell monolayer linearly increased between 1 and 3 h of incubation, and transport rates [pmol/(h⋅mg cellular protein)] were calculated using linear regression analysis (r2 > 0.97) (Table 1). The addition of EGCG to the uptake buffer decreased the rate of 55Fe transfer across the cell monolayer in a dose-dependent manner (P < 0.05) (Table 1). The inhibitory effects of EGCG, GSE, and GT on the transepithelial 55Fe transport were associated with the doses of these polyphenols, which had half-maximal inhibitory concentrations (IC50) values (95% CI) of 0.4 (0.3–0.6), 21.2 (11.2–40.3), and 14.0 (5.3–37.1) mg/L, respectively, when 10 μmol/L of Fe3+(NTA)2 was applied (Fig. 1A–C). During the 3-h incubation, the transepithelial iron transfer across the cell monolayer decreased with the addition of 0.46, 4.6, and 46 mg/L (1, 10, and 100 μmol/L, respectively) of EGCG (51, 93, and 96%, respectively) (P < 0.001) (Fig. 1A). The transepithelial iron transport was also decreased in the presence of 4.6 and 46 mg/L GSE (19 and 90%, respectively) during the 3-h transport assay (P < 0.05) (Fig. 1B). Similarly, iron transport was decreased with the addition of 4.6 and 46 mg/L GT (31 and 90%, respectively) during the 3-h incubation (P < 0.05) (Fig. 1C). The addition of bioactive dietary polyphenols did not alter transepithelial electric resistance values, which confirmed the integrity of the monolayer for the EGCG-, GSE-, or GT-added cells.
FIGURE 1.
A dose-dependent inhibitory effect of EGCG (A), GSE (B), and GT (C) on iron transport across the fully differentiated Caco-2 cell monolayers. Values are means ± SEM, n = 6 or 3 (EGCG). Means at a time without a common letter differ, P < 0.05. *Different from the preceding time point within a treatment, P < 0.05.
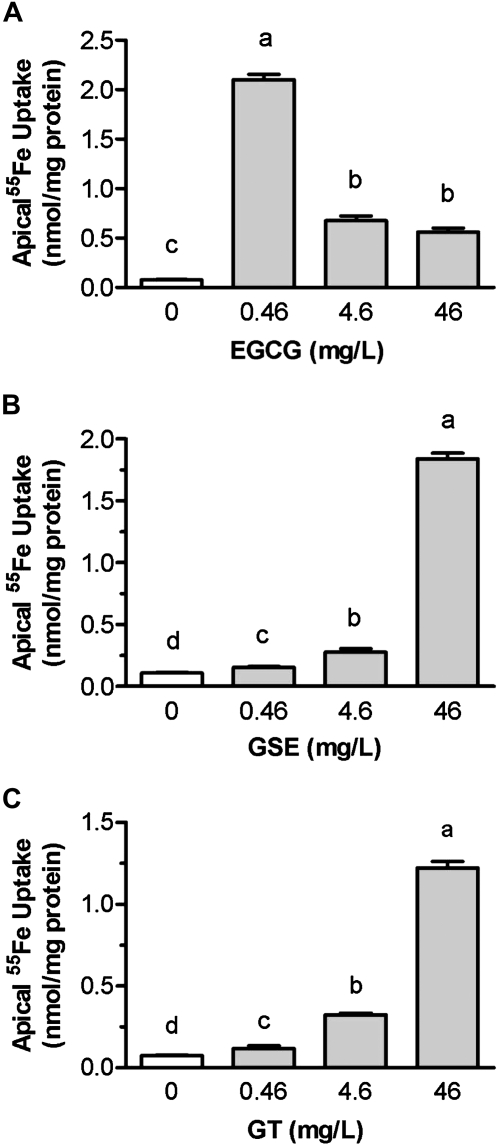
GSE and GT increase apical iron uptake in a dose-dependent manner.
The addition of EGCG, GSE, and GT to the iron uptake solution enhanced iron uptake across the brush border membrane and its assimilation by Caco-2 cells during the 3-h incubation (P < 0.001) (Fig. 2). The addition of GSE and GT enhanced the apical iron uptake in a dose-dependent manner (P < 0.001). The apical iron uptake increased in the presence of 0.46, 4.6, and 46 mg/L of GSE (0.4-, 1.5-, and 16.2-fold, respectively) compared with the control values (P < 0.001) (Fig. 2B). Similarly, the apical iron uptake was enhanced by the addition of 0.46, 4.6, and 46 mg/L of GT (0.6-, 3.3-, and 15.3-fold, respectively) (P < 0.001) (Fig. 2C). The apical iron uptake was tremendously increased in cells that were exposed to very small amounts of EGCG (0.46 mg/L) (26.3-fold of control) (P < 0.001), but it was enhanced by a much smaller amount in cells that were exposed to 4.6 or 46 mg/L of EGCG (7.8- and 6.3-fold, respectively) (P < 0.001) (Fig. 2A).
FIGURE 2.
A dose-dependent effect of EGCG (A), GSE (B), and GT (C) on the apical iron uptake in fully differentiated Caco-2 cells during 3-h incubation. Values are means ± SEM, n = 6 or 3 (EGCG). Means without a common letter differ, P < 0.05.
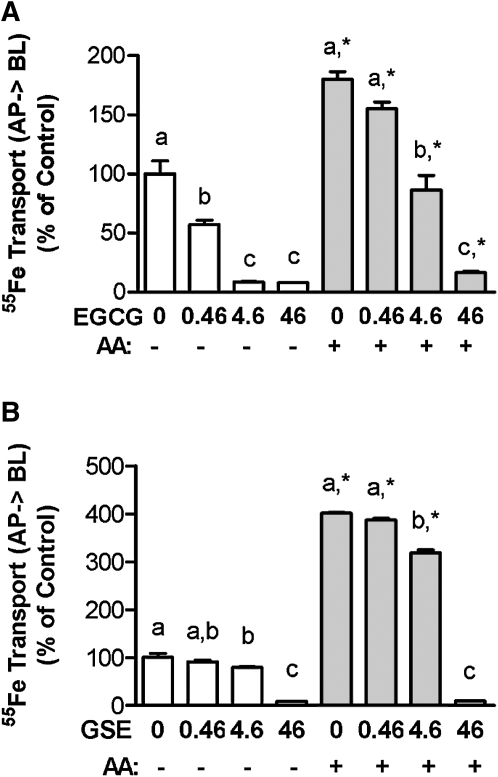
Ascorbic acid offsets the inhibitory action of polyphenols on iron transport across the cell monolayer.
The solubility of Fe was the same when ascorbic acid was also added to the uptake buffer, and it was not changed when EGCG and GSE were added to the uptake buffer containing ascorbic acid (data not shown). The transepithelial transport and apical uptake of iron were not affected by the order of addition of polyphenols and ascorbic acid to the uptake buffer. The addition of 500 μmol/L ascorbic acid offset the inhibitory action of EGCG on iron transfer across the cell monolayer without modulating apical iron uptake. Ascorbic acid completely reversed the inhibitory effect of EGCG on iron transport when a low concentration of EGCG (0.46 mg/L = 1 μmol/L) was applied. The transepithelial iron transport was decreased by 91 and 93% in the presence of 4.6 and 46 mg/L EGCG, respectively, compared with the control cells without ascorbic acid (P < 0.001). However, the inhibitory action was alleviated to 13 and 83% with the addition of 500 μmol/L ascorbic acid during the 3-h incubation (Fig. 3A). The transepithelial iron transport was enhanced by ascorbic acid in the presence of 0.46 or 4.6 mg/L of GSE (2.9- and 2.2-fold, respectively) compared with the control cells without ascorbic acid (Fig. 3B). The apical uptake of 55Fe was markedly increased by ascorbic acid in the presence of lower concentrations of GSE (≤4.6 mg/L) (P < 0.001) (data not shown). Although the iron transport across the cell monolayer was not affected by small amounts of polyphenols (0.46 mg/L) in the presence of ascorbic acid, the transepithelial iron transport was still decreased when the levels of polyphenols were ≥4.6 mg/L. The addition of ascorbic acid increased both the transepithelial transport and the apical uptake of iron in the control samples (P < 0.001).
FIGURE 3.
Ascorbic acid offsets the inhibitory effects of EGCG (A) and GSE (B) on iron transport across the Caco-2 cell monolayers. Ascorbic acid (500 μmol/L) was added into the apical compartment containing various concentrations (0–46 mg/L) of EGCG and GSE during a 3-h transport assay. Values are expressed as percent of control and means ± SEM, n = 4–5. Within −AA (ascorbic acid) and + AA groups, means without a common letter differ, P < 0.05. *Different from corresponding −AA, P < 0.05.
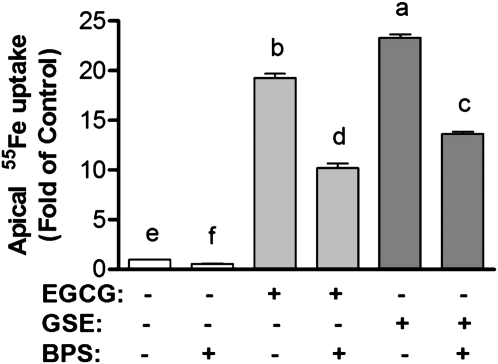
Addition of a Fe2+chelator reduces EGCG- and GSE-mediated apical iron uptake.
The addition of a membrane-impermeable Fe2+ chelator, BPS, reduced the apical 55Fe uptake in the presence and absence of EGCG and GSE during the 3-h incubation period (P < 0.001) (Fig. 4). Similarly, the addition of FZ, another membrane-impermeable Fe2+ chelator, also reduced the apical 55Fe uptake in the presence of EGCG and GSE (P < 0.001) (data not shown).
FIGURE 4.
Effects of Fe2+ chelator on EGCG- and GSE-mediated apical iron uptake in human intestinal Caco-2 cells. BPS (100 μmol/L) was added in the apical compartment containing 10 μmol/L Fe3+(NTA)2 and 46 mg/L EGCG and GSE during 1-h incubation. Values are expressed as means ± SEM, n = 4. Means without a common letter differ, P < 0.05.
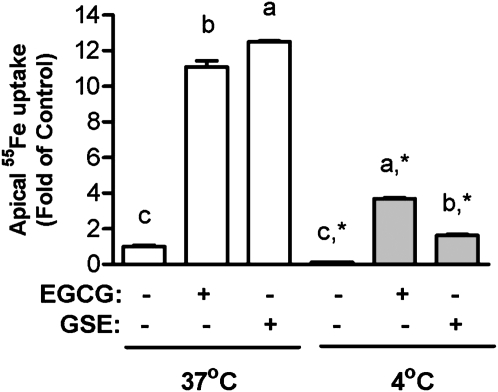
EGCG and GSE enhance apical iron uptake at 4#x00B0C.
The apical iron uptake decreased by 90% in cells incubated at 4°C compared with that at 37°C in control cells (P < 0.001). The EGCG- and GSE-mediated apical 55Fe uptake also decreased when cells were incubated at 4°C compared with that at 37°C (P < 0.0001). The EGCG- and GSE-mediated apical iron uptake at 4°C was still 269 and 62% higher, respectively, than the control values at 37°C (P < 0.0001) (Fig. 5).
FIGURE 5.
Effects of temperature on EGCG- and GSE-mediated apical iron uptake in fully differentiated Caco-2 cells. Incubations included 10 μmol/L Fe3+(NTA)2 and 46 mg/L EGCG or GSE. Values are means ± SEM, n = 4. Within 37°C and 4°C groups, means without a common letter differ, P < 0.05. *Different from corresponding 37°C, P < 0.05.
Effects of ascorbic acid and polyphenols on the expression of ferroportin-1 in Caco-2 cells.
The expression of ferroportin-1 was not changed by ascorbic acid, EGCG, GSE, GT, or a cocktail of ascorbic acid and each of these polyphenols (data not shown).
Discussion
Flavonoids can form 1:1, 2:1, and 3:1 complexes with iron, and the relative proportion of each form depends on the concentration of both metal and flavonoids and the pH of the solution. Under the current experimental condition of 10 μmol/L iron, the 2:1 species would predominate when 100 μmol/L flavonoids are provided. Many flavonoids, such as EGCG and ECG, possess more than 1 metal-binding site and, therefore, are capable of concatenation of monomeric flavonoids with the metal cations, which results in flavonoids that are less prone to partition into the membrane (26). The metal cations that link the flavonoid subunits together will also be unable to enter the cell through metal ion transporters. However, EGCG, GT, and GSE increased the apical iron uptake in the current study. A plausible explanation for the increased apical iron uptake is that membrane-permeable flavonoid-iron complexes are formed in addition to membrane-impermeable flavonoid-iron polymers/oligomers. Iron may also initially form the 2:1 iron:EGCG complex, which may be able to enter the cell. A well-controlled kinetic study showed that the complex formation reactions of Fe3+ with EGCG resulted in the formation of 2:1 metal EGCG complexes when the ratio of [Fe]:[EGCG] was 10:1 (27). In this case, electron transfer can result in the formation of Fe2+ and EGCG can form complexes with Fe2+. However, EGCG and other flavonoids with appreciable affinity for Fe3+ can facilitate the autoxidation of Fe2+ at a neutral pH (28).
In this study, the apical iron uptake was much higher when EGCG was added at a low concentration than at higher concentrations. It is possible that at low EGCG concentrations most EGCG may form complexes with iron (as Fe2+ or Fe3+) as 2:1 iron:EGCG complexes, and at high EGCG concentrations, EGCG may form either 2:1 iron:EGCG complexes or polymerized forms of the 1:2 iron EGCG complex that cannot enter the cell. Thus, at a low EGCG concentration, iron may enter the cell as either free Fe2+ or as 2:1 iron:EGCG complexes. Conversely, at high EGCG concentrations (≥4.6 mg/L), iron may enter the cells as 2:1 iron:EGCG complexes and, possibly, as less polymerized forms of the 1:2 iron EGCG:complex. These iron atoms that enter the cells complexed with flavonoids will add to the pool of labile iron and, once the flavonoid has been metabolized, to the free iron pool. It can be either Fe2+ or Fe3+. This increase in free iron should not suffice to produce significant increases in the total basolateral iron release, as observed in cells treated with EGCG, GSE, and GT.
GT and GSE contain various catechins and procyanidins (22, 23). GT contains 97% pure polyphenols, 65% of which are catechins, including ≥38% of EGCG, ≥15% of ECG, ≥6% of EGC, epicatechin, gallocatechin gallate, gallocatechin, catechingallate, and catechin. GSE consists of a mixture of many polyphenols with procyanidin dimers as a major component (62% by weight), followed by catechin (~13%), procyanidin trimer (~11%), epicatechin (~8%), procyanidin tetramer (~6%), and ECG (~1%). Whereas the apical iron uptake was positively associated with the amounts of GT and GSE in the uptake buffer, the basolateral iron transport was negatively associated with these amounts. A plausible explanation for the minimal effects of GT and GSE on the apical uptake and transepithelial transport of iron at a low level (0.46 mg/L) of GT and GSE may be due to the low concentration of polyphenols in these compounds.
Interestingly, the inhibitory effect of EGCG at 0.46 mg/L was much higher than that of GT at 4.6 mg/L, which contains ∼4 μmol/L EGCG. The apical iron uptake was much higher in the presence of 0.46 mg/L EGCG than that in the presence of 4.6 mg/L GT, which contains an ignorable amount of ascorbic acid. A plausible explanation for this unexpected result is that once iron is reduced to Fe2+ by polyphenols in GT, it may form complexes with the smaller flavonoids, such as catechin and epicatechin, and these iron-flavonoid complexes enter the cells. Inside the cell, Fe2+ may be released from the flavonoids and be transported across the basolateral membrane.
Ascorbic acid is known to enhance nonheme iron absorption by enhancing the apical iron uptake by reducing dietary ferric to ferrous ion in the gastrointestinal lumen. However, the addition of ascorbic acid increased the basolateral iron release by 1.7-, 8.9-, and 1.1-fold without changing the apical iron uptake in the presence of 0.46, 4.6, and 46 mg/L EGCG, respectively. Under the given experimental conditions, 10 μmol/L Fe3+ was completely reduced to Fe2+ in the presence of 500 μmol/L ascorbic acid (29). EGCG was capable of chelating 42 and 78% of Fe2+ at 10 and 100 μmol/L concentrations, respectively, when 10 μmol/L Fe2+ was provided (20). Thus, most of the iron that entered the cell was Fe2+ bound to EGCG or free iron. The iron that entered the cell as free Fe2+ can be transferred across the basolateral membrane of the enterocyte. In addition, Fe2+ that entered the cell as iron complexed with EGCG will be released to the iron pool after EGCG has been metabolized, and then it will be exported through the basolateral membrane. The ratio of total iron atoms:EGCG-iron–binding sites would be higher than 1 inside the cell.
As previously reported (20), the apical iron uptake was increased in Caco-2 cells by dietary polyphenolic compounds. The effects of EGCG, GSE, and GT on the apical iron uptake were comparable to that of ascorbic acid. Ascorbic acid stimulated the apical iron uptake by reducing dietary ferric ion to ferrous ion, and the ascorbic acid-mediated apical iron uptake was completely blocked by a high affinity chelator of Fe2+ (9). To examine whether the increased apical iron uptake from solution containing the polyphenolic compounds was associated with a change in the ratio of Fe3+:Fe2+, a membrane-impermeable, high affinity chelator of Fe2+, BPS, was added to iron uptake solutions containing either EGCG or GSE. The EGCG- and GSE-mediated apical iron uptake was decreased by 42 and 47%, respectively, by BPS. Similarly, FZ, another membrane-impermeable chelator of Fe2+, also reduced EGCG- and GSE-mediated apical iron uptake (P < 0.001). We previously reported that the addition of 46 mg/L EGCG and GSE reduced 14.3 and 15.4% of Fe3+, respectively, when 10 μmol/L Fe3+(NTA)2 was applied (20). The present findings indicate that EGCG and GSE induce apical iron uptake partially by reducing Fe3+ to Fe2+. The free Fe2+ may enter the cell via an active iron transport pathway through divalent metal transporter-1 and may form complexes with polyphenols inside the cell. Another possible mechanism by which polyphenolic compounds increase the apical iron uptake is that polyphenols may form complexes with Fe2+ or Fe3+ in the apical compartment and enter the cell as complexes, as discussed above. As we previously reported (20), EGCG and polyphenols in GSE are able to chelate metals, including iron (5, 26, 30–33).
Because the polyphenol-mediated apical iron uptake was only partially reduced with the addition of the Fe2+ chelator, we evaluated the apical uptake of 55Fe at 4°C to address the question of whether Fe-polyphenol complexes can enter the cells across the apical membrane of enterocytes at a low temperature. The apical iron uptake was almost completely blocked when cells were incubated at 4°C compared with the control cells that were incubated at 37°C in the absence of polyphenols (Fig. 5). The polyphenol-mediated apical iron uptake was also reduced when cells were incubated at 4°C (P < 0.001). However, the EGCG- and GSE-mediated apical iron uptake at 4°C was still higher than the control values at 37°C (P < 0.001), indicating that some of polyphenol-iron complexes enter the cell via an energy-independent pathway. It was previously suggested that EGCG uptake mainly occurs by a passive diffusion process in HT 29 cells (34). The apical EGCG uptake was concentration dependent and did not plateau even at a concentration of 294 mg/L (34). These data, along with the current results, suggest that polyphenol-iron complexes enter the cell through a facilitated diffusion pathway.
The addition of ascorbic acid offset the inhibitory effects of polyphenolic compounds on the basolateral iron release without modulating the apical iron uptake. Because iron is released across the basolateral membrane of the enterocyte by ferroportin-1, the expression of ferroportin-1 was assessed in cells exposed to uptake solution that contained ascorbic acid, polyphenols, or a cocktail of ascorbic acid and polyphenolic compounds during the iron transport study. Neither ascorbic acid nor polyphenols altered ferroportin-1 expression. These findings suggest that the addition of ascorbic acid increases the basolateral iron release without modulating the expression of the iron-exporting protein, possibly by increasing the cellular iron pool, which is available for the basolateral iron exporter, ferroportin-1, in the enterocyte. The levels of hephaestin and ferritin proteins were not changed by ascorbic acid, EGCG, GSE, or GT during the iron transport study (Q. Ma and O. Han, unpublished data).
The present study shows that dietary bioactive polyphenols inhibit iron transport in a dose-dependent manner. Small amounts of polyphenolic compounds are also able to reduce iron transport across the intestinal enterocyte. Interestingly, the inhibitory effect of EGCG on iron transport was alleviated when EGCG was added with other catechins, such as GT. The inhibitory effect of dietary polyphenolic compounds on nonheme iron transport was offset by ascorbic acid. Our current results suggest that polyphenols enhance apical iron uptake partially by reducing ferric to ferrous ion and by increasing the uptake of polyphenol-iron complexes via an energy-independent pathway. Interestingly, ascorbic acid enhances iron transport across the cell monolayers without changing the apical iron uptake and ferroportin-1 expression in the presence of EGCG. The interactions of ascorbic acid and polyphenols with iron inside the cell remain to be explored. The mechanism by which polyphenols enhance the apical iron uptake and decrease the basolateral iron release should be further defined.
Acknowledgments
O.H. designed the research, E-Y.K., S-K.H., D.B., Q.M., and O.H. conducted the research; Q.M. and O.H. analyzed the data; O.H. wrote the paper; and O.H. was responsible for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by the College of Human and Health Development at the Pennsylvania State and National Center for Complementary and Alternative Medicine: R21AT005006 (to O.H.).
Abbreviations used: BPS, bathophenanthroline disulfonate; ECG, epicatechin gallate; EGCG, (-) -epigallocatechin-3-gallate; FZ, ferrozine; GSE, grape seed extract; GT, green tea extract; NTA, nitrilotriacetic acid.
Literature Cited
- 1.Basu A, Rhone M, Lyons TJ. Berries: emerging impact on cardiovascular health. Nutr Rev. 2010;68:168–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frederiksen H, Mortensen A, Schroder M, Frandsen H, Bysted A, Knuthsen P, Rasmussen SE. Effects of red grape skin and seed extract supplementation on atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Mol Nutr Food Res. 2007;51:564–71 [DOI] [PubMed] [Google Scholar]
- 4.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta. 1996;1304:210–22 [DOI] [PubMed] [Google Scholar]
- 6.Apak R, Guclu K, Ozyurek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 2004;52:7970–81 [DOI] [PubMed] [Google Scholar]
- 7.Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, Bezwoda WR, Mills W, Charlton RW, Mayet F. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br J Nutr. 1983;49:331–42 [DOI] [PubMed] [Google Scholar]
- 8.Hallberg L, Hulthen L. Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. Am J Clin Nutr. 2000;71:1147–60 [DOI] [PubMed] [Google Scholar]
- 9.Han O, Failla ML, Hill AD, Morris ER, Smith JC., Jr Reduction of Fe(III) is required for uptake of nonheme iron by Caco-2 cells. J Nutr. 1995;125:1291–9 [DOI] [PubMed] [Google Scholar]
- 10.Nunez MT, Alvarez X, Smith M, Tapia V, Glass J. Role of redox systems on Fe3+ uptake by transformed human intestinal epithelial (Caco-2) cells. Am J Physiol. 1994;267:C1582–8 [DOI] [PubMed] [Google Scholar]
- 11.McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–9 [DOI] [PubMed] [Google Scholar]
- 12.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8 [DOI] [PubMed] [Google Scholar]
- 13.Fleming MD, Trenor CC III, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–6 [DOI] [PubMed] [Google Scholar]
- 14.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–81 [DOI] [PubMed] [Google Scholar]
- 15.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309 [DOI] [PubMed] [Google Scholar]
- 16.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–12 [DOI] [PubMed] [Google Scholar]
- 17.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–9 [DOI] [PubMed] [Google Scholar]
- 18.Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J Agric Food Chem. 2001;49:5340–7 [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Moreno C, Cao G, Ou B, Prior RL. Anthocyanin and proanthocyanidin content in selected white and red wines. Oxygen radical absorbance capacity comparison with nontraditional wines obtained from highbush blueberry. J Agric Food Chem. 2003;51:4889–96 [DOI] [PubMed] [Google Scholar]
- 20.Kim EY, Ham SK, Shigenaga MK, Han O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J Nutr. 2008;138:1647–51 [DOI] [PubMed] [Google Scholar]
- 21.Ma Q, Kim EY, Han O. Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J Nutr. 2010;140:1117–21 [DOI] [PubMed] [Google Scholar]
- 22.Tsang C, Auger C, Mullen W, Bornet A, Rouanet JM, Crozier A, Teissedre PL. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br J Nutr. 2005;94:170–81 [DOI] [PubMed] [Google Scholar]
- 23.Lu QY, Jin YS, Pantuck A, Zhang ZF, Heber D, Belldegrun A, Brooks M, Figlin R, Rao J. Green tea extract modulates actin remodeling via Rho activity in an in vitro multistep carcinogenic model. Clin Cancer Res. 2005;11:1675–83 [DOI] [PubMed] [Google Scholar]
- 24.Burton DD, Kim HJ, Camilleri M, Stephens DA, Mullan BP, O'Connor MK, Talley NJ. Relationship of gastric emptying and volume changes after a solid meal in humans. Am J Physiol Gastrointest Liver Physiol. 2005;289:G261–6 [DOI] [PubMed] [Google Scholar]
- 25.Han O, Kim EY. Colocalization of ferroportin-1 with hephaestin on the basolateral membrane of human intestinal absorptive cells. J Cell Biochem. 2007;101:1000–10 [DOI] [PubMed] [Google Scholar]
- 26.Hider RC, Liu ZD, Khodr HH. Metal chelation of polyphenols. Methods Enzymol. 2001;335:190–203 [DOI] [PubMed] [Google Scholar]
- 27.Ryan P, Hynes MJ. The kinetics and mechanisms of the complex formation and antioxidant behaviour of the polyphenols EGCg and ECG with iron(III). J Inorg Biochem. 2007;101:585–93 [DOI] [PubMed] [Google Scholar]
- 28.Harris DC, Aisen P. Facilitation of Fe(II) autoxidation by Fe(3) complexing agents. Biochim Biophys Acta. 1973;329:156–8 [DOI] [PubMed] [Google Scholar]
- 29.Han O, Failla ML, Hill AD, Morris ER, Smith JC., Jr Ascorbate offsets the inhibitory effect of inositol phosphates on iron uptake and transport by Caco-2 cells. Proc Soc Exp Biol Med. 1995;210:50–6 [DOI] [PubMed] [Google Scholar]
- 30.Ohyoshi E, Hamada Y, Nakata K, Kohata S. The interaction between human and bovine serum albumin and zinc studied by a competitive spectrophotometry. J Inorg Biochem. 1999;75:213–8 [DOI] [PubMed] [Google Scholar]
- 31.Kumamoto M, Sonda T, Nagayama K, Tabata M. Effects of pH and metal ions on antioxidative activities of catechins. Biosci Biotechnol Biochem. 2001;65:126–32 [DOI] [PubMed] [Google Scholar]
- 32.Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. J Alzheimers Dis. 2004;6:367–77 [DOI] [PubMed] [Google Scholar]
- 33.Inoue MB, Inoue M, Fernando Q, Valcic S, Timmermann BN. Potentiometric and (1)H NMR studies of complexation of Al(3+) with (-)-epigallocatechin gallate, a major active constituent of green tea. J Inorg Biochem. 2002;88:7–13 [DOI] [PubMed] [Google Scholar]
- 34.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–6 [PubMed] [Google Scholar]