Abstract
Potential antiinflammatory and antioxidant effects were recently ascribed to naturally occurring micronutrients. The extent and magnitudes of their differential effects on the metabolic syndrome (MetS) are still unknown. We examined the association between serum antioxidant status and MetS. NHANES 2001–2006 cross-sectional data among adults aged 20–85 y were analyzed (n = 3008–9099). MetS was defined with the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and also by elevated homeostatic model assessment insulin resistance (HOMA-IR), C-reactive protein (CRP) and hyperuricemia. Serum antioxidants included retinol, retinyl esters, carotenoids [α-carotene, β-carotene (cis+trans), β-cryptoxanthin, lutein+zeaxanthin, total lycopene], vitamin E, and vitamin C. MetS (NCEP ATP III) prevalence in U.S. adults was 32.0% among men and 29.5% among women. Adults with MetS had consistently lower serum carotenoid concentrations compared with those without MetS, even after controlling for total cholesterol and TG among other potential confounders. Vitamin E had no significant relationship with MetS in the full multiple logistic regression model, whereas retinol+retinyl esters were inversely related to MetS among men only. The latter were also inversely related to elevated CRP and positively associated with hyperuricemia. Vitamin C exhibited a similar pattern to serum carotenoids with an inverse linear association with MetS (binary), HOMA-IR, and hyperuricemia. Future intervention studies of dietary and lifestyle change must be conducted to assess the utility of modifying serum antioxidant concentrations, especially carotenoids, given their suboptimal levels among U.S. adults with MetS, for the prevention of type 2 diabetes and various cardiovascular endpoints.
Introduction
Conceptualized as a clustering of several cardio-metabolic conditions (1), metabolic syndrome (MetS)8 includes abdominal obesity, high blood pressure (BP), hyperglycemia, and 2 types of dyslipidemia, elevated fasting TG and low levels of HDL-cholesterol (HDL-C) (2). Other MetS conditions include elevated C-reactive protein (CRP) level, high homeostatic model assessment insulin resistance (HOMA-IR), hyperuricemia, and hemostasis. MetS has been consistently associated with increased risks of type 2 diabetes, cardiovascular disease (CVD), and all-cause mortality (3–8).
Potential beneficial antiinflammatory and antioxidant effects were recently ascribed to naturally occurring micronutrients. These micronutrients may reduce oxidative stress triggered by injury characterizing pathogenesis of many chronic diseases, including type 2 diabetes, CVD, rheumatological conditions and carcinogenesis (9). Antioxidants, including carotenoids, in serum reflected their dietary intakes in several studies (10, 11). Although the primary dietary sources of carotenoids are fruits and vegetables (FV), they are also found in bread, eggs, beverages, fats, and oils (12). Among >40 carotenoids in the human diet, only β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin are ubiquitous in human serum (12).
Some observational studies showed inverse associations between carotenoids and CVD (13) and type 2 diabetes (14–18), but findings were inconsistent (13, 19–22). Importantly, only 1 study using national U.S. data (NHANES III, 1988–1994) examined the association between antioxidant status and MetS (23), and 4 studies looked at serum or dietary carotenoids in particular in relation to MetS (23–26). Despite accumulating evidence for potential beneficial effects of antioxidants for preventing MetS and its complications, more research is needed to differentiate the effects of major serum antioxidants on MetS and its components. Importantly, no previous study controlled for key confounders, including serum 25-hydroxyvitamin D [25(OH)D], which was shown to reduce the risk of MetS and its components (27–40). We used recent nationally representative data (NHANES 2001–2006) from U.S. adults aged 20–85 y to examine the association between serum antioxidant status and MetS. As a secondary objective, we also explored socio-demographic, lifestyle, and dietary predictors of serum antioxidant status.
Materials and Methods
Database and study population
The NHANES are a series of cross-sectional surveys of nationally representative samples, using a stratified, multistage probability cluster design and covering the U.S. noninstitutionalized population. NHANES consist of in-home interviews followed by health examinations in a mobile examination center (MEC) that include anthropometric, BP, and laboratory measurements (41). Procedures complied with ethical standards and approval was obtained from the relevant committee on human participants welfare (42).
NHANES data from adults aged 20–85 y from 2001–2006 were analyzed. Of 15,431 adults (n = 7341 men and n = 8090 women) with complete basic demographic data (Sample 1), 11,845 had complete serum antioxidant status (retinol, retinyl esters, total carotenoids, and vitamin E) and dietary data (total energy intake). Among those, physical activity (PA), smoking status, other dietary data, and supplement use data were complete for 8950 participants (Sample 2). In Sample 2, those with complete data on MetS components, including the National Cholesterol Education Program, Adult Treatment Panel III (NCEP ATP III) (2) and other components (Sample 3a–h) ranged between 4312 and 8949, whereas those with complete data on all NCEP ATP III components combined (Sample 4) had a sample size of 3008. In Sample 4, only 1574 had complete data on serum vitamin C levels (2003–2004 and 2005–2006 waves). The present study was approved for ethical treatment of participants by the Institutional Review Board of the National Institute on Aging, Intramural Research Program.
Outcome assessment
Anthropometric measures.
Waist circumference, among anthropometric measures in the MEC, was measured for all adults (43). While the adult person is not holding his/her breath, a tape measure is wrapped around the waist starting from the hip bone in a medium loose fashion and is kept parallel to the floor.
Blood pressure.
The mean of 3 blood pressure (systolic and diastolic) measurements (during MEC examinations for younger adults; during home visits for adults ≥ 50 y) using a mercury sphygmomanometer (44).
Laboratory tests.
Specimen collection and processing protocols for NHANES have been published (45). Fasting blood glucose was determined with a reaction between glucose and ATP catalyzed by hexokinase; serum or plasma TG was enzymatically measured with coupled reactions in which TG was hydrolyzed to produce glycerol; HDL-C was measured using 2 methods: A heparin-manganese (Mn) precipitation method or a direct immunoassay technique (in case of limited specimen sample). Total cholesterol was measured with coupled reactions using cholesteryl ester hydrolase, cholesterol oxidase, and peroxidase (Roche Hitachi Models 717 and 912). Fasting serum insulin was measured using RIA reagent (Pharmacia Diagnostics); uric acid level was measured by oxidization with uricase to form allantoin and H2O2 (Hitachi Model 737 Multichannel Analyzer, Boehringer Mannheim Diagnostics) and high-sensitivity CRP was quantified by latex-enhanced nephelometry (Behring Diagnostics).
Outcome variables: classification of MetS and its components.
Based on the NCEP ATP III (2005) (2), MetS was positive (MetS+) when 3 or more of the following conditions occurred simultaneously (46): 1) abdominal obesity defined by waist circumference ≥ 102 cm (40 inches, men), ≥ 88 cm (35 inches, women) (47); 2) dyslipidemia: TG ≥ 1.695 mmol/L (150 mg/dL); 3) dyslipidemia: HDL-C < 40 mg/dL (<1.036 mmol/L, male), <1.295 mmol/L (<50 mg/dL, female); 4) BP ≥ 130/85 mm Hg; 5) fasting plasma glucose ≥ 5.55 mmol/L (100 mg/dL) (2). The number of metabolic disturbances was also examined in its continuous form (MetS count score ranging between 0 and 5). Additionally, we examined: 1) elevated HOMA-IR (>2.61) reflecting insulin resistance (48, 49); 2) hyperuricemia [>416.36 μmol/L (7 mg/dL) in men and >356.88 μmol/L (>6 mg/dL) in women] (50); 3) elevated CRP defined as >2.11 mg/L (0.211 mg/dL) (51).
Exposure assessment: serum concentrations of key antioxidants
Serum concentrations of key antioxidants were measured using HPLC with photodiode array detection. In this study, retinol and retinyl esters (sum of retinyl palmitate and retinyl stearate) were of interest, separately as well as their sum. Carotenoids were grouped as α-carotene, β-carotene (cis+trans), β-cryptoxanthin, lutein+zeaxanthin, lycopene, or as total carotenoids. Vitamin E was defined as the sum of α- and γ-tocopherol. Although carotenoids, retinol+retinyl esters and vitamin E were available for all 3 NHANES waves (2001–2002, 2003–2004, and 2005–2006), vitamin C was available only for 2003–2004 and 2005–2006. A sensitivity analysis was therefore conducted that included vitamin C as an exposure.
Covariates
Socio-demographic.
Socio-demographic covariates included age, sex, race/ethnicity [non-Hispanic (NH) whites, NH blacks, Mexican Americans, and other ethnicities], education (<high school (HS), HS, >HS), marital status (married vs. other), and family income [poverty income ratio (PIR): <100, 100–200, >200%].
Lifestyle factors. Physical activity and cigarette smoking.
Physical activity (PA) was constructed based on cumulative self-reported leisure-time activities. Intensity scores were computed from the product of each activity’s metabolic equivalent (MET), the duration of the activity, and each activity’s frequency per week. Cumulative MET⋅h⋅wk−1 for each participant depended on the number of leisure-time PA elicited by this participants: those not eliciting any activity were considered sedentary (score = 0) (52–54) and others were categorized as 0 to <5, 5–10, and >10 in the main part of the analysis. Cigarette smoking status was defined as never, former, or current smoker.
Dietary intakes.
All participants were eligible for two 24-h recalls in the 2003–2004 and 2005–2006 waves and for one 24-h recall in the 2001–2002 wave. The first recall was administered during MEC examinations and the second 3–10 d later by telephone interview. The mean of two 24-h recalls was examined from which nutrient intakes were estimated using a revised nutrient database (55). Total dietary intakes of energy (1 kcal = 4.187 kJ), fiber, alcohol, caffeine, and selected antioxidants (β-carotene, vitamin C, and vitamin E) were included as potential confounders (continuous variables) based on their putative associations with MetS (19, 20, 26, 56–58). Dietary supplement use over the past 30 d was summarized as 0, nonuser; 1, using 1 supplement; 2, using 2 supplements or more.
Other nutritional biomarkers.
Serum folate and vitamin B-12 were measured by using a Bio-Rad Laboratories Quantaphase II Folate RIA kit (45); (1 μg/L of folate = 2.266 nmol/L; 1 pg/mL of vitamin B-12 = 0.738 pmol/L). Total homocysteine (tHcy) in plasma was measured using a Abbott Homocysteine (HCY) assay, a fully automated technique (59, 60); (1 mg/L of tHcy = 7.397 μmol/L). Serum 25(OH)D was measured by RIA (Diasorin) [1 μg/L of 25(OH)D = 2.496 nmol/L] (45). These nutritional biomarkers were considered as potential confounders and entered into models in their continuous forms.
Statistical methods
Using Stata 11.0 (61), we first characterized our study sample by sex and MetS status. Differences in means across groups and associations between categorical variables were tested with t and design-based χ2 tests, respectively. Secondly, multiple linear ordinary least square (OLS) regression models were conducted in which we assessed potential socio-demographic, lifestyle, and dietary predictors of serum antioxidants (continuous retinol+retinyl esters, total carotenoids and Loge-transformed vitamins E and C) using the Wald test. Third, multiple logistic regression models were conducted to test associations between antioxidant status (standardized Z-scores computed from the total NHANES sample aged 20–85 y with available antioxidant data) and MetS (and components) binary outcomes, controlling for confounders and stratifying by sex. These associations were also tested with MetS count (range: 0–5) using multiple zero-inflated Poisson (ZIP) regression models. Significance of regression coefficients was assessed using the Wald test. Finally, to test dose-response relationships, quartiles of main antioxidants were entered into multiple logistic and ZIP regression models as ordinal variables, and P-trends were computed from the Wald test. In all analyses, sampling design complexity was taken into account using Stata survey commands. The 2-y fasting half-sample MEC exam weights were used for most sample estimations (high BP and abdominal obesity excepted where full MEC exam weights were used) and masked variance units allowed estimating variances using the Taylor series linearization method. P-values presented are 2-tailed and the α error was 0.05.
Results
MetS prevalence was estimated at 32.0% among men and 29.5% among women. Participants with MetS (MetS+) were generally older and had higher serum retinol+retinyl ester and vitamin E but lower total carotenoid and vitamin C compared with MetS− participants (P < 0.05). Among men, MetS+ consisted of a higher proportion of NH whites, married participants, former and current smokers, and participants with lower mean PA levels compared with MetS− participants. Among women, MetS+ participants had higher educational attainment compared with MetS− participants. In addition to differences in dietary intakes, MetS+ participants (compared with MetS−) had significantly lower serum 25(OH)D and higher tHcy, whereas vitamin B-12 was significantly lower in MetS+ participants among men only (Table 1). Men had worse metabolic profiles than women (except for MetS binary status, P > 0.05), although MetS status clearly discriminated between poor and better metabolic profile on all measures in both sexes (Supplemental Table 1).
TABLE 1.
Selected baseline characteristics of NHANES 2001–2006 participants by sex and MetS status (n = 3202)1
| Socio-demographic, lifestyle factors | Men |
Women |
P2 |
|||||||
| MetS− | MetS+ | All men | MetS− | MetS+ | All women | Men vs. women | MetS− vs. MetS+ among men | MetS− vs. MetS+ among women | ||
| n | 1063 | 534 | 1597 | 1091 | 515 | 1606 | ||||
| Age, y | 42.4 ± 0.7 | 49.1 ± 0.8 | 44.6 ± 0.6 | 42.2 ± 0.8 | 53.6 ± 0.8 | 45.6 ± 0.7 | 0.001 | <0.001 | <0.001 | |
| Race/ethnicity, % | ||||||||||
| NH white | 72.7 ± 2.2 | 78.5 ± 3.0 | 74.6 ± 2.2 | 75.1 ± 2.0 | 73.8 ± 3.4 | 74.7 ± 2.0 | 0.627 | 0.037 | 0.797 | |
| NH black | 10.5 ± 1.1 | 6.2 ± 9.7 | 9.1 ± 0.9 | 9.5 ± 1.2 | 9.8 ± 1.6 | 9.6 ± 1.2 | ||||
| Mexican American | 8.2 ± 1.0 | 6.9 ± 1.1 | 7.7 ± 0.9 | 6.4 ± 0.6 | 7.5 ± 1.2 | 6.7 ± 0.7 | ||||
| Other ethnicity | 8.5 ± 1.3 | 8.4 ± 2.3 | 8.4 ± 1.4 | 9.0 ± 1.5 | 9.0 ± 2.7 | 9.0 ± 1.6 | ||||
| Married, % | 56.5 ± 2.0 | 67.3 ± 2.7 | 60.0 ± 1.5 | 56.5 ± 1.9 | 62.5 ± 2.6 | 58.2 ± 1.6 | 0.297 | 0.004 | 0.065 | |
| Education, % | ||||||||||
| <HS | 5.0 ± 0.7 | 7.2 ± 1.0 | 5.7 ± 0.6 | 4.6 ± 0.7 | 8.9 ± 1.3 | 5.9 ± 0.6 | 0.052 | 0.057 | <0.001 | |
| HS | 36.6 ± 1.6 | 39.8 ± 2.4 | 37.6 ± 1.6 | 27.4 ± 1.6 | 47.1 ± 2.5 | 33.2 ± 1.5 | ||||
| >HS | 58.4 ± 1.9 | 53.1 ± 2.6 | 56.7 ± 1.7 | 68.0 ± 1.8 | 43.9 ± 2.3 | 60.9 ± 1.5 | ||||
| PIR, % | ||||||||||
| <100 | 8.9 ± 1.0 | 8.1 ± 1.2 | 8.7 ± 0.9 | 10.3 ± 1.4 | 13.3 ± 2.6 | 11.2 ± 1.3 | 0.001 | 0.371 | 0.412 | |
| 100 to <200 | 16.0 ± 1.5 | 18.9 ± 2.2 | 16.8 ± 1.3 | 20.4 ± 1.5 | 21.8 ± 2.5 | 20.8 ± 1.2 | ||||
| ≥200 | 75.2 ± 1.9 | 73.0 ± 2.7 | 74.5 ± 1.7 | 69.3 ± 2.4 | 64.9 ± 3.2 | 68.0 ± 1.9 | ||||
| Smoking status, % | ||||||||||
| Never smoker | 64.5 ± 2.1 | 61.6 ± 2.7 | 63.6 ± 1.9 | 75.1 ± 1.8 | 70.9 ± 2.4 | 73.9 ± 1.7 | <0.001 | 0.191 | 0.065 | |
| Former smoker | 17.6 ± 2.0 | 22.3 ± 2.1 | 19.1 ± 1.6 | 12.0 ± 1.3 | 16.7 ± 2.0 | 13.4 ± 1.2 | ||||
| Current smoker | 17.9 ± 1.6 | 16.0 ± 2.3 | 17.3 ± 1.5 | 12.9 ± 1.4 | 12.3 ± 1.8 | 12.7 ± 1.2 | ||||
| PA, − | 13.3 ± 0.9 | 9.1 ± 0.6 | 12.0 ± 0.7 | 9.3 ± 0.6 | 9.6 ± 2.0 | 9.4 ± 0.7 | <0.001 | 0.007 | 0.758 | |
| Serum antioxidant, μmol/L | ||||||||||
| Total retinol+retinyl esters | 2.35 ± 0.02 | 2.41 ± 0.03 | 2.37 ± 0.02 | 2.02 ± 0.02 | 2.30 ± 0.03 | 2.10 ± 0.02 | <0.001 | <0.001 | <0.001 | |
| Retinol | 2.26 ± 0.02 | 2.31 ± 0.03 | 2.27 ± 0.02 | 1.95 ± 0.02 | 2.19 ± 0.03 | 2.02 ± 0.02 | <0.001 | <0.001 | <0.001 | |
| Retinyl esters | 0.087 ± 0.003 | 0.095 ± 0.005 | 0.090 ± 0.003 | 0.078 ± 0.002 | 0.089 ± 0.007 | 0.081 ± 0.003 | 0.569 | 0.053 | 0.012 | |
| Total carotenoids | 1.35 ± 0.03 | 1.14 ± 0.02 | 1.28 ± 0.03 | 1.50 ± 0.03 | 1.19 ± 0.03 | 1.41 ± 0.03 | <0.001 | <0.001 | <0.001 | |
| α-Carotene | 0.079 ± 0.005 | 0.056 ± 0.004 | 0.072 ± 0.004 | 0.110 ± 0.007 | 0.070 ± 0.004 | 0.098 ± 0.005 | <0.001 | <0.001 | <0.001 | |
| β-Carotene | 0.35 ± 0.02 | 0.24 ± 0.01 | 0.31 ± 0.01 | 0.48 ± 0.02 | 0.32 ± 0.01 | 0.43 ± 0.02 | <0.001 | <0.001 | <0.001 | |
| β-Cryptoxanthin | 0.172 ± 0.006 | 0.136 ± 0.004 | 0.161 ± 0.004 | 0.181 ± 0.005 | 0.155 ± 0.007 | 0.173 ± 0.005 | 0.001 | <0.001 | 0.013 | |
| Lutein+zeaxanthin | 0.285 ± 0.006 | 0.267 ± 0.008 | 0.279 ± 0.005 | 0.295 ± 0.007 | 0.253 ± 0.009 | 0.283 ± 0.006 | 0.235 | 0.063 | <0.001 | |
| Lycopene | 0.464 ± 0.011 | 0.440 ± 0.011 | 0.456 ± 0.008 | 0.435 ± 0.008 | 0.390 ± 0.010 | 0.422 ± 0.007 | 0.003 | 0.003 | <0.001 | |
| Vitamin E | 28.5 ± 0.5 | 33.8 ± 0.9 | 30.2 ± 0.5 | 28.2 ± 0.5 | 35.3 ± 0.9 | 30.3 ± 0.5 | 0.028 | <0.001 | <0.001 | |
| Vitamin C3 | 50.9 ± 1.4 | 43.7 ± 1.5 | 48.6 ± 1.0 | 57.7 ± 1.2 | 52.1 ± 2.4 | 56.1 ± 1.4 | <0.001 | <0.001 | 0.001 | |
| Dietary intakes | ||||||||||
| Total energy,4kcal/d | 2,748 ± 39.0 | 2,585 ± 64.7 | 2,695 ± 35.7 | 1,851 ± 22.1 | 1,744 ± 32.3 | 1820 ± 18.8 | <0.001 | <0.001 | ||
| Alcohol, g/d | 18.4 ± 1.5 | 13.9 ± 1.3 | 16.9 ± 1.1 | 16.7 ± 0.6 | 4.0 ± 0.9 | 5.9 ± 0.5 | <0.001 | 0.085 | ||
| Caffeine, mg/d | 218.2 ± 8.6 | 217.8 ± 10.8 | 218.1 ± 7.2 | 159.9 ± 9.2 | 160.9 ± 10.3 | 160.2 ± 8.6 | <0.001 | 0.677 | ||
| Fiber, g/d | 18.3 ± 0.4 | 17.1 ± 0.5 | 17.9 ± 0.3 | 14.8 ± 0.3 | 14.2 ± 0.4 | 14.6 ± 0.2 | <0.001 | 0.018 | ||
| β-Carotene, mg/d | 2.22 ± 0.16 | 1.90 ± 0.15 | 2.11 ± 0.12 | 2.20 ± 0.09 | 2.08 ± 0.14 | 2.17 ± 0.08 | 0.885 | 0.112 | ||
| Vitamin C, mg/d | 96.5 ± 3.3 | 95.3 ± 5.9 | 96.1 ± 3.0 | 85.4 ± 2.8 | 81.2 ± 3.9 | 84.2 ± 2.3 | 0.022 | 0.297 | ||
| Vitamin E, mg/d | 8.2 ± 0.2 | 8.0 ± 0.2 | 8.2 ± 0.2 | 6.6 ± 0.1 | 6.1 ± 0.2 | 6.4 ± 0.1 | <0.001 | 0.060 | ||
| Dietary supplement use | <0.001 | 0.460 | ||||||||
| None | 44.9 ± 1.9 | 40.6 ± 2.5 | 43.5 ± 1.3 | 30.5 ± 1.7 | 33.0 ± 2.5 | 31.2 ± 1.4 | ||||
| One | 27.4 ± 1.7 | 30.2 ± 2.4 | 28.3 ± 1.3 | 28.7 ± 1.4 | 27.8 ± 2.3 | 28.4 ± 1.3 | ||||
| ≥2 | 27.7 ± 1.7 | 29.2 ± 2.4 | 28.2 ± 1.3 | 40.8 ± 2.1 | 39.2 ± 2.5 | 40.3 ± 1.8 | ||||
| Other serum measures | ||||||||||
| Folate, nmol/L | 28.3 ± 0.4 | 28.5 ± 0.9 | 28.4 ± 0.4 | 28.5 ± 0.9 | 31.6 ± 0.6 | 32.1 ± 0.6 | <0.001 | 0.200 | ||
| Vitamin B-12, pmol/L | 380.3 ± 4.5 | 358.2 ± 9.2 | 373.2 ± 4.8 | 372.5 ± 6.4 | 370.2 ± 10.6 | 371.8 ± 5.6 | 0.705 | 0.012 | ||
| tHcy, μmol/L | 8.8 ± 0.1 | 9.5 ± 0.2 | 9.0 ± 0.1 | 7.4 ± 0.1 | 8.0 ± 0.2 | 7.6 ± 0.1 | <0.001 | <0.001 | ||
| 25(OH)D,5μg/L | 24.4 ± 0.5 | 22.6 ± 0.5 | 23.8 ± 0.4 | 25.1 ± 0.5 | 21.6 ± 0.6 | 24.1 ± 0.5 | 0.120 | 0.004 | ||
Values are mean ± SEM or percent ± SE of the proportion, = 3203. Sampling design complexity is taken into account in all analyses. This analysis was done among participants with complete data for MetS screening using the NCEP ATP III criteria and other key variables of interest.
-value was based on t test when row variable is continuous and design-based χ2 test when row variable is categorical.
Vitamin C was available only for the 2003–2004 and 2005–2006 NHANES waves and thus sample size in this analysis was restricted to = 895 men (288 are MetS+) and 849 women (244 are MetS+).
1 kcal = 4.187 kJ.
To convert to mol/L, multiply by 2496.
Findings from OLS multiple regression models (Table 2) indicated a higher antioxidant status with age (retinol+retinyl esters, carotenoids, and vitamin E) among women (total carotenoids and vitamin C) and among men (retinol+retinyl esters) and an independent predictive value for race/ethnicity (all 4 antioxidants). Higher serum carotenoid and retinol+retinyl ester concentrations were found among married adults. Education was positively associated with serum retinol+retinyl esters and vitamin E and PIR was directly related to carotenoids, vitamin E, and vitamin C. Current smoking status was inversely related to serum carotenoids and vitamin C. PA was positively related and energy intake was negatively related to most antioxidant levels (retinol+retinyl esters excepted). Carotenoids were also significantly predicted by higher alcohol, fiber, β-carotene, and vitamin C intakes and by supplement use. Furthermore, vitamin C status was associated with supplement use, higher alcohol, fiber, and vitamin C intakes, and lower caffeine intake. Supplement use was positively associated with all other antioxidant variables, with a clear dose-response relationship. Retinol+retinyl esters were positively associated with alcohol intake, whereas serum vitamin E was inversely related to energy and caffeine intakes and positively associated with fiber, alcohol, and vitamin C intakes.
TABLE 2.
Socio-demographic, lifestyle, and dietary predictors of serum antioxidant concentrations of U.S. adults (NHANES 2001–2006): OLS multiple regression models1
| Retinol+retinyl esters, μmol | Total carotenoids, μmol | Loge (vitamin E), μmol | Loge (vitamin C)3, μmol | |
| β ± SEE | ||||
| n | 9099 | 9099 | 9099 | 5145 |
| Age | +0.0086 ± 0.00052 | +0.0020 ± 0.00062 | +0.0083 ± 0.00032 | +0.0008 ± 0.0008 |
| Sex, women vs. men | −0.2658 ± 0.01772 | +0.1119 ± 0.01852 | −0.0023 ± 0.0093 | +0.1424 ± 0.02882 |
| NH white | Ref | Ref | Ref | Ref |
| NH black | −0.2328 ± 0.02302 | +0.1004 ± 0.02722 | −0.0776 ± 0.01052 | +0.0462 ± 0.0336 |
| Mexican American | −0.1676 ± 0.02042 | +0.1574 ± 0.03312 | +0.0459 ± 0.00932 | +0.0903 ± 0.04102 |
| Other ethnicity | −0.1127 ± 0.02862 | +0.0803 ± 0.0411 | +0.0153 ± 0.0159 | +0.0223 ± 0.0371 |
| Married | −0.0476 ± 0.01552 | +0.0634 ± 0.01532 | +0.0244 ± 0.0089 | +0.0005 ± 0.0214 |
| Education, <HS | Ref | Ref | Ref | Ref |
| HS | +0.0803 ± 0.02352 | −0.0719 ± 0.03372 | +0.0344 ± 0.01662 | −0.0512 ± 0.0377 |
| >HS | +0.0736 ± 0.02622 | +0.0343 ± 0.0392 | +0.0384 ± 0.01772 | −0.0278 ± 0.0472 |
| PIR, <100% | Ref | Ref | Ref | Ref |
| 100 to <200% | +0.0257 ± 0.0292 | +0.0155 ± 0.0313 | −0.0050 ± 0.0178 | +0.0273 ± 0.0309 |
| ≥200% | +0.0355 ± 0.0247 | +0.0956 ± 0.02622 | +0.0346 ± 0.01622 | +0.0812 ± 0.03222 |
| Never smoker | Ref | Ref | Ref | Ref |
| Former smoker | −0.0249 ± 0.0175 | −0.0112 ± 0.0335 | −0.0069 ± 0.0106 | −0.0178 ± 0.0303 |
| Current smoker | −0.0378 ± 0.0269 | −0.1652 ± 0.02212 | −0.0163 ± 0.0109 | −0.3035 ± 0.03292 |
| PA, Met · hr· wk-1, 0 to 5 | Ref | Ref | Ref | Ref |
| 5–10 | 0.0118 ± 0.0203 | +0.0900 ± 0.02522 | +0.0477 ± 0.01132 | +0.1259 ± 0.04682 |
| >10 | 0.0285 ± 0.0265 | +0.1144 ± 0.02612 | +0.0418 ± 0.01132 | +0.1394 ± 0.04852 |
| Total energy intake, per 100 kcal/d | −0.0013 ± 0.0012 | −0.0083 ± 0.00112 | −0.0016 ± 0.00072 | −0.0056 ± 0.00132 |
| Alcohol intake, per 10 g/d | +0.0030 ± 0.00032 | +0.0058 ± 0.00252 | +0.0034 ± 0.0018 | +0.0173 ± 0.00612 |
| Caffeine intake, per 10 mg/d | −0.0000 ± 0.0000 | −0.0006 ± 0.0004 | −0.0002 ± 0.0002 | −0.0029 ± 0.00052 |
| Fiber, per g/d | +0.0008 ± 0.0010 | +0.0143 ± 0.00132 | +0.0019 ± 0.00062 | +0.0079 ± 0.00172 |
| β-Carotene, per 1 mg/d | +0.0012 ± 0.0023 | +0.0363 ± 0.00002 | −0.0013 ± 0.0016 | +0.0014 ± 0.0043 |
| Vitamin C, per 10 mg/d | +0.0015 ± 0.0017 | +0.0093 ± 0.00142 | +0.0013 ± 0.00062 | +0.0186 ± 0.00152 |
| Vitamin E, per mg/d | +0.0021 ± 0.0017 | +0.0041 ± 0.0023 | +0.0035 ± 0.00132 | +0.0048 ± 0.0027 |
| Dietary supplement use, none | Ref | Ref | Ref | Ref |
| One | +0.1073 ± 0.01702 | +0.0423 ± 0.02022 | +0.1027 ± 0.01022 | +0.1788 ± 0.03082 |
| ≥2 | +0.1960 ± 0.01792 | +0.2009 ± 0.02262 | +0.2804 ± 0.01112 | +0.4326 ± 0.03292 |
Values are β ± SEE. Sampling design complexity is taken into account in all analyses. All models were multivariate-adjusted for all variables included in this table. This analysis was performed on the sample with complete antioxidant status, demographic, lifestyle factors, and dietary data. Ref, referent category.
< 0.05 for null hypothesis that β = 0 based on Wald test.
Vitamin C was available only for the 2003–2004 and 2005–2006 NHANES waves and thus sample size in this analysis was restricted to = 5145.
Adjusted associations between selected serum antioxidant levels and MetS (binary and counts) were tested (Table 3). In model 1, which controlled for socio-demographic, lifestyle, and dietary factors, the odds of MetS among men and women for each SD of vitamin E were increased by 94%, whereas for β-carotene, the odds were reduced by 45% [model 1 adjusted OR = 0.55 (95% CI = 0.40–0.74)]. These significant associations were found consistently with MetS count outcome. Inverse relationships between other carotenoids and MetS were found in men only (β-cryptoxanthin) or in women only (lutein+zeaxanthin), without significant effect modification by sex. Retinol was positively associated with MetS binary outcome and count score among women. After additionally controlling for serum cholesterol and TG in model 2, the positive association between vitamin E and MetS binary outcome became null but remained significant among women for the count score. In contrast, retinol and retinyl esters had significant inverse associations with MetS binary outcome among men. Although associations for β-carotene and lutein+zeaxanthin (both sexes combined) were markedly attenuated by adding serum cholesterol and TG as covariates, they remained significant by reducing the odds of MetS by 18% with each 1-SD increase in their respective values. In model 3, there was an inverse association between total carotenoids and MetS (both sexes combined). However, when adding serum cholesterol and TG as covariates (model 4), this association was markedly attenuated among women [OR = 0.45 (model 3) vs. 0.69 (model 4); P < 0.05]. When model 4 was reexamined (both sexes combined) by adding serum vitamin C among main exposures (n = 1574), vitamin C and carotenoids were both inversely related to MetS count and binary outcomes (P < 0.05). In particular, a 1-SD increase (~28.5μmol/L) in vitamin C was associated with a 24% lower prevalence odds of MetS (OR = 0.76, 95% CI = 0.58–0.98) (data not shown).
TABLE 3.
Associations between concentrations of selected serum antioxidants (per 1-SD increase) and MetS: multiple logistic and ZIP regression models (NHANES 2001–2006)1
| MetS+ vs. MetS− |
MetS count score |
|||||
| All, n = 3008 | Men, n = 1483 | Women, n = 1525 | All, n = 3008 | Men, n = 1483 | Women, n = 1525 | |
| OR(95% CI) | β ± SEE | |||||
| Model 13 | ||||||
| Retinol, per 0.63 μmol/L | 1.13 (0.99; 1.29) | 0.95 (0.80; 1.13) | 1.342 (1.06; 1.70) | +0.04 ± 0.022 | +0.02 ± 0.02 | +0.07 ± 0.032 |
| Retinyl esters, per 0.09 μmol/L | 1.16 (0.97; 1.38) | 1.08 (0.89; 1.32) | 1.40 (0.92; 2.13) | +0.01 ± 0.01 | +0.05 ± 0.022 | −0.00 ± 0.01 |
| α-Carotene, per 0.11 μmol/L | 0.95 (0.74; 1.20) | 1.01 (0.72; 1.42) | 0.82 (0.59; 1.17) | −0.01 ± 0.03 | −0.03 ± 0.04 | −0.00 ± 0.05 |
| β-Carotene, per 0.41 μmol/L | 0.552 (0.40; 0.74) | 0.542 (0.32; 0.93) | 0.562 (0.37; 0.83) | −0.19 ± 0.042 | −0.16 ± 0.052 | −0.20 ± 0.052 |
| β-Cryptoxanthin, per 0.16 μmol/L | 0.86 (0.69; 1.08) | 0.702 (0.50; 0.97) | 1.01 (0.77; 1.33) | −0.08 ± 0.032 | −0.12 ± 0.042 | −0.05 ± 0.03 |
| Lutein+zeaxanthin, per 0.16 μmol/L | 0.762 (0.63; 0.91) | 0.89 (0.70; 1.13) | 0.672 (0.51; 0.88) | −0.08 ± 0.022 | −0.05 ± 0.03 | −0.02 ± 0.03 |
| Lycopene, per 0.20 μmol/L | 1.02 (0.88; 1.20) | 1.05 (0.84; 1.32) | 0.93 (0.76; 1.15) | +0.01 ± 0.02 | +0.02 ± 0.02 | −0.02 ± 0.03 |
| Vitamin E, per 14.6 μmol/L | 1.942 (1.63; 2.30) | 1.792 (1.35; 2.35) | 2.152 (1.69; 2.74) | +0.19 ± 0.012 | +0.16 ± 0.032 | 0.19 ± 0.022 |
| Model 24 | ||||||
| Retinol, per 0.63 μmol/L | 0.93 (0.79; 1.10) | 0.762 (0.60; 0.95) | 1.09 (0.86; 1.39) | +0.02 ± 0.02 | +0.01 ± 0.02 | +0.03 ± 0.02 |
| Retinyl esters, per 0.09 μmol/L | 0.832 (0.73; 0.95) | 0.522 (0.38; 0.70) | 1.12 (0.77; 1.63) | −0.05 ± 0.03 | −0.11 ± 0.032 | −0.03 ± 0.02 |
| α-Carotene, per 0.11 μmol/L | 0.96 (0.80; 1.15) | 1.06 (0.83; 1.37) | 0.82 (0.64; 1.05) | −0.01 ± 0.03 | −0.00 ± 0.04 | −0.01 ± 0.05 |
| β-Carotene, per 0.41 μmol/L | 0.822 (0.69; 0.98) | 0.87 (0.66; 1.15) | 0.802 (0.68; 0.95) | −0.13 ± 0.032 | −0.10 ± 0.042 | −0.14 ± 0.052 |
| β-Cryptoxanthin, per 0.16 μmol/L | 0.88 (0.70; 1.11) | 0.692 (0.51; 0.94) | 1.14 (0.86; 1.53) | −0.07 ± 0.022 | −0.11 ± 0.042 | −0.03 ± 0.03 |
| Lutein+zeaxanthin, per 0.16 μmol/L | 0.822 (0.68; 0.99) | 0.95 (0.76; 1.20) | 0.79 (0.60; 1.05) | −0.08 ± 0.022 | −0.06 ± 0.032 | −0.08 ± 0.032 |
| Lycopene, per 0.20 μmol/L | 1.04 (0.88; 1.23) | 1.14 (0.92; 1.42) | 0.98 (0.77; 1.23) | +0.01 ± 0.02 | +0.04 ± 0.02 | −0.04 ± 0.03 |
| Vitamin E, per 14.6 μmol/L | 1.01 (0.87; 1.17) | 0.87 (0.66; 1.15) | 1.18 (0.88; 1.60) | +0.05 ± 0.032 | −0.03 ± 0.03 | +0.06 ± 0.032 |
| Model 35 | ||||||
| Total retinol+retinyl esters, per 0.66 μmol/L | 1.192 (1.05; 1.36) | 0.98 (0.82; 1.17) | 1.472 (1.18; 1.82) | +0.06 ± 0.012 | +0.03 ± 0.02 | +0.08 ± 0.022 |
| Total carotenoids, per 0.73 μmol/L | 0.502 (0.43; 0.58) | 0.542 (0.43; 0.68) | 0.452 (0.37; 0.55) | −0.24 ± 0.022 | −0.21 ± 0.032 | −0.27 ± 0.032 |
| Vitamin E, per 14.6 μmol/L | 1.962 (1.68; 2.30) | 1.892 (1.45; 2.46) | 2.102 (1.70; 2.60) | +0.19 ± 0.012 | +0.18 ± 0.022 | +0.18 ± 0.022 |
| Model 46 | ||||||
| Total retinol+retinyl esters, per 0.66 μmol/L | 0.90 (0.76; 1.07) | 0.702 (0.55; 0.88) | 1.12 (0.89; 1.41) | +0.02 ± 0.02 | +0.00 ± 0.03 | +0.02 ± 0.02 |
| Total carotenoids, per 0.73 μmol/L | 0.672 (0.58; 0.77) | 0.702 (0.55; 0.88) | 0.692 (0.57; 0.83) | −0.22 ± 0.022 | −0.18 ± 0.032 | −0.23 ± 0.032 |
| Vitamin E, per 14.6 μmol/L | 0.99 (0.84; 1.15) | 0.83 (0.63; 1.10) | 1.15 (0.86; 1.54) | +0.04 ± 0.022 | +0.01 ± 0.03 | +0.05 ± 0.03 |
Values are OR with 95% CI or β ± SEE. An appreciable drop in sample size (vitamin C available only for the 2003–2004 and 2005–2006 waves of NHANES). A sensitivity analysis was conducted to examine the association between quartiles of vitamin C and MetS (and its components). See Figure 1 and Table 5, Results section.
< 0.05 for null hypothesis that β = 0 or Loge(OR) = 0 based on Wald test.
Model 1 included all antioxidant exposures simultaneously and adjusted for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level, PIR, and other potential confounders: lifestyle and health-related factors (smoking status, PA: Met⋅h⋅wk−1, recoded as 0 to <5, 5–10, >10) and dietary intakes (total energy intake, alcohol, caffeine, β-carotene, vitamin C, vitamin E, and dietary supplement use), serum levels of folate, tHcy, vitamin B-12, and 25(OH)D.
Model 2 is model 1 but additionally adjusted for serum total cholesterol and triglycerides, given the high correlation between lipid-soluble vitamins and serum lipids.
Model 3 is model 1 but with main exposures being the total retinol+retinyl esters, total carotenoids and vitamin E.
Model 4 is model 3 but additionally adjusted for serum total cholesterol and triglycerides, given the high correlation between lipid-soluble vitamins and serum lipids.
Associations between components of MetS and serum antioxidant status controlling for potential confounders, including serum cholesterol and TG (except when the outcome was hypertriglyceridemia, whereby only serum cholesterol was included), were further tested (Table 4). In the total U.S. adult population (both sexes combined), inverse associations between retinol+retinyl esters and MetS components were noted for abdominal obesity, low HDL-C, and elevated CRP, and there was a positive association for hypertriglyceridemia and hyperuricemia. Moreover, there was an inverse relationship between total carotenoids and all MetS components. Vitamin E had an inverse relationship with elevated HOMA-IR among women only but a positive association with hypertriglyceridemia.
TABLE 4.
Associations between concentrations of selected serum antioxidants (per 1-SD increase) and MetS components: multiple logistic regression models (NHANES 2001–2006)12
| MetS binary components |
||||||
| All |
Men |
Women |
||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| NCEP ATP III components4 | ||||||
| Abdominal obesity | n = 4218 | n = 2053 | n = 2165 | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 0.883 | (0.79; 0.97) | 0.823 | (0.70; 0.95) | 0.92 | (0.79; 1.07) |
| Total carotenoids, per 0.73 μmol/L | 0.583 | (0.51; 0.65) | 0.593 | (0.50; 0.68) | 0.603 | (0.49; 0.74) |
| Vitamin E, per 14.6 μmol/L | 1.03 | (0.92; 1.15) | 0.93 | (0.78; 1.11) | 1.09 | (0.90; 1.31) |
| Hypertriglyceridemia | n = 4322 | n = 2099 | n = 2223 | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 1.813 | (1.64; 2.01) | 1.933 | (1.61; 2.30) | 1.763 | (1.53; 2.01) |
| Total carotenoids, per 0.73 μmol/L | 0.393 | (0.34; 0.45) | 0.383 | (0.30; 0.47) | 0.403 | (0.32; 0.50) |
| Vitamin E, per 14.6 μmol/L | 3.143 | (2.51; 3.92) | 3.813 | (2.69; 5.39) | 2.783 | (2.13; 3.64) |
| Low HDL-C | n = 4322 | n = 2099 | n = 2223 | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 0.763 | (0.65; 0.89) | 0.673 | (0.54; 0.84) | 0.823 | (0.68; 1.00) |
| Total carotenoids, per 0.73 μmol/L | 0.653 | (0.55; 0.77) | 0.673 | (0.50; 0.88) | 0.653 | (0.53; 0.81) |
| Vitamin E, per 14.6 μmol/L | 1.01 | (0.85; 1.22) | 0.88 | (0.58; 1.38) | 1.12 | (0.92; 1.38) |
| High blood pressure | n = 3071 | n = 1510 | n = 1561 | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 1.09 | (0.99; 1.21) | 0.99 | (0.85; 1.10) | 1.263 | (1.06; 1.49) |
| Total carotenoids, per 0.73 μmol/L | 0.783 | (0.68; 0.90) | 0.773 | (0.61; 0.97) | 0.733 | (0.59; 0.91) |
| Vitamin E, per 14.6 μmol/L | 0.89 | (0.75; 1.04) | 0.80 | (0.62; 1.04) | 1.00 | (0.79; 1.26) |
| Hyperglycemia | n = 4315 | n = 2094 | n = 2221 | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 1.03 | (0.92; 1.15) | 1.01 | (0.87; 1.19) | 1.01 | (0.85; 1.20) |
| Total carotenoids, per 0.73 μmol/L | 0.803 | (0.70; 0.92) | 0.92 | (0.80; 1.07) | 0.733 | (0.62; 0.87) |
| Vitamin E, per 14.6 μmol/L | 0.95 | (0.85; 1.05) | 0.94 | (0.78; 1.13) | 0.93 | (0.74; 1.16) |
| Other components4 | ||||||
| Elevated HOMA-IR | n = 4305 | n = 2089 | n = 2216 | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 0.99 | (0.89; 1.10) | 0.92 | (0.79; 1.15) | 1.08 | (0.91; 1.29) |
| Total carotenoids, per 0.73 μmol/L | 0.623 | (0.55; 0.70) | 0.653 | (0.55; 0.77) | 0.613 | (0.51; 0.73) |
| Vitamin E, per 14.6 μmol/L | 0.89 | (0.75; 1.04) | 0.95 | (0.79; 1.15) | 0.753 | (0.60; 0.95) |
| Elevated CRP | n = 4322 | n = 2099 | n = 2223 | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 0.513 | (0.36; 0.72) | 0.423 | (0.22; 0.83) | 0.593 | (0.38; 0.93) |
| Total carotenoids, per 0.73 μmol/L | 0.353 | (0.22; 0.55) | 0.323 | (0.15; 0.68) | 0.363 | (0.21; 0.62) |
| Vitamin E, per 14.6 μmol/L | 1.29 | (0.93; 1.78) | 1.46 | (0.89; 2.40) | 1.34 | (0.87; 2.07) |
| Hyperuricemia | n = 3284 | n = 1580 | n = 1704 | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 1.403 | (1.24; 1.60) | 1.303 | (1.07; 1.58) | 1.513 | (1.21; 1.87) |
| Total carotenoids, per 0.73 μmol/L | 0.583 | (0.46; 0.74) | 0.663 | (0.47; 0.91) | 0.493 | (0.36; 0.68) |
| Vitamin E, per 14.6 μmol/L | 0.88 | (0.73; 1.05) | 0.81 | (0.63; 1.02) | 0.89 | (0.69; 1.15) |
Values are OR with 95% CI. Sampling design complexity is taken into account in all analyses. Vitamin C was not included in this analysis due to an appreciable drop in sample size (vitamin C available only for the 2003–2004 and 2005–2006 waves of NHANES). A sensitivity analysis was conducted to examine the association between quartiles of vitamin C and MetS (and its components). See Table 5 and Figure 1 results section.
Models included the 3 main antioxidant status exposures and adjusted for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level, PIR, and other potential confounders: lifestyle and health-related factors (smoking status, PA: Met⋅h⋅wk−1, recoded as 0 to <5, 5–10, >10) and dietary intakes (total energy intake, alcohol, caffeine, β-carotene, vitamin C, vitamin E ,and dietary supplement use), serum levels of folate, tHcy, vitamin B-12, 25(OH)D, total cholesterol, and TG. Note that in models with hypertriglyceridemia as the outcome, only serum cholesterol was controlled for among serum lipids.
< 0.05 for null hypothesis that Loge(OR) = 0 based on Wald test.
See Methods for definition of each component.
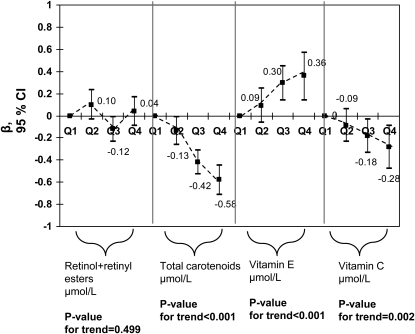
We additionally examined the dose-response relationship between antioxidant status and MetS by expressing antioxidant groups (including vitamin C) as quartiles. Models were similar to model 4 of Table 3 and Table 4 models. Carotenoid quartiles exhibited a linear dose-response inverse association with MetS (binary and count), elevated HOMA-IR, elevated CRP, and hyperuricemia (P-trend < 0.05). Vitamin C exhibited a similar pattern of association with MetS (binary and count), HOMA-IR, and hyperuricemia (P-trend < 0.05) and retinol+retinyl esters with CRP (P-trend < 0.001). However, retinol+retinyl esters were linearly and positively associated with hyperuricemia (P-trend = 0.001) and vitamin E was directly related to MetS count outcome (P-trend < 0.001) (Fig. 1;Table 5).
FIGURE 1.
Major serum antioxidant concentrations by quartiles (Q2, Q3, Q4 vs. Q1) and MetS (NCEP ATP III definition) score count outcome among U.S. adults (NHANES 2003–2006); n = 1,574. Values are adjusted β (95% CI). Ranges for each antioxidant quartile is as follows in μmol/L: retinol+retinyl esters (Q1: 0.066–1.703; Q2: 1.703–2.083; Q3: 2.084–2.521; Q4: 2.522–8.860); total carotenoids (Q1: 0.057–0.863; Q2: 0.863–1.183; Q3: 1.183–1.622; Q4: 1.62–10.114); vitamin E (Q1: 0.16–21.66; Q2: 21.67–27.35; Q3: 27.37–35.94; Q4: 35.95–303.81); and vitamin C (Q1: 0.6–34.6; Q2: 35.2–54.5; Q3: 55.1–70.4; Q4: 71.0–274.2). Analyses were based on multiple ZIP regression models that simultaneously included all antioxidant exposures and adjusted for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level, and PIR and other potential confounders, including: lifestyle and health-related factors (smoking status, PA: Met⋅h⋅wk−1, recoded as 0 to <5, 5–10, >10) and dietary intakes (total energy intake, alcohol, caffeine, β-carotene, vitamin C, vitamin E, and dietary supplement use), serum levels of folate, tHcy, vitamin B-12, 25(OH)D, total cholesterol, and TG.
TABLE 5.
Associations between concentrations of selected serum antioxidants (expressed as quartiles) and MetS (NCEP ATP III) and other MetS components: multiple logistic regression models (NHANES 2003–2006)12
| Serum antioxidant quartiles |
|||||||
| Q2 vs. Q1 |
Q3 vs. Q1 |
Q4 vs. Q1 |
P-trend | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| MetS (NCEP ATP III),4n = 1574 | μmol/L | ||||||
| Total retinol+retinyl esters | 1.83 | (1.00; 3.35) | 0.83 | (0.46; 1.51) | 1.22 | (0.63; 2.36) | 0.091 |
| Total carotenoids | 0.73 | (0.41; 1.28) | 0.363 | (0.22; 0.58) | 0.323 | (0.18; 0.56) | <0.001 |
| Vitamin E | 0.79 | (0.41; 1.52) | 1.54 | (0.84; 2.84) | 1.09 | (0.44; 2.67) | 0.489 |
| Vitamin C | 0.98 | (0.62; 1.53) | 0.523 | (0.28; 0.98) | 0.52 | (0.25; 1.10) | 0.033 |
| Other components4 | |||||||
| Elevated HOMA-IR, n = 2366 | |||||||
| Total retinol+retinyl esters | 1.14 | (0.85; 1.52) | 0.87 | (0.58;1.32) | 1.07 | (0.70; 1.65) | 0.905 |
| Total carotenoids | 0.633 | (0.47; 0.83) | 0.533 | (0.40; 0.70) | 0.313 | (0.23; 0.42) | <0.001 |
| Vitamin E | 0.99 | (0.71; 1.37) | 1.21 | (0.87; 1.68) | 0.77 | (0.40; 1.46) | 0.538 |
| Vitamin C | 0.71 | (0.48; 1.04) | 0.623 | (0.41; 0.92) | 0.64 | (0.40; 1.00) | 0.039 |
| Elevated CRP, n = 2382 | |||||||
| Total retinol+retinyl esters | 0.373 | (0.19; 0.68) | 0.143 | (0.04; 0.44) | 0.173 | (0.06; 0.51) | <0.001 |
| Total carotenoids | 0.50 | (0.23; 1.08) | 0.313 | (0.13; 0.73) | 0.083 | (0.03; 0.25) | <0.001 |
| Vitamin E | 2.65 | (0.92; 7.67) | 1.94 | (0.58; 6.48) | 7.593 | (1.35; 42.65) | 0.075 |
| Vitamin C | 0.78 | (0.43; 1.42) | 0.393 | (0.19; 0.78) | 0.48 | (0.19; 1.30) | 0.075 |
| Hyperuricemia, n = 1354 | |||||||
| Total retinol+retinyl esters | 3.513 | (1.57; 7.84) | 2.463 | (0.89; 6.78) | 5.303 | (2.71; 10.39) | 0.001 |
| Total carotenoids | 0.67 | (0.35; 1.28) | 0.393 | (0.18; 0.83) | 0.413 | (0.20; 0.85) | 0.026 |
| Vitamin E | 1.05 | (0.59; 1.84) | 1.31 | (0.73; 2.33) | 1.39 | (0.61; 3.17) | 0.383 |
| Vitamin C | 0.47 | (0.87; 1.60) | 0.66 | (0.34; 1.30) | 0.443 | (0.20; 0.98) | 0.043 |
Values are OR with 95% CI. Sampling design complexity is taken into account in all analyses. Vitamin C was not included in this analysis due to a appreciable drop in sample size (vitamin C available only for the 2003–2004 and 2005–2006 waves of NHANES).
Models included the 4 main antioxidant status exposures and adjusted for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level and PIR, and other potential confounders: lifestyle and health-related factors (smoking status, PA: Met⋅h⋅wk−1, recoded as 0 to <5, 5–10, >10) and dietary intakes (total energy intake, alcohol, caffeine, β-carotene, vitamin C, vitamin E ,and dietary supplement use), serum levels of folate, tHcy, vitamin B-12, 25(OH)D, total cholesterol, and TG.
< 0.05 for null hypothesis that Loge(OR) = 0 based on Wald test.
See Methods section for definition of each component and Figure 1 footnotes for range of serum antioxidant quartiles Q1 through Q4.
Discussion
Contributing to a growing body of literature, this study examined the relationship between MetS and antioxidant status using a recent nationally representative sample of U.S. adults. Several key findings emerged. First, MetS prevalence (NHANES 2001–2006: 32.0% among men and 29.5% among women) was much higher than that in NHANES III (22% overall) (62). Second, MetS+ U.S. adults had lower serum antioxidant levels, particularly total carotenoids. After controlling for TC and TG, vitamin E had no significant relationship with MetS, whereas retinol+retinyl esters were inversely related to MetS among men only. Retinol+retinyl esters were also inversely related to elevated CRP and positively associated with hyperuricemia (both sexes) with a linear dose-response relationship. Among serum carotenoids, β-carotene (both sexes), β-cryptoxanthin (men), and lutein+zeaxanthin (women) were significantly and inversely related to MetS (binary). Vitamin C exhibited a similar pattern to serum carotenoids with an inverse linear association with MetS (binary), HOMA-IR, and hyperuricemia.
Previous observational studies and 1 randomized controlled trial (RCT) consistently found an inverse association between type 2 diabetes or MetS (or components) and carotenoid levels (15, 17, 23–25, 63, 64). For instance, a cross-sectional study of Australian adults (n = 1597) suggested an inverse association between serum carotenoids and type 2 diabetes (15) and these findings were also replicated for MetS (25). Similar results were obtained with MetS using NHANES III cross-sectional data (23). In a large prospective cohort study (CARDIA) with 15 y of follow-up, lower risks of type 2 diabetes and insulin resistance were found with higher baseline serum carotenoids, although only among nonsmokers (17). Conversely, serum carotenoids exhibited a significant inverse relationship with MetS only in smokers in a cross-sectional study of 1073 Japanese adults (24). A case-control study found that lutein+zeaxanthin and cryptoxanthin were lower in coronary artery disease patients compared with controls and were inversely related to a number of cardiovascular risk factors (63). A recent RCT found that baseline serum concentrations of β-carotene and vitamin C were inversely related to the incidence of MetS after 7.5 y of follow-up (64). In contrast, in a nested case-control study, levels of vitamin E and β-carotene were inversely related to type 2 diabetes only prior to control for other cardiovascular risk factors (18). Similarly, no association was detected between plasma lycopene (or other carotenoids) and type 2 diabetes among middle-aged and older women in a recent prospective cohort study (21).
Other observational studies examining dietary intakes of carotenoids and total antioxidant capacity of the diet found an inverse relationship with MetS (or its components, including CRP) (26, 65, 66) and type 2 diabetes (14). However, large RCT did not find any long-term benefits of antioxidant supplements, particularly vitamin E and β-carotene, in the prevention of type 2 diabetes (19, 20, 67) or MetS (64). Thus, even though epidemiological studies pointed to an inverse relationship between serum carotenoids and MetS or type 2 diabetes, RCT have shown that supplementing the diet with β-carotene or α-tocopherol had no effect on the incidence of type 2 diabetes or MetS.
Emerging evidence of cardioprotective effects of lycopene and other carotenoids, however, indicated that there is more to be learned about carotenoids and their individual associations with CVD and its risk factors (68). Our study uncovered some of those research gaps and indicated that serum total carotenoids, rather than β-carotene by itself, may have a cardio-protective potential. In fact, a previous study indicated that total antioxidant capacity was an independent predictor of β-carotene level, suggesting that a number of antioxidants may interact to increase the level of each of the carotenoids in serum. This may partly explain the failure of β-carotene supplements by themselves to reduce the risk of MetS (69). Finally, a few previous studies have also shown that retinol binding protein increases among type 2 diabetes patients (70) and is associated with renal dysfunction and hyperuricemia among type 2 diabetes patients (71, 72). The latter finding of high concentration of retinol and retinyl esters among hyperuricemic participants was replicated in our present study. Our finding of retinol’s inverse relationship with CRP was also replicated by others (72).
It is suggested that individuals with MetS have elevated levels of oxidative stress markers like singleton oxygen, peroxyl molecules (73, 74). Thus, this group has a higher requirement of antioxidants like carotenoids, due to which there is an inverse association between carotenoids and MetS components. Vitamin C acts by sparing and increasing the levels of plasma-reduced glutathione (GSH) and reducing the ratio of glutathione disulfide (GSSG):GSH [GSSG being the oxidized form]. This GSSG:GSH ratio might be linked to the physical-chemical integrity of the plasma membrane viscosity (75). Thus, the role of glucose transporter might be compromised if there are low levels of plasma vitamin C and play a role in insulin resistance. Retinol is mainly stored in liver and excess retinol is released in the blood stream as retinyl ester. High levels of retinol and retinyl ester are thus seen in conditions where liver function is impaired (23), a possibility during insulin resistance and MetS.
Our study has many strengths, including national representativeness and large sample size. However, we note several limitations. First, no accurate data on micronutrient supplementation were available, although use of dietary supplements over a 30-d period was considered a proxy measure (prevalence of supplement use in our sample of adults was ~57% and approximately one-third used ≥2 supplements). Second, not all serum antioxidant concentrations were available for the recent NHANES waves, particularly vitamin C, which was available only for the 2003–2004 and 2005–2006 waves. To reduce selection bias, we only included serum vitamin C as an exposure in part of the analysis. Third, the cross-sectional design of our study precludes inference about temporality of associations. Hence, it was not possible to conclude from our study whether lower serum antioxidant concentrations, particularly carotenoids, were the result of oxidative stress caused by MetS or whether they were the direct outcome of lower FV intake, mediating the relationship between FV and the pathogenesis of MetS. Fourth, some of our significant findings (especially with a P-value between 0.05 and 0.01) may be due to chance, given multiple comparisons, although most of our consistent main findings had P-values < 0.001, reducing this possibility. Finally, residual confounding by unmeasured covariates cannot be totally discounted (see Supplement 1 for description of main confounders in the final models).
The U.S. adult population has witnessed an appreciable surge in the prevalence of obesity and MetS over the past few decades, a trend paralleled in other developed and developing countries. Preventive efforts aiming at modifiable risk factors for obesity and its related disorders are greatly warranted. It is clear from previous studies that oxidative stress is associated with incidence of type 2 diabetes and cardiovascular mortality and morbidity (76). Our study adds to the accumulating evidence that a higher level of oxidative stress also accompanies obesity-related disorders, which may be the causative agent behind further complications related to MetS, including the development of atherosclerosis. In particular, we found that the total serum carotenoid level was inversely associated with MetS NCEP ATP III status, CRP, and hyperuricemia and that the vitamin C level tended to follow a comparable trend to that of carotenoids. Future intervention studies related to dietary and lifestyle change need to be conducted to assess the utility of modifying serum concentrations of antioxidants, especially carotenoids, given their suboptimal levels among U.S. adults with MetS, for the prevention of type 2 diabetes and various cardiovascular endpoints.
Acknowledgments
We thank Dr. Joshua Goh and Dr. Wayne Chan for their thoughtful comments on the manuscript. M.A.B. wrote and revised the manuscript, planned analysis, performed data management and statistical analysis, and had primary responsibility for the final content; M.R.S. wrote and revised parts of the manuscript and participated in literature review and plan of analysis; X.C. participated in literature search and review and in the revision of the manuscript; H.A.B. wrote and revised parts of the manuscript and participated in literature search and plan of analysis; Y.W. participated in the plan of analysis and revised the manuscript; A.B.Z. participated in the plan of analysis and revised the manuscript. All authors read and approved the final manuscript.
Footnotes
Supported by the National Institute on Aging, Intramural Research Program. X.C. and Y.W.'s related work was supported by the NIH/NIDDK (R01DK81335-01A1).
Supplemental Text 1 and Table 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: 25(OH)D, 25-hydroxyvitamin D; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; FV, fruits and vegetables; GSH, plasma-reduced glutathione; GSSG, glutathione disulfide; HDL-C, HDL cholesterol; HOMA-IR, homeostatic model assessment insulin resistance; HS, high school; MEC, mobile examination center; MET, metabolic equivalent; MetS, metabolic syndrome; MetS+, participants with MetS; MetS−, participants without MetS; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; NH, non-Hispanic; OLS, ordinary least square; PA, physical activity; PIR, poverty income ratio; Q, quartile; RCT, randomized controlled trial; Ref, referent category; tHcy, total homocysteine; ZIP, zero-inflated Poisson.
Literature Cited
- 1.Meigs JB. Epidemiology of the metabolic syndrome, 2002. Am J Manag Care. 2002;8:S283–92; quiz S93–6 [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52 [DOI] [PubMed] [Google Scholar]
- 3.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9 [DOI] [PubMed] [Google Scholar]
- 4.Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol. 1998;148:958–66 [DOI] [PubMed] [Google Scholar]
- 5.Wilson PW, Kannel WB, Silbershatz H, D'Agostino RB. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–9 [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes. 1992;41:715–22 [DOI] [PubMed] [Google Scholar]
- 7.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16 [DOI] [PubMed] [Google Scholar]
- 8.Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–76 [DOI] [PubMed] [Google Scholar]
- 9.Soory M. Relevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: potential for therapeutic targeting. Infect Disord Drug Targets. 2009;9:400–14 [DOI] [PubMed] [Google Scholar]
- 10.Campbell DR, Gross MD, Martini MC, Grandits GA, Slavin JL, Potter JD. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev. 1994;3:493–500 [PubMed] [Google Scholar]
- 11.Stryker WS, Kaplan LA, Stein EA, Stampfer MJ, Sober A, Willett WC. The relation of diet, cigarette smoking, and alcohol consumption to plasma beta-carotene and alpha-tocopherol levels. Am J Epidemiol. 1988;127:283–96 [DOI] [PubMed] [Google Scholar]
- 12.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–16 [DOI] [PubMed] [Google Scholar]
- 13.Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr. 2006;83:1265–71 [DOI] [PubMed] [Google Scholar]
- 14.Montonen J, Knekt P, Jarvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27:362–6 [DOI] [PubMed] [Google Scholar]
- 15.Coyne T, Ibiebele TI, Baade PD, Dobson A, McClintock C, Dunn S, Leonard D, Shaw J. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr. 2005;82:685–93 [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Will JC, Bowman BA, Narayan KM. Diabetes mellitus and serum carotenoids: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;149:168–76 [DOI] [PubMed] [Google Scholar]
- 17.Hozawa A, Jacobs DR, Jr, Steffes MW, Gross MD, Steffen LM, Lee DH. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: interaction with smoking: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2006;163:929–37 [DOI] [PubMed] [Google Scholar]
- 18.Reunanen A, Knekt P, Aaran RK, Aromaa A. Serum antioxidants and risk of non-insulin dependent diabetes mellitus. Eur J Clin Nutr. 1998;52:89–93 [DOI] [PubMed] [Google Scholar]
- 19.Kataja-Tuomola M, Sundell JR, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2008;51:47–53 [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Ajani U, Chae C, Hennekens C, Buring JE, Manson JE. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA. 1999;282:1073–5 [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Liu S, Pradhan AD, Manson JE, Buring JE, Gaziano JM, Sesso HD. Plasma lycopene, other carotenoids, and the risk of type 2 diabetes in women. Am J Epidemiol. 2006;164:576–85 [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Liu S, Manson JE, Gaziano JM, Buring JE, Sesso HD. The consumption of lycopene and tomato-based food products is not associated with the risk of type 2 diabetes in women. J Nutr. 2006;136:620–5 [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346–52 [DOI] [PubMed] [Google Scholar]
- 24.Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Matsumoto H, Ando F, Shimokata H, Yano M. Associations of serum carotenoid concentrations with the metabolic syndrome: interaction with smoking. Br J Nutr. 2008;100:1297–306 [DOI] [PubMed] [Google Scholar]
- 25.Coyne T, Ibiebele TI, Baade PD, McClintock CS, Shaw JE. Metabolic syndrome and serum carotenoids: findings of a cross-sectional study in Queensland, Australia. Br J Nutr. 2009;102:1668–77 [DOI] [PubMed] [Google Scholar]
- 26.Sluijs I, Beulens JW, Grobbee DE, van der Schouw YT. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J Nutr. 2009;139:987–92 [DOI] [PubMed] [Google Scholar]
- 27.Reis JP, von Muhlen D, Miller ER III. Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol. 2008;159:41–8 [DOI] [PubMed] [Google Scholar]
- 28.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vazquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–80 [DOI] [PubMed] [Google Scholar]
- 29.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–61 [DOI] [PubMed] [Google Scholar]
- 30.Looker AC. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab. 2005;90:635–40 [DOI] [PubMed] [Google Scholar]
- 31.Gannage-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol. 2009;160:965–71 [DOI] [PubMed] [Google Scholar]
- 32.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–9 [DOI] [PubMed] [Google Scholar]
- 33.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305 [DOI] [PubMed] [Google Scholar]
- 34.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–65 [DOI] [PubMed] [Google Scholar]
- 35.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9 [DOI] [PubMed] [Google Scholar]
- 37.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8 [DOI] [PubMed] [Google Scholar]
- 38.Chonchol M, Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int. 2007;71:134–9 [DOI] [PubMed] [Google Scholar]
- 39.Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann K, Janssen OE. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577–83 [DOI] [PubMed] [Google Scholar]
- 40.Beydoun MA, Boueiz A, Shroff MR, Beydoun HA, Wang Y, Zonderman AB. Associations among 25-hydroxyvitamin D, diet quality, and metabolic disturbance differ by adiposity in United States adults. J Clin Endocrinol Metab. 2010;95:3814–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 42.CDC. National Health and Nutrition Examination Survey 2006 [cited 2006 Sep 25]. Available from: http://www.cdc.gov/nchs/nhanes.htm.
- 43.CDC. NHANES III anthropometric procedures video [cited 2007 Sep 25]. Available from: http://www.cdc.gov/nchs/about/major/nhanes/avideo.htm.
- 44.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–70 [DOI] [PubMed] [Google Scholar]
- 45.CDC. National Health and Nutrition Examination Survey 2007 [cited 2006 Sep 25]. Available from: http://www.cdc.gov/nchs/nhanes.htm.
- 46.Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999;83:F25–9 [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Beydoun MA. The obesity epidemic in the United States: gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28 [DOI] [PubMed] [Google Scholar]
- 48.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95 [DOI] [PubMed] [Google Scholar]
- 49.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 50.Handbook of diagnostic tests. 3rd ed Philadelphia: Lipincott Williams; & Wilkins; 2003 [Google Scholar]
- 51.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9 [DOI] [PubMed] [Google Scholar]
- 52.Cheng YJ, Gregg EW, De Rekeneire N, Williams DE, Imperatore G, Caspersen CJ, Kahn HS. Muscle-strengthening activity and its association with insulin sensitivity. Diabetes Care. 2007;30:2264–70 [DOI] [PubMed] [Google Scholar]
- 53.Lagerros YT, Lagiou P. Assessment of physical activity and energy expenditure in epidemiological research of chronic diseases. Eur J Epidemiol. 2007;22:353–62 [DOI] [PubMed] [Google Scholar]
- 54.McCullough ML, Feskanich D, Rimm EB, Giovannucci EL, Ascherio A, Variyam JN, Spiegelman D, Stampfer MJ, Willett WC. Adherence to the dietary guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr. 2000;72:1223–31 [DOI] [PubMed] [Google Scholar]
- 55.USDA, Agriculture Research Service, Food Surveys Research Group. Food and Nutrient Database for Dietary Studies, 3.0 [cited 2008 Mar]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=17031.
- 56.Alkerwi A, Boutsen M, Vaillant M, Barre J, Lair ML, Albert A, Guillaume M, Dramaix M. Alcohol consumption and the prevalence of metabolic syndrome: a meta-analysis of observational studies. Atherosclerosis. 2009;204:624–35 [DOI] [PubMed] [Google Scholar]
- 57.Hino A, Adachi H, Enomoto M, Furuki K, Shigetoh Y, Ohtsuka M, Kumagae S, Hirai Y, Jalaldin A, et al. Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome: an epidemiological study in a general Japanese population. Diabetes Res Clin Pract. 2007;76:383–9 [DOI] [PubMed] [Google Scholar]
- 58.Aleixandre A, Miguel M. Dietary fiber in the prevention and treatment of metabolic syndrome: a review. Crit Rev Food Sci Nutr. 2008;48:905–12 [DOI] [PubMed] [Google Scholar]
- 59. Abbott Homocysteine (HCY) assay package insert for IMX Analyzer. [Google Scholar]
- 60.Pernet P, Lasnier E, Vaubourdolle M. Evaluation of the AxSYM homocysteine assay and comparison with the IMx homocysteine assay. Clin Chem. 2000;46:1440–1 [PubMed] [Google Scholar]
- 61.STATA Statistics/data analysis: release 11.0. College Station (TX): Stata Corporation; 2009 [Google Scholar]
- 62.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9 [DOI] [PubMed] [Google Scholar]
- 63.Lidebjer C, Leanderson P, Ernerudh J, Jonasson L. Low plasma levels of oxygenated carotenoids in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2007;17:448–56 [DOI] [PubMed] [Google Scholar]
- 64.Czernichow S, Vergnaud AC, Galan P, Arnaud J, Favier A, Faure H, Huxley R, Hercberg S, Ahluwalia N. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am J Clin Nutr. 2009;90:329–35 [DOI] [PubMed] [Google Scholar]
- 65.Brighenti F, Valtuena S, Pellegrini N, Ardigo D, Del Rio D, Salvatore S, Piatti P, Serafini M, Zavaroni I. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br J Nutr. 2005;93:619–25 [DOI] [PubMed] [Google Scholar]
- 66.Oliveira A, Rodriguez-Artalejo F, Lopes C. The association of fruits, vegetables, antioxidant vitamins and fibre intake with high-sensitivity C-reactive protein: sex and body mass index interactions. Eur J Clin Nutr. 2009;63:1345–52 [DOI] [PubMed] [Google Scholar]
- 67.Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, Hercberg S. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr. 2006;84:395–9 [DOI] [PubMed] [Google Scholar]
- 68.Sesso HD. Carotenoids and cardiovascular disease: what research gaps remain? Curr Opin Lipidol. 2006;17:11–6 [DOI] [PubMed] [Google Scholar]
- 69.Valtuena S, Del Rio D, Pellegrini N, Ardigo D, Franzini L, Salvatore S, Piatti PM, Riso P, Zavaroni I, et al. The total antioxidant capacity of the diet is an independent predictor of plasma beta-carotene. Eur J Clin Nutr. 2007;61:69–76 [DOI] [PubMed] [Google Scholar]
- 70.Basualdo CG, Wein EE, Basu TK. Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr. 1997;16:39–45 [DOI] [PubMed] [Google Scholar]
- 71.Chang YH, Lin KD, Wang CL, Hsieh MC, Hsiao PJ, Shin SJ. Elevated serum retinol-binding protein 4 concentrations are associated with renal dysfunction and uric acid in type 2 diabetic patients. Diabetes Metab Res Rev. 2008;24:629–34 [DOI] [PubMed] [Google Scholar]
- 72.Chen CC, Wu JY, Chang CT, Tsai FJ, Wang TY, Liu YM, Tsui HC, Chen RH, Chiou SC. Levels of retinol-binding protein 4 and uric acid in patients with type 2 diabetes mellitus. Metabolism. 2009;58:1812–6 [DOI] [PubMed] [Google Scholar]
- 73.Park K, Gross M, Lee D-H, Holvoet P, Himes JH, Shikany JM, Jacobs DR. Oxidative stress and insulin resistance. Diabetes Care. 2009;32:1302–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmieri VO, Grattagliano I, Portincasa P, Palasciano G. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J Nutr. 2006;136:3022–6 [DOI] [PubMed] [Google Scholar]
- 75.Paolisso G, D'Amore A, Balbi V, Volpe C, Galzerano D, Giugliano D, Sgambato S, Varricchio M, D'Onofrio F. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am J Physiol. 1994;266:E261–8 [DOI] [PubMed] [Google Scholar]
- 76.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–12 [DOI] [PubMed] [Google Scholar]