Abstract
Caspase-8 has two opposing biological functions - it promotes cell death by triggering the extrinsic pathway of apoptosis, but also has a survival activity, as it is required for embryonic development1, T lymphocyte activation2, and resistance to necrosis induced by Tumor Necrosis Factor-α (TNF) and related family ligands3,4. Here we show that development of caspase-8-deficient mice is completely rescued by ablation of Receptor Interacting Protein Kinase-3 (RIPK3). Adult animals lacking both caspase-8 and RIPK3 display a progressive lymphoaccumulative disease resembling that seen with defects in CD95 or CD95-ligand, and resist the lethal effects of CD95 ligation in vivo. We have found that caspase-8 prevents RIPK3-dependent necrosis without inducing apoptosis by functioning in a proteolytically active complex with FLICE-Like Inhibitory Protein Long (FLIPL), and this complex is required for the protective function.
The death receptor pathway of apoptosis is induced by ligation of a subset of tumor necrosis factor (TNF) receptor super-family members (the death receptors)5. This pathway involves the recruitment of an adapter molecule, FADD, to the intracellular region of the receptor; FADD, in turn binds and thereby activates caspase-8 to initiate apoptosis. Cell death in this pathway is antagonized by another protein, FLIPL (herein called FLIP), which resembles caspase-8 but lacks a catalytic site5.
Intriguingly, genetic ablation of caspase-81, FADD6 or FLIP7results in embryonic lethality around day e10.5, revealing that these proteins function in cell survival as well as cell death. This is supported by the finding that caspase-8 deficiency by siRNA knock-down or gene ablation sensitizes fibroblasts for necrotic cell death in response to TNF3(Fig. S1a). Necrosis induced by TNF in the presence of caspase inhibitors is dependent on the kinase activity of Receptor-Interacting Protein Kinase-1 (RIPK1)4,8 and RIPK39–11, although the mechanisms remain obscure.
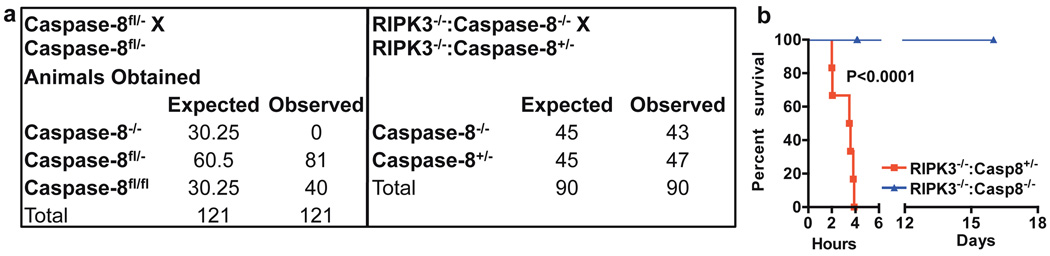
To determine if RIPK3-dependent necrosis contributes to the embryonic lethality of caspase-8-deficient mice, we generated caspase-8−/−: RIPK3−/−double knock-out (DKO) animals. Although, as expected, we were unable to obtain viable caspase-8−/− :RIPK3WT mice, caspase-8−/−:RIPK3−/− DKO mice were born at the expected frequency (Fig. 1a). These animals displayed nogross developmental abnormalities (Fig. S1b), and their mass at different ages was indistinguishable from that of heterozygous mice (Fig. S1c), as described for the RIPK3−/− mouse12, despite lacking detectable caspase-8 or RIPK3 (Fig. S1d). Thymocytes from these animals underwent apoptosis in response to several agents known to induce the mitochondrial pathway of apoptosis, but were resistant to apoptosis induced by ligation of the death receptor, CD95 (Fig. S1e). We examined the latter effect in more detail, as injection of agonistic anti-CD95 antibody is known to trigger hepatocyte apoptosis, liver damage, and death in WT mice13. While anti-CD95 caused liver destruction and mortality in heterozygous caspase-8+/−:RIPK3−/− animals, caspase-8−/−:RIPK3−/− mice were completely resistant to this insult (Fig. 1b, S1f-h).
Figure 1. RIPK3−/−:Caspase-8−/− mice are viable and functionally deficient for caspase-8.
a. Expected and observed frequency of caspase-8 status in offspring from crosses of mice with the indicated genotypes. “Fl” indicates an allele of caspase-8 that is present but flanked with LoxP sites. (p<0.0001 left, p=0.6733 right) b. Effect of tail vein injection of 15µg per animal of the CD95-activating antibody Jo2 on mice of the indicated genotypes. (n=6 RIPK3−/−:Casp8+/−, 8 DKO)
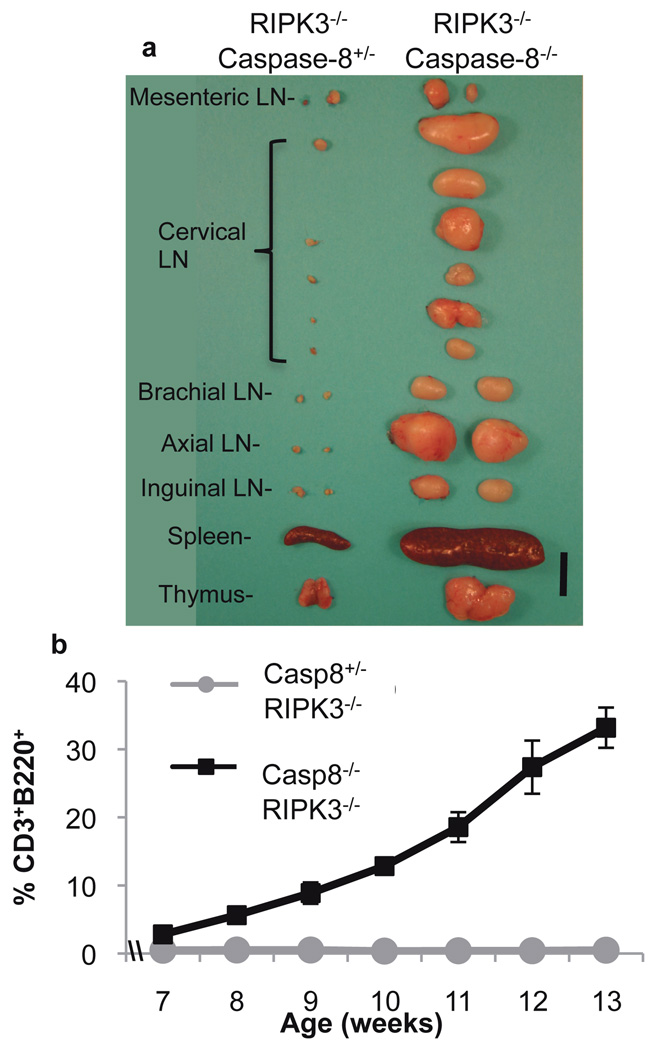
In young caspase-8−/−:RIPK3−/− DKO mice, lymphoid organs appeared overtly normal (Fig. S2a), T lymphocyte proliferation in response to activation was identical to that of heterozygote littermates (Fig. S2b), and T cells from these animals displayed expansion and subsequent peripheral deletion in vivo when challenged with the bacterial superantigen staphylococcus enterotoxin B (SEB)(Fig. S2c). However, we noted that older DKO mice displayed a marked lymphoaccumulation (Fig. 2a), resembling that described in animals lacking either CD95 or CD95-ligand14. The latter are known to accumulate an unusual population of B220+, CD3+, CD4−, CD8− T lymphocytes, also seen in humans with defective CD95 or CD95-ligand14. While young (1 mo) caspase-8−/−:RIPK3−/− DKO mice showed normal mature T lymphocyte subsets, we observed a dramatic increase in B220+, CD3+ cells as the animals aged (Fig. 2b, S2d).
Figure 2. RIPK3−/−:Caspase-8−/− mice display progressive severe lymphoaccumulation.
a. Lymphoid organs removed from 15 week old littermate mice of the indicated genotypes. LN is Lymph Node. Scale bar is 1cm. b. Percentage of total blood cells (following red blood cell lysis) that are B220+CD3+ in mice of the indicated genotypes and ages. (Error bars are s.d., n=3 each genotype). Both a and b are representative of similar results obtained from all sampled mice of the indicated genotypes.
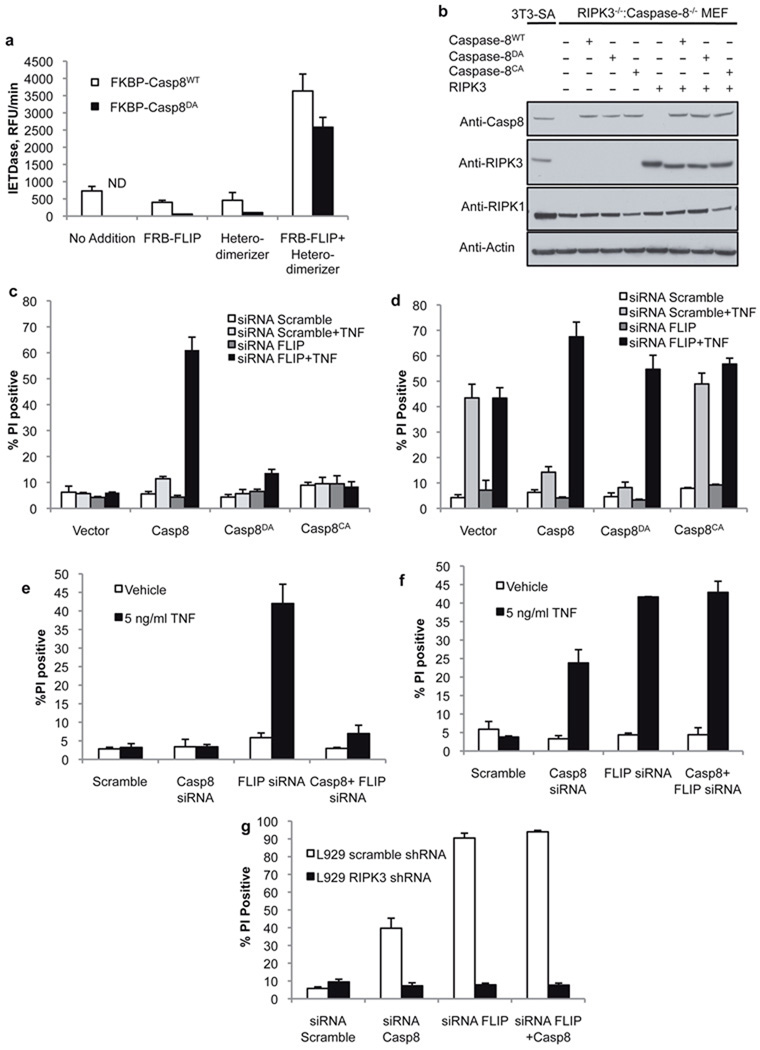
The ability of RIPK3 ablation to rescue the lethal phenotype of caspase-8 deletion strongly suggests that caspase-8-mediated inhibition of RIPK3-dependent necrosis is necessary for embryonic development, and that this is the primary protective role of caspase-8 in development. This raises the question of how caspase-8 can mediate this effect without itself engaging apoptotic cell death in the cells in which it manifests this protective function. A clue is provided by the observation that a mutant of caspase-8, lacking the cleavage site between the large and small subunits of the mature enzyme, rescued survival of caspase-8-deficient animals when expressed as a bacterial artificial chromosome (BAC)-transgene15. Such “non-cleavable” caspase-8 has been shown to be unable to restore death receptor-induced apoptosis in caspase-8-deficient cells16,17. Biochemical and structural studies have indicated that FLIP can heterodimerize with caspase-8 in kosmotropic salt, and that this complex may be able to impart catalytic activity on caspase-8 in the absence of interdomain cleavage18,19. We sought to test this directly by examining enzymatic activity of non-cleavable caspase-8DA when dimerized with FLIP in vitro. We generated caspase-8 or non-cleavable caspase-8DA, as well as FLIP, in which the prodomains were replaced by FKBP or FRB domains respectively, allowing enforced homo-or hetero-dimerization upon addition of specific FK506 derivatives20. Although dimerization of FKBP-caspase-8DA was catalytically inactive under physiological buffer conditions (Fig. S3a)16, hetero-dimerization of this non-cleavable caspase-8 with FRB-FLIP was enzymatically active (Fig. 3a). Thus, FLIP can impart catalytic activity to non-cleavable caspase-8.
Figure 3. Both catalytically active caspase-8 and FLIP are required for suppression of TNF-induced RIPK3-dependent death.
a. Fluorogenic substrate cleavage activity of recombinant purified FKBP-caspase-8WT or non-cleavable FKBP-caspase-8DA in the presence of recombinant purified FRB-FLIP, a compound that induces FKBP-FRB heterodimers (heterodimerizer), or FRB-FLIP and heterodimerizer. ND indicates none detected. (Error bars are s.d., n=3) b. Western blot analysis of RIPK3−/−:Caspase-8−/− (DKO) MEF stably expressing the indicated mutants of caspase-8 and RIPK3. Caspase-8CA indicates catalytically inactive caspase-8. c, d. Cell death assessed by propidium iodide (PI) uptake of DKO MEF expressing the indicated caspase-8 mutants in the absence (c) or presence (d) of stably expressed RIPK3, following transfection with scramble or FLIP-targeted siRNA, and 12 hours TNF treatment. (Error bars are s.d., n=3) e, f. Cell death (PI uptake) of SVEC 4–10 cells stably expressing RIPK3 specific (e) or scramble (f) shRNA, transfected with the indicated siRNAs and treated with TNF for 12 hours. (Graph is mean of 2 independent experiments, error bars indicate range) g. Cell death (PI uptake) of L929 cells expressing scramble or RIPK3-specific shRNA, transfected with siRNAs specific for caspase-8 and/or FLIP as indicated. Death was assessed 48h post-transfection. (Error bars are s.d., n=3.) The data presented are representative of results obtained with either of 2 separate siRNAs to both caspase-8 and FLIP.
Since ablation of FLIP leads to developmental defects similar to those observed upon caspase-8 ablation, and since expression of non-cleavable caspase-8DA allows normal development, we hypothesized that FLIP may activate caspase-8 to allow suppression of RIPK3-dependent death. To investigate this possibility, we generated MEF from caspase-8−/−:RIPK3−/− DKO embryos and reconstituted them with caspase-8, non-cleavable caspase-8DA, or catalytically inactive caspase-8CA, plus or minus RIPK3 (Fig. 3b). We then knocked down FLIP expression by siRNA (Fig. S3b). Cells lacking RIPK3 underwent cell death upon treatment with TNF only if they expressed WT caspase-8, and only if FLIP was lacking (Fig. 3c). Conversely, cells expressing RIPK3 died upon TNF exposure if they lacked caspase-8 or expressed catalytically inactive caspase-8CA (Fig. 3d). Importantly, cells expressing non-cleavable caspase-8DA were resistant to TNF-induced, RIPK3-dependent death, but became sensitive to this form of cell death upon knock-down of FLIP (Fig. 3d). Thus, FLIP expression prevents caspase-8-dependent, RIPK3-independent death (apoptosis), and prevents RIPK3-dependent, necrotic cell death in a caspase-8 dependent manner. Apoptosis mediated by caspase-8 depends on cleavage of the caspase16,17, while inhibition of RIPK3-mediated cell death does not.
We obtained similar results in several cell lines. In murine SVEC cells, knockdown of caspase-8 or FLIP promoted TNF-induced cell death, which in the case of caspase-8 was completely blocked by concomitant knock-down of RIPK3 (Fig. 3e,f, S3c). In contrast, TNF-induced cell death promoted by knock-down of FLIP was only partially dependent on RIPK3, presumably due to compensation by caspase-8-mediated apoptosis. This was supported by the combined knock-down of caspase-8 and FLIP, in which TNF-induced cell death was entirely RIPK3 dependent (Fig. 3e,f). Similar results were obtained in two 3T3 cell lines with (SA) or without (NIH) RIPK3 (Fig. S3d-f).
In L929 cells, treatment with caspase inhibitor alone has been shown to cause RIPK3-dependent death due to autocrine production of TNF16. In these cells, knock-down of either caspase-8 or FLIP led to cell death that was fully dependent on RIPK3 (Fig. 3g, S3g). For unknown reasons, no RIPK3-independent, caspase-8-dependent apoptosis was observed in these cells. However, together with the results obtained in caspase-8−/−, RIPK3−/− DKO MEF expressing non-cleavable caspase-8DA (discussed above), these observations suggest that rather than simply functioning to dampen caspase-8 activation, FLIP is an important component of the protective effect.
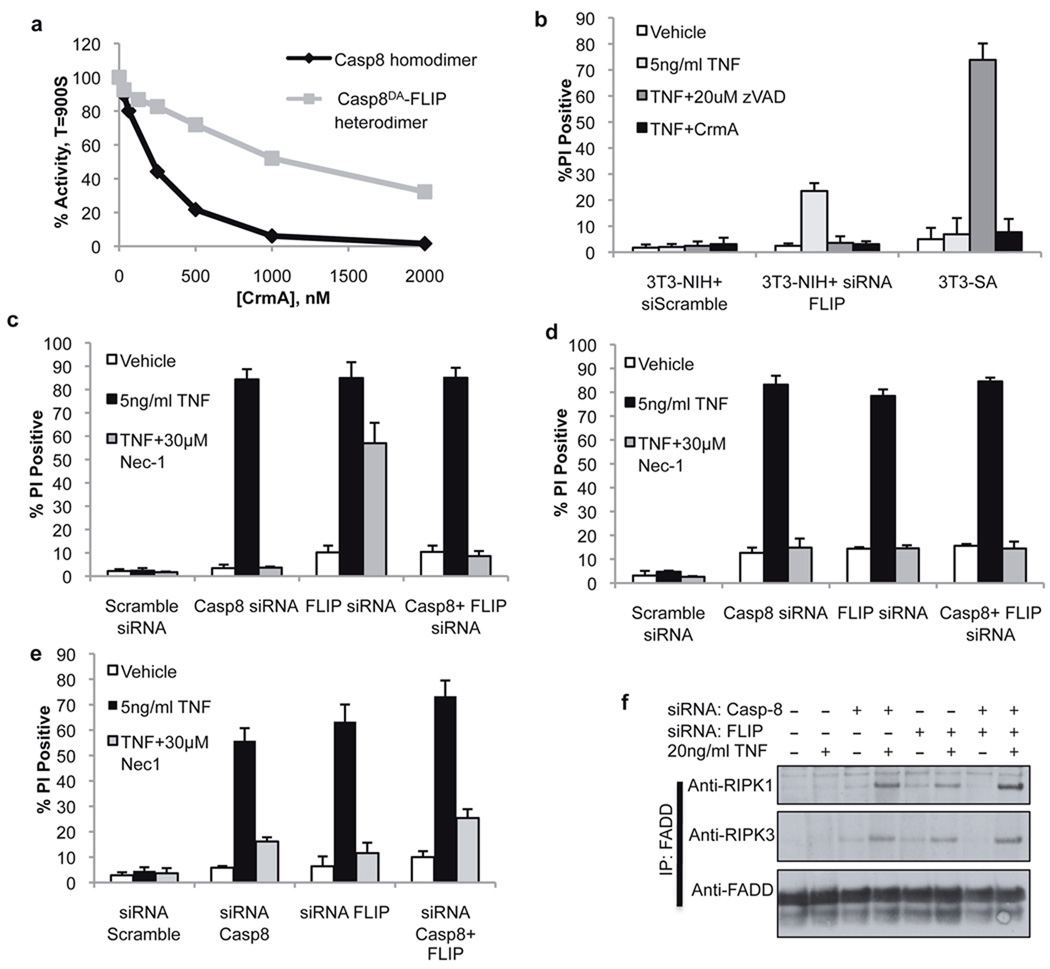
CrmA is a pox-virus protein that potently inhibits the enzymatic activity of caspase-8 homodimers21(Fig. 4a). Remarkably, the ability of CrmA to inhibit the activity of caspase-8DA-FLIP heterodimers was strikingly attenuated (Fig. 4a). Hypothesizing that this property would allow discrimination between the cellular roles of caspase-8 homodimers and caspase-8-FLIP heterodimers, we expressed CrmA in RIPK3-deficient 3T3-NIH cells or RIPK3-expressing 3T3-SA cells and examined the response to TNF (Fig. 4b). CrmA blocked the RIPK3-independent, caspase-8-dependent apoptotic death induced by TNF treatment of 3T3-NIH cells following FLIP knockdown. However, CrmA did not sensitize RIPK3-expressing 3T3-SA cells to TNF-induced death (Fig. 4b), demonstrating that the inhibitor is not able to influence the “survival” role of caspase-8. However, 3T3-SA cells expressing CrmA were readily sensitized to undergo TNF-dependent death by knockdown of caspase-8, FLIP, or both (Fig. 4d). Importantly, death induced by FLIP knock-down in 3T3-SA cells was only partially inhibited by the RIPK1 inhibitor necrostatin-1 (Nec1) or by stable knock-down of RIPK3, presumably due to contribution from caspase-8 dependent, RIPK3 independent apoptotic death under these conditions (Fig. 4c, Fig. S4a). However, in CrmA expressing 3T3-SA cells, the death induced by FLIP knockdown and TNF treatment was entirely inhibitable by Nec1 (Fig. 4d), presumably due to CrmA-mediated blockade of caspase-8-dependent apoptosis in these cells. These observations provide further support for the idea that the caspase-8-FLIP heterodimer is functionally active in inhibiting RIPK3-dependent necrosis without promoting apoptosis.
Figure 4. The FLIP-Caspase-8 heterodimeric complex suppresses RIPK3-dependent cell death.
a. Inhibitory effect of CrmA on FKBP-Caspase-8 homodimers or caspase-8DA-FLIP heterodimers induced by dimerizer treatment of purified recombinant protein. b. Cell death (PI uptake) of RIPK3-deficient (NIH) or RIPK3 expressing (SA) 3T3 cells treated as indicated for 12h. (Error bars are s.d., n=3) c, d, e. Cell death (PI uptake) of 3T3-SA cells stably expressing vector (c) or CrmA (d), or anti-apoptotic Bcl-XL (e) following transfection with the indicated siRNA and treatment with TNF and the RIPK1 inhibitor Nec-1 as indicated for 24h. (Error bars are s.d., n=3) f. 3T3-SA cells expressing Bcl-XL were subjected to immunoprecipitation of FADD following transfection of siRNAs to caspase-8 and FLIP and treatment with TNF for 90 minutes as indicated. Immune complexes were resolved by western blotting with the indicated antibodies. The data presented are representative of results obtained with either of 2 separate siRNAs to both caspase-8 and FLIP.
We next sought to determine whether FLIP itself is required for the suppression of RIPK3-dependent TNF induced death, or whether it is merely required to dampen the apoptotic effect of caspase-8. To differentiate between these possibilities, we blocked the mitochondrial pathway of apoptosis in 3T3-SA cells by expression of the anti-apoptotic Bcl-2 family member Bcl-XL, which blocks caspase-8-mediated apoptosis in many cells22. Strikingly, knock-down of FLIP in these cells strongly sensitized them to TNF-induced cell death, an effect that was completely inhibited by Nec1 or concomitant RIPK3 knockdown (Fig. 4e, S4b). Western blot analysis showed appearance of fully-processed caspase-8 upon FLIP knockdown and TNF treatment in 3T3-SA cells with or without overexpression of Bcl-XL (Fig. S4c). However, Bcl-XL expression blocked appearance of fully processed caspase-3 under these conditions, consistent with inhibition of the mitochondrial pathway of apoptosis (Fig. S4c). Therefore, cells in which caspase-8 is activated but apoptosis is blocked nevertheless require FLIP for effective suppression of RIPK3-mediated death. Immunoprecipitation of FADD following TNF treatment of Bcl-XL expressing 3T3 cells did not co-precipitate RIPK1 or RIPK3 (Fig. 4f). However, when caspase-8, FLIP, or both were knocked down in these cells, association of both RIPK1 and RIPK3 with FADD was observed (Fig. 4f). Cleaved caspase-8 was observed in whole lysates from these cells upon FLIP knockdown and TNF treatment, indicating that active caspase-8 was present in these cells, but was unable to prevent formation of the RIPK1-RIPK3 complex in the absence of FLIP (fig. S4d). Therefore, caspase-8-FLIP heterodimer, but not caspase-8 homodimer, prevents the stable association of FADD, RIPK1, and RIPK3, and thereby prevents necrotic death.
The death of mice homozygous for caspase-8 deletion at e10.5 has been traced to a failure in early vascularization and hematopoietic development1. Our results demonstrate that this requisite role for caspase-8 in embryogenesis is eliminated by concurrent ablation of RIPK3. As RIPK3 is essential for programmed necrosis, the likely role of caspase-8 in development is to suppress the lethal effects of RIPK3, probably associated with necrotic death of endothelial and hematopoietic cells. Consistent with this idea, we observed RIPK3 expression in hematopoietic tissues (Fig. S1d). Further, we found that the ability of caspase-8 to inhibit RIPK3-dependent necrosis depends on the expression of FLIP. Caspase-8-FLIP heterodimers are enzymatically active, and this does not require cleavage of caspase-8 between its large and small subunits. In contrast, apoptosis induced by caspase-8 depends on such cleavage, which stabilizes the homo-dimer15–17,23. This explains the ability of non-cleavable caspase-8 to rescue development in caspase-8 deficient mice without restoring sensitivity to death receptor-induced apoptosis15. This also explains the observation that activation of caspase-8-deficient T-cells causes cell death that is inhibitable by Nec-124. Our finding that T-cells lacking both caspase-8 and RIPK3 proliferate normally is consistent with the idea that caspase-8 functions to suppress RIPK1-RIPK3 dependent cell death. Demonstration that non-cleavable caspase-8 can rescue T-cell proliferation15, along with our finding that FLIP can impart catalytic activity to this form of caspase-8, further supports an essential role for the FLIP-caspase-8 complex in this process. Furthermore, since FLIP is a well known transcriptional target of NF-kB25, the suppression of RIPK3-dependent necrosis may represent a novel role for NF-kB signaling in immune cell proliferation. Taken together, these results support the idea that the main non-apoptotic function of caspase-8 is to suppress RIPK3-dependent necrosis during development and immune cell proliferation, and that it does so in complex with FLIP. While caspase-8 may yet prove to have roles in other cellular processes, such as NF-kB activation26 and cell motility27, our results do not provide support for such activities in development.
Based on the phenotypes of the caspase-81, FADD6, and FLIP7–deficient animals, it is likely that the complex that protects cells from RIPK3-dependent necrosis includes all three of these components. Such complexes readily form in response to death receptor signaling28. At present, it is not known if development of caspase-8 deficient animals can be rescued by deletion of TNFR1, CD95, or the murine TRAIL receptor, all of which are capable of triggering RIPK-dependent necrosis in the absence of caspase-84. It is also possible that these (and their ligands) function redundantly to cause necrosis in caspase-8-deficient endothelium and hematopoietic cells, or that other death or immune receptors cause this effect.
The ability of CrmA to block caspase-8-mediated apoptosis but not the protective effect of caspase-8-FLIP suggests that some viruses can subvert this system to prevent both apoptosis and necrosis of infected cells. Consistent with our findings, T cells expressing a CrmA transgene were observed to resist death receptor-induced apoptosis, but unlike caspase-8-deficient T cells, showed no proliferative defects in response to activation29. In contrast, in one study CrmA over-expression was observed to sensitize L929 cells to TNF-induced necrosis; it is likely that in this case CrmA was expressed at high enough levels that its relatively weak inhibition of caspase-8-FLIP activity was sufficient to disrupt protection3.
The precise mechanism by which the catalytic activity of the caspase-8-FLIP complex is engaged to prevent RIPK3-dependent necrosis without triggering apoptosis is not presently known. Our data indicate that both caspase-8 and FLIP are required to disrupt formation of a stable complex containing FADD, RIPK1 and RIPK3 following TNF ligation. In cell-free systems, caspase-8-FLIP heterodimers display less proteolytic activity than caspase-8 homodimers on apoptotic substrates such as Bid and caspase-317,30, while maintaining a low but perceptible ability to cleave RIPK130. It is therefore possible that FADD-dependent formation of caspase-8-FLIP heterodimers prevents stable RIPK1-RIPK3 association by cleaving RIPK1. However, the target and efficiency of this protease in vivo is likely to be determined by its recruitment to specific complexes, and the presence and availability of substrates therein, making it difficult to draw firm conclusions from biochemical studies. It remains possible that downstream targets of RIPK3 that cause necrosis (currently unknown) are the relevant substrates. NADPH-oxidase, mitochondrial ROS production3, and metabolic enzymes9 have been suggested to be possible downstream effectors of RIPK3, but at present, we do not know how these may be engaged by this kinase following its activation by RIPK1.
Methods Summary
Mice and treatments
Mice with a deleted allele of caspase-8 were generated by germ line deletion of a previously-described caspase-8flox allele2. RIPK3 deficient animals were obtained from Vishva Dixit21. Genotypes were confirmed by tail snip PCR as previously described. For Jo2 injections, animals were injected via tail vein with 15µg purified Jo2 in LPS-free PBS per animal. Liver enzymes were assayed using a Trilogy Multi-Purpose Analyzer System from Drew Scientific, and liver sections were created and stained with hematoxylin and eosin, in the St Jude Veterinary Pathology Core facility. For SEB injections, 50µg SEB (Toxin Technology Inc.) per animal was injected via tail vein and T-cell populations were monitored by retro-orbital bleed and FACS as detailed previously. The St. Jude Institutional Animal Care and Use Committee approved all procedures in accordance with the Guide for the Care and Use of Animals.
Methods
siRNAs and DNA constructs
SiRNAs against caspase-8 (catalog# J-043044-05 and J-043044-06) and FLIP (J-041091-05 and J-041091-08) as well as scramble siRNA control (D-001810-01) were ordered from Dharmacon, and introduced into cells using Lipofectamine RNAiMAX (Invitrogen) according to manufacturer’s guidelines. Caspase-8 constructs were created by cloning full-length untagged murine caspase-8 upstream of a T2A ribosome-skipping sequence followed by GFP, and introducing this construct into the pBabe-puro retroviral vector. The indicated D387A and C362A mutants were introduced using the QuikChange Mutagenesis kit from Aligent. For in cellulo experiments, CrmA was cloned into the pLNCX vector upstream of an IRES-GFP sequence, and cells transduced with this construct or vector control were sorted to for GFP expression via FACS. Bcl-XL-expressing cell lines were produced by retroviral transduction of Bcl-XL-GFP fusion protein in pLZRS vector, followed by FACS sorting to achieve homogeneous expression.
Cell lines
L929, SVEC 4–10 and 3T3-SA cell lines stably expressing scrambled or RIPK3-specific short hairpin RNAs were produced using the TRCN0000022535 retroviral construct from OpenBiosystems or contol shRNA as previously described.31 These cell lines were maintained in DMEM (Invitrogen) supplemented with 10% FCS, L-glutamine, and pen/strep. MEF were isolated from RIPK3−/−:Caspase-8−/− embryos, transformed using E1A12S and H-RasG12V in pWZL-Hygro and pBabe-Puro retroviral vectors respectively, then selected in 0.5µg/ml puromycin and 40µg/ml hygromycin. These transformed cells were then transduced with the murine caspase-8 constructs described in above, and sorted by FACS to achieve homogenous caspase-8 expression, then transduced with full-length untagged murine RIPK3 in the pLZRS retroviral vector and selected in 100µg/ml zeocin. MEF were maintained in DMEM as described above, but also supplemented with 55µM β-mercaptoethanol, 1mM sodium pyruvate and non-essential amino acids (Gibco). Caspase-8flox/flox:RosaCreER MEF were transformed as described above, and Cre recombinase was activated by culturing cells in 100nM 4-hydroxytamoxifen for 48h, followed by culture for 1 week to establish a stable population. Caspase-8 deletion was confirmed by PCR and western blot (not shown).
Immuoprecipitation of FADD
3T3-SA cells stably overexpressing Bcl-XL were transfected with siRNAs specific to FLIP, Caspase-8, or with a scrambled control siRNA as described. 48h post-transfection, cells were treated with 20ng/ml recombinant murine TNF-α for 90 minutes. Immuoprecipitation of DISC-associated complexes was carried out using buffer and lysis conditions previously described32. FADD was immunoprecipitated using the M19 polyclonal anti-FADD antibody conjugated to Protein A/G-PLUS Agarose beads, also from Santa Cruz. Immune complexes were eluted by boiling in reducing western blot loading buffer and resolved by western blot using the antibodies described.
Immune cell staining, cell death and activation assays
For immune cell staining, spleen, thymus and lymph node were harvested from animals and single cell suspensions were generated. For immune cells staining from the blood, blood was harvested weekly from the retrorbital sinus from animals anesthetized with 2–2.5% isoflurane in 1L oxygen. Red blood cells were lysed in hypotonic buffer and samples were stained with the appropriate antibodies as described below. Data was acquired using a FACsCalibur or LSRII using CellQuest Pro and FACsDiva software respectively. Data analysis was performed using FlowJo (Tree Star). For activation assays, splenic T cells were isolated from whole spleen using magnetic separation (Pan T cell isolation kit II, Miltenyi Biotec (130-095-130)). Cells were stained with CFSE and plated at 1×105 cells/well in 96 wells plates with 10µg/mL plate-bound anti-CD3 and 10µg/mL soluble anti-CD28. For thymocyte death assays, single cell suspensions of thymocytes were plated at 1.5×105 cells/well in 96 wells plates and treated with the various apoptosis inducers with or without 20µM qVD. Cells were harvested at 8h (or 22h for Jo2), stained with AnnexinV and 7-AAD, and assayed for viability using flow cytometry. Splenocytes were cultured in RPMI 1640 (Invitrogen) supplemented as described for MEF above. Thymocytes were cultured in this media as well, but charcoal-stripped FBS was used.
Compounds, antibodies and cytokines
Antibodies used for western blot were: Anti-RIPK1 from BD (610458), anti-RIPK3 from Imgenex (IMG-5523-2), anti-Caspase-8 (1G12) and anti-FLIP (Dave-2) both from Alexis, and anti-Actin (C4) from MP, anti-Caspase-3 from Cell Signaling (9662), and anti-Bcl-XL(S18) and anti-FADD(M19) both from Santa Cruz. Murine TNF-α came from Peprotech. Necrostatin-1 came from Enzo, and zVAD-fmk came from SM Biochemicals. Purified anti-murine CD95 (clone Jo2), antiCD3 (clone 145-2C11), and anti-CD28 (clone 37.51), and fluorescent-conjugated anti-B220-PE (clone RA3-6B2), anti-CD4-PerCp-Cy5.5 (clone RM4-5), anti-Vβ6-FITC and anti-Vβ8-PE came from BD Biosciences. Anti-CD3-FITC (clone 145-2C11) and anti-CD8-APC (clone 53-6.7), as well as 7-AAD came from eBioscience. Annexin-V-APC came from Invitrogen. Dexamethasone was from APP Pharmaceuticals, staurosporine, etoposide and ionomycin were from Sigma. UV irradiation was accomplished using a UV Stratalinker 2400 from Stratagene, while γ-irradiation used a JL Shepard Mark 1 Cesium 137 irradiator.
Protein purification and in vitro assays
Production of CrmA33 and FKBP-Caspase-834 were accomplished as previously described. FRB-FLIPL mutants were expressed as C-terminal His-tagged proteins in pET29b. Upon induction with 1 mM IPTG, cells were grown at 25 °C for 16 h and purified by Ni-affinity chromatography. Homodimerization and kosmotrope assays have been described3. Heterodimerization experiments were performed similarly, except that FRB-FLIPL and the heterodimerization drug AP21967 (provided, along with the homodimerization drug AP20187, by Ariad Pharmaceuticals, http://www.ariad.com/regulationkits) were added in 4–5 fold molar excess to FKBP-caspase-8. For CrmA inhibition studies, homo or heterodimeric activated caspase-8 (10–50 nM) diluted in caspase buffer (10 mM Pipes pH 7.2, 0.1 M NaCl, 1 mM EDTA, 10% sucrose, 0.05% CHAPS, 5 mM DTT)was added to a mixture of serially diluted CrmA (30-2000 nM). Caspase-8 activity was monitored using Ac-IETD-afc; indicated activities represent linear rates of IETDase activity between 850 and 950 seconds after addition of CrmA, as a percentage of activity without CrmA addition.
Supplementary Material
Acknowledgements
The authors thank W.J. Kaiser and E.S. Mocarski of Emory University for their discussions and material support. We thank Ariad Pharmaceutical for providing the homo-and heterodimerization reagents. We thank the members of the St Jude Immunology FACS core facility, as well as Melissa Johnson, Dr. Laura Janke and the St Jude Veterinary Pathology Core. We also thank the St Jude Hartwell Center. This work was supported by NIH grant AI44828 to D.R.G and CA69381 to G.S.S, as well as CIHR grant MOP 36537 to R.H. This work was also supported by ALSAC.
Footnotes
Author Contributions: A.O. and D.R.G conceived the study and designed the experiments. C.P.D and L.L.M. designed and conducted mouse breedings. C.P.D., R.W. and A.O. carried out all experiments involving mice and tissues from mice. A.O. and R.W. carried out experiments involving cell lines and produced western blots. P.F. provided essential logistical and administrative support. C.P. and G.S.S. conceived, designed and carried out in vitro dimerization assays and CrmA inhibition studies. R.H. produced the caspase-8flox animals that made the study possible.
Author Information: Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Varfolomeev EE, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9(2):267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 2.Salmena L, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17(7):883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vercammen D, et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187(9):1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 5.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10(4):348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 6.Yeh WC, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279(5358):1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 7.Yeh WC, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12(6):633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 8.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 10.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24(4):1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogasawara J, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 14.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1(6):469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 15.Kang TB, et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181(4):2522–2532. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- 16.Oberst A, et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 2010;285(22):16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes MA, et al. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell. 2009;35(3):265–279. doi: 10.1016/j.molcel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Chang DW, et al. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21(14):3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382(Pt 2):651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang DW, Yang X. Activation of procaspases by FK506 binding protein-mediated oligomerization. Sci STKE. 2003;2003(167):PL1. doi: 10.1126/stke.2003.167.pl1. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Q, et al. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272(12):7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 22.Scaffidi C, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17(6):1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pop C, Fitzgerald P, Green DR, Salvesen GS. Role of proteolysis in caspase-8 activation and stabilization. Biochemistry. 2007;46(14):4398–4407. doi: 10.1021/bi602623b. [DOI] [PubMed] [Google Scholar]
- 24.Ch'en IL, et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci U S A. 2008;105(45):17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21(16):5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su H, et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307(5714):1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 27.Scharner D, et al. Caspase-8 is involved in neovascularization-promoting progenitor cell functions. Arterioscler Thromb Vasc Biol. 2009;29(4):571–578. doi: 10.1161/ATVBAHA.108.182006. [DOI] [PubMed] [Google Scholar]
- 28.Geserick P, et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol. 2009;187(7):1037–1054. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith KG, Strasser A, Vaux DL. CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO J. 1996;15(19):5167–5176. [PMC free article] [PubMed] [Google Scholar]
- 30.Pop C, et al. FLIPL induces caspase-8 activity in the absence of interdomain caspase-8 cleavage and alters substrate specificity. Biochem J. 2011 doi: 10.1042/BJ20101738. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geserick P, et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol. 2009;187:1037–1054. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan LT, Caputo A, Bleackley RC, Pickup DJ, Salvesen GS. Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J Biol Chem. 1995;270:10377–10379. doi: 10.1074/jbc.270.18.10377. [DOI] [PubMed] [Google Scholar]
- 34.Oberst A, et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 285:16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.