Abstract
Primary biliary cirrhosis (PBC) is characterized by chronic non-suppurative destructive cholangitis (CNSDC) associated with destruction of small bile ducts. Although there have been significant advancements in the dissection of the adaptive immune response against the mitochondrial autoantigens, there is increasing data that suggests a contribution of innate immune mechanisms in inducing chronic biliary pathology. We have taken advantage of our ability to isolate subpopulations of liver mononuclear cells (LMC) and examined herein the role of toll-like receptors (TLR), their ligands and natural killer (NK) cells in modulating cytotoxic activity against biliary epithelial cells (BECs). In particular, we demonstrate that toll-like receptor 4 ligand (TLR4-L) stimulated NK cells destroy autologous BECs in the presence of interferon (IFN)-α synthesized by TLR 3 ligand (TLR3-L) stimulated monocytes (Mo). Indeed, IFN-α production by hepatic Mo is significantly increased in patients with PBC compared to disease controls. There were also marked increases in the cytotoxic activity of hepatic NK cells from PBC patients compared to NK cells from controls but only when the NK cells were prepared following ligation of both TLR3-L and TLR4-L stimulated LMC. These functional data are supported by the immunohistochemical observation of an increased presence of CD56 positive NK cells scattered around destroyed small bile ducts more frequently in liver tissues from PBC patients than controls. In conclusion, these data highlight critical differences in the varied roles of Mo and NK cells following TLR3-L and TLR4-L stimulation.
Keywords: natural killer cells, monocytes, biliary epithelial cells, toll-like receptors, IFN-α, primary biliary cirrhosis
Introduction
The cholangitis of PBC has been coined as an orchestrated immune attack, including involvement of autoantibodies, CD4+ and CD8+ T cells (1, 2). This concept has led to the thesis that a multi-lineage response against the immunodominant autoantigen PDC-E2 is an essential component of disease pathogenesis (3). It is unclear whether the natural history of PBC is “entirely” secondary to adaptive auto-immune responses; epidemiologic analysis has suggested a role of transient exposure to environmental agents in the etiology of PBC (4). The data presented herein suggests that innate immune mechanisms contribute to the pathology characteristic of PBC by either accelerating disease or by specific chronic destruction of small bile duct epithelial cells (5). Indeed, one paradox in PBC has been the relative lack of a therapeutic response to the various immunosuppressive drugs that have been administered to PBC patients, despite the observation that PBC is a model autoimmune disease (6). A more detailed analysis of the effector mechanisms involved in the pathogenesis of human PBC has led us to suggest that in addition to the documented adaptive autoimmune responses there is also a direct role of innate immune responses in the biliary pathology of PBC (2, 5, 7-9).
The studies described herein take advantage of our ability to culture primary human biliary epithelial cells (BEC) in vitro as well as to isolate sub-populations of liver infiltrating mononuclear cells (8, 10, 11). While there are significant numbers of NK cells present around small bile ducts, especially during the early stages of PBC (12), we note there are NK cells present throughout the disease course. Importantly, we have focused on these NK cells and report herein that such NK cells are highly cytotoxic for autologous BEC following ligation of the toll like receptor 4 (TLR4) expressed by NK cells in the presence of interferon-α (IFN-α). Furthermore, this function of NK cells is dependent on the activation of Mo via toll like receptor 3 (TLR3). We submit that activation of Mo and their cross talk with NK cells contribute to the pathology of PBC. The data supporting this view are the basis of the present report.
Materials and Methods
Subjects and Protocol
A total of 22 explanted liver tissues constitute the present study. Eight of these 22 liver tissues were from patients with PBC, 3 from patients with hepatitis B virus infection, 8 with hepatitis C virus infection, and 3 with alcoholic liver disease. The term control diseases in this report refers to patients with diseases other than PBC. All patients had end-stage liver cirrhosis without detectable signs of other acute liver injury from an unrelated cause. The diagnosis of PBC was based on established criteria (2) and sera from each of these patients had readily detectable high titers of anti-mitochondrial antibodies (2). The immunohistochemical studies reported herein were performed on fresh tissue samples from wedge biopsies of 47 patients including 11 normal controls with metastatic liver disease, 14 patients with PBC, 16 with hepatitis C and 6 with primary sclerosing cholangitis (PSC). All of the tissues from patients used herein for immuno-histological studies were classified as early stage without detectable signs of cirrhosis. Samples were obtained and studied after informed consent of the donor, and all experimental protocols were approved by the Research Ethics Committee of Kyushu University and the University of California at Davis. The isolation, verification of purity and the specific protocols used are described below.
Isolation of intrahepatic biliary epithelial cells (BEC) and liver-infiltrating mononuclear cells (LMC)
The liver mononuclear cell populations were isolated as previously described in detail by our laboratory (7). Briefly, liver specimens were first digested with 1 mg/ml of collagenase type I. Cells from the digested tissue were purified using a Ficoll-hypaque gradient to obtain LMC (9). The LMC were allowed to adhere by incubating the cells overnight in tissue culture plates and an enriched population of adherent cells harvested. This adherent cell population was maintained in tissue culture until the cells reached full confluence, usually by day 14, and the non-adherent cell population aspirated, washed and cryopreserved in media containing 7.5% DMSO and stored in liquid nitrogen.
BEC were separated from adherent cells using CD326 (EpCAM) conjugated MicroBeads (Miltenyi Biotec) specific for epithelial cells. Cells were then re-suspended in media consisting of a 1:1 mixture of Ham’s F12 and DMEM, supplemented with 5% FCS, epithelial growth factor (10 ng/ml), cholera toxin (10 ng/ml), hydrocortisone (0.4 μg/ml), tri-iodothyronine (1.3 μg/l), transferrin (5 μg/ml), insulin (5 μg/ml), adenine (24.3 μg/ml), and 10 ng/ml hepatocyte growth factor (R&D systems, Minneapolis, MN) and cultured (7). The purity of the cells was verified by immunohistochemical examination of an aliquot of these cells for the expression of cytokeratins 7 and 19 using appropriate antibodies (Dako, Glostrup, Denmark) and only cultures that were > 90% positive for these cytokeratins and > 95% viable (as determined by trypan blue) utilized for the studies reported herein. The cultures used in the studies herein were between 4 to 6 passages to exclude the possibility for potential loss of phenotype after prolonged in vitro culture.
Isolation of T cells, Mo, NK cells, myeloid dendritic cells (mDC), plasmacytoid DC (pDC) and natural killer T (NKT) cells
As previously reported (8), the T cells utilized for the studies were isolated from LMC using a Pan T cell isolation kit II (Miltenyi Biotec) (8). Similarly the highly enriched population of Mo and NK cells utilized were purified using Mo and NK cell isolation kits, respectively (Miltenyi Biotec) (8). The purity of the CD3+ T cells, Mo and NK cells utilized were >90% as determined by flow cytometric analysis of an aliquot from each isolation. In efforts to ensure the purity of the cell population being studied, the population of T cells, Mo, or NK cells were each harvested separately. In addition, the same assay was performed following depletion of each of the 3 cell lineages from LMC’s in efforts to confirm that the data obtained was indeed the function of the lineage being studied. The mDCs (BDCA-1+), pDC (BDCA-2+) and NKT cells were isolated using the mDC, pDC and NKT cell isolation kits (Mitenyi Biotec), respectively, which included two magnetic separation steps. The purity of BDCA-1+ mDCs and the CD3+ CD56+ NKT cells were each >80% as determined by flow cytometric analysis of an aliquot of the cell preparation utilized for the study. Enriched population of mDC and NKT cells were harvested separately and, once again, the same assay was performed following depletion of the specific cell population in efforts to confirm that the function identified was due to the specific cell lineage being studied.
Cytotoxicity assay against autologous BEC
The cytotoxic activity of LMC was assessed using an 8 hr 51Cr release assay using autologous BEC as target cells (7). Briefly, the detached BEC were labeled with 2μCi/ml 51Cr (Amersham) overnight, washed x3 in media and 5 × 103 51Cr labeled cells dispensed into individual wells of a 96 well round-bottom plate. The non-stimulated, the IL-2 or TLR activated LMC were added to triplicate wells at an effector to target cell ratio of 20:1 in a total volume of 200μl of complete RPMI medium. The IL-2 stimulated effector LMC used for the assay were stimulated for 3 days with IL-2 (100 units/mL) and the TLR activated LMC comprised of a series of cell cultures incubated with a single or mixture of TLR ligands each at a pre-determined optimal concentration of 2-10 μg/ml of the appropriate TLR-L prior to their addition to the target cells. The TLR ligands utilized included TLR2 ligand (lipoteichoic acid, LTA: TLR2-L), TLR3 ligand (polyinosine-polycytidylic acid, poly(I:C): TLR3-L), TLR4 ligand (lipopolysaccharide, LPS: TLR4-L), TLR5 ligand (Flagellin: TLR5-L), TLR7/8 ligand (CL097: TLR7/8-L), TLR9 ligand type A (ODN2216, CpG type A: TLR9-LA) and TLR9 ligand type B(ODN2006, CpG type B: TLR9-LB). The combination of TLR ligands used for activation of LMC included a) TLR2-L + the ligands for either TLR3, 4, 5, 7/8, 9-LA or 9-LB b) TLR3-L + the ligands for either TLR4, 5, 7/8, 9-LA, or 9-LB c) TLR4-L + ligands for either TLR5, 7/8, 9-LA, or 9-LB d) theTLR5-L + the ligands for either 7/8, 9-LA, or 9-LB e) TLR7/8-L + the ligands of either 9-LA to TLR9-LB f) TLR9-LA + TLR9-LB. The TLR ligands were purchased from Invitrogen (San Diego, CA). Controls consisted of triplicate wells containing target cells cultured in media alone and target cells which were incubated with 10% triton X-100 to determine spontaneous and maximal 51Cr release, respectively. Following incubation of the co-cultures of the effector with target cells for 8 hr, 100μl of supernatant fluid was collected from each well and counted and the percentage of specific 51Cr release calculated as (cpm of experimental release - cpm of spontaneous release) / (cpm of maximal release - cpm of spontaneous release) × 100). Experiments using the combination of TLR3-L and TLR4-L were performed on aliquots of samples at least three times from each of the patients. As further controls, polymyxin B and chloroquine were used as specific inhibitors of LPS and poly I:C, respectively, for assays involving TLR4 and TLR3 induced activation. While polymyxin B was added at the time of TLR4 activation, chloroquine was added 2 hours prior to the activation of the TLR3 pathway for the cytotoxicity assay.
Hepatic Mo, T cells, and NK cells were isolated from LMC following in vitro activation with TLR3-L and TLR4-L for 3 days. Subsequently, highly enriched populations of Mo, T cells, NK cells and LMC depleted of Mo, T cells and NK cells were assessed for their cytotoxic activity against autologous BEC at an effector to target cell ratio of 5:1. Thence enriched populations of NK cells and LMC were stimulated with several combinations of TLR3-L and TLR4-L in the presence of a variety of supernatant fluids prepared as described above. The combinations included a) activation of the appropriate cell cultures with TLR3-L and TLR4-L in the presence of supernatant of unfractionated LMC b) the activation of the appropriate cell cultures with TLR3-L in the presence of supernatant of TLR4-L activated LMC, c) activation of the appropriate cell cultures with TLR4-L in the presence of supernatant of TLR3-L activated LMC, and d) activation of the appropriate cell cultures with supernatants of TLR3-L and TLR4-L stimulated LMC. The stimulated NK and LMC were then assessed for cytotoxicity against autologous BEC. Finally, unfractionated LMC, and highly enriched populations of mDC, Mo, NKT or LMC depleted of mDC, Mo or NKT cells were cultured at 1×105/200 μl in 96 well plates for 48 hours in the presence of either TLR3-L or supernatant fluids obtained from cultures of NK cells stimulated with TLR4-L. The cultures were then assessed for cytotoxicity against autologous BEC.
In efforts to study the influence of IFN-α, an additional cytotoxicity assay was performed in which highly enriched populations of NK cells were stimulated with TLR4-L in the presence or absence of recombinant IFN-α. In parallel, the supernatant fluids from TLR3-L stimulated Mo in the presence or absence of anti IFN-α antibody (Abcam, Cambridge, MA) were studied. Similarly, in nested experiments, anti TNF-related apoptosis inducing ligand (TRAIL) monoclonal antibody (mAb) (R&D Systems, final concentration: 1μ g/ml), anti Fas-L mAb (R&D Systems, final concentration: 1μg/ml) or Granzyme B inhibitor (BioVision, final concentration: 10μM) were used in the same cytotoxicity assay in attempts to identify the effector molecules involved. Importantly, each of these experiments was performed on samples from all PBC patients and control liver disease patients at least three times.
IL-12, IL-15, IL-18 and IFN-α production from Mo
In efforts to identify the nature of the cytokines that were involved in promoting NK cell effector function, supernatants from the TLR3-L stimulated hepatic Mo cultured for 3 days were analyzed for levels of IL-12, IL-15, IL-18 and IFN-α. These cytokines were selected based on previously published data that has reported their involvement in NK cell functional activity (13). Assays were performed using a sandwich ELISA (R&D Systems, Minneapolis, MN), using a combination of unlabeled and biotin- or enzyme-coupled monoclonal antibody to each cytokine. Data reported herein represent results obtained from each of the experiments performed on samples from all patients at least three times.
Isolation and quantitation of mRNA for select markers
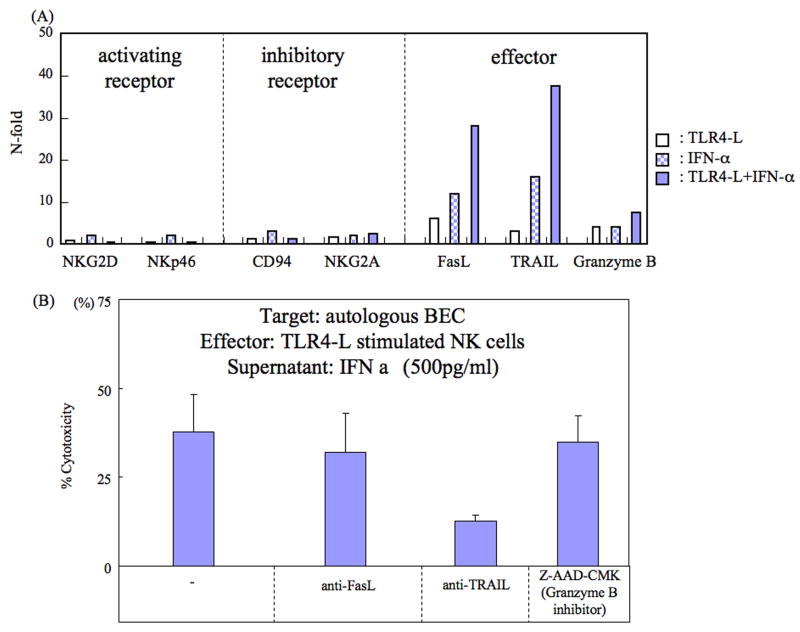
Aliquots of NK cells from PBC patients and disease controls were cultured in media alone (unstimulated) or cultured in the presence of TLR4-L, IFN-α, or the combination of TLR4-L and IFN-α for 24 hours. Total RNA was isolated from the cultured NK cells using RNAeasy columns (Qiagen, Valencia, CA) and quantitative analyses carried out utilizing a real-time PCR assay using SYBR Green PCR Master Mix (Invitrogen) and an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Tokyo, Japan). The relative levels of NKG2D and NKp56 (activating receptors), CD94 and NKG2A (inhibitory receptors), and FasL, TRAIL and Granzyme B (effector function markers) were determined using the primers noted in Table 1. Data are expressed as the fold-change in levels of mRNA vs. unstimulated NK cells.
Immuno-histochemical staining of human liver specimens for CD56 expression
De-paraffinized and rehydrated sections, and frozen sections of liver tissues from 11 normal controls with a diagnosis of metastatic liver disease, 14 patients with PBC, 16 with hepatitis C and 6 with primary sclerosing cholangitis (PSC), were used for the detection of CD56 expressing cells using standard immunostaining. Endogenous peroxidase was blocked using normal goat serum diluted 1:10 (Vector Lab, Burlingame, CA) for 20 min; CD56 was diluted 1:100 (Dako) and immunostaining was performed on coded sections and the data interpreted by a “blinded” pathologist.
Statistical analysis
All experiments were performed in triplicate and data points shown are the mean values of results of these triplicates. Comparisons between the points for certain data sets were expressed as mean ± standard deviation (SD), and the significance of differences was determined by Student’s t test. All analyses were two-tailed and p values <0.05 were considered significant. Statistical analyses were performed using Intercooled Stata 8.0 (Stata Corp, College Station, TX).
Results
Autologous BEC killing assay by LMC
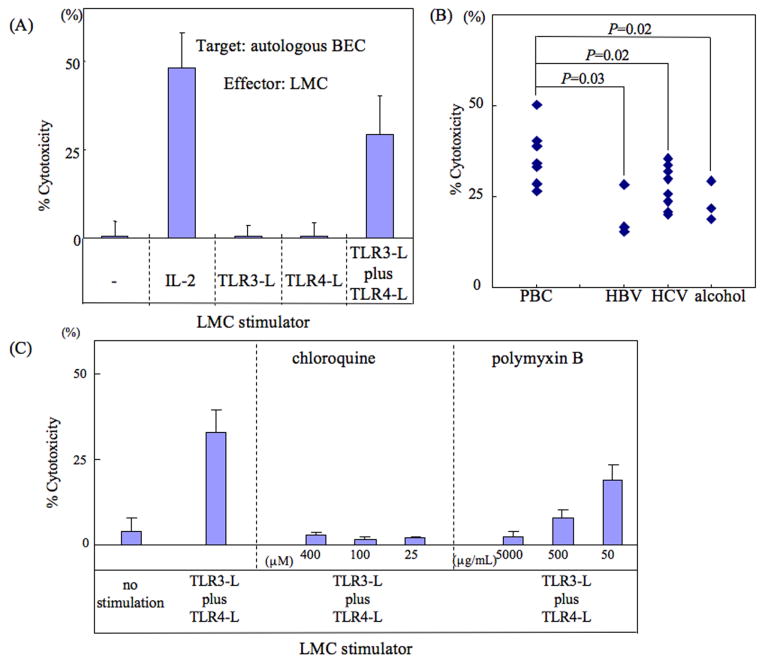
As noted in Fig. 1A and as expected, LMC when co-cultured with autologous BEC demonstrated no detectable cytotoxicity (0.5 ± 4.3%). However, following incubation of LMC’s with IL-2 (100 u/ml) a marked increase in cytotoxic activity against autologous BEC was observed (48.3 ± 9.7%). It is well known that innate immune effector cells can be activated in vitro via a number of TLR pathways besides IL-2. Thus, we studied a variety of TLR ligands either individually or in various combinations as outlined in the methods section above. Firstly, while LMC did not demonstrate any detectable cytotoxicity against autologous BEC following ligation of any single TLR ligand (for example the CTL activity following TLR3-L ligation was 0.5 ± 3.1% and following TLR4 ligation was 0.6 ± 3.9%) (Figure 1A and supplemental figure 1A), use of the combination of TLR3-L and TLR4-L led to significant cytotoxicity against autologous BEC (CTL activity; 29.3 ± 11.1%). Importantly, LMC did not induce significant cytotoxicity against autologous BEC using any other combination of TLR ligands (supplemental figure 1B). To exclude the possibility that the cytotoxicity noted using the combination of TLR3-L + TLR-L was not due to the direct effect of the TLR ligands on BEC instead of LMC, we co-cultured BEC with TLR3-L and TLR4-L in a similar cytotoxic assay described above. However, no detectable cytotoxic activity was found (data not shown).
Figure 1.
(A) In vitro activation requirements of LMC for cytotoxicity against BEC. LMC isolated from 8 patients with PBC and 14 control patients were cultured in vitro with either IL-2, TLR3-L alone, TLR4-L alone or a mixture of TLR3-L + TLR4-L for 3 days and then washed and assayed for cytotoxicity against autologous BEC using the standard 51Cr release assay. LMC cultured in media alone served as a negative control. The assay was performed in triplicate for each activation agent and expressed as mean +/- S.D. Representative data from one PBC patient is shown. (B) The net cytotoxicity for LMC against BEC was performed. There were statistical differences in the degree of net cytotoxicity induced by TLR3-L and TLR4-L activation of LMC in cells from PBC when compared to other control liver diseases. (C) The use of inhibitors of the TLR3 and TLR4 signaling pathways on the cytotoxicity of activated LMC against autologous BEC. LMC from 8 PBC patients and 14 control patients were activated in vitro with TLR3-L + TLR4-L in the presence of various concentrations of either chloroquine (TLR3 pathway inhibitor) or polymyxin B (TLR4 pathway inhibitor) and tested for cytotoxicity against autologous BEC. The left panel shows the control cytotoxicity data of LMC cultured in media alone or following activation with TLR3-L and TLR4-L. The middle and right panels reflect data obtained on aliquots of the same LMC activated using TLR3-L and TLR4-L but cultured in the presence of chloroquine or polymyxin B, respectively. Each culture was performed in triplicate and the data shown are mean +/- S.D. The data shown are from one PBC patient but is representative.
Studies were then carried out to evaluate the differences if any in the cytotoxicity of BEC following TLR3-L and TLR4-L stimulation of LMC from PBC as compared with LMC isolated from other disease controls. The net cytotoxicity of LMC’s from PBC patients (n=8) against BEC was 36.4 ± 7.5. In the case of LMC’s from HBV (n=3), HCV (n=8), and alcohol related cirrhosis (n=3) controls, the net cytotoxicity was 20.2 ± 7.1, 27.7 ± 5.9 and 23.4 ± 5.5, respectively, as shown in figure 1B. There were statistical differences in the degree of net cytotoxicity induced by TLR3-L+TLR4-L activation of LMC in cells from PBC when compared to similarly activated LMC’s from other control liver diseases (PBC vs HBV related cirrhosis: p=0.03, PBC vs HCV related cirrhosis: p=0.02, PBC vs alcohol related cirrhosis: p=0.02). Subsequently in efforts to confirm that the activation by TLR4-L (LPS) and TLR3-L (poly I:C) was indeed induced via the respective TLR pathways, use was made of pre-treatment of the activation agents with previously defined optimum concentrations of polymyxin B for LPS and chloroquine for poly I:C. As shown in Figure 1C, polymyxin B inhibited CTL activity in a dose dependent manner and chloroquine inhibited CTL activity even at the lowest concentration utilized.
NK cells are cytotoxic for autologous BEC in the presence of TLR3-L+TLR4-L stimulated LMC
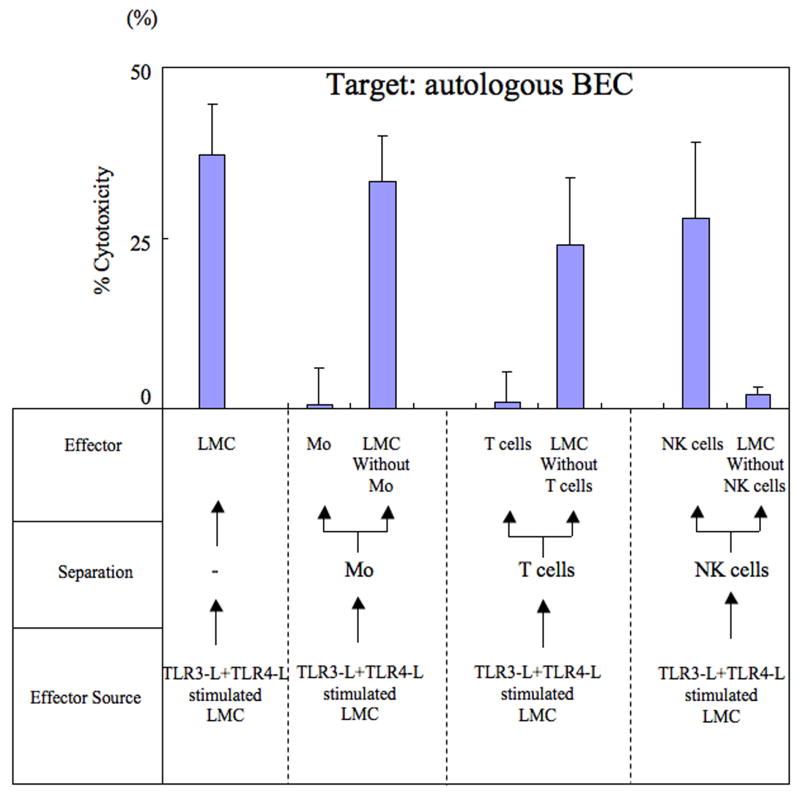
The ability of cells to induce cytotoxic activity against autologous BEC following the ligation of TLR3-L+TLR4-L was next examined. Cultures of LMC, stimulated with TLR3-L+TLR4-L were utilized to either isolate enriched populations of Mo, T cells, NK cells or isolate cultures depleted of each of these cell lineages. These enriched and depleted cell cultures were assessed for their cytotoxicity against autologous BEC. Unfractionated TLR3-L+TLR-4 activated LMC were used for purposes of a positive control. As shown in Figure 2, while Mo did not demonstrate any significant cytotoxicity against autologous BEC (CTL activity; 0.6 ± 5.4%), LMC depleted of Mo demonstrated significant cytotoxicity against autologous BEC (CTL activity; 33.2 ± 6.8%). Similarly while T cells did not demonstrate significant cytotoxicity against autologous BEC (CTL activity; 0.8 ± 4.5%), LMC depleted of T cells had significant cytotoxicity against autologous BEC (CTL activity; 24.0 ± 10.0%). On the other hand, while NK cells demonstrated significant cytotoxicity against BEC (CTL activity; 28.0 ± 11.0%), LMC depleted of NK cells did not show significant cytotoxicity against autologous BEC (CTL activity; 2.0 ± 1.1%). These data indicate that it is the NK cell lineage following TLR3-L and TLR4-L stimulation that is responsible for significant cytotoxic activity against autologous BEC. Represented data from one PBC patient is shown in Figure 2.
Figure 2.
Identification of the cell lineage within LMC that mediate cytotoxicity against autologous BEC. Cultures of LMC were activated in vitro with TLR3-L and TLR4-L and then aliquots assayed for cytotoxicity against autologous BEC (control) or used to isolate or deplete specific cell lineages. Thus, LMC were either enriched for Mo or depleted of Mo, enriched for T cells or depleted of T cells and enriched for NK cells or depleted of NK cells and each of these tested for cytotoxicity against autologous BEC. Results of mean cytotoxicity (mean +/- S.D.) of data obtained on one PBC patient is displayed.
TLR4-L stimulated NK cells with supernatants from TLR3-L stimulated LMC are cytotoxic for autologous BEC
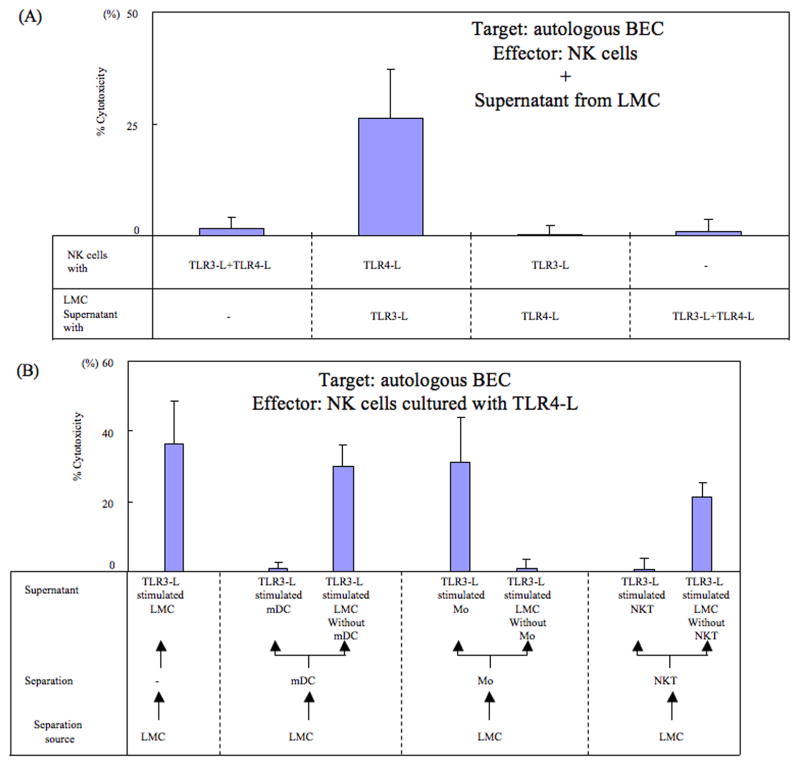
In efforts to identify the potential mechanisms by which activation of TLR3-L+TLR4-L in cultures of LMC generate cytotoxic activity of NK cells against autologous BEC, data obtained in preliminary studies showed that the activation of enriched population of NK cells with TLR3-L+TLR4-L did not lead to significant cytotoxicity against autologous BEC (see Fig. 3A). These data indicate that the generation of cytotoxic activity against autologous BEC was likely due to the presence of a second population of cells. Experiments were thus carried out to clarify the relationship of NK cells, LMC, TLR3-L and TLR4-L. We prepared supernatant fluids from LMC cultured in the presence of the appropriate ligands for either TLR3, TLR4 or TLR3 + TLR4. As shown in Fig. 3A, NK cells only demonstrated cytotoxicity against autologous BEC when cultured in the presence of TLR4-L and supernatant fluids prepared from TLR3-L activated LMC (CTL activity; 26.3 ± 11.0%), but not when cultured in the presence of TLR3-L and supernatant fluids prepared from LMC with TLR4-L (CTL activity; 0.2 ± 2.1%). The NK cells, in addition, did not kill autologous BEC in the presence of supernatant from TLR3-L and TLR4-L stimulated LMC (CTL activity; 0.8 ± 2.8%) as shown in Figure 3A. These data indicate that NK cells cytotoxicity against autologous BEC requires not only the activation of TLR4-L but also cytokines which are synthesized by LMC upon TLR3-L activation.
Figure 3.
A. The activation requirements of NK cells in mediating cytotoxicity against autologous BEC. Highly enriched population of NK cells were cultured in vitro in the presence of (i) TLR3-L+TLR4-L, (ii) TLR4-L and supernatant fluid from LMC cultured in the presence of TLR3-L, (iii) TLR3-L and supernatant fluid from LMC cultured in the presence of TLR4-L and (iv) supernatant fluids from LMC cultured in the presence of TLR3-L+TLR4-L. Cultures were performed in triplicate and the mean +/- S.D. of the net % cytotoxicity calculated. The data shown are from one PBC patient and is representative.
B. Identification of the cell lineage that is the source of the factor required to mediate cytotoxicity of autologous BEC by TLR4-L activated NK cells. A pool of a highly enriched population of NK cells was cultured with TLR4-L in the presence of supernatant fluids from a) unfractionated LMC cultured with TLR3-L (control), b) highly enriched populations of mDC or LMC depleted of mDC stimulated with TLR3-L, c) highly enriched population of Mo or LMC depleted of Mo stimulated with TLR3-L and d) highly enriched population of NKT cells to LMC depleted of NKT cells stimulated with TLR3-L. These cultures were tested for cytotoxicity against autologous BEC. Each culture was performed in triplicate and the data shown reflect mean +/- S.D. of net % cytotoxicity of the triplicate cultures. The data shown are from one PBC patient and is representative.
The supernatant from TLR3-L stimulated Mo induces NK cell cytotoxicity
We next carried out studies in efforts to identify the cell lineage that was the source of the cytokine(s) in the supernatant fluids from TLR3-L activated unfractionated LMC that induced TLR4-L stimulated NK cell cytotoxicity against autologous BEC. Highly enriched populations of mDC, Mo, NKT cells and the corresponding population of LMC’s depleted of mDC, Mo and NKT cells were stimulated with TLR3-L and the supernatant harvested; insufficient quantities were available to study the pDC fraction. NK cells were cultured with TLR4-L in the presence or absence of each of these supernatant fluids and analyzed for cytotoxicity against autologous BEC as described in the methods. As noted in Fig. 3B, while TLR4-L stimulated NK cells cultured in the presence of supernatant fluids from TLR3-L unfractionated LMC demonstrated significant cytotoxicity; similarly TLR4-L stimulated NK cells, when cultured with supernatant fluids of TLR3-L, stimulated mDC, and NKT cells did not demonstrate detectable cytotoxicity against autologous BEC. However, the TLR4-L activated NK cells, cultured in the presence of TLR3-L activated Mo, readily demonstrated cytotoxicity. The identification of Mo as the source of the cytokine required for TLR4-L activated NK cells to induce cytotoxicity against autologous BEC was confirmed by results obtained with supernatant fluids from TLR3-L stimulated LMC depleted of mDC, and NKT cells, respectively.
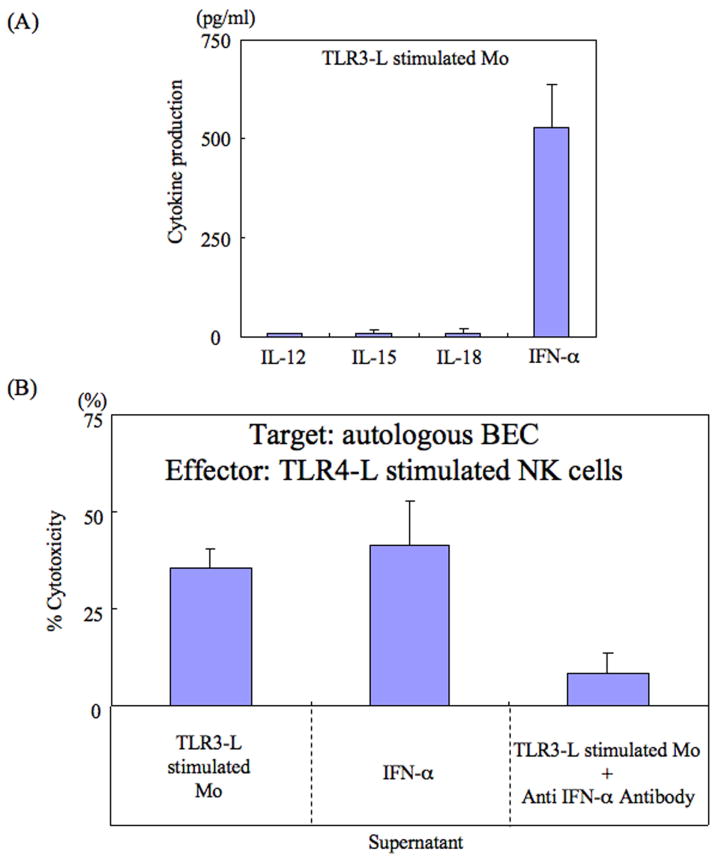
TLR3-L stimulated Mo produce IFN-α
The nature of the cytokine synthesized by TLR3-L activated Mo that promoted cytotoxicity in TLR4-L activated NK cells was studied next. We reasoned that the cytokine responsible for this activity was most likely IL-12, IL-15, IL-18 or IFN-α which have previously been shown to generally activate NK cells. As seen in Fig. 4A, while TLR3-L stimulated Mo produced low but detectable levels of IL-12 (7.9 ± 3.4 pg/ml), IL-15 (9.8 ± 8.0 pg/ml) and IL-18 (10.0 ± 9.6 pg/ml), the major cytokine synthesized was shown to be IFN-α (530.1 ± 106.2 pg/ml). In efforts to confirm that it was indeed IFN-α that was responsible for inducing TLR4-L activated NK cell cytotoxicity, aliquots of TLR4-L activated NK cells were cultured in the presence or absence of various concentrations of either IL-12, IL-18, IL-15 or IFN-α (see Fig. 4A). Data derived from such studies demonstrated that while TLR4-L activated NK cells cultured in the presence of IL-12, IL-18 or IL-15 (10-20 pg/ml) had no detectable cytotoxicity (data not shown), TLR4-L activated NK cells cultured in the presence of recombinant IFN-α (500 pg/ml) readily induced cytotoxicity against autologous BEC (cytotoxicity; 41.2 ± 11.4%) (Fig 4B). The identity of IFN-α as the cytokine responsible for inducing cytotoxicity in cultures of TLR4-L activated NK cells was confirmed with the use of anti IFN-α antibody. Thus, pre-treatment of supernatant fluids from TLR3-L activated Mo with anti-IFN-α reduced the cytotoxicity of TLR-4 stimulated NK cells against autologous BEC (cytotoxicity; 8.5 ± 5.2%). We also examined the relative levels of IFN-α synthesized by TLR3-L activated Mo from patients with other diseases as compared with Mo from PBC patients in efforts to determine whether there was a qualitative and/or quantitative difference in the synthesis of this cytokine. IFN-α production from TLR3-L activated Mo from PBC patients (n=8; 355 ± 132 pg/ml) was significantly higher than similarly activated Mo from HBV related cirrhosis (n=3; 175 ± 74 pg/ml: p<0.03), HCV related cirrhosis (n=8; 175 ± 57 pg/ml: p<0.01) or those from alcohol related cirrhosis (n=3; 180 ± 54 pg/ml: p<0.03).
Figure 4.
(A) Analysis of cytokines synthesized by in vitro TLR3-L activated hepatic Mo. Mo from the liver of the 22 patients included in the present study were isolated and cultured in vitro and the supernatant fluids analyzed for levels of IL-12, IL-15, IL-18 and IFN-α. Cultures were performed in triplicate and the data displayed represents mean +/- S.D. of values obtained from cultures from one representative patient. Statistical differences between PBC patients and disease controls are described in the text.
(B) IFN-α is required by TLR4-L stimulated NK cells to mediate cytotoxicity against autologous BEC. Aliquots of TLR4-L stimulated NK cells were cultured in the presence of either supernatant fluids from TLR3-L stimulated hepatic Mo (control), IFN-α, or supernatant fluids from TLR3-L stimulated Mo incubated with previously determined optimum concentration of anti-IFN-α monoclonal antibody. Cultures were performed in triplicate and assayed for cytotoxicity against autologous BEC. Data displayed are net % cytotoxicity and the data shown are from one representative PBC patient.
Contribution of other molecules to liver NK cell cytotoxicity against autologous BEC
While the above studies identified IFN-α as the cytokine synthesized by TLR4-L activated Mo, we next attempted to identify the nature of the molecules synthesized by NK cells that were potentially involved in mediating cytotoxicity against autologous BEC. First, we evaluated the expression of activating receptors, inhibitory receptors and effectors utilizing RT-PCR methods on mRNA isolated from unstimulated NK cells, TLR4-L stimulated NK cells, IFN-α stimulated NK cells and the combination of TLR4-L and IFN-α stimulated NK cells. As shown in Figure 5A, based on the activation signals the cultured cells expressed effector molecules such as FasL, TRAIL and/or Granzyme B. Among these effector molecules, TRAIL appeared to be the molecule involved in promoting the cytotoxicity of TLR4-L activated NK cells. Thus, as shown in Figure 5B, the addition of monoclonal anti-TRAIL antibody but not anti-FasL antibody or anti-Granzyme B significantly reduced the cytotoxicity of TLR4-L activated NK cells. These data indicate that IFN-α from Mo and TLR4-L activated NK cells induce TRAIL to mediate cytotoxicity against liver BEC.
Figure 5.
A. Expression of activating receptor, inhibitory receptor and effectors based upon TLR4-L, IFN-α or a combination of TLR4-L and IFN-α stimulation. Activating receptors and inhibitory receptors were not expressed following stimulation from TLR4-L, IFN-α or their combination. Effectors such as FasL, TRAIL and Granzyme B were synergistically expressed dependent on the stimulation. Data displayed are from one representative PBC patient.
B. Contribution of TRAIL to liver NK cell cytotoxicity against autologous BEC. Aliquots of NK cells were cultured in the presence of TLR4-L and 500 pg/ml of IFN-α alone (control) or with the addition of pre-determined optimum concentrations of anti-FAS-L, anti-TRAIL or Z-AAD-CMK (inhibitor of Granzyme B) and then assayed for cytotoxicity against autologous BEC. Cultures were performed in triplicate and the data displayed reflects net % cytotoxicity expressed as mean +/- S.D. of the triplicate cultures. The data shown are from one PBC patient and is representative.
NK cells around BEC in the liver
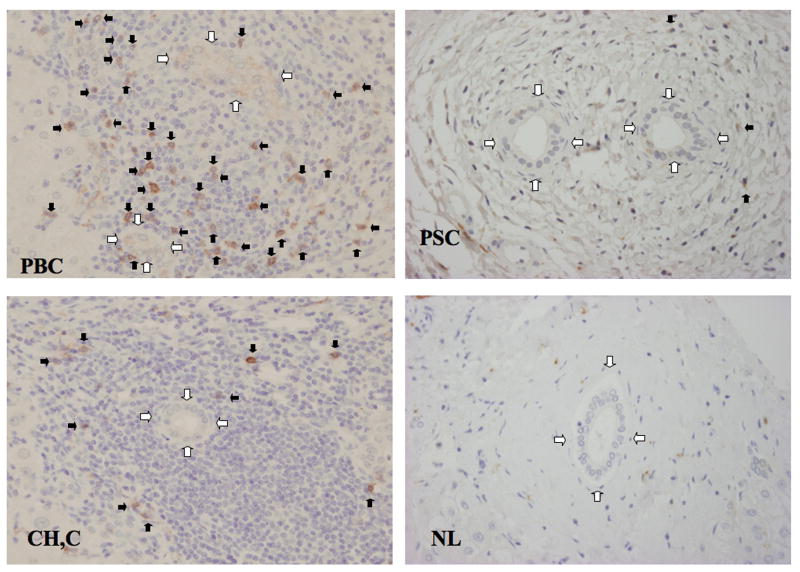
Finally we investigated the relative levels of NK cells around bile ducts in sections of liver by immunohistochemistry. Comparative analyses of sections of liver from PBC patients and patients with liver diseases other than PBC demonstrated that CD56+ NK cells predominantly invaded the portal area only in sections from PBC patients. Thus while the number of CD56+ NK cells invading portal areas was determined to be 8±4.4 cells per small bile duct from PBC patients, those for sections of liver from patients with hepatitis C gave values of 2.7±2.1 CD56+NK cells per small bile duct (p< 0.01), those from PSC gave values of 1.1±1.2 CD56+NK cells (p< 0.01), and those from normal liver gave a value of 0.8±1.0 CD56+NK cells (p< 0.01). Representative histochemical images are displayed in Figure 6.
Figure 6.
Immunohistochemistry of CD56 positive cells in liver. Mononuclear cells expressing CD56 are seen in the biliary epithelial layer and periductal tissue. In PBC, CD56+ cells are seen within the biliary epithelium (black arrow) and also at high density around the bile ducts (white arrow). In PSC and hepatitis C, CD56+ cells are scattered, and in normal liver CD56+ cells are rare around the bile ducts. Statistical differences between PBC patients and controls are described in the text.
Discussion
Studies of the mechanisms of a variety of autoimmune diseases, including PBC, have predominantly focused on the contributory role of adaptive T and B cell responses in the pathogenesis of disease (14-16). It is thus generally assumed that the major effector mechanisms that induce tissue pathology are those mediated by autoantigen specific CD8+ T cells and autoantigen specific antibodies that directly and/or indirectly contribute to tissue pathology. Interestingly, the institution of immunosuppressive agents that predominantly target pathways involved in the activation and effector mechanisms employed by cells of the adaptive immune system have so far failed to result in clear therapeutic benefit in patients with PBC. This therapeutic failure of inhibiting adaptive immunity in patients with chronic autoimmune diseases such as PBC has prompted a need for the re-evaluation of this line of thinking. Thus, it is reasonable to consider that alternate immune effector mechanisms are functioning and contributing to the pathogenesis of human PBC.
We submit that the involvement of innate immune effector mechanisms in any chronic disease including autoimmune diseases such as PBC needs to be considered and evaluated. Thus while it is easy to visualize a role for innate immune involvement in the initial stages of the disease process followed by the emergence of adaptive immune responses, it is clear that destruction of tissues during the chronic stages must require removal of dying cells and products of lytic cells. The removal of such unwanted tissues in addition to autophagy, must involve the function of innate immune mechanisms. It naturally follows that the activated state of the innate immune system must result in pro-inflammatory cascades contributing to the pathology of the autoimmune disease. It should also be noted that the adaptive immune system has been shown to affect the character and magnitude of innate inflammatory responses (17).
One of the major cell lineages of the innate immune system that is known to mediate target cell destruction are cells of the NK cell lineage (18). Our previous findings of a high frequency of NK cells within cellular infiltrates around small bile duct cells of the liver in PBC patients (12) prompted us to examine the potential role this cell lineage plays in the pathogenesis of human PBC. Data presented herein demonstrates that NK cells from TLR3-L and TLR4-L stimulated LMC kill autologous BEC, especially in PBC patients when compared to other control liver diseases; there have been descriptions of cross talk between NK cells and other innate immune populations via TLRs (13, 19). One explanation for this observation is the finding of high levels of IFN-α in the sera of patients with PBC as compared with sera from patients with other liver diseases and otherwise control individuals. Thus IFN-α is known to activate NK cell and contributes to enhance NK cell mediated cytotoxicity (supplemental figure 2 highlights these pathways).
The data herein also demonstrate that CD56 expressing NK cells upon ligation of TLR4 in the presence of IFN-α activates NK cells (20) and induces TRAIL (21). The function of NK cells appears to vary depending on the disease process (22). For example, the phenotypes of NK cells in patients with inflammatory bowel disease are different from those from normal intestinal mucosa (23). NK cell activation receptor NKp46 positive NK cells have been shown to recognize and destroy beta cells in type I diabetes (24). However, as previously shown, NKp46 is not induced on NK cells by TLR-4L in the presence of IFN-α. Hence, it is our working hypothesis that the function of local resident NK cells in CNSDC is distinct from that noted in patients with PBC as exemplified by the unique expression of TRAIL in the latter but not the former.
There was no detectable cytotoxic effect when BEC were cultured with either TLR3-L or TLR4-L alone in our assay. Upregulation of NK cell activating ligands has been reported in several liver subpopulations, including BEC and has been implicated in liver injury (25). It is not clear whether NK cell activating ligands are also upregulated on BEC in PBC and involved in the increased sensitivity to NK cell killing. Studies are in progress to define the relative sensitivity of BEC to NK cell cytotoxicity.
NK cells are cytotoxic for autologous BEC in the presence of TRAIL. The fact that human cholangiocytes constitutively express death receptor 5 which is the natural receptor for TRAIL, coupled with the finding of elevated levels of TRAIL expression and apoptosis in cholangiocytes of PBC patients (26), suggests that TRAIL/Death receptor5-mediated apoptosis may be the major pathway involved in the pathogenesis of chronic cholestatic disease.
Our data indicate that there are two requirements for NK cell mediated cytotoxicity. One requirement depends on the source of TLR4 ligation and the other is the source of IFN-α, reportedly elevated in CNSDC (27). Indeed, IFN-α appears to be derived from a monocytoid lineage, potentially plasmacytoid dendritic cells (pDC) (28); in our study we were not able to address this issue because of insufficient quantities of pDC in this experimental protocol. This is an issue that should be examined in the future. An additional remaining question is the identification of the source of the ligands for TLR3 and TLR4 that activate Mo and NK cells, respectively. Thus, whereas exogenous sources of ligands for TLR3 and 4 were utilized herein, it will be important to identify the natural ligands that are functional in patients with PBC since such data will be of major clinical importance. In this regard, it is of interest to note that there is a correlation of urinary tract infections and PBC (29, 30); that could be the candidate source for TLR-L.
Sera from patients with autoimmune diseases often reflect the presence of elevated levels of inflammatory cytokines, including type 1 and 2 interferons (IFN), TNF-α and IL-12 (31-33). IFN is induced by both a TLR-dependent and independent pathway in systemic autoimmunity (34). Additionally, activation and proliferation of both autoantigen specific and non-specific CD8 T cell responses are characterized by the expression of CD38 and Ki-67 expression (35). Previous work has demonstrated that pDC is a major source of type 1 IFN in response to ligation of TLR7 (36). In this regard, the characteristics of pDC that contribute to their pathogenic role include the observation that TRAIL-expressing pDC induces death of CD4 T cells that express TRAIL-associated death receptors (37). In addition, pDC inhibit T cell proliferation through an indoleamine oxidase (IDO)-dependent pathway (38) and, finally, pDC rapidly migrate to the site of autoimmune mediated injury and/or infection and attract CD4+ T cells to the site (39). We should note that in this study we did not evaluate IFN production from pDC in the presence of TLR7/8-L (CL097), but we did note the absence of cytolytic activity of LMC incubated with TLR4-L and TLR7/8-L (CL097).
Finally, it has also been demonstrated that CX3CL1 is expressed by BEC from patients with PBC and appears involved in the recruitment of intra-hepatic lymphocytes into bile ducts (8, 40). This interaction promotes NK cell activation (41). In conclusion, therefore, there is a complex but nonetheless well-defined relationship between liver mononuclear cell subpopulations and the biliary cell pathology of PBC. These interactions provide several steps that can potentially be modulated to reduce inflammation and will be the focus of further studies.
Supplementary Material
Supplementary figure legend 1. Cytotoxicity assay against autologous BEC with TLR-L. (A) TLR2-L, TLR3-L, TLR4-L, TLR5-L, TLR7/8-L, TLR9-LA or TLR9-LB were used individually as activation agents of LMC which were then tested for cytotoxicity against autologous BEC using a standard cytotoxic assay. No TLR-L induced detectable cytotoxicity alone. (B) Various combinations of two TLR ligands were used as agents for activation of LMC which were then tested for cytotoxicity against autologous BEC. Only the combination of TLR3-L and TLR4-L induced detectable cytotoxicity.
Supplemental figure legend 2. A summarized scheme of the interactions that involve TLR4-L stimulated NK cells producing cytotoxicity of autologous biliary epithelial cells in the presence of IFN-α, produced by TLR3-L stimulated Mo.
Acknowledgments
Financial support provided by Grant-in-Aid for Scientific Research(C) (Kakenhi 22590739) and National Institutes of Health grant DK39588.
List of Abbreviations
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
- CNSDC
chronic non-suppurative destructive cholangitis
- LMN
liver mononuclear cells
- BEC
biliary epithelial cells
- NK cells
natural killer cells
- Mo
monocytes
- NKT cells
natural killer T cells
- mDC
myeloid dendritic cells
- pDC
plasmacytoid dendritic cells
- TLR
toll-like receptor
- TLR-L
toll-like receptor ligand
- IFN
interferon
- TRAIL
TNF-related apoptosis inducing ligand
- mAb
monoclonal antibody
Contributor Information
Kenichi Harada, Email: kenichih@med.kanazawa-u.ac.jp.
Hiroaki Niiro, Email: hniiro@cancer.med.kyushu-u.ac.jp.
Ken Shirabe, Email: kshirabe@surg2.med.kyushu-u.ac.jp.
Akinobu Taketomi, Email: taketomi@surg2.med.kyushu-u.ac.jp.
Yoshihiko Maehara, Email: maehara@surg2.med.kyushu-u.ac.jp.
Koichi Tsuneyama, Email: ktsune@med.u-toyama.ac.jp.
Yasuni Nakanuma, Email: pbcpsc@kenroku.kanazawa-u.ac.jp.
Patrick Leung, Email: psleung@ucdavis.edu.
Aftab A. Ansari, Email: pathaaa@emory.edu.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
Koichi Akashi, Email: akashi@med.kyushu-u.ac.jp.
References
- 1.Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, Coppel RL. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–225. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 3.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNally RJ, Ducker S, James OF. Are transient environmental agents involved in the cause of primary biliary cirrhosis? Evidence from space-time clustering analysis. Hepatology. 2009;50:1169–1174. doi: 10.1002/hep.23139. [DOI] [PubMed] [Google Scholar]
- 5.Lleo A, Bowlus C, Yang G-X, Invernizzi P, Podda M, Van de Water J, Ansari A, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van de Water J, Ishibashi H, Coppel RL, Gershwin ME. Molecular mimicry and primary biliary cirrhosis: premises not promises. Hepatology. 2001;33:771–775. doi: 10.1053/jhep.2001.23902. [DOI] [PubMed] [Google Scholar]
- 7.Kamihira T, Shimoda S, Nakamura M, Yokoyama T, Takii Y, Kawano A, Handa M, et al. Biliary epithelial cells regulate autoreactive T cells: implications for biliary-specific diseases. Hepatology. 2005;41:151–159. doi: 10.1002/hep.20494. [DOI] [PubMed] [Google Scholar]
- 8.Shimoda S, Harada K, Niiro H, Taketomi A, Maehara Y, Tsuneyama K, Kikuchi K, et al. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51:567–575. doi: 10.1002/hep.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, et al. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawano A, Shimoda S, Kamihira T, Ishikawa F, Niiro H, Soejima Y, Taketomi A, et al. Peripheral tolerance and the qualitative characteristics of autoreactive T cell clones in primary biliary cirrhosis. J Immunol. 2007;179:3315–3324. doi: 10.4049/jimmunol.179.5.3315. [DOI] [PubMed] [Google Scholar]
- 11.Shimoda S, Ishikawa F, Kamihira T, Komori A, Niiro H, Baba E, Harada K, et al. Autoreactive T-cell responses in primary biliary cirrhosis are proinflammatory whereas those of controls are regulatory. Gastroenterology. 2006;131:606–618. doi: 10.1053/j.gastro.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, Gershwin ME. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 14.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Palmer MT, Weaver CT. Autoimmunity: increasing suspects in the CD4+ T cell lineup. Nat Immunol. 2010;11:36–40. doi: 10.1038/ni.1802. [DOI] [PubMed] [Google Scholar]
- 16.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 17.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, et al. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med. 2010;16:558–564. doi: 10.1038/nm.2142. 551p following 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 21.Stegmann KA, Bjorkstrom NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 22.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–1160. 1160 e1151–1157. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 23.Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume M, Chang J, Matuzaki Y, et al. Imbalance of NKp44+NKp46- and NKp44-NKp46+ natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology. 2010;139:882–892. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Gur C, Porgador A, Elboim M, Gazit R, Mizrahi S, Stern-Ginossar N, Achdout H, et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat Immunol. 2010;11:121–128. doi: 10.1038/ni.1834. [DOI] [PubMed] [Google Scholar]
- 25.Gao B. Natural killer group 2 member D, its ligands, and liver disease: good or bad? Hepatology. 2010;51:8–11. doi: 10.1002/hep.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda K, Kojima Y, Ikejima K, Harada K, Yamashina S, Okumura K, Aoyama T, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci U S A. 2008;105:10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takii Y, Nakamura M, Ito M, Yokoyama T, Komori A, Shimizu-Yoshida Y, Nakao R, et al. Enhanced expression of type I interferon and toll-like receptor-3 in primary biliary cirrhosis. Lab Invest. 2005;85:908–920. doi: 10.1038/labinvest.3700285. [DOI] [PubMed] [Google Scholar]
- 28.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 29.Hopf U, Moller B, Stemerowicz R, Lobeck H, Rodloff A, Freudenberg M, Galanos C, et al. Relation between Escherichia coli R(rough)-forms in gut, lipid A in liver, and primary biliary cirrhosis. Lancet. 1989;2:1419–1422. doi: 10.1016/s0140-6736(89)92034-5. [DOI] [PubMed] [Google Scholar]
- 30.Butler P, Valle F, Hamilton-Miller JM, Brumfitt W, Baum H, Burroughs AK. M2 mitochondrial antibodies and urinary rough mutant bacteria in patients with primary biliary cirrhosis and in patients with recurrent bacteriuria. J Hepatol. 1993;17:408–414. doi: 10.1016/s0168-8278(05)80225-9. [DOI] [PubMed] [Google Scholar]
- 31.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 35.Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. 2005;17:624–631. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Rajagopal D, Paturel C, Morel Y, Uematsu S, Akira S, Diebold SS. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of Toll-like receptor 7 agonists. Blood. 2010;115:1949–1957. doi: 10.1182/blood-2009-08-238543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stary G, Klein I, Kohlhofer S, Koszik F, Scherzer T, Mullauer L, Quendler H, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 38.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 39.Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends in Immunology. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Isse K, Harada K, Zen Y, Kamihira T, Shimoda S, Harada M, Nakanuma Y. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41:506–516. doi: 10.1002/hep.20582. [DOI] [PubMed] [Google Scholar]
- 41.Pallandre JR, Krzewski K, Bedel R, Ryffel B, Caignard A, Rohrlich PS, Pivot X, et al. Dendritic cell and natural killer cell cross-talk: a pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood. 2008;112:4420–4424. doi: 10.1182/blood-2007-12-126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure legend 1. Cytotoxicity assay against autologous BEC with TLR-L. (A) TLR2-L, TLR3-L, TLR4-L, TLR5-L, TLR7/8-L, TLR9-LA or TLR9-LB were used individually as activation agents of LMC which were then tested for cytotoxicity against autologous BEC using a standard cytotoxic assay. No TLR-L induced detectable cytotoxicity alone. (B) Various combinations of two TLR ligands were used as agents for activation of LMC which were then tested for cytotoxicity against autologous BEC. Only the combination of TLR3-L and TLR4-L induced detectable cytotoxicity.
Supplemental figure legend 2. A summarized scheme of the interactions that involve TLR4-L stimulated NK cells producing cytotoxicity of autologous biliary epithelial cells in the presence of IFN-α, produced by TLR3-L stimulated Mo.