Abstract
Rationale
Mesolimbic dopamine (DA), particularly in the nucleus accumbens, importantly regulates activational aspects of maternal responsiveness. DA antagonism and accumbens DA depletions interfere with early postpartum maternal motivation by selectively affecting most forms of active maternal behaviors, while leaving nursing behavior relatively intact. Considerable evidence indicates that there is a functional interaction between DA D2 and adenosine A2A receptors in striatal areas, including the nucleus accumbens.
Objective
This study was conducted to determine if adenosine A2A receptor antagonism could reverse the effects of DA receptor antagonism on early postpartum maternal behavior.
Methods
The adenosine A2A receptor antagonist MSX-3 (0.25–2.0 mg/kg, IP) was investigated for its ability to reverse the effects of the DA D2 receptor antagonist haloperidol (0.1 mg/kg, IP) on the maternal behavior of early postpartum female rats.
Results
Haloperidol severely impaired the expression of active maternal components, including retrieval and grouping the pups at the nest site, pup licking, and nest building. Co-administration of MSX-3 (0.25–2.0 mg/kg, IP) with haloperidol produced a dose-related attenuation of the haloperidol-induced behavioral deficits in early postpartum females. Doses of MSX-3 that effectively reversed the effects of haloperidol (0.5, 1.0 mg/kg), when administered in the absence of haloperidol, did not affect maternal responding or locomotor activity.
Conclusions
Adenosine and DA systems interact to regulate early postpartum maternal responsiveness. This research may potentially contribute to the development of strategies for treatments of psychiatric disorders during the postpartum period, with particular emphasis in maintaining or restoring the mother–infant relationship.
Keywords: Anergia, Haloperidol, Maternal motivation, Mother–infant relationship, Behavioral activation, Postpartum psychiatric disorders
Introduction
Early postpartum is a period of heightened vulnerability for the development or exacerbation of a number of psychiatric disturbances. The occurrence of maternal psychiatric disorders during the postpartum period not only has deleterious effects on the mother, but also poses risks for the mother–infant relationship and ultimately infant developmental outcome (Goodman and Gotlib 1999; Lyons-Ruth 2008). Symptoms related to reduced behavioral activation such as impaired motivational arousal and energy-related dysfunctions like psychomotor slowing, anergia, and fatigue are well-recognized aspects of postpartum depression (Paris et al. 2009; Weinberg and Tronick 1998), and are typical side effects of commonly used antipsychotic treatments for postpartum psychosis and schizophrenia (Bosanac et al. 2003). Importantly, it is recognized that these symptoms have profound disruptive effects on the quality of maternal behavior (Bosanac et al. 2003; Campbell et al. 2007; Field 2010; Murray et al. 1996; Wan and Green 2009). Specifically, mothers with such symptoms show a lack of attunement to their child's particular needs and signals, are less proactive, and are less likely to engage in social contact with their infants relative to healthy mothers (Carter et al. 2001; Jameson et al. 1997).
Impairments in behavioral activation and energy-related functions in humans, which are evident in depression and other psychiatric disorders, are associated with diminished dopamine (DA) neurotransmission (D'Aquila et al. 2000; Demyttenaere et al. 2005; Dunlop and Nemeroff 2007; Stahl 2002). Preclinical studies indicate that DA neurotransmission, particularly in the nucleus accumbens (NA), is a critical component of the brain circuitry regulating behavioral activation and effort-related processes involved in the performance of motivated behavior (Floresco et al. 2008; Ikemoto and Panksepp 1999; Salamone et al. 2006). Manipulation of accumbens DA powerfully influences the exertion of effort in motivated behavior, and alters effort-related decisions that are based upon cost–benefit analyses (Aberman and Salamone 1999; Bardgett et al. 2009).
In common with other motivated behaviors, the activational aspects of rat maternal behavior depend upon accumbens DA neurotransmission. Release of DA in the NA of postpartum female rats, as measured by in vivo neurochemical procedures, was reported to be enhanced during maternal interactions with pups (Champagne et al. 2004; Hansen et al. 1993). On the other hand, interference with DA neurotransmission, following either depletion of accumbens DA, as well as both systemic and intra-NA administration of relatively low doses of either DA D1 or D2 receptor antagonists, all selectively and severely disrupt most forms of active maternal behaviors in early postpartum female rats, including retrieval and grouping of the pups at the nest site, pup licking, and nest building, while general motor abilities remain unaffected. In contrast, such manipulations fail to impair, or even facilitate, nursing behaviors. Furthermore, the time postpartum females spend in close contact with the pups is not affected by DA antagonism and accumbens DA depletions, indicating that the mothers remain directed toward approaching the pups and maintaining physical contact with them (Hansen et al. 1991a,b; Numan et al. 2005; Pereira and Ferreira 2006; Silva et al. 2003).
Notably, the behavioral deficits related to DA dysfunction observed in postpartum mother rats resemble those of depressed and antipsychotic-treated human mothers. Moreover, postpartum Flinders sensitive line (FSL) female rats, a genetic animal model of depression (Overstreet 1993), showed reduced expression of active maternal behaviors correlated with reduced accumbens DA release while interacting with their pups compared to control mother rats (Lavi-Avnon et al. 2008). Furthermore, systemic treatment with clinically relevant doses of commonly used typical and atypical antipsychotics similarly disrupted active components of early postpartum maternal behavior in female rats (Li et al. 2004).
Recent studies have focused upon the involvement of the purine nucleoside adenosine and adenosine A2A receptors in functions associated with the DAergic system, including motor, cognitive, and motivational processes (Ferré et al. 1997; Mingote et al. 2008a; O’Neill and Brown 2006; Pinna et al. 2005; Salamone and Correa 2009). Adenosine A2A receptors are almost exclusively concentrated in striatal areas, including the NA, particularly on the dendrites of GABAergic striatopallidal neurons (Schiffmann et al. 1991), where they are predominantly co-localized with DA D2 receptors (DeMet and Chicz-DeMet 2002; Fink et al. 1992; Hettinger et al. 2001). Considerable evidence indicates that there is a functional antagonistic interaction between DA D2 receptors and adenosine A2A receptors at the cellular level (Chen et al. 2001; Ferré 1997; Fuxe et al. 2003; Svenningsson et al. 1999). Recent studies have begun to examine the role of DA-adenosine receptor–receptor interactions in behavioral procedures that assess activational aspects of motivation. For instance, adenosine A2A antagonism has been shown to reverse the behavioral effects of DA antagonism on behavioral output and effort-related decision-making using instrumental tasks that involve food-seeking behaviors (Farrar et al. 2010; Mott et al. 2009; Worden et al. 2009). Furthermore, injections of the adenosine A2A agonist CGS 21680 into the NA produced behavioral deficits that closely resembled those of accumbens DA depletion or antagonism (Barraco et al. 1993; Font et al. 2008; Mingote et al. 2008a).
Based upon these studies of DAergic involvement in maternal behavior, and recent findings showing a specific involvement of A2A receptors in the modulation of DA-mediated mesolimbic functions, the present study was conducted to examine the role of DA/adenosine A2A receptor interactions in maternal responsiveness. To this aim, the selective adenosine A2A receptor antagonist MSX-3 was assessed for its ability to reverse the behavioral effects on early postpartum maternal behavior induced by the DA receptor antagonist haloperidol, a reference antipsychotic currently in clinical use.
Materials and methods
Animals
Primiparous postpartum Sprague–Dawley female rats (original stock from Charles River Laboratories, Kingston, NY, USA), bred in our colony at the AAALAC-accredited Rutgers University Laboratory Animal Facility, were used in this study. Pregnant females were housed in individual transparent cages (48.5 cm long×38.5 cm wide×20.5 cm high) lined with fresh woodchip bedding (Beta chip, Northeastern Products, Warrensburg, NY, USA) and containing shredded paper towels as nest-building material. The floors of these cages were divided into four equal compartments by 5-cm-high Plexiglas dividers. All females were kept on a 12-h light/dark cycle (light on at 0700 AM) at 22±1°C, with ad libitum access to water, rat chow (PMI Lab Diet 5008, Nutrition International, LLC, Brentwood, MO, USA) and sunflower seeds. On postpartum day 1 (birth=day 0), litters were culled to four male and four female pups per dam. All animal care and experimental procedures performed in this study followed the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003), and were reviewed and approved by the Rutgers University Animal Care and Facilities Committee.
Pharmacological agents and selection of doses
Haloperidol (Sigma Chemical, St. Louis, MO, USA) was dissolved in a 0.3% tartaric acid solution (pH=4.0), which also was used as the vehicle condition for haloperidol. The haloperidol dose and injection time (0.1 mg/kg IP; 50 min before testing) chosen for the present study were selected on the basis of previously published reports, showing that this dose effectively disrupts active maternal behaviors, but does not induce locomotor suppression or cataleptic effects (Li et al. 2004; Pereira and Ferreira 2006; Stern and Keer 1999). Furthermore, at this dose, haloperidol is highly selective for DA D2 receptors, as demonstrated by ex vivo and in vivo binding studies (McCormick et al. 2010; Schotte et al. 1996; Zhang and Bymaster 1999), producing striatal DA D2 receptor occupancy levels (50–70%) in rodents that are consistent with occupancy levels observed with clinically effective doses of commonly used antipsychotics in humans (Kapur et al. 2003).
The adenosine A2A receptor antagonist prodrug MSX-3 ((E)-phosphoric acid mono-[3-[8-[2-(3-methoxyphenyl) vinyl]-7-methyl-2,6-dioxo-1-prop-2-ynyl-1,2,6,7-tetrahydropurin-3-yl]propyl] ester) was synthesized in the laboratory of Christa E. Müller (Hockemeyer et al. 2004) at the Pharmazeutisches Institut, Universität Bonn (Bonn, Germany). MSX-3 is also commercially available from Sigma. MSX-3 (free acid) was dissolved in 0.9% saline, and the pH of the MSX-3 solution was adjusted by adding 1.0 N NaOH until the drug was completely in solution (pH 7.4–7.8). Doses of MSX-3 were selected on the basis of previous research (Farrar et al. 2007; Worden et al. 2009) and on pilot studies.
Experimental design
Effects of MSX-3 on haloperidol-induced maternal behavior deficits in early postpartum female rats
This study investigated the ability of the adenosine A2A receptor antagonist MSX-3 (0.25–2.0 mg/kg, IP) to reverse the behavioral impairments in early postpartum maternal behavior induced by 0.1 mg/kg IP of the DA receptor antagonist haloperidol.
All behavioral testing was conducted on postpartum day 7, during the light phase of the light/dark cycle. Separate groups of postpartum females were randomly assigned to receive only one of the following treatments: (1) VEH/VEH: tartaric acid vehicle (50 min before testing) plus saline vehicle IP (20 min before testing); (2) HP/VEH: 0.1 mg/kg haloperidol IP (50 min before testing) plus saline vehicle IP (20 min before testing); (3) HP/MSX-3: 0.1 mg/kg haloperidol IP (50 min before testing) plus various doses of MSX-3 injected IP (0.25, 0.5, 1.0, and 2.0 mg/kg given 20 min before testing); and (4) VEH/MSX-3: tartaric acid vehicle (50 min before testing) plus various doses of MSX-3 injected IP (0.5, 1.0, and 2.0 mg/kg given 20 min before testing).
Fifty minutes before the test, postpartum females were removed from their cages, received the first injection, either haloperidol or the same volume of corresponding vehicle, and were immediately returned to their home cage. Thirty minutes later, both mother and litter were removed, the mothers received the second injection treatment, either MSX-3 or the same volume of corresponding vehicle, and they were immediately returned to their home cage. The eight-pup litters were individually housed in small cages until testing.
Maternal behavior testing
At the beginning of the test, 20 min after the second injection, each female's eight-pup litter was scattered in the home cage opposite the female's nest site. The frequency of each of the following maternal behavioral components was continuously recorded for 30 min: retrievals of the pups into the nest, mouthings (oral repositioning of the pups into the nest), full body and anogenital lickings, and nest building. In addition, the total duration of huddling behaviors, including hovering over the pups in the nest while actively performing other behaviors (i.e., licking of pups or self grooming), and the nursing posture kyphosis, a quiescent upright crouching over pups were recorded. Total time in contact with pups was the summed durations of huddling plus nursing behaviors. Also, the latencies to begin retrieving pups, to reunite of the entire litter into the nest, and to begin hovering over and nursing were registered. The latency to begin hovering over or nursing the pups was the first occurrence of a bout of each behavior ≥2 min in duration. A latency of 1,800 s was given for any category of behavior that was not initiated within the 30-min observation period. Other behaviors recorded included general exploration (line crosses and rearings), self-grooming, and eating/drinking.
Statistical analyses
Maternal behavior data are expressed as median (semiinterquartile ranges [SIQR]). As variances were not homogeneous, data were analyzed by means of non-parametric tests (Siegel 1956). Kruskal–Wallis one-way analysis of variance by ranks was used first for comparisons of multiple independent groups, and if an overall significant difference was detected, Mann–Whitney U tests were conducted to estimate differences between pairs of groups. Statistical significance in all cases was P<0.05, two-tailed probabilities.
Results
Haloperidol severely impaired active components of early postpartum maternal behavior
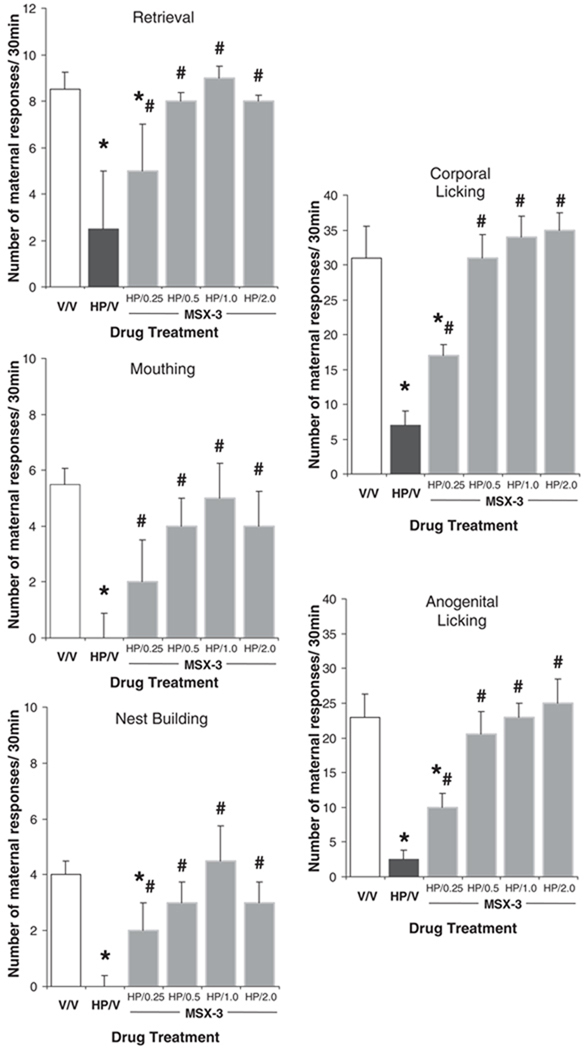
Early postpartum females receiving haloperidol treatment exhibited severe deficits in their active maternal behaviors. As shown in Fig. 1, haloperidol-treated postpartum females (HP/VEH, n=10) exhibited significantly fewer retrievals (HP/VEH vs VEH/VEH: U=3.5, P<0.001; Mann–Whitney U test) and mouthings (U=1.5, P<0.001) compared to the vehicle-treated control group (VEH/VEH, n=11). In fact, following haloperidol treatment, only one of the 10 postpartum females completed retrieving and grouping the pups into the nest; four of 10 did not retrieve any pups, and the remaining five retrieved ≤4 pups of their eight-pup litter. This is in contrast to vehicle-treated postpartum females who all completed retrieving and grouped the entire litter into the nest (1/10 vs 11/11, respectively— Fisher exact probability test P<0.05). The number of corporal (U=0.0, P<0.001) and anogenital lickings (U=0.0, P<0.001) was also dramatically reduced, and nest building was virtually absent (U=0.0, P<0.01) in haloperidol-treated females, highly contrasting with the typical levels exhibited by early postpartum control females (Fig. 1).
Fig. 1.
Effect of MSX-3 on haloperidol-induced changes in performance of active maternal behaviors. Median (SIQR) number of maternal responses of postpartum female rats following treatment either with: (1) vehicle + vehicle (V/V), (2) haloperidol + vehicle (HP/V), or (3) haloperidol plus various doses of MSX-3 (HP/0.25 MSX-3, HP/0.5 MSX-3, HP/1.0 MSX-3, and HP/2.0 MSX-3). Asterisk denotes significant difference in responding relative to V/V group and sharp sign indicates significant difference in responding relative to HP/V group
Haloperidol facilitated nursing behaviors
Although the majority of haloperidol-treated postpartum females did not group their pups into the nest, they did approach and investigate them, and eventually hovered over the pups and nursed them. Females in both groups spent a similar total amount of time in contact with their pups (U=54.0, P=ns); however, administration of haloperidol resulted in a more rapid onset (U=2.0, P<0.001) and a longer duration of nursing (U=13.0, P<0.01) compared to vehicle (Table 1). There were no significant differences between haloperidol-treated and vehicle control groups for any other behavior measured, including general locomotor activity, as measured by line crosses across the different quadrants of the test cage (HP/VEH vs VEH/VEH:U=33.0, P=ns; Mann–Whitney U test), rearings (U=46.5, P=ns), and self-grooming (U=42.0, P=ns) (Table 1).
Table 1.
Effects of MSX-3 on latencies and durations of maternal behaviors in haloperidol-treated early postpartum female rats
| Drug treatment | ||||||
|---|---|---|---|---|---|---|
| VEH/VEH | HP/VEH | Dosage of MSX-3 (mg/kg) | ||||
| HP/0.25 | HP/0.5 | HP/1.0 | HP/2.0 | |||
| Latency (seconds) | ||||||
| First retrieval | 2.0±1.5 | 608.5±886.8* | 11.0±7.0*# | 4.0±1.6# | 5.0±4.0# | 15.0±11.7# |
| Reunion litter | 73.0±25.5 | 1,800.0±0.0* | 175.0±842.0*# | 83.5±42.7# | 146.0±106.0*# | 156.0±96.5*# |
| Hover over | 220.0±102.3 | 329.5±104.6 | 289.0±132.0 | 274.0±151.6 | 235.0±78.5 | 347.0±63.0 |
| Nursing posture | 966.0±110.5 | 489.5±114.2* | 629.0±201.0* | 866.0±192.8# | 949.0±171.3# | 1,631.0±362.0# |
| Duration (seconds) | ||||||
| Hover over | 811.0±81.8 | 442.5±91.2* | 436.0±88.0* | 616.0±49.1*# | 753.0±140.3# | 626.0±54.7*# |
| Nursing posture | 570.0±184.5 | 935.5±73.2* | 1,055.0±93.0* | 750.5±140.8# | 481.0±177.8# | 237.0±204.5*# |
| Total time with pups | 1,368.0±62.5 | 1,357.5±96.6 | 1,441.0±30.5 | 1,417.0±82.4 | 1,254.0±133.8 | 1,010.0±365.0 |
| Locomotor activity (No. of responses/30 min) | ||||||
| Crossings | 26.0±1.8 | 25.0±2.4 | 27.0±2.5 | 27.0±2.8 | 35.0±9.8 | 36.0±4.5*# |
| Rearings | 9.0±2.3 | 9.5±2.4 | 7.0±1.5 | 8.5±2.75 | 13.0±9.3 | 18.0±5.5*# |
| Self-grooming | 3.0±1.0 | 4.5±1.5 | 4.0±1.0 | 4.0±1.3 | 2.0±1.25 | 7.0±1.8* |
Data are expressed as median ± SIQR. Kruskal–Wallis one-way analysis of variance followed by Mann–Whitney U-test
Significant difference in responding relative to the vehicle-treated group
Significant difference in responding relative to haloperidol-treated postpartum females
MSX-3 dose-dependently reversed the haloperidol-induced disruption of early postpartum maternal behavior
As shown in Fig. 1, co-administration of MSX-3 with haloperidol produced a statistically significant dose-related increase in all active components of maternal behavior relative to the haloperidol-treated postpartum group (HP/VEH vs HAL/0.5–2.0 MSX-3: all Ps<0.05; Mann–Whitney U test). Specifically, females receiving the lowest dose of MSX-3 (HP/0.25 MSX-3, n=9) exhibited a partial reversal of the effects of haloperidol, with mean number of behaviors between those of HP/VEH- and VEH/VEH-treated postpartum groups. Co-administration of 0.25 mg/kg of MSX-3 with haloperidol increased the number of all active maternal behaviors of postpartum females compared to the haloperidol-treated group (HP/0.25 MSX-3 vs HP/VEH, all Ps<0.05; Mann–Whitney U test). Furthermore, all the postpartum females receiving 0.25 mg/kg of MSX-3 along with haloperidol retrieved pups, five of nine completed retrieving and grouping the pups into the nest, and the remaining four retrieved ≤5 pups of their eight-pup litter, in contrast to the haloperidol-treated group (5/9 and 1/10, respectively; Fisher exact probability test P<0.05). Thus, the latency to begin (U=17.5, P<0.05) and to complete (U=22.0, P<0.05) retrieving was significantly shorter in those haloperidol-treated females receiving 0.25 mg/kg MSX-3 than vehicle. On the other hand, this dose of MSX-3 was not sufficient to completely restore behaviors to levels characteristic of early postpartumfemales (HP/0.25MSX-3 vs VEH/VEH: all Ps<0.05—Mann–Whitney U test; percentage of mothers grouping all their pups 5/9 and 11/11, respectively—Fisher exact probability test P<0.05). The remaining three doses of MSX-3, 0.5, 1.0, and 2.0 mg/kg (n=10, n=8 and n=7, respectively), effectively reversed the behavioral deficit in all active maternal components induced by haloperidol (HP/VEH vs HP/0.5–2.0 MSX-3: all Ps<0.05; Mann–Whitney U test), to levels characteristic of early postpartum (VEH/VEH vs HP/0.5–2.0 MSX-3: all Ps=ns; Mann–Whitney U test—Fig. 1).
In addition to reversing the effects of haloperidol on active components of maternal behavior, co-administration of MSX-3 with haloperidol also produced a dose-related reversal of the haloperidol-induced effects on hovering over and nursing behaviors (Table 1). Thus, following MSX-3 administration, haloperidol-treated postpartum females dose-dependently increased the duration of hovering over and consequently decreased the duration of nursing, with the 1.0 mg/kg dose of MSX-3 completely reversing haloperidol-induced effects to levels characteristic of control postpartum females (HP/1.0 MSX-3 vs HP/VEH: both Ps<0.05 and HP/1.0 MSX-3 vs VEH/VEH: both Ps=ns; Mann–Whitney U test—Table 1).
Only the highest dose of MSX-3 used, however, had minor motor stimulant effects on haloperidol-treated postpartum females, such as increased line crosses (VEH/VEH vs HP/2.0 MSX-3: U=4.0, P<0.01; Mann–Whitney U test) and rearings (U=6.0, P<0.01), compared to the vehicle-treated control group. This increased exploratory behavior mostly affected nursing behaviors by reducing the time females spent nursing their pups (U=16.0, P<0.05) compared to the control group. Furthermore, at 2.0 mg/kg, MSX-3 induced the expression of mild oral stereotypy, mostly sniffing, in four of seven haloperidol-treated females, although this did not appear to be disruptive to maternal behavior.
Effects ofMSX-3 alone on early postpartummaternal behavior
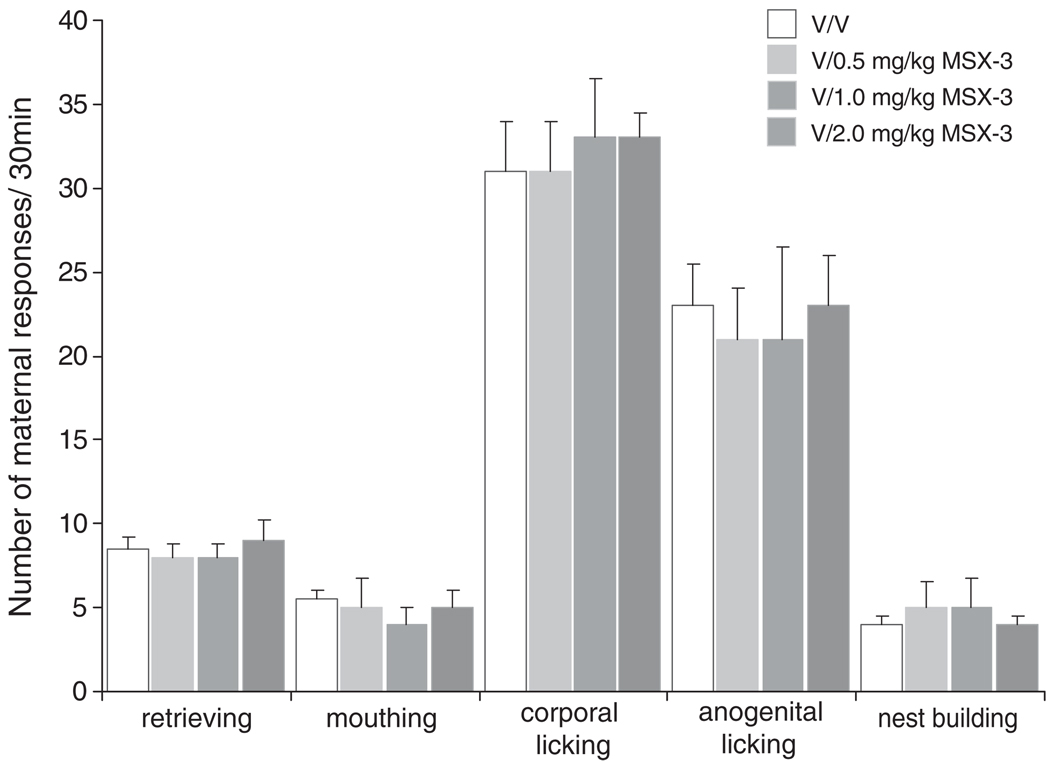
As shown in Fig. 2, administration of either 0.5 or 1.0 mg/kg of MSX-3 alone (without co-administration of haloperidol; n=7 and n=7, respectively) had no effect on any maternal component measured (Fig. 2 and Table 2). Thus, early postpartum females receiving either dose of MSX-3 exhibited full maternal behavior that was not different from the control group (VEH/VEH vs VEH/0.5 or 1.0 MSX-3: all Ps=ns; Mann–Whitney U test).
Fig. 2.
Effect of MSX-3 on early postpartum maternal behavior in the absence of haloperidol. Median (SIQR) number of maternal responses of postpartum female rats that received treatment either with vehicle (V/V) or vehicle plus various doses of MSX-3 (V/0.5 MSX-3, V/1.0 MSX-3, and V/2.0 MSX-3)
Table 2.
Effects of MSX-3 administered in the absence of haloperidol on latencies and durations of maternal behaviors in early postpartum female rats
| Drug treatment | ||||
|---|---|---|---|---|
| VEH/VEH | Dosage of MSX-3 (mg/kg) | |||
| VEH/0.5 | VEH/1.0 | VEH/2.0 | ||
| Latency (seconds) | ||||
| First retrieval | 2.0±1.5 | 3.0±1.5 | 2.0±2.2 | 9.0±5.75* |
| Reunion litter | 73.0±25.5 | 73.0±12.7 | 95.0±19.2 | 127.0±46.2* |
| Hover over | 220.0±102.3 | 251.0±54.5 | 247.0±62.2 | 383.0±157.0 |
| Nursing posture | 966.0±110.5 | 975.0.0±163.0 | 983.0±171.2 | 1,571.0±137.7* |
| Duration (seconds) | ||||
| Hover over | 811.0±81.8 | 867.0±62.2 | 820.0±89.2 | 801.0±180.7 |
| Nursing posture | 570.0±184.5 | 552.0±81.7 | 511.0±74.0 | 270.0±86.0* |
| Total time with pups | 1,368.0±62.5 | 1,403.0±130.7 | 1,368.0±51.2 | 926.0±289.2* |
| Locomotor activity (No. of responses/30 min) | ||||
| Crossings | 26.0±1.8 | 27.0±2.0 | 31.0±1.7 | 37.0±2.2* |
| Rearings | 9.0±2.3 | 8.0±1.5 | 15.0±4.0 | 19.0±4.0* |
| Self-grooming | 3.0±1.0 | 5.0±0.7 | 3.0±1.7 | 8.0±1.5* |
Data are expressed as median ± SIQR. Kruskal–Wallis one-way analysis of variance followed by Mann–Whitney U-test
Significant difference in responding relative to the vehicle-treated group
As was the case when co-administered with haloperidol (see above), the highest dose of MSX-3 (2.0 mg/kg, n=7) increased line crosses (VEH/VEH vs VEH/2.0 MSX-3: U=0.5, P<0.05; Mann–Whitney U test), rearings (U=3.0, P< 0.05), and self-grooming (U=1.5, P<0.05), and induced stereotyped sniffing when administered in the absence of haloperidol (Table 2). This increase in exploratory behavior significantly affected the latency and duration of huddling and nursing behaviors. Specifically, the latency to begin nursing (U=2.0, P<0.001) was significantly longer, while the total time spent with the litter (U=9.0, P<0.01) and time spent nursing (U=0.0, P<0.001) were significantly shorter compared to the control group.
Discussion
The present study demonstrates that the adenosineA2A receptor antagonist MSX-3 completely reversed the disruptive effects of the DA D2 antagonist haloperidol on early postpartum maternal responsiveness. In agreement with previous findings (Pereira and Ferreira 2006; Silva et al. 2001; Stern and Keer 1999), a low, sub-cataleptic dose of haloperidol produced a substantial disruption in all active components of maternal behavior, while facilitating nursing behavior.
Co-administration of the adenosine A2A receptor antagonist MSX-3 with haloperidol dose-dependently increased and eventually completely restored all active components of maternal behaviors to levels characteristic of early postpartum. The intermediate doses of MSX-3 (0.5 and 1.0 mg/kg) also reversed the increase in nursing behaviors induced by haloperidol. Thus, the effect of haloperidol was to decrease all active components of maternal behavior while concurrently facilitating nursing behavior, and MSX-3 substantially attenuated both effects.
The highest dose of MSX-3 used in the present study (2.0 mg/kg) produced mild locomotor stimulation in postpartum females, slightly increasing exploratory behaviors and inducing very mild oral stereotypies, mostly sniffing, which consequently affected maternal behavior. Consistent with this finding, it has been reported that systemic and intra-accumbens injections of adenosine A2A receptor antagonists stimulate locomotor and stereotyped activity (El Yacoubi et al. 2000; Nagel et al. 2003) and reverse the locomotor suppression induced by DA antagonists (Hauber et al. 2001; Ishiwari et al. 2007). Furthermore, the adenosine A2A receptor agonist CGS 21680 was shown to suppress locomotion when injected systemically (Mingote et al. 2008b) or directly into the NA (Barraco et al. 1993; Hauber and Münkle 1997). Additionally, treatments that enhance DA neurotransmission, including systemic apomorphine treatment and DA transporter knockout mutation are associated with hyperlocomotion and stereotypy and consequently disrupt maternal behavior in rodents (Pereira and Ferreira, submitted for publication; Spielewoy et al. 2000; Stern and Protomastro 2000). It is important to emphasize, however, that MSX-3 fully reversed the effects of haloperidol at dosage levels (0.5–1.0 mg/kg) that did not affect locomotion, suggesting that the effective dose range of MSX-3 for reversing haloperidol-induced deficits in maternal behavior is well below any potentially disruptive stimulant-like dose. The present results are consistent with previous findings demonstrating that adenosine A2A receptor blockade can reverse the behavioral effects of D2 antagonism (Correa et al. 2004; Farrar et al. 2007; Hauber et al. 2001).
The mechanism by which adenosine A2A antagonists reverse the behavioral effects of DA D2 antagonists is thought to occur at the cellular level. Adenosine A2A receptors are almost exclusively co-localized with DA D2 receptors on GABAergic striatopallidal neurons, whereas adenosine A1 and DA D1 receptors tend to be co-localized on striatonigral neurons (Fink et al. 1992; Schiffmann et al. 1991). Converging evidence indicates that there is a functional antagonistic interaction between adenosine A2A and DA D2 receptors that strongly modulates the function of GABAergic striatopallidal neurons. Specifically, there is evidence for the coexistence of two reciprocal antagonistic interactions between A2A and D2 receptors in the same neuron: an antagonistic A2A–D2 intramembrane receptor interaction, which depends on A2A–D2 receptor heteromerization and G(q/11)-PLC signaling (Azdad et al. 2009), and an antagonistic A2A–D2 receptor interaction at the adenylylcyclase level, as both receptors converge onto the same intracellular signaling transduction mechanism (Ferré et al. 2008; Svenningsson et al. 1999). In support of this, it has been shown that blockade of D2 receptors by haloperidol and other DA D2 antagonists, as well as activation of A2A receptors, increases expression of c-Fos-IR in striatopallidal neurons of the NA (MacGibbon et al. 1994; Robertson and Fibiger 1992) that can be counteracted by blockade of A2A receptors and activation of D2 receptors (Boegman and Vincent 1996; Pinna et al. 1999).
Consistent with a functional interaction between A2A and D2 receptors, recent behavioral studies have demonstrated that A2A receptor blockade preferentially reverses the effects of D2, but not D1, antagonism on effort-related tasks (Nunes et al. 2010; Worden et al. 2009). These findings, together with binding studies demonstrating that, at the dose used in the present study, haloperidol is highly selective for DA D2 receptors (McCormick et al. 2010; Schotte et al. 1996; Zhang and Bymaster 1999), strongly suggest that the disruptive effects of haloperidol on maternal behavior are primarily mediated by blockade of DA D2 receptors. This is further supported by studies showing that the D2 receptor agonist, quinpirole, reversed both the haloperidol-induced deficits in maternal behavior and haloperidol-induced c-Fos expression in striatal areas (Zhao and Li 2010).
Considerable evidence suggests that the NA is an important locus at which DAergic manipulations influence active components of maternal behavior. The selective suppression of active maternal behaviors by systemic haloperidol is also observed in postpartum females following transient functional inactivation of the ventral tegmental area (VTA) (Numan et al. 2009; Seip and Morrell 2009), depletion of accumbens DA (Hansen et al. 1991a,b), and intra-accumbens administration of DA D2 antagonists (Parada et al. 2008; Silva et al. 2003—although see Numan et al. 2005), but not after local DA depletion or antagonism in dorsal striatal sites (Hansen et al. 1991a; Keer and Stern 1999). Given the role of accumbens DA in maternal responsiveness, coupled with evidence of direct D2–A2A receptor–receptor interactions at the cellular level, the present results raise the possibility that NA might be an important brain locus for D2–A2A receptor interactions involved in activational aspects of maternal motivation. This idea is consistent with a recent study demonstrating that systemic and intra-accumbens co-administration of MSX-3 reversed the behavioral effects and attenuated the local increase in c-Fos expression induced by intra-accumbens eticlopride (Farrar et al. 2010). Ongoing experiments are being conducted to more precisely determine the anatomical locus of D2–A2A receptor interactions in maternal behavior.
Conclusions
The present results indicate that MSX-3 can exert a relatively specific reversal of the effects induced by DA D2 receptor antagonism on maternal behavior. These results are in agreement with, and extend the findings of, previous studies demonstrating that DA and adenosine systems in the brain interact to regulate activational aspects of motivation. Adenosine A2A receptor antagonists have been proposed as putative candidates to be used for the treatment of symptoms such as psychomotor slowing, anergia, and fatigue, which are evident in depression and other psychiatric disorders (Salamone et al. 2006). The present results suggest that adenosine A2A antagonists may be potentially useful for ameliorating motivational disruptions in mother–infant interactions that are caused by postpartum depression or exacerbated by antipsychotic medications used clinically during postpartum to treat psychoses and schizophrenia.
Acknowledgements
This research was supported by NARSAD Young Investigator Award and NIH SOAR DA027945 awarded to MP, NIMH MH078023 awarded to JDS, and NIH DA014025 awarded to JIM. The authors thank the Laboratory Animal Facility staff at Rutgers University, Newark Campus, for animal breeding and care.
Contributor Information
Mariana Pereira, Email: pereiram@andromeda.rutgers.edu, Center for Molecular and Behavioral Neuroscience, Rutgers, The State University of New Jersey, 197 University Avenue, Newark, NJ 07102, USA.
Andrew M. Farrar, Center for Molecular and Behavioral Neuroscience, Rutgers, The State University of New Jersey, 197 University Avenue, Newark, NJ 07102, USA
Jörg Hockemeyer, Pharma-Zentrum Bonn, Pharmazeutisches Institut, Pharmazeutische Chemie I, Universität Bonn, Bonn, Germany.
Christa E. Müller, Pharma-Zentrum Bonn, Pharmazeutisches Institut, Pharmazeutische Chemie I, Universität Bonn, Bonn, Germany
John D. Salamone, Department of Psychology, Division of Behavioral Neuroscience, University of Connecticut, Storrs, CT 06269-1020, USA
Joan I. Morrell, Center for Molecular and Behavioral Neuroscience, Rutgers, The State University of New Jersey, 197 University Avenue, Newark, NJ 07102, USA
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A–D2 receptor heteromerization. Neuropsychopharmacology. 2009;34(4):972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behav Neurosci. 2009;123:463–467. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraco RA, Martens KA, Parizon M, Normile HJ. Adenosine A2A receptors in the nucleus accumbens mediate locomotor depression. Brain Res Bull. 1993;31:397–404. doi: 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- Boegman RJ, Vincent SR. Involvement of adenosine and glutamate receptors in the induction of c-fos in the striatum by haloperidol. Synapse. 1996;22:70–77. doi: 10.1002/(SICI)1098-2396(199601)22:1<70::AID-SYN8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Bosanac P, Buist A, Burrows G. Motherhood and schizophrenic illnesses: a review of the literature. Aust N Z J Psychiatry. 2003;37(1):24–30. doi: 10.1046/j.1440-1614.2003.01104.x. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Matestic P, von Stauffenberg C, Mohan R, Kirchner T. Trajectories of maternal depressive symptoms, maternal sensitivity, and children’s functioning at school entry. Dev Psychol. 2007;43(5):1202–1215. doi: 10.1037/0012-1649.43.5.1202. [DOI] [PubMed] [Google Scholar]
- Carter AS, Garrity-Rokous FE, Chazan-Cohen R, Little C, Briggs-Gowan MJ. Maternal depression and comorbidity: predicting early parenting, attachment security, and toddler social-emotional problems and competencies. J Am Acad Child Adolesc Psychiatry. 2001;40(1):18–26. doi: 10.1097/00004583-200101000-00012. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hacket E, Fink JS, Low MJ, Ongini E, Schwarzschild MA. The role of the D2 dopamine receptor (D2R) in A2A adenenosine-receptor (A2AR) mediated behavioral and cellular responses as revealed by A2A and D2 receptor knockout mice. Proc Natl Acad Sci USA. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa M, Wisniecki A, Betz A, Dobson DR, O’Neill MF, O’Neill MJ, Salamone JD. The adenosine A2A antagonist KF17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to Parkinsonism. Behav Brain Res. 2004;148:47–54. doi: 10.1016/s0166-4328(03)00178-5. [DOI] [PubMed] [Google Scholar]
- D'Aquila PS, Collu M, Gessa GL, Serra G. The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol. 2000;405(1–3):365–373. doi: 10.1016/s0014-2999(00)00566-5. [DOI] [PubMed] [Google Scholar]
- DeMet EM, Chicz-DeMet A. Localization of adenosine A2A receptors in rat brain with [3H]ZM-241385. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:478–481. doi: 10.1007/s00210-002-0613-3. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois J. SCH 58261 and ZM 241385 differentially prevent the motor effects of CGS 21680 in mice: evidence for a functional ‘atypical’ adenosine A2A receptor. Eur J Pharmacol. 2000;401(1):63–77. doi: 10.1016/s0014-2999(00)00399-x. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Müller CE, Salamone JD. Adenosine A2A receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology (Berl) 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Segovia KN, Randall PA, Nunes EJ, Collins LE, Stopper CM, Port RG, Hockemeyer J, Müller CE, Correa M, Salamone JD. Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience. 2010;166(4):1056–1067. doi: 10.1016/j.neuroscience.2009.12.056. [DOI] [PubMed] [Google Scholar]
- Ferré S. Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology (Berl) 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev. 2010;33(1):1–6. doi: 10.1016/j.infbeh.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8(4):375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar AM, Pereira M, Worden L, Stopper C, Port RG, Salamone JD. Intra-accumbens injections of the adenosine A2A agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacology (Berl) 2008;199:515–526. doi: 10.1007/s00213-008-1174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferre S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61:S19–S23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991a;105(4):588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991b;39(1):71–77. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45(3):673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hauber W, Münkle M. Motor depressant effects mediated by dopamine D2 and adenosine A2A receptors in the nucleus accumbens and the caudate-putamen. Eur J Pharmacol. 1997;323:127–131. doi: 10.1016/s0014-2999(97)00040-x. [DOI] [PubMed] [Google Scholar]
- Hauber W, Neuscheler P, Nagel J, Müller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A2A receptors in the caudate-putamen of rats. Eur J Neurosci. 2001;14:1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hockemeyer J, Burbiel JC, Müller CE. Multigram-scale syntheses, stability, and photoreactions of A2A adenosine receptor antagonists with 8-styrylxanthine structure: potential drugs for Parkinson’s disease. J Org Chem. 2004;69(10):3308–3318. doi: 10.1021/jo0358574. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Madson LJ, Farrar AM, Mingote SM, Valenta JP, DiGianvittorio MD, Frank LE, Correa M, Hockemeyer J, Müller C, Salamone JD. Injections of the selective adenosine A2A antagonist MSX-3 into the nucleus accumbens core attenuate the locomotor suppression induced by haloperidol in rats. Behav Brain Res. 2007;178:190–199. doi: 10.1016/j.bbr.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson PB, Gelfand DM, Kulcsar E, Teti DM. Mother–toddler interaction patterns associated with maternal depression. Dev Psychopathol. 1997;9(3):537–550. [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305(2):625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67(5):659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Lavi-Avnon Y, Weller A, Finberg JP, Gispan-Herman I, Kinor N, Stern Y, Schroeder M, Gelber V, Bergman SY, Overstreet DH, Yadid G. The reward system and maternal behavior in an animal model of depression: a microdialysis study. Psychopharmacology (Berl) 2008;196(2):281–291. doi: 10.1007/s00213-007-0961-2. [DOI] [PubMed] [Google Scholar]
- Li M, Davidson P, Budin R, Kapur S, Fleming AS. Effects of typical and atypical antipsychotic drugs on maternal behavior in postpartum female rats. Schizophr Res. 2004;70(1):69–80. doi: 10.1016/j.schres.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K. Contributions of the mother–infant relationship to dissociative, borderline, and conduct symptoms in young adulthood. Infant Ment Health J. 2008;29(3):203–218. doi: 10.1002/imhj.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon GA, Lawlor PA, Bravo R, Dragunow M. Clozapine and haloperidol produce a differential pattern of immediate early gene expression in rat caudate-putamen, nucleus accumbens, lateral septum and islands of Calleja. Brain Res Mol Brain Res. 1994;23:21–32. doi: 10.1016/0169-328x(94)90207-0. [DOI] [PubMed] [Google Scholar]
- McCormick PN, Kapur S, Graff-Guerrero A, Raymond R, Nobrega JN, Wilson AA. The antipsychotics olanzapine, risperidone, clozapine, and haloperidol are D2-selective ex vivo but not in vitro. Neuropsychopharmacology. 2010;35(8):1826–1835. doi: 10.1038/npp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, Port RG, Sink KS, Bunce JG, Chrobak JJ, Salamone JD. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008a;28:9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Pereira M, Farrar AM, McLaughlin PJ, Salamone JD. Systemic administration of the adenosine A2A agonist CGS 21680 induces sedation at doses that suppress lever pressing and food intake. Pharmacol Biochem Behav. 2008b;89(3):345–351. doi: 10.1016/j.pbb.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a T-maze cost/benefit procedure. Psychopharmacology (Berl) 2009;204:103–112. doi: 10.1007/s00213-008-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, Cooper P. The impact of postnatal depression and associated adversity on early mother–infant interactions and later infant outcome. Child Dev. 1996;67(5):2512–2526. [PubMed] [Google Scholar]
- Nagel J, Schladebach H, Koch M, Schwienbacher I, Müller CE, Hauber W. Effects of an adenosine A2A receptor blockade in the nucleus accumbens on locomotion, feeding, and prepulse inhibition in rats. Synapse. 2003;49(4):279–286. doi: 10.1002/syn.10240. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119(6):1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS, Dellevigne AA, Correnti CM, Numan MJ. Temporary inactivation of ventral tegmental area neurons with either muscimol or baclofen reversibly disrupts maternal behavior in rats through different underlying mechanisms. Behav Neurosci. 2009;123(4):740–751. doi: 10.1037/a0016204. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Santerre JL, Given AB, Sager TN, Correa M, Salamone JD. Differential effects of selective adenosine antagonists on the effort-related impairments induced by dopamine D1 and D2 antagonism. Neuroscience. 2010;170(1):268–280. doi: 10.1016/j.neuroscience.2010.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M, Brown VJ. The effect of the adenosine A2A antagonist KW-6002 on motor and motivational processes in the rat. Psychopharmacology (Berl) 2006;184(1):46–55. doi: 10.1007/s00213-005-0240-z. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17(1):51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- Parada M, King S, Li M, Fleming AS. The roles of accumbal dopamine D1 and D2 receptors in maternal memory in rats. Behav Neurosci. 2008;122(2):368–376. doi: 10.1037/0735-7044.122.2.368. [DOI] [PubMed] [Google Scholar]
- Paris R, Bolton RE, Weinberg MK. Postpartum depression, suicidality, and mother–infant interactions. Arch Womens Ment Health. 2009;12(5):309–321. doi: 10.1007/s00737-009-0105-2. [DOI] [PubMed] [Google Scholar]
- Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175(1):139–148. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Pinna A, Wardas J, Cozzolino A, Morelli M. Involvement of adenosine A2A receptors in the induction of c-fos expression by clozapine and haloperidol. Neuropsychopharmacology. 1999;20:44–51. doi: 10.1016/S0893-133X(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Pinna A, Wardas J, Simola N, Morelli M. New therapies for the treatment of parkinson’s disease: adenosine A2A receptor antagonists. Life Sci. 2005;77:3259–3267. doi: 10.1016/j.lfs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Fibiger HC. Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience. 1992;46:315–328. doi: 10.1016/0306-4522(92)90054-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Dopamine/adenosine interactions involved in effort-related aspects of food motivation. Appetite. 2009;53:422–425. doi: 10.1016/j.appet.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications for understanding anergia and psychomotor slowing in depression. Curr Psych. 2006;2:267–280. [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Bonaventure P, Leysen JE. Endogenous dopamine limits the binding of antipsychotic drugs to D3 receptors in the rat brain: a quantitative autoradiographic study. Histochem J. 1996;28(11):791–799. doi: 10.1007/BF02272152. [DOI] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine-paired contexts. Behav Neurosci. 2009;123(6):1325–1338. doi: 10.1037/a0017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- Silva MR, Bernardi MM, Felicio LF. Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacol Biochem Behav. 2001;68(3):461–468. doi: 10.1016/s0091-3057(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Silva MR, Bernardi MM, Cruz-Casallas PE, Felicio LF. Pimozide injections into the Nucleus accumbens disrupt maternal behaviour in lactating rats. Pharmacol Toxicol. 2003;93(1):42–47. doi: 10.1034/j.1600-0773.2003.930106.x. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol. 2000;11(3–4):279–290. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. The psychopharmacology of energy and fatigue. J Clin Psychiatry. 2002;63(1):7–8. doi: 10.4088/jcp.v63n0102. [DOI] [PubMed] [Google Scholar]
- Stern JM, Keer SE. Maternal motivation of lactating rats is disrupted by low dosages of haloperidol. Behav Brain Res. 1999;99(2):231–239. doi: 10.1016/s0166-4328(98)00108-9. [DOI] [PubMed] [Google Scholar]
- Stern JM, Protomastro M. Effects of low dosages of apomorphine on maternal responsiveness in lactating rats. Pharmacol Biochem Behav. 2000;66(2):353–359. doi: 10.1016/s0091-3057(00)00180-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Wan MW, Green J. The impact of maternal psychopathology on child-mother attachment. Arch Womens Ment Health. 2009;12(3):123–134. doi: 10.1007/s00737-009-0066-5. [DOI] [PubMed] [Google Scholar]
- Weinberg MK, Tronick EZ. The impact of maternal psychiatric illness on infant development. J Clin Psychiatry. 1998;59(2):53–61. [PubMed] [Google Scholar]
- Worden LT, Shahriari M, Farrar AM, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: differential interaction with D1 and D2 family antagonists. Psychopharmacology (Berl) 2009;203(3):489–499. doi: 10.1007/s00213-008-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Bymaster FP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology (Berl) 1999;141(3):267–278. doi: 10.1007/s002130050834. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li M. c-Fos identification of neuroanatomical sites associated with haloperidol and clozapine disruption of maternal behavior in the rat. Neuroscience. 2010;166(4):1043–1055. doi: 10.1016/j.neuroscience.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]