Abstract
African American race is an independent risk factor for enhanced oxidative stress and inflammation. We sought to examine whether oxidative‐stress and inflammatory markers that are typically measured in humans also differ by race in cell culture. We compared levels between African American and Caucasian young adults and then separately in human umbilical vein endothelial cells (HUVECs) from both races. We found heightened oxidative stress and inflammation in the African Americans both in vitro and in vivo. African American HUVECs showed higher nitric oxide (NO) levels (10.8 ± 0.4 vs. 8.8 ± 0.7 μmol/L/mg, p = 0.03), Interleukin‐6 (IL‐6) levels (61.7 ± 4.2 vs. 23.9 ± 9.0 pg/mg, p = 0.02), and lower superoxide dismutase activity (15.6 ± 3.3 vs. 25.4 ± 2.8 U/mg, p = 0.04), and also higher protein expression (p < 0.05) of NADPH oxidase subunit p47phox, isoforms NOX2 and NOX4, endothelial nitric oxide synthase (NOS), inducible NOS, as well as IL‐6. African American adults had higher plasma protein carbonyls (1.1 ± 0.1 vs. 0.8 ± 0.1 nmol/mg, p = 0.01) and antioxidant capacity (2.3 ± 0.2 vs. 1.1 ± 0.3 mM, p = 0.01). These preliminary translational data demonstrate a racial difference in HUVECs much like that in humans, but should be interpreted with caution given its preliminary nature. It is known that racial differences exist in how humans respond to development and progression of disease, therefore these data suggest that ethnicity of cell model may be important to consider with in vitro clinical research. Clin Trans Sci 2011; Volume 4: 32–37
Keywords: African American, NADPH oxidase, HUVECs
Introduction
African American ethnicity is an independent risk factor for exaggerated oxidative stress, inflammation, hypertension, and cardiovascular disease. 1 , 2 Clinical and epidemiological studies have reported significant racial differences among African American, Caucasian, and Mexican populations. Oxidative stress arises from either an increased production of reactive oxygen species (ROS), through augmented release of the superoxide (O2 −) radical, or from an incapability of the antioxidant system to effectively remove the ROS. Increased oxidative stress and inflammation have independently been associated with chronic disease, but it remains to be established which one is the predecessor, and this may very well depend on external factors. 3 , 4 , 5 Research has also established that racial differences exist in the development, progression, and treatment of disease, however in molecular biology studies the racial origins of cultured cells have commonly been ignored. Therefore, it may be plausible that the complex mechanisms mediating the in vivo racial variation could also exist in vitro between cell cultures of different races. Thus, it is critical to measure the innate oxidative‐stress and inflammation levels of cultured cells and determine whether these levels actually differ between racial groups.
Over the past several decades, there has been a growing body of evidence defining the value of using cell culture as an appropriate in vitro model in order to elucidate mechanisms associated with the in vivo adaptations to various pathophysiologies. 6 Human umbilical vein endothelial cells (HUVECs) have been used to study many different biological processes because cells isolated from umbilical cords are typically free from pathogen and pathophysiology. It is believed that the HUVEC responses to any stimuli closely mimic those responses of true in vivo endothelial cells and also that HUVECs can serve as a suitable model for studying in vivo endothelial function.
As human clinical studies continue, translating to an appropriate in vitro model will be an important step in understanding the underlying mechanisms in the observed epidemiological differences. Therefore, we sought to examine whether common oxidative‐stress and inflammatory markers that are typically measured in humans also differ by race in cell culture. Using standard assays, we compared oxidative‐stress and inflammation levels between African American and Caucasian young adults. Separately we then used a parallel cell culture experimental design to compare oxidative‐stress and inflammation levels in HUVECs from both races.
Methods
Subjects included college‐aged self‐reported African American and Caucasian young adults, 18–25 years. All were apparently healthy and free of cardiovascular risk factors as assessed by completion of an extensive health history form during an initial laboratory visit. The subjects were asked to refrain from vitamin supplements for 2 weeks prior, from caffeine, alcohol, or exercise for 24 hours prior, and to fast for at least 10 hours the night before the study. All females were tested on days 1–5 of their menstrual cycle, during early follicular phase, to eliminate hormonal interference on oxidative stress. No females were on oral contraceptives. On the morning of the study, height and weight were measured, blood was drawn from the antecubital vein into ethylenediamine tetraacetic acid (EDTA) and Sodium‐Heparin tubes, and plasma separated by centrifugation and stored at −80°C until assay. Written, informed consent was obtained from each subject prior to participation. The Institutional Review Board of Temple University approved this study, and all protocols conformed to guidelines as set forth by the Declaration of Helsinki.
Cell culture
African American (N = 3) and Caucasian (N = 3) HUVECs were obtained from Lonza (Walkersville, MD, USA) and cultured in parallel, in endothelial growth medium (EGM) complete medium supplemented with 2% fetal bovine serum at 37°C in a 95% air‐5% CO2 atmosphere, following methods by Lonza. All experiments were completed three times for confirmation, all HUVECs were treated identically, and samples used for all assays were tested in duplicate. All Lonza (Clonetics) HUVECs were characterized by both morphological observation through serial passaging, and endothelial cells tested positive for von Willebrand Factor VIII and Aceltylated low density lipoprotein (LDL) uptake, and all tested negative for α‐smooth muscle Actin.
The supernatant from confluent HUVECs, passages three to five, was removed and immediately stored at −80°C until assay. Cell lysate was collected by fractionation. Briefly, cells were washed once with ice‐cold phosphate buffer, harvested using a rubber scraper, and resuspended in 2 mL cold 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (HEPES) (Lonza, Walkersville, MD, USA). Cells were centrifuged (600 g, 10 minutes, 4°C), cell pellet was resuspended in 500 μL cold HEPES, and then transferred to a Teflon glass coupling potter. Cell solution was homogenized (1,600 rpm, 30 strokes) while on ice and then immediately centrifuged (1,500 g, 5 minutes, 4°C). Supernatant aliquots were stored at −80°C until assay. The remaining supernatant was then centrifuged (10,000 g, 15 minutes, 4°C) for measurement of cytoplasmic superoxide dismutase (SOD) (SOD1). Protein concentration was measured by Bradford assay.
Western blot
Confluent HUVECs were washed twice in ice‐cold Hanks buffered saline solution, lysed in Radio‐Immunoprecipitation Assay Buffer with Roche protease inhibitor, and centrifuged (16,000 g, 10 minutes, 4°C). Protein content was measured by Bradford assay. Twenty microgram of protein was separated by electrophoresis through 10% SDS‐polyacrylamide gel, proteins transferred to nitrocellulose filter membranes, which were blocked with non‐fat dry milk in Tris‐buffered saline and incubated overnight with primary antibodies at 4°C. Immunoreactive proteins were detected by chemiluminescence with Thermo Scientific SuperSignal (Pierce Biotechnology, Rockford, IL, USA). Band densitometry analysis was performed with NIH ImageJ software. Primary antibodies were Anti‐NOX4 (Thermo Scientific/Pierce Biotechnology, Rockford, IL, USA), Anti‐gp91phox (NOX2) and Anti‐p47phox (BD Transduction Labs, San Jose, CA, USA), Anti‐SOD2 (AbFrontier, Rantoul, IL, USA), Anti‐inducible nitric oxide synthase (iNOS) and Anti‐endothelial nitric oxide synthase (eNOS) (BD Transduction Labs, San Jose, CA, USA), Anti‐(IL‐6) (Abcam, Inc., Cambridge, MA, USA). Actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was the internal control.
Oxidative stress and inflammation measurements
For SOD activity, plasma samples were diluted 1:5 in sample buffer; for total antioxidant capacity (TAC), plasma samples were diluted 1:20 in Assay buffer; while HUVEC cell lysate samples were not diluted for either assay. For nitric oxide (NO) measurements, HUVECs were grown to confluence in phenol‐red free EGM medium. Human plasma samples and HUVEC supernatant were ultrafiltered through a 10,000 MWCO Amicon Ultra Filter (Millipore, Billerica, MA, USA) by microcentrifuge (14,000 g, 30 minutes, 4°C). Levels of NO end products were measured using a modified Griess assay (Assay Designs, Ann Arbor, MI, USA), while both SOD activity and TAC were measured by assay kit (Cayman Chemical, Ann Arbor, MI, USA), as previously reported. 7 Interassay and intraassay coefficients of variation were 7.6% and 10.6% (NO); 5.9% and 12.4% (SOD); and 6.7% and 9.2% (TAC), respectively.
Average human plasma protein levels were determined to be 6 g/dL by Bradford assay. Protein Carbonyl (PC) formation was determined with the Oxiselect Protein Carbonyl enzyme‐linked immunosorbent assay (ELISA) (Cell Biolabs, Inc., San Diego, CA, USA). Interassay and intraassay coefficients of variation were 5.5% and 7.8%, respectively.
IL‐6 levels were measured by ELISA (Thermo Scientific/Pierce Biotechnology). Interassay and intraassay coefficients of variation were 8.6% and 10%, respectively.
Statistical analysis
Data are presented as means ± SE and significance was set at p < 0.05. The distribution of all variables was examined using the Shapiro‐Wilk test of normality, and homogeneity of variances was determined using Levene’s test. All data were normal. Independent t‐tests and analysis of variance (ANOVA) were used to determine if there were significant differences between ethnic groups. Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Eighteen adults participated in this study (nine African Americans, nine Caucasians, 21 ± 0.4 years, body mass index 25.8 ± 1.1 kg/m2, and blood pressure 123.8 ± 2.6/78.4 ± 2.2 mmHg. For analyses, participants were grouped by race. Table 1 shows the subject characteristics grouped by race. No significant difference existed between the groups.
Table 1.
Subject characteristics by ethnic group.
| African Americans (N= 9) | Caucasians (N= 9) | p‐Value | |
|---|---|---|---|
| Age, years | 21.5 ± 0.4 | 20.6 ± 0.6 | 0.25 |
| BMI, kg/m2 | 25.6 ± 0.8 | 26.1 ± 2.0 | 0.85 |
| Systolic BP, mmHg | 121.6 ± 4.0 | 126.2 ± 3.5 | 0.41 |
| Diastolic BP, mmHg | 80.6 ± 3.3 | 76.0 ± 2.9 | 0.32 |
Data are presented as mean ± SE. N = sample size; BMI = body mass index; BP = blood pressure.
Racial differences in NO production and NOS protein expression levels
No difference existed in plasma NO levels between the African American and Caucasian adults ( Figure 1A ). However, African American HUVECs produced significantly higher NO levels than Caucasian HUVECs (10.8 ± 0.4 vs. 8.8 ± 0.7 nmol/L/mg, p = 0.03) ( Figure 1B ). In order to further assess in vitro NO production, protein expression of both eNOS and iNOS was measured and found to be significantly higher in African American HUVECs compared to Caucasian HUVECs (p < 0.01) ( Figure 1C ).
Figure 1.
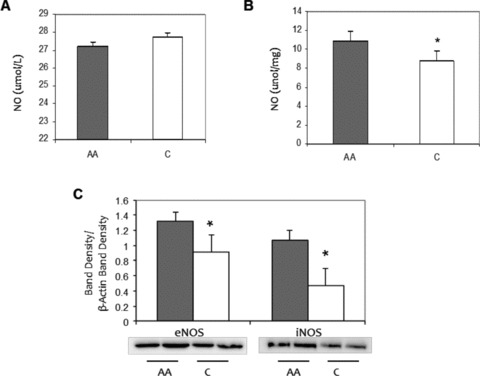
Racial differences in NO end product, eNOS and iNOS protein expression levels. (A) African American (AA) and Caucasian (C) human plasma. (B) Basal HUVEC culture, AA and C. Values normalized to protein count. (C) eNOS and iNOS protein expression levels in AA (shaded) and C (open) HUVECs, basal levels. ImageJ densitometric analysis of bands expressed in relation to b‐actin. Bars show mean ± SE. *p < 0.05 between ethnic group.
Racial differences in IL‐6
Because of the higher iNOS protein expression in African American HUVECs, we then measured IL‐6 levels in both the adult’s and HUVEC supernatant to determine if a heightened inflammation level exists. No difference existed between the African American and Caucasian adults ( Figure 2A ). However, African American HUVECs had higher IL‐6 levels compared to Caucasian HUVECs (61.7 ± 4.2 vs. 23.9 ± 9.0 pg/mg, p = 0.02) ( Figure 2B ). This was further confirmed by higher IL‐6 protein expression (p < 0.01) ( Figure 2C ).
Figure 2.
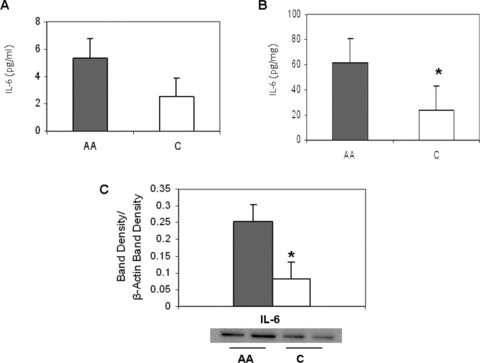
Racial differences in Interleukin‐6 (IL‐6) levels. (A) African American (AA) and Caucasian (C) human plasma. (B) Basal HUVEC culture, AA and C. Values normalized to protein content. (C) IL‐6 protein expression levels in AA (shaded) and C (open) basal HUVEC culture. ImageJ densitometric analysis of bands expressed in relation to b‐actin. Bars show mean ± SE. *p < 0.05 between ethnic group.
Racial differences in SOD activity
We found that African American adults had higher plasma SOD activity compared to Caucasian adults (5.4 ± 0.4 vs. 3.6 ± 0.7U/mL, p = 0.04) ( Figure 3A ). However, we found lower total SOD activity in African American HUVECs (15.6 ± 3.3 vs. 25.5 ± 2.8 U/mg, p = 0.04) compared to Caucasian HUVECs ( Figure 3B ). Also, we found that African American HUVECs tend to have lower levels of SOD1 activity (11.1 ± 3.9 vs. 19.0 ± 5.0 U/mg, p = 0.15) ( Figure 3C ). Further analysis of protein expression of the mitochondrial SOD isoform, SOD2, showed that African American HUVECs also tended to have lower SOD2 expression (p = 0.23) ( Figure 3D ).
Figure 3.
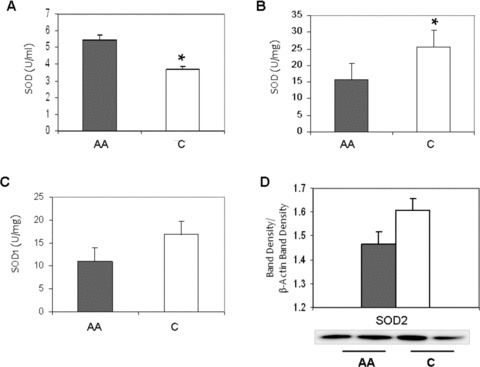
Racial differences in total Superoxide Dismutase (SOD) activity. (A) African American (AA) and Caucasian (C) human plasma. (B) Basal HUVEC culture, AA and C. (C) SOD1 activity basal HUVEC culture, AA and C. Values normalized to protein content. SOD2 protein expression levels in AA (shaded) and C (open) HUVECs, basal levels. ImageJ densitometric analysis of bands expressed in relation to b‐actin. Bars show mean ± SE. *p < 0.05 between ethnic group.
Racial differences in TAC and PC
Figure 4A shows that African American adults had higher plasma TAC levels compared to Caucasian adults (2.3 ± 0.2 vs. 1.1 ± 0.3 mM, p = 0.01). Higher TAC activity was also measured in African American HUVECs (1.3 ± 0.4 vs. 0.5 ± 0.1 μM/mg, p = 0.04) ( Figure 4B ). Confirming this heightened oxidative stress, we found higher plasma PC levels in African American adults compared to Caucasian adults (1.1 ± 0.1 vs. 0.8 ± 0.1 nmol/mg, p = 0.005) ( Figure 4C ).
Figure 4.
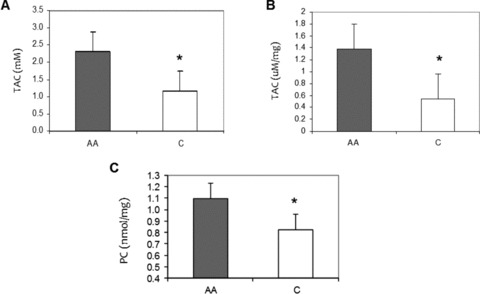
Racial differences in oxidative‐stress markers. (A) Total antioxidant capacity (TAC) levels in African American (AA) and Caucasian (C) human plasma. (B) TAC levels in basal AA and C HUVEC culture. (C) Plasma Protein Carbonyl (PC) levels in AA and C human plasma. Values normalized to protein content. Bars show mean ± SE. *p < 0.05 between ethnic group.
Racial differences in NADPH oxidase subunit expression
To assess the potential for superoxide production, we measured protein expression of NADPH oxidase subunits and isoforms ( Figure 5 ). We found higher protein expression of p47phox subunit as well as the NOX2 and NOX4 isoforms in African American HUVECs compared to those in Caucasian HUVECs (p < 0.01).
Figure 5.
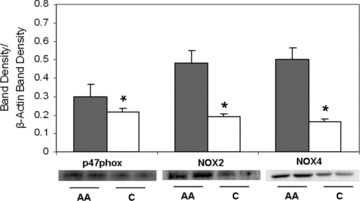
NADPH oxidase subunit protein expression levels in African American (shaded) and Caucasian (open) HUVECs, basal levels. ImageJ densitometric analysis of bands expressed in relation to b‐actin. Bars show mean ± SE. *p < 0.05 between ethnic group.
Discussion
In this preliminary observational study, we sought to examine whether the known in vivo racial differences that exist in plasma oxidative‐stress and inflammatory markers also exist in vitro in primary endothelial culture. A primary finding was that heightened oxidative stress and inflammation exists in primary culture in African Americans just as it does in vivo. Oxidative stress plays a critical role in the pathology and progression of cardiovascular disease and hypertension, and recent evidence suggests that low‐grade systemic inflammation may also contribute to development of said diseases. 8 , 9 Here we have shown that an in vitro racial difference exists in cultured endothelial cells in oxidative‐stress and inflammation levels. Recognizing that this difference exists in HUVECs, could provide new directions for mechanistic and therapeutic research.
Over the past several decades, investigators have used the in vitro cell‐culture model as a research tool, yet in vitro research on racial differences is scarce. In 1995, Frist et al. determined that African American HUVECs expressed higher levels of fibrinolytic proteins when compared to Caucasian HUVECs. 10 But to the best of our knowledge, only one study has measured oxidative stress in African American and Caucasian HUVECs. In 2004, Kalinowski et al. reported upregulated eNOS and NADPH oxidase subunit expression for p67phox, p47phox, and p22phox in the African American HUVECs. Their data led them to conclude that the steady‐state NO/O2 − balance in African Americans may be kept closer to the characteristic redox state found in endothelial dysfunction. 11 We also report that African American HUVECs have higher levels of oxidative stress than Caucasian HUVECs do. Our study adds to previous findings by showing that African American HUVECs may be predisposed to inflammation more than Caucasian HUVECs are. To the best of our knowledge, this finding is novel.
Research has suggested that the observed racial differences in prevalence of cardiovascular diseases and hypertension could be attributed in part to a blunted NO‐dependent vasodilation in African Americans when compared to Caucasians. 12 , 13 , 14 It has been shown that vascular reactivity is altered in African Americans compared to Caucasians, but only a few studies have directly measured plasma NO end products to assess potential racial differences and limited studies have done so in HUVECs of unreported origin. 15 , 16 We found no difference between races in human plasma NO end‐product levels, but this could be attributed to low sample size or could be because our human subjects were healthy young adults. In African American HUVECs, we found significantly higher level of NO end products in cell supernatant suggesting an augmented NOS production in response to high O2 −.
In order to confirm the heightened NO production in African American HUVECs, protein expression of two NO synthase isoforms, eNOS and iNOS, were measured. In both, we found significantly higher expression in the African American HUVECs. The eNOS isoform is constitutively expressed in the endothelium, while activation of iNOS is usually cytokine induced. It has been reported that NADPH oxidase activity is associated with eNOS expression and is required for iNOS expression. 17 , 18 It also has been suggested that iNOS expression serves a protective role against proinflammatory cytokine conditions, with specific association to IL‐6. 19 Taking this into consideration, we then measured IL‐6 levels and protein expression and confirmed a potential proinflammatory state in African American compared to Caucasian HUVECs. Further research should use mechanistic studies to understand this in vitro racial difference in inflammation.
Heightened oxidative stress has been found to be associated with augmented antioxidant activity as scavenging increases to eliminate the rise in O2 − levels, and the SOD enzyme serves as the main protective antioxidant scavenger. Three main SOD isoforms exist— manganese SOD (SOD2) in the mitochondria, copper/zinc SOD (SOD1) in the cytoplasm, and extracellular SOD (SOD3). In most tissues, SOD3 exists in very small amounts, but in the circulation it can represent up to half the total SOD activity. 20 Therefore in controlled cell‐culture conditions, endothelial cell lysate would not have the same inherent percentages of SOD3. In HUVECs, we found African Americans had significantly lower levels of total SOD activity, while in human plasma we found African American adults to have significantly higher SOD activity when compared to Caucasians. This difference could be attributed to the large contributions from SOD3 to the total SOD measure in plasma from the African American adults. To confirm, we then used cell fractionation to compare isoform levels between racial groups and found that African American HUVECs tend to exhibit lower SOD1 activity and also lower SOD2 protein expression. Together, our data suggest that total SOD activity is lower in African American HUVECs compared to Caucasian HUVECs.
Measuring TAC levels in conjunction with other biomarkers can provide a relative indicator of the antioxidant defense and therefore, in vitro studies that include TAC measures as part of their oxidative‐stress cascade could provide potential insight into cellular antioxidant activity. We measured TAC as the capacity of all antioxidants in the sample to prevent ABTS® (2,29‐azino‐bis [3‐ethylbenzthiazoline‐6‐sulphonic acid]) oxidation and found that both African American HUVECs and African American humans had heightened antioxidant activity when compared to Caucasians.
NADPH oxidase‐mediated radical production plays a vital role in the pathophysiology of endothelial dysfunction, inflammation, cardiovascular diseases, and angiogenesis. 21 , 22 , 23 , 24 , 25 We found greater expression levels of p47phox, NOX2, and NOX4 in African American HUVECs than in Caucasian HUVECs. It has been reported that in primary endothelial cultures NOX2 and NOX4 serve as primary sources of NADPH‐derived oxidative stress, with some reports suggesting that NOX4 is the major source. 26 , 27 Our findings are the first report of a racial difference in NOX4 expression. Recently, in contrast to other NOX proteins, NOX4 has been suggested to directly produce hydrogen peroxide, 28 , 29 and given the higher NOX4 seen in African American HUVECs, this should be investigated further. Further studies will better characterize the in vivo African American endothelial phenotype as well as the associated molecular mechanisms underlying the development of endothelial dysfunction so that proper therapeutic interventions can be implemented.
In conclusion, these preliminary observational data show that relative to Caucasian HUVECs, African American HUVECs exhibit enhanced oxidative‐stress and inflammation levels through increased expression of NADPH oxidase, IL‐6, eNOS and iNOS, higher NO end‐product production, and lower SOD activity. We have demonstrated an in vitro racial difference in basal HUVECs much like that found in vivo. However, given the small sample sizes and the preliminary nature of these data, results should be interpreted with caution. Nevertheless, it is known that racial differences exist in how humans respond to development and progression of disease, so these data suggest that ethnicity of cell model may be important to consider with in vitro clinical research.
Acknowledgements
This research was supported by NIH/NHLBI Grant RO1 HL085497 (PI, Michael Brown) and by NIH/NIA Grant KO1 AG019640 (PI, Michael Brown).
References
- 1. Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects–atherosclerosis risk in communities study. Arch Intern Med. 2007; 167: 573–579. [DOI] [PubMed] [Google Scholar]
- 2. Carroll JF, Fulda KG, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Cardarelli R. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring). 2009; 17: 1420–1427. [DOI] [PubMed] [Google Scholar]
- 3. Vaziri ND, Rodriguez‐Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006; 2: 582–593. [DOI] [PubMed] [Google Scholar]
- 4. Chen X, Andresen BT, Hill M, Zhang J, Booth F, Zhang C. Role of reactive oxygen species in tumor necrosis factor‐alpha induced endothelial dysfunction. Curr Hypertens Rev. 2008; 4: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Csanyi G, Taylor WR, Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med. 2009; 47: 1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kvietys PR, Granger DN. Endothelial cell monolayers as a tool for studying microvascular pathophysiology. Am J Physiol. 1997; 273: G1189–G1199. [DOI] [PubMed] [Google Scholar]
- 7. Feairheller DL, Sturgeon KM, Diaz KM, Veerabhadrappa P, Williamson ST, Crabbe DL, Brown MD. Prehypertensive African‐American women have preserved nitric oxide and renal function but high cardiovascular risk. Kidney Blood Press Res. 2010; 33: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010; 10: 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 10. Frist ST, Taylor HA Jr, Kirk KA, Grammer JR, Li XN, Grenett HE, Booyse FM. Expression of PAI‐1, t‐PA and u‐PA in cultured human umbilical vein endothelial cells derived from racial groups. Thromb Res. 1995; 77: 279–290. [DOI] [PubMed] [Google Scholar]
- 11. Kalinowski L, Dobrucki IT, Malinski T. Race‐specific differences in endothelial function. Predisposition of African Americans to vascular diseases. Circulation. 2004; 109: 2511–2517. [DOI] [PubMed] [Google Scholar]
- 12. Perregaux D, Chaudhuri A, Rao S, Airen A, Wilson M, Sung BH, Dandona P. Brachial vascular reactivity in blacks. Hypertension. 2000; 36: 866–871. [DOI] [PubMed] [Google Scholar]
- 13. Cardillo C, Kilcoyne CM, Cannon RO 3rd, Panza JA. Attenuation of cyclic nucleotide‐mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator function. Circulation. 1999; 99: 90–95. [DOI] [PubMed] [Google Scholar]
- 14. Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium‐dependent and ‐independent dilation of conductance arteries in African Americans. J Am Coll Cardiol. 2002; 40: 754–760. [DOI] [PubMed] [Google Scholar]
- 15. Mata‐Greenwood E, Chen DB. Racial differences in nitric oxide‐dependent vasorelaxation. Reprod Sci. 2008; 15: 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lazzerini G, Del Turco S, Basta G, O’Loghlen A, Zampolli A, Caterina RD. Prominent role of NF‐kappaB in the induction of endothelial activation by endogenous nitric oxide inhibition. Nitric Oxide. 2009; 21: 184–191. [DOI] [PubMed] [Google Scholar]
- 17. Ding H, Aljofan M, Triggle CR. Oxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cells. J Cell Physiol. 2007; 212: 682–689. [DOI] [PubMed] [Google Scholar]
- 18. Wu F, Tyml K, Wilson JX. iNOS expression requires NADPH oxidase‐dependent redox signaling in microvascular endothelial cells. J Cell Physiol. 2008; 217: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hemmrich K, Suschek CV, Lerzynski G, Kolb‐Bachofen V. iNOS activity is essential for endothelial stress gene expression protecting against oxidative damage. J Appl Physiol. 2003; 95: 1937–1946. [DOI] [PubMed] [Google Scholar]
- 20. Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004; 24: 1367–1373. [DOI] [PubMed] [Google Scholar]
- 21. Meyer JW, Schmitt ME. A central role for the endothelial NADPH oxidase in atherosclerosis. FEBS Letters. 2000; 472: 1–4. [DOI] [PubMed] [Google Scholar]
- 22. Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003; 285: R277–R297. [DOI] [PubMed] [Google Scholar]
- 23. Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox‐containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004; 109: 1795–1801. [DOI] [PubMed] [Google Scholar]
- 24. Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007; 27: 2319–2324. [DOI] [PubMed] [Google Scholar]
- 25. Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004; 122: 339–352. [DOI] [PubMed] [Google Scholar]
- 26. Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004; 109: 227–233. [DOI] [PubMed] [Google Scholar]
- 27. Van Buul JD, Fernandez‐Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005; 7: 308–317. [DOI] [PubMed] [Google Scholar]
- 28. Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007; 406: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II‐stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008; 45: 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]


