Abstract
Objective. To investigate whether advanced glycation end products (AGEs) induce the expression of IL-6 and IL-8 through the receptor for AGEs (RAGE)-activated pathways in human OA chondrocytes.
Methods. OA chondrocytes were stimulated with AGE-modified BSA (AGE-BSA). Gene expression of IL-6 and IL-8 was quantified by TaqMan assays and the production was determined using ELISAs. Immunoblotting was used to analyse the activation of mitogen-activated protein kinases (MAPKs) and the degradation of IκBα. Activation of NF-κB was determined using an ELISA. Pharmacological studies to elucidate the involved pathways were executed using transfection with small interfering RNAs (siRNAs), inhibitors of MAPKs and NF-κB.
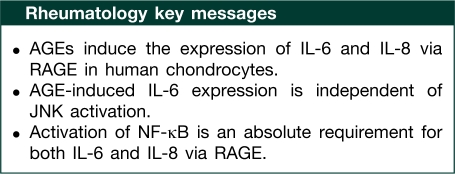
Results. AGE-BSA induced the expression of IL-6 and IL-8 in OA chondrocytes, which was inhibited by pre-treatment with soluble RAGE (sRAGE) or RAGE knockdown by siRNAs. Treatment with SB202190 (p38-MAPK inhibitor) or PD98059 (ERK inhibitor) inhibited AGE-BSA-induced IL-6 and IL-8 expression. However, SP600125 (JNK inhibitor) had no effect on AGE-BSA-induced IL-6 expression but inhibited the expression of IL-8. Treatment with NF-κB inhibitors suppressed AGE-BSA-induced IL-6 and IL-8 expression.
Conclusions. This is the first study to demonstrate that AGEs induce the expression of IL-6 and IL-8 in OA chondrocytes. A novel finding of our studies is that in OA chondrocytes, AGE-BSA-induced expression of IL-6, but not of IL-8, was independent of the JNK pathway. Activation of NF-κB was an absolute requirement for both IL-6 and IL-8 expression. These results demonstrate that AGE-BSA-induced expression of IL-6 and IL-8 via RAGE is mediated through different MAPK signalling pathways in OA and possibly in other degenerative diseases.
Keywords: Osteoarthritis, Advanced glycation end products, Receptor for advanced glycation end product, mitogen-activated protein kinases, nuclear factor-κB, Interleukin-6, Interleukin-8
Introduction
World population is ageing rapidly, and consequently age-related diseases significantly impact the quality of life of the elderly. OA is one of the most prevalent and disabling chronic conditions affecting the elderly and poses a significant public health challenge [1]. The most prominent feature of OA is the progressive destruction of articular cartilage, resulting in impaired joint motion, severe pain and ultimately disability [2]. Although the aetiology of OA remains largely unknown, the incidence of OA increases with age and >50% of the population >60 years of age is affected [3, 4]. Advanced glycation end products (AGEs) are produced by the non-enzymatic glycation of macromolecules and accumulate in a number of different tissues [5–7]. In articular cartilage, relatively high levels of AGEs accumulate with increasing age [8–10] and result in stiffening of the articular collagen network [11], consistent with published reports [12, 13]. Furthermore, accumulation of AGEs makes the collagen network brittle in articular cartilage thus increasing the risk for the development of OA [14]. The biological activities of AGEs are thought to be mediated by specific receptors for AGE (RAGE) and activation of RAGE engages critical signalling pathways linked to pro-inflammatory responses and activation of various inflammatory genes [15, 16]. RAGE signalling has also been found to be stimulated by members of the S100 family of proteins, including S100A4 (15–17), which has been shown to be present in articular cartilage and was up-regulated in tissues from patients with OA or RA [16, 17]. In OA, activation of RAGE has been shown to stimulate chondrocytes, resulting in increased production of MMPs, TNF-α, cyclo-oxygenase (COX)-2 and microsomal prostaglandin E synthase I (mPGES-1) [17–21].
Recent progress in cytokine and chemokine biology has provided a considerable amount of evidence for the pathological role of IL-6 and IL-8 in inflammatory arthritis [22–34]. IL-6 is a pleiotropic cytokine with a wide range of biological activities, including immunoregulation, mediation of acute-phase responses and effects on bone metabolism [26]. Dysregulated overproduction of IL-6 is suspected in the systemic inflammatory manifestations observed in patients with arthritis [27]. This gets support from studies showing that IL-6 knockout mice were resistant to inflammatory arthritis and had reduced levels of serum TNF-α [33]. Furthermore, the elevated levels of IL-6 in serum and SF of arthritis patients have been shown to correlate with clinical and laboratory indices of disease activity [28]. OA exhibits increased IL-6 levels and these are associated with inflammation and cartilage/bone destruction in arthritic joints [25, 31]. Blocking IL-6 with specific antibody (tocilizumab), or its interactions with IL-6 receptor by IL-6R antagonists reduces cartilage/bone destruction and invasion of cartilage by synovium in animal models and in human patients [33, 34]. Like IL-6, IL-8 is produced in arthritic joints by activated synovial cells and infiltrating macrophages and both IL-6 and IL-8 are considered to be potent catabolic factors in arthritic joints [23, 24, 30–32]. It is now well established that in arthritic joints, IL-8 induces a massive accumulation of neutrophils, which produce neutrophil elastase, leading to cartilage destruction [35]. In these studies, it was also shown that an inhibitor of neutrophil elastase (ONO-5046) prevented the destruction of the cartilage [35]. Injections of IL-8 also induced the expression of bioactive and immunoreactive IL-1β and IL-1 receptor antagonist (IL-1Ra) in the joint cavity [35]. In neutrophil-depleted rabbits, IL-8 induced far lower concentrations of IL-1β and IL-1Ra and no cartilage destruction [35]. High levels of IL-8 have also been detected in serum and in SF of arthritic patients [32]. Thus, IL-8 could also be implicated in inflammation and cartilage/bone destruction in arthritis [29–32].
Although AGEs have been shown to induce the inflammatory response in different cell types, including chondrocytes, whether they induce the expression of IL-6 and IL-8 in human chondrocytes has not yet been reported. Expression of IL-6 and IL-8 proceeds via the activation of mitogen-activated protein kinases (MAPKs) and nuclear factor (NF)-κB in response to various stimuli [36]. The current study was designed to examine whether AGEs activate the MAPKs and NF-κB signalling cascade through RAGE to stimulate IL-6 and IL-8 expression and production by human OA chondrocytes. Our results demonstrate a role of RAGE, MAPKs and NF-κB in AGE-stimulated differential expression and production of IL-6 and IL-8 in primary human OA chondrocytes.
Methods
Cartilage selection and chondrocyte preparation
After the study was approved by the Institutional Review Board of Metrohealth Medical Center, Cleveland, OH, USA, discarded and de-identified human cartilage samples were obtained from the knee or hip joints of 17 OA patients aged 39–67 years [13 female and 4 male Caucasians; mean (s.d.) age 49 (28) years] who underwent joint replacement surgery at Metrohealth Medical Center, Cleveland, OH. Chondrocytes were prepared from macroscopically unaffected cartilage by enzymatic digestion essentially as previously described [37–40]. Isolated chondrocytes were plated at a density of 1.2 × 106/ml in 35-mm tissue culture dishes (Corning, NY, USA) in complete DMEM/Ham’s F-12 medium as previously described [36–40]. Only primary OA chondrocytes were used in studies described here.
AGE-BSA preparation
AGE-BSA was prepared by reacting BSA (Sigma Chemical Co., St Louis, MO, USA) with glycoaldehyde (Sigma Chemical Co.), as previously described [40, 41]. The reaction was terminated by removing non-reacted glycoaldehyde by dialysing extensively against PBS.
Treatment of OA chondrocytes with AGE-BSA, antagonists of RAGE, MAPKs and NF-κB inhibitors
Human OA chondrocytes (1.2 × 106/ml) were plated in 35-mm culture dishes in complete DMEM/Ham's F-12 medium and serum-starved for 12 h/overnight and then treated with 5–100 µg/ml AGE-BSA for 0–24 h. Primary chondrocytes were pre-treated with soluble RAGE (sRAGE; R&D System, MN, USA) for 2 h or different MAPKs or NF-κB inhibitors for 1 h prior to stimulation with AGE-BSA. Chondrocytes cultured without AGE-BSA or cultured with native BSA served as controls.
Stimulation of human OA cartilage explants with AGE-BSA or S100A4
Human OA cartilage explants (3 × 3 mm) were prepared by our standard method and plated in 24-well culture plates in complete DMEM. Explants were serum-starved for 18 h and then treated with AGE-BSA (100–200 µg/ml) or recombinant human S100A4 protein (R&D Systems, St Paul, MN, USA; 25–50 ng/ml) for 24 h. Production of IL-6 and IL-8 was analysed by cytokine/chemokine-specific ELISA (R&D Systems). Explants cultured without AGE-BSA or recombinant human S100A4 protein served as controls.
RAGE knockdown by transfection with small interfering RNA
Human OA chondrocytes were transfected with an On-Target SMART Pool (Dharmacon RNA Technologies, Lafayette, CO, USA) human RAGE-specific small interfering RNA (siRNAs) or with human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) On-target SMART pool siRNA (Dharmacon) as a negative control using Amaxa Human Chondrocytes Nucleofector Kit (Lonza, Walkersville, MD, USA) according to the manufacturer’s instructions. In brief, human OA chondrocytes (1.4 × 106) were transfected with 30–100 nM siRNAs and with 100 µl of nucleofector solution using electroporation program U-024 and the transfected OA chondrocytes were seeded in 6-well plates. After 24 h, the culture medium was changed to serum-free medium for the experiments using AGE-BSA. Transfection efficiency was monitored with red fluorescent siRNA oligonucleotides (siGLO red indicator, catalogue #D0016300220; Dharmacon). Approximately 70–80% of the OA chondrocytes emitted red fluorescent signal when transfected with siGLO.
Cytotoxicity assay
Human OA chondrocytes were treated with various doses of AGE-BSA (5–200 μg/ml), inhibitors (10–100 μM) and sRAGE (10 µg/ml) for 24 h and the cytotoxicity was examined using the Cytotoxicity Assay Kit according to the instructions of the manufacturer (Promega, Madison, WI, USA).
Real-time PCR
Real-time quantitative RT–PCR (qRT–PCR) was used to quantify the expression of mRNAs for IL-6 and IL-8 with expression of GAPDH or β-actin as endogenous control. Total RNA was prepared from OA chondrocytes using the Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. First-strand cDNA was synthesized using 1 µg of total RNA and the First-Strand cDNA Synthesis Kit (Invitrogen). Primers used for PCR-assisted amplification were: IL-6 (NM_000600.2, F 5′-AAA TTC GGT ACA TCC TCG ACG GCA-3′; R 5′-AGT GCC TCT TTG CTG CTT TCA CAC-3′); IL-8 (NM_000584, F 5′-AGA AAC CAC CGG AAG GAA CCA TCT-3′; R 5′-AGA GCT GCA GAA ATC AGG AAG GCT-3′); and GAPDH (NM_002046.3, F 5′-TCG ACA GTC AGC CGC ATC TTC TTT-3′; R 5′-ACC AAA TCC GTT GAC TCC GAC CTT-3′). PCR amplification was carried out using the core kit for SYBR Green (Applied Biosystems, Foster City, CA, USA) and the Step One Real Time PCR System (Applied Biosystems). Typical profile times used were initial step, 95°C for 10 min, followed by a second step at 95°C for 15 s and 60°C for 60 s for 40 cycles with melting curve analysis. The level of target mRNA was normalized to the level of GAPDH and compared with control (untreated sample). Data were analysed using a comparative ΔΔCT method [42].
Western immunoblotting
Stimulated and control primary human OA chondrocytes were washed with cold PBS and lysed using the cell lysis buffer (50 mM Tris–HCl, pH 7.4; 150 mM NaCl; 1% Triton X-100; 0.1% SDS; 0.5% sodium deoxycholate; 1 mM EDTA; 1 mM EGTA; protease and phosphatase inhibitors) as previously described [40]. Whole chondrocytes lysate protein (35 µg/lane) was resolved by SDS–PAGE (10% resolving gel with 4% stacking) and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with blocking buffer containing non-fat dry milk powder in Tris-buffered saline containing 0.1% Tween-20 (TBS-T), and probed with 1 : 200–1 : 1000 diluted primary antibodies (Cell Signaling Technologies, Beverley, MA, USA; Santa Cruz Biotechnology, Santa Cruz, CA, USA) specific for the target protein. Immunoreactive proteins were visualized by using 1 : 1000 diluted horseradish peroxidase (HRP)-linked secondary antibodies and enhanced chemiluminescence (GE Healthcare, Milwaukee, WI, USA).
ELISA
Human OA chondrocytes and cartilage explants were stimulated with or without AGE-BSA (5–100 µg/ml) for 0–24 h. IL-6 and IL-8 produced in the culture medium was quantified using commercially available IL-6 and IL-8-specific ELISA kits according to the instructions of the manufacturer (R&D Systems). Plates were read at 450 nm using Synergy HT microplate reader (Biotek Instruments, Winooski, VT, USA).
NF-κB DNA binding activity assay
Activation of NF-κB p65 in AGE-BSA-stimulated human OA chondrocytes was determined using a highly sensitive Transcription Factor ELISA kit according to the instructions of the manufacturer (Assay Designs, Ann Arbor, MI, USA). The assay uses streptavidin-coated plates with bound NF-κB-biotinylated consensus sequence to capture the active form of NF-κB, incubated with HRP-labelled anti-NF-κB p65 antibody. The assay was developed with a chemiluminescent substrate and the signal was detected using a Multimode Dectector (DTX-880; Beckman Coulter, Brea, CA, USA), and the values are expressed as relative light units.
Densitometric analysis
Densitometric measurements of the scanned bands were performed using UN-SCAN-IT software (Silk Scientific Corporation, Orem, UT, USA). Each band was scanned five times, and the mean band intensity (pixels per band) was obtained. Data were normalized to suitable loading controls and expressed as mean (s.d.).
Statistical analysis
All measurements were performed in duplicate and repeated at least three times using chondrocytes prepared from different age- and sex-matched OA cartilage samples. All statistical analyses were performed using Origin 6.1 software (Origin, Northampton, MA, USA) (one-paired two-tailed t-test with one-way ANOVA and Tukey’s post hoc analysis) and P < 0.05 was considered to be statistically significant. Values shown are mean (s.e.m.) unless stated otherwise.
Results
AGE-BSA was not toxic to human OA chondrocytes in vitro
Previously characterized AGE-BSA was used in these studies [40] and it was found that AGE-BSA up to 200 µg/ml had no significant cytotoxic effects on OA chondrocytes compared with controls treated with 200 µg/ml native BSA (P > 0.05, data not shown).
Induction of IL-6 and IL-8 expression by AGE-BSA in primary human OA chondrocytes
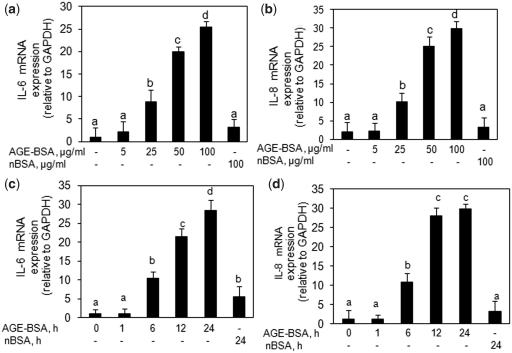
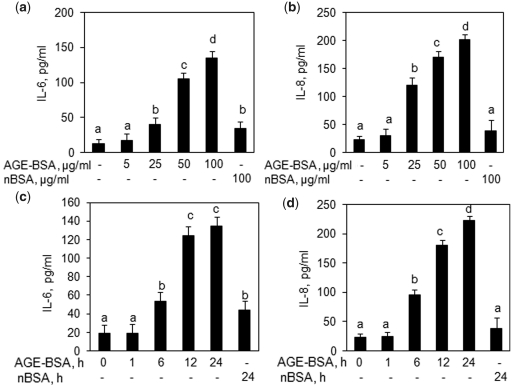
Based on the results of cytotoxicity assays, we treated primary human OA chondrocytes with AGE-BSA (5–100 µg/ml) for 0–24 h and the gene expression of IL-6 and IL-8 was quantified by a qRT–PCR method and compared with the levels in control chondrocytes. Our results showed that AGE-BSA significantly up-regulated the mRNA expression of IL-6 and IL-8 in a dose– (Fig. 1a and b) and time-dependent manner (Fig. 1c and d) (P < 0.05). To determine whether the up-regulation of IL-6 or IL-8 mRNA also affects protein levels, culture supernatants were assayed for IL-6 and IL-8 protein using specific ELISA assays. As shown in Fig. 2, AGE-BSA significantly induced IL-6 and IL-8 production in a dose-dependent (Fig. 2a and b) and time-dependent manner (Fig. 2c and d) in primary human OA chondrocytes (P < 0.05). Treatment of primary human OA chondrocytes with native BSA (100 µg/ml) for 24 h showed a slight increase in IL-6 and IL-8 gene and protein expression compared with controls (P < 0.05) but the increase in the case of IL-8 was statistically insignificant (P > 0.05).
Fig. 1.
Expression of IL-6 and IL-8 in AGE-BSA-stimulated primary human OA chondrocytes. Effect of AGE-BSA on the gene expression of IL-6 (a) and IL-8 (b) in primary human OA chondrocytes. Primary human OA chondrocytes were treated with AGE-BSA (5–100 µg/ml) and native BSA (100 µg/ml) for 24 h. Time-dependent effect of AGE-BSA on the gene expression of IL-6 (c) and IL-8 (d) in human OA chondrocytes. Primary chondrocytes were treated with AGE-BSA (100 µg/ml) and native BSA (100 µg/ml) for 0–24 h. Expression of IL-6 or IL-8 mRNA was determined by real-time qRT–PCR using comparative ΔΔCT method. Incubation with native BSA (nBSA) was used as control. Results are representative [mean (s.e.m.)] of duplicate experiments with OA chondrocytes obtained from five age- and sex-matched OA donors and differ without a common letter; P < 0.05.
Fig. 2.
Enhanced production of IL-6 and IL-8 by AGE-BSA-stimulated primary human OA chondrocytes. Effect of AGE-BSA on the protein production of IL-6 (a) and IL-8 (b) in primary human OA chondrocytes. Primary human OA chondrocytes were treated with AGE-BSA (5–100 µg/ml) and native BSA (100 µg/ml) for 24 h. Kinetics of AGE-BSA-induced production of IL-6 (c) and IL-8 (d) in primary human OA chondrocytes. Primary human OA chondrocytes were treated with AGE-BSA (100 µg/ml) and native BSA (100 µg/ml) for 0–24 h. Production of IL-6 or IL-8 was determined by a sandwich ELISA. Native BSA (nBSA) was used as control. Results are representative [mean (s.e.m)] of duplicate experiments with OA chondrocytes obtained from five age- and sex-matched OA donors and differ without a common letter; P < 0.05.
Necessity of RAGE for AGE-BSA or S100A4-mediated stimulation of IL-6 and IL-8 in human OA chondrocytes
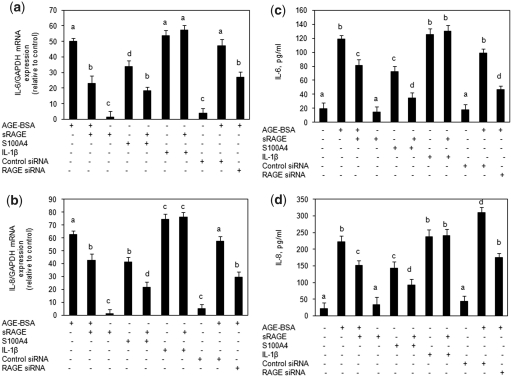
To investigate whether AGE-BSA- or S100A4-induced expression of IL-6 and IL-8 in primary human OA chondrocytes was mediated via binding to RAGE, we used excess sRAGE to block their binding to RAGE. Primary human OA chondrocytes were pre-incubated with sRAGE for 2 h prior to AGE-BSA or S100A4 stimulation. Real-time qPCR data showed that the induction of IL-6 and IL-8 gene expression was significantly inhibited in cultures pre-incubated with sRAGE and then stimulated with AGE-BSA (P < 0.05) (Fig. 3a and b). Inhibition of gene expression also significantly blocked AGE-BSA or S100A4-induced IL-6 and IL-8 protein production in the culture supernatant (P < 0.05) (Fig. 3c and d). Treatment of primary human OA chondrocytes with IL-1β (20 ng/ml) for 24 h showed significant increase in IL-6 or IL-8 gene and protein expression compared with untreated chondrocytes (P < 0.05), but sRAGE pre-treatment had no effect on IL-1β-induced or native BSA-induced IL-6 or IL-8 gene expression and protein production (P > 0.05) (Fig. 3). The effect of RAGE on the expression of IL-6 and IL-8 in response to stimulation with AGE-BSA in human OA chondrocytes was further investigated with siRNA-mediated knockdown of RAGE expression. Transfection of human OA chondrocytes with RAGE-specific siRNAs knocked down the expression of RAGE and inhibited AGE-BSA-induced IL-6 and IL-8 gene expression and protein production (Fig. 3). Taken together these findings provide strong evidence of the involvement of RAGE in AGE-BSA- or S100A4 protein-mediated stimulation of IL-6 or IL-8 expression in human OA chondrocytes.
Fig. 3.
Effect of sRAGE or RAGE-knockdown on the AGE-BSA or S100A4 protein-induced expression of IL-6 and IL-8 in primary human OA chondrocytes. Primary OA chondrocytes were pre-treated with sRAGE (200 µg/ml) for 2 h and then stimulated with AGE-BSA (50 µg/ml) or S100A4 protein (25 ng/ml) or IL-1β (20 ng/ml) for 24 h. Human OA chondrocytes were transfected with RAGE-siRNA or control siRNA and then stimulated with AGE-BSA for 24 h. Expression of IL-6 (a) and IL-8 (b) mRNA was determined by real-time qPCR and normalized to GAPDH and compared with the levels present in untreated chondrocytes using a comparative ΔΔCT method. Production of IL-6 (c) and IL-8 (d) in the culture medium was quantified by IL-6- or IL-8-specific sandwich ELISA. Results are representative [mean (s.e.m)] of duplicate experiments with human OA chondrocytes obtained from two age- and sex-matched OA donors and differ without a common letter; P < 0.05.
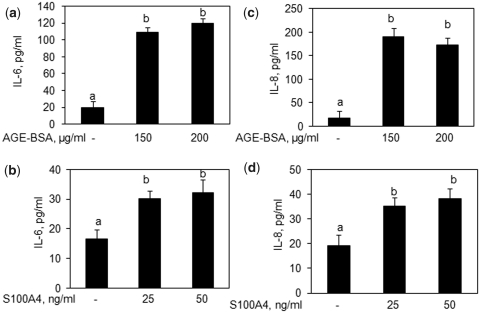
Treatment of human OA cartilage explants with AGE-BSA or S100A4 protein induces the expression of IL-6 and IL-8 in vitro
To investigate whether AGE-BSA or S100A4 protein induced the production of IL-6 and IL-8 in human chondrocytes embedded in the cartilage matrix (3D cultures), we used human OA cartilage explants. Treatment of cartilage explants with either AGE-BSA or recombinant human S100A4 protein for 24 h significantly increased the production of IL-6 and IL-8 in the culture medium compared with controls (Fig. 4; P < 0.05).
Fig. 4.
AGE-BSA or S100A4 enhances the production of IL-6 and IL-8 in human OA cartilage explants. Human OA cartilage explants in serum-free DMEM were stimulated with AGE-BSA or S100A4 protein for 24 h at 37°C. Production of IL-6 (a, b) and IL-8 (c, d) in the culture medium was quantified by IL-6- or IL-8-specific sandwich ELISA. Results are representative [mean (s.e.m)] of duplicate experiments with human OA chondrocytes obtained from two age- and sex-matched OA donors and differ without a common letter; P < 0.05.
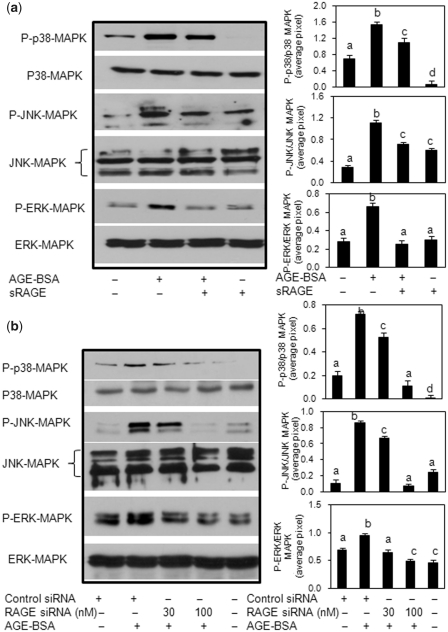
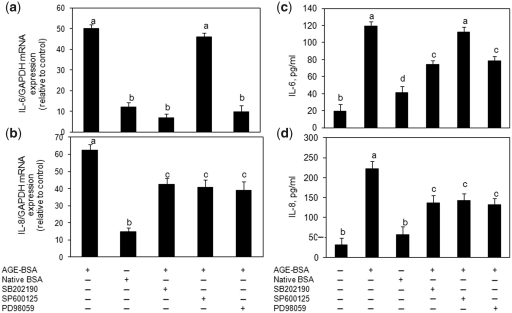
RAGE-mediated activation of MAPKs in human OA chondrocytes is required for AGE-BSA-induced IL-6 and IL-8 expression
Primary human OA chondrocytes were pre-treated with sRAGE for 2 h or RAGE expression was knocked down by transfection with specific siRNAs and then human OA chondrocytes were stimulated with AGE-BSA for 45 min, and chondrocyte lysates were analysed by western immunoblotting. Treatment with sRAGE or knockdown of RAGE expression with RAGE-specific siRNAs attenuated the AGE-BSA-induced phosphorylation of p38-MAPK, JNK-MAPK and ERK-MAPK in human OA chondrocytes (Fig. 5a and b) indicating that AGE-BSA is a ligand for RAGE and activate the MAPKs through its binding to RAGE in human OA chondrocytes. To determine the relationship of p38-MAPK, JNK, ERK inhibition by treatment with sRAGE or RAGE siRNA-mediated knockdown of RAGE expression and pro-inflammatory mediators IL-6 and IL-8 expression in AGE-BSA-stimulated human OA chondrocytes, we used pharmacological agents that inhibit p38-MAPK (SB202190), JNK (SP600125) and ERK (PD89059). Analysis using real-time qRT–PCR and cytokine/chemokine-specific ELISAs showed that pre-treatment with SB202190 and PD98059 caused significant decrease in mRNA expression and protein production of IL-6 (Fig. 6a and c) as well as IL-8 (Fig. 6b and d) in AGE-BSA-stimulated primary human OA chondrocytes (P < 0.05). In contrast, treatment with the JNK inhibitor SP600125 had no significant effect on AGE-BSA-induced IL-6 mRNA expression or protein production compared with controls (Fig. 6a and c; P > 0.05). However, IL-8 mRNA expression and protein production was significantly and equally inhibited in primary human OA chondocytes treated with the MAPK inhibitors (Fig. 6b and d; P < 0.05). Taken together, these results suggested that p38-MAPK and ERK-MAPK are involved in RAGE-mediated AGE-BSA-stimulated gene expression and protein production of IL-6 and IL-8, whereas activation of JNK-MAPK in addition to p38-MAPK and ERK is required for AGE-BSA-induced IL-8 expression and production in primary human OA chondrocytes.
Fig. 5.
Activation of RAGE-mediated MAPK signalling by AGE-BSA in primary human OA chondrocytes. Effect of sRAGE (a) and RAGE knockdown (b) on MAPK phosphorylation in AGE-BSA-stimulated OA chondrocytes. After pre-treatment with sRAGE (200 µg/ml) for 2 h at 37°C or RAGE-siRNA (30–100 nM) transfection, human OA chondrocytes (70–80% confluent) were incubated with AGE-BSA (50–100 µg/ml) for 45 min, then the phosphorylation (P) of p38-MAPK, JNK and ERK was determined by western immunoblot analysis. Band images were digitally captured and the band intensities were obtained using UN-San-It software and are expressed in average pixels. Data shown are cumulative of two experiments. Average pixel values presented as mean (s.e.m); data without a common letter differ; P < 0.05.
Fig. 6.
Different MAPKs regulate AGE-BSA-induced expression of IL-6 and IL-8 in primary human OA chondrocytes. Effect of MAPK inhibition on the gene expression of IL-6 (a) and IL-8 (b) in AGE-BSA-stimulated OA chondrocytes. Primary human OA chondrocytes were pre-treated with inhibitors of p38 (SB202190), JNK (SP600125) and ERK (PD98059) MAPKs for 1 h and then stimulated with AGE-BSA (100 µg/ml) for 24 h. Expression of IL-6 or IL-8 mRNA was determined by real-time qPCR and normalized to GAPDH and compared with the levels present in control using the comparative ΔΔCT method. Effect of MAPK inhibition on the protein production of IL-6 (c) and IL-8 (d) in culture medium of AGE-BSA-stimulated OA chondrocytes. Primary human OA chondrocytes were pre-treated with MAPK inhibitors SB202190, SP600125, PD98059 for 1 h and then stimulated with AGE-BSA (100 µg/ml) for 24 h. Production of IL-6 or IL-8 was quantified by a sandwich ELISA. Primary OA chondrocytes incubated with native BSA (100 µg/ml) were used as control. The concentration of SB202190, SP600125 and PD98059 used in these studies was 100, 10 and 50 µM, respectively. Results are representative [mean (s.e.m)] of duplicate experiments with OA chondrocytes obtained from five age- and sex-matched OA donors and differ without a common letter; P < 0.05.
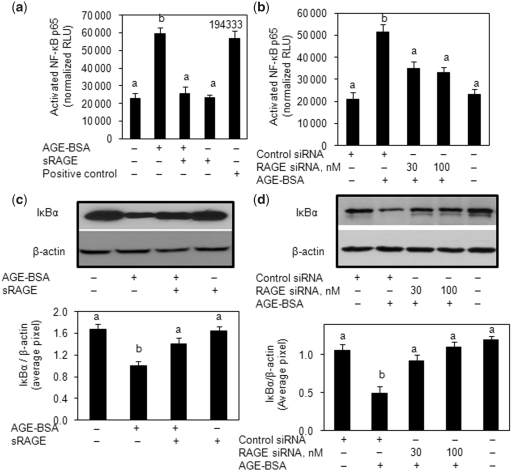
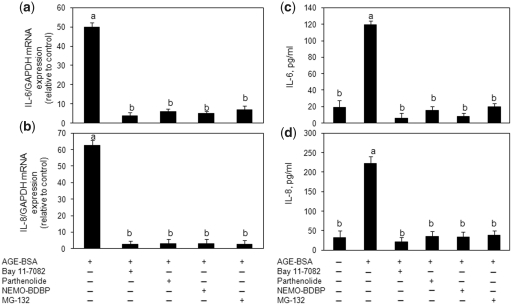
RAGE-mediated NF-κB activation is associated with IL-6 and IL-8 expression in primary human OA chondrocytes
Stimulation of human OA chondrocytes with AGE-BSA induced the degradation of NFκB inhibitorα (IκBα) and nuclear translocation of NF-κB (Fig. 7). Pre-treatment with sRAGE or RAGE knockdown significantly inhibited the AGE-BSA-induced degradation of IκBα (P < 0.05) (Fig. 7b and d). We also used a highly sensitive ELISA method to determine the activation of NF-κB by AGE-BSA in human OA chondrocytes (Fig. 7a and c). Treatment of human OA chondrocytes with sRAGE or transfection with RAGE-specific siRNA significantly inhibited the activation and DNA binding activity of NF-κB p65 (P < 0.05) (Fig. 7a and c). To determine the relation of the NF-κB pathway and the AGE-BSA-induced expression of IL-6 and IL-8, we next investigated the effect of pharmacological agents—Bay 11-7082 (IKKα/β inhibitor), parthenolide (IKKβ inhibitor), NF-κB essential modifier (NEMO)-BDBP (IKKγ inhibitor) and MG-132 (proteasome inhibitor)—to treat the human OA chondrocytes prior to stimulation with AGE-BSA. AGE-BSA-induced IL-6 or IL-8 expression and production was almost completely inhibited in human OA chondrocytes treated with any one of the NF-κB inhibitors [Bay 11-7082 (P < 0.0001), parthenolide (P < 0.0001), NEMO-BDBP (P < 0.0001) or MG-132 (P < 0.0001)] (Fig. 8). Together these results indicated that AGE-BSA-induced IL-6 and IL-8 expression in human OA chondrocytes was dependent on the activation of NF-κB.
Fig. 7.
Activation of RAGE-mediated NF-κB signalling by AGE-BSA in primary human OA chondrocytes. (a) Primary chondrocytes were pre-treated with sRAGE (200 µg/ml) for 2 h prior to AGE-BSA (50 µg/ml) stimulation or (b) RAGE-siRNA-transfected OA chondrocytes were stimulated with AGE-BSA (100 µg/ml) for 45 min and NF-κB p65 was determined in cell extracts (3 µg) by highly sensitive and specific ELISA. TNF-α-treated HeLa cell extract (supplied with kit) was used as a positive control. The assay is developed with a chemiluminescent substrate and the signal is detected using a multimode detector (DTX-880, Beckman Coulter). NF-κB p65 activity was expressed as relative light units (RLUs) and normalized with negative control. Results are representative [mean (s.e.m)] of three independent experiments and differ without a common letter; P < 0.05. (c, d) IκBα degradation was analysed by western immunoblotting using antibodies specific for IκBα (Santa Cruz Biotechnology). β-actin was used as protein-loading control. Band images were digitally captured and the band intensities (pixels/band) were obtained using the UN-Scan-It software and are expressed in average pixels. Data shown are cumulative of three experiments and the OD values are mean (s.e.m) and differ without a common letter; P < 0.05.
Fig. 8.
Activation of NF-κB is required for AGE-BSA-induced expression of IL-6 and IL-8 in primary human OA chondrocytes. Primary human OA chondrocytes were pre-treated with inhibitors specific for IKKα/β (Bay 11-7082), IKKβ (parthenolide), IKKγ (NEMO-BDBP) or proteasome (MG-132) for 1h and then stimulated with AGE-BSA (100 µg/ml) for 24 h. Expression of (a) IL-6 mRNA or (b) IL-8 mRNA was determined as described under Fig. 5. Effect of specific inhibitors of NF-κB on the production of IL-6 (c) and IL-8 (d) in AGE-BSA-stimulated primary human OA chondrocytes. Primary human OA chondrocytes were pre-treated with Bay 11-7082, parthenolide, NEMO-BDBP or MG-132 for 1 h and then stimulated with AGE-BSA (100 µg/ml) for 24 h. Level of IL-6 or IL-8 was quantified by a sandwich ELISA. Primary human OA chondrocytes incubated with native BSA (100 µg/ml) were used as control. Concentration of Bay 11-7082, parthenolide, NEMO-BDBP and MG-132 used was 50, 50, 50 and 100 µM, respectively. Results are representative [mean (s.e.m)] of duplicate experiments with OA chondrocytes obtained from age- and sex-matched five OA donors and differ without a common letter P < 0.05.
Discussion
Accumulation of AGEs in articular cartilage has been proposed as a mechanism for the age-related development of OA [43, 44]. Studies have also shown that ‘still-healthy’ cartilage of patients with a focal degenerative cartilage lesion elsewhere in the joint has higher AGE levels than healthy cartilage from controls in which there are no signs of OA [45]. The age-related accumulation of AGEs results in cross-linking of cartilage matrix components suggesting a putative molecular mechanism for the development of OA. In diabetic patients, AGE levels tend to be increased, since hyperglycaemia accelerates AGE formation [46]. The correlation between diabetes mellitus and OA is supported by previous findings showing that radiographic OA is more common, more severe and present earlier in diabetic patients [47–49]. Therefore, the levels of AGEs might predict susceptibility for developing OA. Elevated levels of AGEs may also trigger the activation of catabolic pathways through RAGE in chondrocytes and synoviocytes [15–17]. The activation of RAGE stimulates critical signalling pathways linked to the activation and expression of various inflammatory genes [15]. Pro-inflammatory cytokines and chemokines are known to induce degradative enzymes in cartilage itself and in synovial tissue [22, 23]. Studies have shown that abnormally high levels of IL-6 and IL-8 are present in the SF, synovium and cartilage tissues of arthritic patients [22–35]. In the current study, using primary human OA chondrocytes, we have demonstrated that in vitro-generated AGE-BSA binds to RAGE and activates a RAGE-mediated signalling cascade and we show for the very first time that this RAGE-mediated signalling cascade results in increased production of IL-6 and IL-8 in human OA chondrocytes. We used AGE-BSA as a model for AGEs, which is basically a complex that includes N3-carboxymethyllysine (CML), pentosidine and other AGEs, produced by reacting BSA with glycoaldehyde under sterile conditions, followed by extensive dialysis as previously described [40]. We also tested the effect of native BSA treatment on the expression and production of IL-6 and IL-8 in primary human OA chondrocytes and found that higher concentrations of native BSA (100 µg/ml) caused a low level increase in IL-6 and IL-8 expression and production, but this increase was statistically insignificant compared with the levels induced by treatment with AGE-BSA (P > 0.05) in agreement with the previous findings [50]. Several lines of evidence support the notion of an interaction of AGE-BSA with RAGE. Soluble RAGE (sRAGE), a truncated form of RAGE that consists of only the extracellular domain, blocked the expression and production of IL-6 and IL-8 upon AGE-BSA stimulation, suggesting that sRAGE and RAGE on the chondrocytes share a common or overlapping AGE-BSA binding site. In addition, knockdown of RAGE by RAGE-specifc siRNAs significantly inhibited AGE-BSA-induced IL-6 and IL-8 expression and production (P < 0.05). This further confirmed that AGE-BSA stimulates IL-6 and IL-8 induction and expression through a RAGE activated signalling cascade.
S100 family of proteins has been implicated in various inflammatory conditions, including arthritis [15]. S100A4 is a member of the S100 family of proteins and is known to be present in articular cartilage [16] and recently, it was demonstrated that S100A4 protein binds to RAGE and stimulates a RAGE-mediated signalling cascade resulting in the activation of MAPK and NF-κB, leading to increased production of cartilage-degrading enzymes [17]. Here we demonstrate that S100A4 protein stimulates the expression and production of IL-6 and IL-8 in human OA chondrocytes. Treatment of human OA chondrocytes with S100A4 protein significantly up-regulated the gene expression and protein production of IL-6 and IL-8 (P < 0.05) and this up-regulation of IL-6 and IL-8 was inhibited when the chondrocytes were pre-treated with sRAGE (P < 0.05). This indicates that AGE-BSA or S100A4 protein activates the RAGE-mediated signalling that stimulates the expression of IL-6 and IL-8 in human OA chondrocytes. Unlike AGE-BSA, IL-1β did not stimulated RAGE signalling in human OA chondrocytes as treatment of human OA chondrocytes with sRAGE had no effect on IL-1β-induced IL-6 and IL-8 gene or protein expression. We also investigated whether AGE-BSA or S100A4 protein induced the production of IL-6 and IL-8 in 3D cultures using human OA cartilage explants (n = 2). Treatment of human cartilage explants with AGE-BSA or S100A4 protein for 24 h increased the production of IL-6 and IL-8 in the culture medium, indicating that induction of IL-6 and IL-8 expression by AGE-BSA or S100A4 protein was mediated through the same mechanism both in OA chondrocytes in monolayer or when chondrocytes are embedded in a matrix such as in cartilage explants.
In previous studies, it has been shown that AGEs activate MAPK signalling pathways in human OA chondrocytes and induce the expression of MMPs, TNF-α, COX-2 and other mediators of cartilage catabolism [18, 21]. MAPK signalling has been reported to be activated through direct AGE interaction with RAGE [51, 52]. Our data confirm and extend these findings by demonstrating that treatment of primary human OA chondrocytes with sRAGE (which quenches RAGE-activating ligands) or transfection of primary human OA chondrocytes with RAGE-specific siRNAs (which knock down the expression of RAGE) inhibited the AGE-BSA-induced activation of p38-MAPK, JNK and ERK. We have shown that AGE-BSA-stimulated expression and production of IL-6 was mediated by the p38-MAPK and ERK-MAPK pathways as treatment of primary human OA chondrocytes with SB202190 and PD98059 significantly down-regulated AGE-BSA-stimulated IL-6 expression and production. This supports an important role of p38-MAPK and ERK in mediating the AGE-induced expression of IL-6 in human OA chondrocytes. Our results further indicate that the mechanism by which AGE-BSA induces IL-8 expression and production differs from that involved in IL-6 induction, which requires the activation of JNK as well, since treatment with SP600125, a JNK inhibitor, significantly inhibited AGE-BSA-induced IL-8 expression and production in primary human OA chondrocytes.
There are several distinct NF-κB activation pathways. The most frequently observed is the canonical or classical pathway, which is typified by the rapid phosphorylation of IκBα at Ser32 and Ser36 and subsequent ubiquitination and degradation by the 26S proteasome and the release of NF-κB [53, 54]. In the canonical pathway, IκBα phosphorylation depends on IKK complex activation [53]. The IKK complex consists of three core subunits, the catalytic subunits IKKα and IKKβ and several copies of a regulatory subunit called NEMO (also known as IKKγ) [53, 54]. Here, we showed that pre-treatment with IKKα/β inhibitors Bay 11-7082 and parthenolide, IKKγ inhibitor NEMO-BDBP and 26S proteasome inhibitor MG-132 significantly inhibited AGE-BSA-induced gene expression and protein production of IL-6 and IL-8 in primary human OA chondrocytes (P < 0.0001). Treatment of human OA chondrocytes with sRAGE or RAGE knockdown by siRNAs significantly inhibited IκBα degradation (P < 0.05) and NF-κB p65 activation (P < 0.05). These findings support previous reports that showed that AGE-BSA stimulates IκBα degradation and nuclear translocation of NF-κB p65 in human OA chondrocytes [18, 40]. These results clearly indicate that NF-κB-mediated AGE-BSA-induced expression of IL-6 and IL-8 in human OA chondrocytes is via activation of the canonical pathway and involves signalling through RAGE. Ours is the first report to demonstrate that RAGE-mediated signalling cascade up-regulates the production of IL-6 and IL-8 in human OA chondrocytes. These results also demonstrated that p38-MAPK, ERK-MAPK and NF-κB pathways are directly involved in IL-6 and IL-8 expression in AGE-stimulated human OA chondrocytes. In addition, activation of JNK was required for the expression of IL-8 but was not essential for IL-6 expression in AGE-BSA-stimulated human OA chondrocytes.
In conclusion, this study demonstrates that AGE-BSA differentially induce IL-6 and IL-8 expression via RAGE in primary human OA chondrocytes. Activation of RAGE by AGE-BSA triggers a cascade of signalling events, which includes phosphorylation of p38-MAPK, JNK and ERK-MAPKs. Taken together, our data suggest that the signalling pathways activated upon ligand binding to RAGE may play an important role in the degeneration of cartilage in OA and possibly in other degenerative diseases.
Acknowledgements
Z.R. carried out the experimental work, collection, interpretation and manuscript drafting. N.A. carried out the experimental work and collection of data. T.M.H. conceived the study, its design, coordination, data interpretation and manuscript drafting. All authors have read and approved the final manuscript.
Funding: This work was supported in part by the National Institutes of Health grants RO1-AT-003267, RO1-AT-005520 and R21-AT504615 and by funds from the MetroHealth Medical Center, Cleveland, OH, USA.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503–8. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention [review] Arthritis Rheum. 1998;41:1343–55. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999;42:17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix: implication of pentoses in the aging process. J Biol Chem. 1989;264:21597–602. [PubMed] [Google Scholar]
- 6.Makita Z, Vlassara H, Cerami A, Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992;267:5133–8. [PubMed] [Google Scholar]
- 7.Verzijl N, Bank RA, TeKoppele JM, DeGroot J. Ageing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15:616–22. doi: 10.1097/00002281-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Uchiyama A, Ohishi T, Takahashi M, et al. Fluorophores from aging human articular cartilage. J Biochem. 1991;110:714–8. doi: 10.1093/oxfordjournals.jbchem.a123646. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi M, Kushida K, Ohishi T, et al. Quantitative analysis of crosslinks pyridinoline and pentosidine in articular cartilage of patients with bone and joint disorders. Arthritis Rheum. 1994;37:724–8. doi: 10.1002/art.1780370517. [DOI] [PubMed] [Google Scholar]
- 10.Verzijl N, DeGroot J, Oldehinkel E, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350:381–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Bank RA, Bayliss MT, Lafeber FPJG, Maroudas A, TeKoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage: the age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330:345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey AJ, Sims TJ, Avery NC, Miles CA. Chemistry of collagen cross-links: glucose-mediated covalent cross-linking of type-IV collagen in lens capsules. Biochem J. 1993;296:489–96. doi: 10.1042/bj2960489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia. 1996;39:946–51. doi: 10.1007/BF00403914. [DOI] [PubMed] [Google Scholar]
- 14.Chen AC, Temple MM, Ng DM, et al. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002;46:3212–7. doi: 10.1002/art.10627. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 16.Loeser RF, Yammani RR, Carlson CS, et al. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376–85. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–11. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 18.Nah SS, Choi IY, Yoo B, Kim YG, Moon HB, Lee CK. Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett. 2007;581:1928–32. doi: 10.1016/j.febslet.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 19.Martel-Pelletier J, Pelletier JP, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003;33:155–67. doi: 10.1016/s0049-0172(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 20.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cartilage. 2002;10:792–8. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 21.Nah SS, Choi IY, Lee CK, et al. Effects of advanced glycation end products on the expression of COX-2, PGE2 and NO in human osteoarthritic chondrocytes. Rheumatology. 2008;47:425–31. doi: 10.1093/rheumatology/kem376. [DOI] [PubMed] [Google Scholar]
- 22.Scheinecker C, Redlich K, Smolen JS. Cytokines as therapeutic targets: advances and limitations. Immunity. 2008;28:440–4. doi: 10.1016/j.immuni.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Koch AE, Kunkel SL, Burrows JC, et al. Synovial tissue macrophages as a source of the chemotactic cytokine IL-8. J Immunol. 1991;147:2187–95. [PubMed] [Google Scholar]
- 24.Kitahara K, Kusunoki N, Kakiuchi T, Suguro T, Kawai S. Adiponectin stimulates IL-8 production by rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2009;378:218–23. doi: 10.1016/j.bbrc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Fonseca JE, Santos MJ, Canhão H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8:538–42. doi: 10.1016/j.autrev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Akira S, Taga T, Kishimoto T. Interleukin 6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto T. IL-6: from laboratory to bedside. Clin Rev Allergy Immunol. 2005;28:177–86. doi: 10.1385/CRIAI:28:3:177. [DOI] [PubMed] [Google Scholar]
- 28.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritis. Arthritis Rheum. 1988;31:784–8. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 29.Aherne CM, McMorrow J, Kane D, FitzGerald O, Mix KS, Murphy EP. Identification of NR4A2 as a transcriptional activator of IL-8 expression in human inflammatory arthritis. Mol Immunol. 2009;46:3345–57. doi: 10.1016/j.molimm.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Choi HM, Oh DH, Bang JS, Yang HI, Yoo MC, Kim KS. Differential effect of IL-1beta and TNF-alpha on the production of IL-6, IL-8 and PGE(2) in fibroblast-like synoviocytes and THP-1 macrophages. Rheumatol Int. 2010;30:1025–33. doi: 10.1007/s00296-009-1089-y. [DOI] [PubMed] [Google Scholar]
- 31.Sakao K, Takahashi KA, Arai Y, et al. Osteoblasts derived from osteophytes produce interleukin-6, interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J Bone Miner Metab. 2009;27:412–23. doi: 10.1007/s00774-009-0058-6. [DOI] [PubMed] [Google Scholar]
- 32.Kaneko S, Satoh T, Chiba J, Ju C, Inove K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6:71–9. doi: 10.1080/13684730050515796. [DOI] [PubMed] [Google Scholar]
- 33.Alonzi T, Fattori E, Lazzaro D, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6-receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–9. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 35.Matsukawa A, Yoshimura T, Maeda T, Ohkawara S, Takagi K, Yoshinaga M. Neutrophil accumulation and activation by homologous IL-8 in rabbits. IL-8 induces destruction of cartilage and production of IL-1 and IL-1 receptor antagonist in vivo. J Immunol. 1995;154:5418–25. [PubMed] [Google Scholar]
- 36.Rasheed Z, Akhtar N, Anbazhagan AN, Ramamurthy S, Shukla M, Haqqi TM. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP kinases and NF-κB in human KU812 cells. J Inflamm. 2009;6:1. doi: 10.1186/1476-9255-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappa B. Arthritis Rheum. 2002;46:2079–86. doi: 10.1002/art.10443. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Ther. 2004;308:767–73. doi: 10.1124/jpet.103.059220. [DOI] [PubMed] [Google Scholar]
- 39.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–71. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss F, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end products-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res Ther. 2009;11:R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valencia JV, Weldon SC, Quinn D, et al. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem. 2004;324:68–78. doi: 10.1016/j.ab.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verzijl N, Bank RA, TeKoppele JM, DeGroot J. Ageing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15:616–22. doi: 10.1097/00002281-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 44.DeGroot J, Verzijl N, Wenting-van Wijk MJ, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50:1207–15. doi: 10.1002/art.20170. [DOI] [PubMed] [Google Scholar]
- 45.Steenvoorden MM, Huizinga TW, Verzijl N, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–63. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 46.McCance DR, Dyer DG, Dunn JA, et al. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91:2470–8. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waine H, Nevinny D, Rosenthal J, Joffe IB. Association of osteoarthritis and diabetes mellitus. Tufts Folia Med. 1961;7:13–9. [PubMed] [Google Scholar]
- 48.Campbell WL, Feldman F. Bone and soft tissue abnormalities of the upper extremity in diabetes mellitus. Am J Roentgenol Radium Ther Nucl Med. 1975;124:7–16. doi: 10.2214/ajr.124.1.7. [DOI] [PubMed] [Google Scholar]
- 49.Bagge E, Bjelle A, Eden S, Svanborg A. Factors associated with radiographic osteoarthritis: results from the population study 70-year-old people in Göteborg. J Rheumatol. 1991;18:1218–22. [PubMed] [Google Scholar]
- 50.Steenvoorden MM, Huizinga TW, Verzijl N, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–63. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 51.Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 2003;550:107–13. doi: 10.1016/s0014-5793(03)00846-9. [DOI] [PubMed] [Google Scholar]
- 52.Yeh CH, Sturgis L, Haidacher J, et al. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495–504. doi: 10.2337/diabetes.50.6.1495. [DOI] [PubMed] [Google Scholar]
- 53.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 54.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]