Abstract
Recent evidence demonstrates that humans are not the only species to respond negatively to inequitable outcomes which are to their disadvantage. Several species respond negatively if they subsequently receive a less good reward than a social partner for completing the same task. While these studies suggest that the negative response to inequity is not a uniquely human behavior, they do not provide a functional explanation for the emergence of these responses due to similar characteristics among these species. However, emerging data support the hypothesis that an aversion to inequity is a mechanism to promote successful long-term cooperative relationships amongst non-kin. In this paper, I discuss several converging lines of evidence which illustrate the need to further evaluate this relationship. First, cooperation can survive modest inequity; in explicitly cooperative interactions, individuals are willing to continue to cooperate despite inequitable outcomes as long as the partner's overall behavior is equitable. Second, the context of inequity affects reactions to it in ways which support the idea that joint efforts lead to an expectation of joint payoffs. Finally, comparative studies indicate a link between the degree and extent of cooperation between unrelated individuals in a species and that species’ response to inequitable outcomes. This latter line of evidence indicates that this behavior evolved in conjunction with cooperation and may represent an adaptation to increase the payoffs associated with cooperative interactions. Together these data inform a testable working hypothesis for understanding decision-making in the context of inequity and provide a new, comparative framework for evaluating decision-making behavior.
Keywords: inequity, cooperation, non-human primates, apes, monkeys, evolution of behavior, comparative approach
Introduction
When making decisions about resources, humans show an intense interest in how their outcomes compare to those of others. In the laboratory, people will reject absolute gains in order to keep others from receiving more (Guth et al., 1982), even if this results in greater inequity (Yamagishi et al., 2009). This behavior seems to affect offers, too; individuals offer more when their partner has a chance to refuse than when they have no recourse (e.g., comparing the ultimatum to the dictator games; Camerer, 2003). People's reaction to inequity is also highly context dependent. Subjects make different decisions if the right to control distributions must be earned (Hoffman et al., 1994) or if they have an opportunity to respond in some other way (Xiao and Houser, 2005). We do not make decisions in a vacuum, but instead seem to care very much how our outcomes compare to those of our social partners.
Although much of this research is done using the ultimatum game, another important game in this vein is the impunity game. In this game, one individual, the proposer, is given an endowment of money and must decide how to split the money between themselves and another individual, the responder. The responder then has two options; if they accept, the distribution is given to each individual as proposed, as in the ultimatum game, but if they reject, the proposer receives their money, but the responder receives nothing. This game is little studied, likely because refusing results in both less absolute gain and increased relative inequity for the responder, thus it was assumed that no rational actor would refuse (in the Ultimatum game, refusing results in less absolute gain, but increased relative equity, as both participants get nothing; Bolton and Zwick, 1995). Note, too, that peoples’ refusals in this context are not inequity aversion as proposed by Fehr and Schmidt (1999), as individuals are increasing rather than decreasing inequity. However, recent evidence indicates that people routinely refuse unequal offers in the impunity game, making this an important set of circumstances to evaluate more fully (Yamagishi et al., 2009).
Humans are not alone in this. Other species show very similar behaviors. In a game reminiscent of an impunity game, two species of primate have been shown to refuse rewards more often when their partners get better rewards than when their partners receive the same, lower-value rewards (Brosnan, 2006; details in next section). While this behavior seems economically irrational, it is a very consistent response in both these primates and humans. This raises questions about the evolution of this behavior on a number of levels. First, is it possible that the underlying function of this behavior is similar across species, including humans? Second, even if the behavior has been selected for similar reasons, what are the mechanisms that lead to the behavior? Related to this, how do we account for the individual differences seen in the behavior, and are these differences, and their causes, consistent across species?
Although the data we have now provide pieces of the puzzle, there is as yet no satisfying functional explanation, and the very ubiquity of the response demands an answer. One hypothesis is that recognizing and responding to inequitable outcomes increases individuals’ payoffs from cooperation. If this is the case in humans, it may be in other species as well, but only recently has enough data emerged to begin to address this hypothesis in species other than humans. In this paper, I discuss this emerging evidence in non-human primates, and based on three main lines of inquiry put forth the hypothesis that there has been co-evolution between cooperation and inequity across the animal kingdom. I discuss the implications of this hypothesis for other, related, areas of inquiry. Finally, I end with ideas for further tests to evaluate and refine this hypothesis, both in primates and other species.
Inequity paradigms in other species
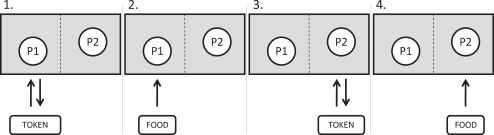
Given inherent species differences, most of the protocols for studying inequity in other species are simpler than those in humans, for instance requiring no verbal instruction. In the typical paradigm, two individuals from the same social group are paired, and they must alternately complete a task to receive a reward (see Figure 1 for details of the experimental procedure). Each can see the others’ performance and the others’ outcomes. In the baseline condition, rewards are the same, but in the inequity condition, one partner receives a reward which is more preferred (e.g., based on previous preference tests between the rewards; see Table 1). Thus we can compare individuals’ reactions to a reward when their partner gets the same reward as they do versus a more preferred one. Additional controls can examine potentially mediating factors such as the role of differential effort or the way in which the mere presence of higher-value rewards (which are not given to a conspecific) may affect reactions. Such studies have now been done in a variety of primates, as have similar studies in other taxa (Heidary et al., 2008; Range et al., 2008).
Figure 1.
A schematic diagram indicating the procedure for each trial of an inequity test. Two primates (P1 and P2) are tested in a pair. For each condition (see Table 1, columns 1 and 2), the primates must sequentially perform a task with the experimenter (typically a token exchange; Table 1, column 3) in order to receive a food reward (Table 1, column 4; see full description of each condition in column 5). An arrow indicates the order in which the object (token or food) moves between the experimenter (E) and the primates in each step of the trial. In all studies in my laboratory, primates are seated side-by-side. Monkeys are separated by a mesh barrier which they can reach through while apes are not separated. The details of the exchange task and the foods given to each primate are determined by the test condition; for details see Table 1.
Table 1.
Description of experimental conditions (for a summary of the procedure, see Figure 1 and associated caption).
| Abbreviation | Condition name | Exchange | Food | Description |
|---|---|---|---|---|
| ETLV | Equity test, low value | Both exchange | Both low value | Both subject and partner exchanged for low value reward. |
| ETHV | Equity test, high value | Both exchange | Both high value | Both subject and partner exchanged for high value reward. |
| FC | Food control | Both exchange | Both see high value before exchange, receive low value following exchange | Prior to exchange, high value reward is held in front of exchanger and then is placed back in container. After successful completion of exchange, exchanger receives low value reward. |
| IT | Inequity test | Both exchange | Subject low value Partner high value | Partner exchanges for high value reward and subject exchanges for low value reward. |
| GR | Gift reward | No exchange | Subject low value Partner high value | Partner is given a high value reward for “free” (e.g., without exchange) and then subject is given a low value reward. |
| DT | Delay test | Both exchange, subject waits 10 s after exchange before receiving food | Both high value | Partner exchanges for a high value reward and subject exchanges and must wait 10 s before receiving high value reward. |
| DETLV | Differential exchange test, low value | Subject exchanges Partner does not exchange | Both low value | Partner is given a low value reward for “free” (e.g., without exchange) and subject must exchange for a low value reward. |
| DETHV | Differential exchange test, high value | Subject exchanges Partner does not exchange | Both high value | Partner is given a high value reward for “free” (e.g., without exchange) and subject must exchange for a high value reward. |
Not all conditions are used in all experiments, but all are provided to give an overview of the types of questions which have been asked. The most critical tests are the ETLV, which collects baseline data on responses to lower-value rewards, the IT, which collects data on responses to lower-value rewards when one's partner receives higher-value ones, and the FC, which is a control to determine reactions to lower-value rewards are present, but no primates receive one. Reprinted with permission from Brosnan et al. (2010a).
Decisions to refuse a reward stem from many factors, nonetheless these results cannot be fully explained by processes other than the aversion to inequitable outcomes. For instance, it has been proposed that refusals of rewards may be due to a “frustration effect,” in which subjects compare their current outcomes to those which they received previously, and protest if the comparison comes up wanting (Roma et al., 2006). Such individual contrast effects are seen in a wide variety of species (Tinklepaugh, 1928; Friedan et al., 2009), however they do not explain these results. In controlled experiments, the mere presence of the higher-value rewards did not cause increased rejections, even when the experimenter called the subjects’ attention to the preferred reward prior to each interaction (Brosnan et al., 2010a). Of course it is likely that these two phenomena are built on similar cognitive underpinnings; in both cases, subjects compare their current outcomes to some other referent and find the current outcome wanting. However, in the case of the “frustration” effect, the referent is individual, or one's own previous outcomes, whereas in the case of inequity, the referent is social, or one's partners’ outcomes. Thus the response to inequity can be thought of as a “social contrast” or “social frustration” effect. Given the ubiquity of individual contrast effects, as well as attention to others’ outcomes (e.g., in the context of social learning), we might expect inequity responses to be widespread.
Primates also show quite a bit of variation in their responses. Considering only chimpanzees, responses vary both within and between experiments. Thus far, factors which have been implicated in this variation include dominance rank, sex, and group identity. Other factors, such as personality and individuals’ relationships are also likely to play a role. However there is not yet consistency in which factors affect behavior, probably due to both interactions between these and other effects and the relatively small sample size which has been tested (approximately three dozen chimpanzees, which is large for primate studies, but too small to pinpoint such interactions). Larger studies are currently underway to investigate this variation. It is also not yet clear how this compares to inter-individual differences in humans, as studies with humans typically focus on mean responses rather than individual behavior.
Hypotheses for the Function of the Response to Inequity
It has been proposed that the inequity response functions to increase the success of long-term cooperative relationships amongst unrelated individuals (hereafter cooperation; Fehr and Schmidt, 1999; Brosnan, 2006). Although this was originally proposed with respect to humans, new evidence provides support for this hypothesis in an evolutionary context. Specifically, the ability to recognize situations in which one is receiving a less good outcome than a partner, or inequity, may allow individuals to determine when their cooperative partners are taking more than their fair share and are thus no longer to one's benefit as a partner. In other words, an aversion to inequity can be a mechanism which encourages individuals to switch to a new partner when they find themselves in a situation which is not to their advantage. This ultimately functions to increase payoffs by encouraging individuals to seek out new partners. If the new partner is more equitable, then there is a benefit and the individual will have an absolute gain, despite temporary costs associated with time spent searching for new partners or potential interludes with other inequitable individuals. This mechanism would be under strong positive selection due to the potential for large fitness gains. Note that while the original inequity aversion formulation assumed that individuals were averse to decisions which increased relative inequity (Fehr and Schmidt, 1999), even reactions that increase short-term inequity (e.g., in the Impunity game) may serve to increase long-term equity by moving actors in to relationships which are more beneficial.
Note, too, that this would not need to be consciously understood by the individual; those who developed an aversion to inequity through whatever mechanism (e.g., an emotional reaction) would be more likely to succeed, increasing the frequency of the reaction in the population. Considering one possible scenario, in most cases in which individuals experience inequity during or immediately following an interaction with another individual (e.g., not just in proximity to another, but after explicitly interacting with them), the inequity is likely to be related to the other's actions. Although there would undoubtedly be a few situations in which this was not the case, this would be somewhat ameliorated if individuals kept track of more than one interaction in the relationship history, as they can do (Brosnan et al., 2006). Thus individuals who happened to respond in these situations could develop the behavior without an understanding of the others intentions or motives (although note that primates likely have the requisite understanding of intentionality and ability to inhibit; see The Context of the Interaction and Inequity and Self-Control). Alternatively, this mechanism could function similarly to that which has been proposed for attitudinal reciprocity, in which individuals base their moves on their current feelings for their partner (Brosnan and de Waal, 2002; Schino and Aureli, 2010). Thus there is no expectation that an inequity response needs to be paired with any higher-order cognition in order to function in this way. In fact, only partner recognition is required, and this is likely widespread throughout the animal kingdom (including invertebrates; Karavanich and Atema, 1998; Tibbets, 2002; Steiger et al., 2008).
This hypothesis also ties in nicely with other hypotheses for why individuals respond negatively to inequity. It has been argued that responding negatively to inequity, including when one is the benefited party, can function as a commitment device (Frank, 1988, 2001; Yamagishi et al., 2009). Responding in this way indicates to both one's current and potential future partners that you are a very good partner (i.e., you do not treat your other partners inequitably), and may also send a signal which increases one's reputation (Frank, 2004). Similarly, refusing absolute gains which are relatively unequal sends a signal to potential partners that they cannot get away with such behavior with you, which may increase future payoffs (Yamagishi et al., 2009). Such behavior represents a short-term cost (in the form of lost immediate gains) for a long-term gain (in the form of long-term beneficial relationships).
Evidence is beginning to emerge to support the hypothesis that cooperation and the response to inequity are linked in species besides humans (see Existing Evidence in Support of the Hypothesis). Such a link may provide evidence for how the response evolved. This question is more than academic. Understanding the evolutionary trajectory of a behavior can help elucidate its evolutionary function. Moreover, while it is often assumed that traits within a taxon are likely to be homologous, this does not need to be the case. My research indicates that responding negatively to receiving less than one's partners is not homologous among primates, but rather is convergent, and the trait which best maps on to it is cooperation. Below I summarize lines of evidence leading to this conclusion, relying on data from a number of different species in several different contexts.
Existing Evidence in Support of the Hypothesis
If cooperation and responding to inequity are linked, a number of predictions emerge. Three among these have already accrued some evidence. First, inequitable outcomes should affect cooperation. Second, negative responses to inequity should be evident in the context of cooperation. Third, species which typically cooperate should show a greater tendency to reject inequitable outcomes than those which do not. Below I explore the evidence which addresses these predictions, focusing on behavior which is disadvantageous to the individual.
The interplay of cooperation and inequity
If cooperation and inequity are related, cooperation should be affected by inequitable outcomes (Brosnan, 2006; de Waal and Suchak, 2010). In fact, this question is of vital importance as it is rare that interactions result in complete equity, at least in the short term (Aghion et al., 1999). Thus any species which both successfully cooperates and responds to inequity must have some capacity for taking into account context or an ability to extrapolate over the longer term in order to maintain cooperative interactions.
Capuchin monkeys are excellent subjects in which to investigate this interaction as they are known to cooperate in many contexts, and to understand the contingencies of cooperation (Brosnan, 2010). Although in most cases cooperation experiments utilize situations in which both individuals receive identical rewards, the few which do not are telling. In a study designed explicitly to address this question, monkeys could cooperate on a mutual task in which joint efforts resulted in rewards to both, but sometimes the rewards were unequal (Brosnan et al., 2006). Unlike in many cooperation experiments, the monkeys were not separated and thus had to decide between themselves which individual would pull which bar and, hence, receive which reward. Since the monkeys determined which one worked for which reward, rather than the experimenter, we could determine whether the presence of inequity affected cooperation, and whether they were able to work around it. In fact, the distribution of rewards (equal or unequal) did not affect cooperation, but the partner's behavior influenced it greatly. If one individual consistently dominated the better rewards, cooperation dropped to almost a third of the rate seen in partnerships in which both monkeys alternated receiving the better rewards.
Intriguingly, this cessation of cooperation happened in all conditions, including equitable ones in which no reward difference was possible. Thus, the monkeys were reacting to their partners’ behavior rather than the distribution of rewards. This is an important point; clearly the monkeys are willing to tolerate inequity on a trial-by-trial basis as long as the overall outcomes are approximately the same. In other words, cooperation only breaks down when faced with long-term inequity, not the occasional unequal outcome. This explains how cooperation can be maintained in tasks such as group hunting or mating coalitions, which may result in unequal outcomes in the short term. Individuals will continue to cooperate despite the occasional lesser reward if their overall rewards remain equitable.
Other studies support this conclusion. Capuchin monkeys will help partners obtain rewards as long as the partner then shares some of the spoils, but, again, cooperation breaks down if no sharing occurs (de Waal and Berger, 2000). Moreover, capuchins appear intrinsically less motivated to cooperate when rewards are easily monopolizable than when rewards are distributed such that neither individual can dominate them (de Waal and Davis, 2002), indicating that they prefer to avoid situations which may result in inequity. Although the same studies have not been run with chimpanzees, these apes are more likely to work to obtain a joint reward when paired with a partner who tolerantly shares food than one who does not (Melis et al., 2006a) and, when given the choice, will choose a more tolerant rather than less tolerant partner with whom to work (Melis et al., 2006b). Together, these studies indicate a close connection between cooperation and inequity, such that cooperation can survive modest or short-term inequity, but greater or more pervasive inequity leads to the cessation of the cooperative interaction. Such a response is indeed advantageous, as it allows individuals to identify partners with whom cooperation is not paying off and alter their choices in the future without forfeiting a beneficial partnership due to one instance of cheating, misunderstanding, or coincidence.
The context of the interaction
In humans, much has been done to investigate the role of the experiment's context on how subjects respond to distributional inequity (although using monetary payoffs rather than food, as in non-human species). One area relevant to cooperation is intentionality. Humans clearly distinguish between acts which were determined by another human being and those which were determined randomly, without human intervention (e.g., by a computer). Humans respond behaviorally, less often refusing unfair outcomes when they are determined by computer (Blount, 1995) and also show differential brain activation between human-initiated and computer-initiated distributional inequity (Knoch et al., 2006). It is typically assumed that the explanation for this is that subjects are sensitive to the intention behind the action; intentional actions must be responded to, while those which were the result of chance were “bad luck” that is not due to ones’ social partners and so do not require a response. This sensitivity to intentionality makes sense in light of cooperation. If your partner gets more than you do because of chance, then there is no reason to go find a new partner; this outcome provides no information at all about their value as a social partner. If, on the other hand, you were working together with a partner who then took a greater share of the benefits reaped, that is a clue about the partner's value, and a sign to find a new partner. In other words, joint efforts should lead to joint outcomes (van Wolkenten et al., 2007).
In primates, there are few studies comparing “intentional” and “accidental” inequity, no doubt in part due to the difficulty of experimentally distinguishing chance occurrence from intentionality in non-verbal species. However, those which do exist indicate behavior similar to that seen in humans. Primates are sensitive to the intentionality of a human experimenter, responding more strongly when they drop a reward intentionally than when it appears to be an accident (Call et al., 2004). Within their own species, chimpanzees react more strongly to punish (e.g., take away access to food) a partner who previously stole that food from them than when it is simply an inequitable distribution (Jensen et al., 2007). In this latter study, chimpanzees had the option to pull down a “table” which held food their partner could access (thus taking away access from the partner). If the partner got the food by taking away access from the subject, subjects were far more likely to pull down the table than if the partner was simply granted access to the food by the experimenter.
Primates are also quite sensitive to how rewards are received. Specifically, primates are most sensitive to inequity in the context of completing a task. Most inequity experiments require the subjects to complete a task (typically an exchange of a token) to receive their rewards (see Figure 1). However in other studies, subjects are handed food rewards alternately, but for “free,” i.e., with no task required to obtain the food (e.g., panels 2 and 4 in Figure 1). Thus far, no reactions to inequity have been found in any study which did not involve a task (Bräuer et al., 2006; Dubreuil et al., 2006; Roma et al., 2006; Fontenot et al., 2007). This includes one study (Dindo and de Waal, 2006) which used the same capuchin subjects which both previously and subsequently responded to inequity in paradigms which involved tasks. Several additional studies have explicitly compared the presence versus absence of a task, three using a within-subjects’ design and one a between-subjects’ design. In a within-subjects’ design, chimpanzees showed no reaction to inequity in the absence of a task (e.g., when rewards were handed out for “free”), while responding when they had to complete the task for their rewards (Brosnan et al., 2010a). In the other three species, all refused more often when no task was used despite subjects not responding differentially between the equity and inequity conditions (Neiworth et al., 2009; Brosnan et al., in review; Talbot et al., in press).
There are several possibilities as to why the primates only respond to inequity when a task is involved. First, despite claims that the presence or absence of a task should be irrelevant (Roma et al., 2006), animals are known to treat rewards which are earned differently than those which are received for “free” (Carder and Berkowitz, 1970). Moreover, since captive animals routinely receive food from keepers in situations which are not equitable, it is possible that they have grown accustomed to inequity in non-task situations, and so do not respond in these situations. Finally, it may also be that the presence of a task mimics a joint activity, despite the sequential nature of the interaction, thus priming the individuals to expect more equitable outcomes (Brosnan et al., 2010a).
Of course, if cooperation is linked with inequity, one would expect other experimental or social contexts to be important as well. Reactions to inequity are affected by group membership (Brosnan et al., 2005), sex (Brosnan et al., 2010a), rank (Bräuer et al., 2006; Brosnan et al., 2010a), and experimental design (Brosnan et al., 2010a) and, as discussed above, tolerance between individuals influences cooperative outcomes (Melis et al., 2006a). Similarly, individuals’ personalities or relationships may play a role. This has not been investigated in great detail in humans, possibly due to the tendency to test subjects in completely anonymous situations, with strangers, in part in an effort to rule out these factors as potential causes. Non-human primate studies offer a wonderful opportunity to investigate these factors in longitudinal studies.
A phylogenetic approach
Not all species of primates respond to inequitable outcomes. A recent surge of studies have provided information on a far wider range of primate species than were initially tested, indicating that the response is likely not a homology among primates. Although any such analysis without information on all species is necessarily limited, we are at the point where it is useful to begin considering the phylogenetic data. Inequity seems to affect cooperative behavior, and the species in which the response to inequity was first documented, capuchins and chimpanzees, are known to cooperate, both in the laboratory (de Waal and Berger, 2000; Melis et al., 2006b) and in the field (Creel and Creel, 1995; Fragaszy et al., 2004; Mitani, 2006; Langergraber et al., 2007), lending credence to this hypothesis. However, while the cooperation hypothesis is compelling, it is not the only possibility. For instance, inequity could be a homology among primates (or a more specialized group within the primate taxon), in which case investigations in to function will necessarily require other taxa. Second, this behavior could be an emergent behavior that arises when a species develops sufficient cognitive abilities to remember and compare outcomes between themselves and others. Third, it could also emerge as a by-product of social living. If individuals were already predisposed to pay attention to their partners’ outcomes, as is required, for instance, in some forms of social learning, they may then begin comparing their outcomes in other situations. Notice an important subtlety in these latter cases; the ability to gain benefits from social comparison may require certain cognitive skills, such as individual recognition, but that is independent of whether cognition is a functional explanation for the behavior. We can address these hypotheses by comparing primates which vary on these dimensions. Data exist for several species of great apes and new world monkeys, providing a more fine-grained analysis.
For these comparisons, it is important to keep the tasks and dependent variables as similar as possible across studies. Given that it is clear that a task is required to elicit inequity (see The Context of the Interaction), in all of the studies done in my laboratory we utilized an exchange-based task (Figure 1). The dependent variables were whether the primate completed the task and whether or not they accepted the reward, combined as a participation measure, and the latency to complete the interaction. Since the latter measure has not been seen to differ between conditions of equity and inequity, I focus here only on whether the subject refused to participate (e.g., refused to complete the task or accept the reward).
First considering the great apes, the response has been well documented in both humans and chimpanzees, as discussed above, although there is variation which needs to be further explored to fully elucidate how context affects the reaction (Brosnan et al., 2005, 2010a; Bräuer et al., 2006, 2009). Considering chimpanzees’ sister species, bonobos, only one study has investigated bonobos in a comparable paradigm to chimpanzees. The bonobos refused twice as often in the inequity (approximately 20%) as compared to the equity (approximately 10%) condition, although this difference was not significant (possibly due to the small sample size; Bräuer et al., 2009). This result rules out none of the potential hypotheses, but the fact that bonobos also cooperate, both in the laboratory (Hare et al., 2007) and the field (e.g., in social relationships; Parrish, 1996; Hohmann and Fruth, 2000; Fruth and Hohmann, 2002), provides support for the cooperation hypothesis.
Orangutans have also been well studied (Bräuer et al., 2006, 2009; Brosnan et al., in review). These apes do not respond negatively to inequity, even in a study which directly replicated the methodology which found a response in chimpanzees (Brosnan et al., in review). These results seem to rule out the possibility that cognitive differences are related to inequity, as orangutans are equally skilful in cognitive (Russon, 1998; Shumaker et al., 2001) and exchange (Flemming et al., in revision) tasks as other apes. Moreover, they support the cooperation hypothesis, as orangutans, while cooperative in experimental studies (Chalmeau et al., 1997; Dufour et al., 2008), are not known to cooperate in the wild to a great degree, possibly due to their more solitary social organization (van Schaik and van Hooff, 1996). These results also support the hypothesis that inequity is a by-product of sociality, as orangutans are less gregarious than other great apes, although they do form relationships and interact much more frequently in some contexts than initially recognized (Edwards and Snowdon, 1980; Singleton and van Schaik, 2002; van Schaik et al., 2009). The orangutan results also indicate that any homology among the apes must be more recent than the orangutan split from the African apes.
Amongst the new world monkeys, brown capuchins (Cebus apella) have been documented to respond negatively to inequity in all but one of four studies which employed a task (Brosnan and de Waal, 2003; van Wolkenten et al., 2007; Fletcher, 2008; Silberberg et al., 2009). Taken with the data on their responses to inequity in the context of cooperation (see The Interplay of Cooperation and Inequity above), it is clear that in many contexts, these monkeys are sensitive to receiving less than their partner. To explicitly address the homology hypothesis, we replicated the test on squirrel monkeys (Saimiri spp.), a species which shares a phylogenetic family, Cebidae, with capuchins. Squirrel monkeys have a smaller brain and neocortex volume per body size than do capuchins (Rilling and Insel, 1999) and cooperate in only limited situations (Boinski, 1987). They are also a highly gregarious, group-living species, and are even sympatric with capuchins in some areas. However, in our study, squirrel monkeys did not respond negatively to inequity, completing the interaction whether or not their partner received a greater reward (Talbot et al., in press). They were sensitive to the experimental paradigm; subjects were more likely to refuse to participate if the rewards violated their expectations than in the control condition. These results indicate that the inequity response is not homologous within the Family Cebidae, and the distribution across new world monkeys and great apes suggests that neither sociality nor cognition are sufficient feature to explain it. Given these data, the one feature in common to all is frequent cooperation among non-kin, indicating the possibility of either a convergence or the secondary loss of the trait in non-cooperative species.
The new world monkeys also introduce an interesting caveat in the form of the Callitrichids. Callitrichids are cooperative breeders, meaning that the parents and, sometimes, adult offspring, work together to rear young. Given this intensive level of cooperation (Cronin et al., 2005), even when confronted with unequal outcomes (Cronin and Snowdon, 2008), one might expect them to be particularly sensitive to inequity. However, among tamarins, there is little, if any, evidence that they refuse to participate when outcomes are unequal (Neiworth et al., 2009). Given the variation seen among species, this result needs to be validated with additional studies of cooperative breeders. Nonetheless, this finding may serve to validate the main tenet of the cooperation hypothesis, which is that responding to inequity serves to increase the long-term gains from cooperation. In the case of cooperative breeders, the interdependency between males and females (the partners in these experiments) may explain these results. If males and females rely on each other for their reproductive fitness, negative responses to minor inequities would end up being more costly than beneficial. Thus, individuals may do best to tolerate minor inequities and respond only when the inequitable outcomes become egregious.
Taken together, the current phylogenetic results most strongly support the hypothesis that inequity and cooperation are interlinked, providing evidence in favor of the cooperation hypothesis for the evolution of the inequity response. Cognition may be necessary, but is not sufficient, as great apes with brain-to-body ratios on par with chimpanzees and capuchins, and comparable performance in cognitive tasks, do not respond negatively to inequity. The response is not a by-product of group-living, as several primates which are gregarious do not respond, including the highly social Callitrichids. However, species which routinely cooperate with non-kin in several different contexts respond negatively to inequity, while those who do not cooperate to this degree fail to do so. Moreover, interdependent species, which may not benefit from such a response, fail to respond to inequity.
Implications of the Hypothesis
In short, there is emerging comparative evidence that links cooperation and inequity. It is interesting to consider further implications of the hypothesized relationship between the negative response to inequity and cooperation.
Inequity and interdependence
One intriguing aspect of the phylogenetic data is the possible role of interdependence in the response. Extreme interdependence occurs among cooperatively breeding species, in which the (unrelated) adults’ genetic fitness is directly tied to the fitness of others. This promotes cooperation (Roberts, 2005) and has been argued to explain the unusually high levels of prosocial behavior among mated pairs in cooperative breeding species (Clutton-Brock, 2002; Hrdy, 2009; Jaeggi et al., 2010; Van Schaik and Burkart, 2010). In other words, the parents are so dependent upon one another for their fitness that it is in their best interests to help each other in most circumstances, regardless of the cost. While there is currently only a single study investigating inequity responses in cooperative breeders, the lack of evidence for an inequity response among mated partners may indicate that interdependence plays a role in inequity. Once the relationship is established, continuing the interaction may be worthwhile even if their partner is getting a better deal. The cost of abandoning one's breeding partner to find a new one due to a small act of inequity would be strongly selected against, and only extreme inequity may be sufficient to change this cost–benefit calculation.
Humans are worth considering in this respect. Humans also appear to be a cooperatively breeding species (Hrdy, 2009), yet we respond to distributional inequity. However, unlike Callitrichids, humans routinely form cooperative relationships with individuals outside of the pair bond, and virtually all experimental tests of inequity involve strangers, and often occur in anonymous settings. Thus, humans may be subject to two selective forces. First, we are likely selected to be sensitive to inequity in interactions with individuals other than our partner, as is likely captured in studies of inequity and cooperation in the laboratory (e.g., with non-pair bonded individuals, who are often both strangers and anonymous). On the other hand, we may be selected to ignore inequity within close relationships, such as the pair bond (Clark and Grote, 2003), as do other cooperatively breeding species. In fact, it is likely that humans are not the only species which will show different behavior depending upon the relationship involved, an area which needs further investigation.
Inequity and relationships
Related to this, it is possible that interactions may differ within the same species depending upon the relationships among the individuals in question. For instance, in species which form mated pairs but nonetheless regularly interact and cooperate with other individuals, one might expect no response to inequity among mated pairs (due to interdependence), but a present response to inequity among non-mated pair adults. For instance, this might describe the interactions we would expect in humans, as discussed above. Humans show reduced sensitivity to inequity in close relationships as compared to more distant ones, which are typically more contingent (Clark and Grote, 2003). As another example, individuals within a cooperative breeding group may have differential investment in the group, or differential cost to finding a new partner. Less invested individuals, or those who can more easily find new partners, should be less tolerant of inequity. Similar patterns may apply in other species which cooperate across many different types of relationships.
Inequity and life history
Another prediction which emerges is that responses to inequity may differ across life history stages. Some species have different relationships with others of their age class depending upon where they are in development. For instance, polygynous species may go through a stage in an adult single-sex group (e.g., a “bachelor group”) prior to becoming the alpha male of a mixed-sex group. In some cases, individuals may even have different relationships with different individuals during the same life history stage, and so show several different behaviors at any given time (but with different partners, e.g., humans; see above sections). Since relationships and the frequency of cooperation differ in these different stages of development, reactions to inequity may vary as well.
An intriguing corollary of this prediction is the possibility of particularly enhanced responses to inequity among individuals who are in the process of forming pair bonds. Given that individuals are in the process of forming a bond which is both critical to reproductive fitness and costly to break, individuals may be best served to be hyper vigilant for signs of inequity. In this way they may be able to predict future behavior from current actions and limit the subsequent costs of either inequity or the requirement to find a new mate. This may also provide a likely opportunity to investigate deception, as there would be strong selection in favor of behaving more equitably in the mating market than once the pair bond was formed. Finally, individuals may be particularly likely to tolerate inequity once there is a reproductive investment in the relationship. Thus inequity may be a mechanism individuals can use to assess partner potential early in a relationship.
Inequity and punishment
While the main purpose of the response to inequity seems to be recognizing when it is time to find a new partner with whom to cooperate, in some situations switching to a new partner may not be an option (e.g., due to low availability of other partners, a high cost to switching, or the difficulty in establishing sufficient trust for cooperation to emerge at the same level). In these cases, the recognition of a partner's low quality that derives from the inequity response may secondarily be used to identify situations in which it is worth an attempt to alter the current partner's behavior, including punishment (Jensen, 2010; Raihani et al., 2010). This provides an alternate option through which individuals can try to increase their benefits for cooperating; if they cannot leave and find a new partner, they may be able to try changing the behavior of the current one. Even the occasional act of punishment may be sufficient to alter the partner's behavior for the better (Bergmüller and Taborsky, 2005).Thus punishment may be more likely in those species which have limited options for finding a new partner or for which such an action is extremely costly, one practical implication of which is that it may be easier to identify punishment in species which are known to react behaviorally to inequity, but in situations in which individuals have limited options for finding new partners.
Inequity and Related Behaviors
Inequity is related to several behaviors, either because these behaviors may contribute to the mechanisms which allow individuals to refuse inequitable outcomes, or because the ability has implications for the behavior. Below I consider two of these.
Inequity and self-control
Refusing a present and available reward seems to require quite a lot of self-control. Primates are known to be good at this (Beran, 2002; Evans and Westergaard, 2006; Dufour et al., 2007), and even use behavioral distraction strategies to assist in refraining from reaching for foods (Evans and Beran, 2007a), both of which indicate that primates have the requisite abilities for the refusals seen in inequity responses. One intriguing possibility is that differences in species’ ability to delay gratification may be related to their tendency to refuse rewards in the context of inequity. Given than there is species variation in the ability to delay gratification, for instance with apes outperforming monkeys (Evans and Beran, 2007b), it will be interesting to see whether this prediction is supported. It may also be that increasing selection for negative reactions to inequity simultaneously selected for increased self-control ability. Ecological forces are known to shape self-control ability in other contexts (Stevens et al., 2005). On the flip side, the cognitive challenge of self-control may serve to limit which species are able to respond to inequity by refusing rewards.
Inequity and prosocial behavior
All of the studies discussed above focus on how individuals respond when they receive less than a partner. However, it is equally interesting to investigate responses when they receive more than a partner. In particular, whether species will bring rewards to their partners has been of interest to investigate the evolution of human social behavior. Presumably the mechanisms which allow individuals to compare their outcomes to those of others and recognize when they receive less would work equally well to identify situations in which they receive more. However, the selective pressures are quite different, thus the behaviors may not manifest equally.
Nonetheless, recent studies indicate that they co-occur and may even interact. Although studies show that chimpanzees are unlikely to bring food rewards to conspecifics (Silk et al., 2005; Jensen et al., 2006; Vonk et al., 2008), they do help other individuals (Warneken and Tomasello, 2006; Warneken et al., 2007). Moreover, in food situations they also notice when they receive more than a partner, refusing preferred rewards more often when their partners receive less preferred ones than when they also receive the preferred fruit (Brosnan et al., 2010a). This indicates that while individuals may not work to improve their partners’ wellbeing, they do notice the disparity.
Capuchins are quite prosocial, possibly moreso than chimpanzees, and bring rewards to conspecific partners in several situations (de Waal et al., 2008; Lakshminarayanan and Santos, 2008). A recent study specifically investigated how prosocial behavior interacted with inequity, and found that capuchins were prosocial (e.g., brought food rewards to partners) even in situations in which their partners received more, or even when the puller received nothing. However, when inequity became greater, prosocial behavior ceased (Brosnan et al., 2010b). Thus, emerging evidence indicates that these two preferences interact to shape behavior. Further research is needed that investigates these two behaviors in additional species, including those which do not respond to inequity, to determine the degree of overlap, and the limits of prosociality.
Thoughts on the Evolution Of Inequity
Why the response makes sense in light of natural selection
Why should we care if another individual gets more than we do? This is particularly true if our outcome is a net gain, or if the response actually increases inequity, a common result in these experiments (see above and Brosnan and de Waal, 2003; Brosnan et al., 2005; Yamagishi et al., 2009). In these cases, it seems particularly surprising that any rational individual would choose an outcome which makes them less well off in the short term. On the other hand, a negative reaction to inequity is not particularly surprising. First of all, despite a focus on cost–benefit analysis in behavioral studies, the entire concept of natural selection is based on relative gains, not absolute ones. Thus, there is every reason to expect natural selection to favor behaviors which increase relative gains, even at the expense of absolute outcomes, or which favor long-term benefits over short-term costs (e.g. Frank, 1988). Note, of course, that this does not mean that the individual must understand this comparison; the beauty of natural selection is that any behavior which increases relative fitness, however inadvertently, will be selected, regardless of the animals’ comprehension of either their behavior, their relative outcomes, or their benefits (see Hypotheses for the Function of the Response to Inequity).
Second, many of the mechanisms for the behavior seem to be in place. As discussed above (see Inequity Paradigms in Other Species), contrast effects occur in a variety of species, and it does not seem to be a great cognitive leap from comparing one's current outcomes to one's own previous outcomes, to comparing one's current outcomes to one's partners’ previous outcomes. Moreover, we already know that many species pay close attention to their partners’ behavioral outcomes. Any species that socially learns receives information from a conspecific which changes their behavior, even if they are not consciously aware of it (as they do not need to be consciously aware of inequity for it to provide a benefit). Social learning is widespread among animals (Zentall et al., 1988; Heyes et al., 1996), and some species are quite nuanced, with individuals paying attention to relevant features of the social partner when determining whether to copy their actions (Swaney et al., 2001; Biro et al., 2003; Perry, 2009; Hopper, 2010; Horner et al., 2010).
A potential evolutionary pathway
Of course, this is a correlative relationship; we do not know which came first, the response to inequity or the tendency to cooperate. Although it is challenging to test experimentally, some evidence provides at least a hint of which direction evolution may have taken. It seems, at this stage, more likely that cooperative behavior evolved first, followed by selection for inequity. Many species cooperate occasionally, and such interactions may lead to small rewards (or small losses) without the risk of a major cost. It is only when cooperation becomes common that some mechanism for avoiding excessive losses becomes essential. Thus, individuals who came under selection for more extensive cooperation because of the benefits such behavior accrued would similarly come under strong selection to limit their cooperative interactions to partners who shared the resulting rewards. Those who managed to do so, by changing partners when outcomes deviated substantially, would have gained far more than those who indiscriminately cooperated with all potential social partners with whom there was a net benefit.
It is worth considering a pathway through which the response to inequity might have evolved. Cooperation occurs when the benefits to both individuals exceed their costs (Bshary and Bergmuller, 2008; Brosnan et al., 2010c). From a purely cost–benefit perspective, it should not matter if A's benefit exceeds B's; it should still be to B's benefit to participate in the interaction. But what if B could reap a far greater benefit by cooperating with C? By recognizing (note, again, that this need not imply cognitive calculation or understanding) only whether the cost exceeds the benefit, B may miss this opportunity. Thus in cooperative interactions there should be pressure to maximize outcomes by evaluating whether there are other, more lucrative options in the environment. Responding to inequity would be a mechanism to do this; situations in which one's payoffs deviate substantially from one's social partners could be a reliable signal to evaluate other options. In this scenario inequity aversion would serve as the mechanism to evaluate when there is the possibility to increase one's benefit from cooperative interactions, and as such function to maximize the outcomes from cooperation.
Note that this will be selected only if the additional gains from a new partner exceed the cost of switching partners (e.g., search costs) and the risk of a worse outcome. The latter occurs either if outcomes with C – the new partner – are worse than those with B or if the initial difference in outcomes between A and B was minimal. Moreover, if the individual accrues indirect benefits from staying (i.e., the partner is related, or is assisting with offspring care), responses to inequity may not be selected. Thus, the theory predicts that responding to inequity may be more beneficial for some types of cooperation than others. For instance, this response may be more likely to be selected in situations with potentially high costs to doing relatively less well than one's partners, for instance in winner-take-all situations such as mating coalitions.
What is Needed Next?
Data from other species and relationships
While this paper is almost exclusively about non-human primates, it will be important to test this theory in other taxa (Drea and Frank, 2003; Bekoff and Pierce, 2010). There are several taxa, for instance canids, cetaceans, and birds, in which some species show a greater tendency to cooperate than others. There are also non-primate cooperative breeders, allowing all three aspects of this hypothesis to be tested more broadly. In particular we need data on not just species which routinely cooperate, but also on closely related species which do not do so in order to further evaluate the link between inequity and cooperation. Finally, it would be useful to test combinations of individuals which are not often addressed (e.g., cooperative breeders which are not part of the same mated pair) in order to address some of the additional implications of the theory, as outlined above.
Data from other contexts
We also need data from a broader variety of situations. For instance, primates respond to differences in outcomes, but do not seem to respond to differences in the effort required to achieve those outcomes (Fontenot et al., 2007; van Wolkenten et al., 2007). However, responses to inequity may occur in situations other than differential food distributions; for instance, several studies have found links between equity and play behavior in non-human species (Bekoff, 2001, 2004; Dugatkin and Bekoff, 2003; van Leeuwen et al., 2010). Despite the fact that food rewards are particularly relevant, making them an easy way to study behavior in experimental situations, some species may respond differently to food than non-food situations (Warneken et al., 2007). Thus it is important to collect data from a broader, and more species-representative, array of situations.
Related to this, measures of inequity other than the rate of reward refusal or the rate of refusal to participate in the task will help clarify the range of situations in which responses to inequity occur. These measures are useful, as they are easy to quantify and the methodology can be used across a wide variety of species. However, giving up a desirably food reward requires quite a cost on the part of the individual, potentially making this less likely to occur than other behaviors. Thus more nuanced behavioral (such as changes in affect) and physiological (such as skin conductance or heart rate) measures may help to uncover situations in which individuals, responses to inequity are not so explicit. This is particularly important in species for which there is no evidence of such explicit responses to inequity.
Finally, there are other situations in which inequity may occur. A recent study finds evidence that in their play behavior, gorillas are sensitive to their immediate social status, and work to maintain social inequities that increase their status with respect to another individual (van Leeuwen et al., 2010). These results point to a promising new avenue for investigating sensitivity to inequity, particularly in species which do not respond to distributional inequity. Such results may also help to distinguish between sensitivity to inequity and reactivity to inequity, the latter being the important criteria for the current hypothesis. These situations may also clarify the range of situations in which responses to inequity may be relevant.
The difference between noticing inequity and responding to inequity
One critical area of study is whether or not individuals notice inequity. Current studies measure whether individuals react to inequity, however it is possible to recognize it in situations in which individuals do not respond. For instance, do cooperative breeders fail to notice that their partner receives more, or have they been selected to not respond because of the high costs of finding a new partner? Are the individual differences in responses due to some individuals failing to notice inequity, or conditions in some situations (e.g., some relationships) not favoring the response? This has important implications for whether behavior will change as the degree of inequity increases. In fact we do know that despite little evidence of prosocial behavior in the form of active giving (Silk et al., 2005; Jensen et al., 2006; Vonk et al., 2008), chimpanzees do alter their behavior when they receive more than a partner (Brosnan et al., 2010a), indicating that they notice, despite not changing their behavior in any way that alters the outcomes. Future studies which can continue to tease apart this difference will be critical for fully understanding the evolution of the response.
Understanding the variation
Another pressing line of research is to better understand the variations in the response among individuals of the same species. Although a variety of factors are known to cause variation in the response (see Inequity Paradigms in Other Species), no factor has yet emerged which explains the variation consistently. Thus, additional research to clarify the role of individual factors, such as sex, age, reproductive status, and personality, as well as social factors, such as the relationships between individuals and group behavior, are required. This is a logistical challenge, as such comparisons require a large sample sizes of individuals who are tested in multiple combinations, which can be difficult to obtain, particularly among larger species such as primates. Nonetheless, research in this area is ongoing and the extra level of control possible in non-human research may yield additional insights to and predictions about human behavior (Brosnan et al., 2009).
Testing the evolutionary model
Finally, it is important to test the proposed evolutionary model of inequity responses. Of course, testing possible evolutionary pathways is by necessity a roundabout approach, but there are some interesting possibilities. First, the experimental economics approach is ideal for designing games which allow for direct comparisons across multiple species or multiple conditions. Among non-humans in particular, it is possible to test the same individuals repeatedly, engaging them in multiple different types of games (with different parameters and payoffs) and with multiple partners over extended periods of time. This has been done successfully using a coordination game (Brosnan et al., 2011) and this approach can be extended to games (e.g., trust, prisoner's dilemma) or payoff structures which address the inequity hypothesis.
Alternatively, many of the parameters of this model could be tested individually. For instance, we know that when given a choice, chimpanzees actively choose partners who share food over those who do not (Melis et al., 2006a,b). Paradigms such as this could be expanded to investigate how increased partner choice affects reactions to inequity. Additional experimental manipulations would also be useful. For instance, the model predicts that individuals would be more likely to choose a new partner as inequity rises, which could be tested by manipulating outcomes (e.g., altering payoffs) and documenting any effects on partner choice. Although each of these may test only a component of the model, taken together they provide validation (or not) for key features which are implicated.
Conclusion
Despite the fact that both humans and other species make decisions which indicate an aversion to inequitable outcomes which are disadvantageous, evidence regarding the function of the behavior has been limited. New evidence discussed in this review develops the hypothesis that recognizing inequity assists individuals in finding new partners with whom they will achieve a greater benefit, increasing the payoffs from cooperation over the long term. In joint situations, individuals cease cooperating with partners who consistently dominate better rewards, giving a selective advantage to those individuals who solicit other, potentially more equitable partners. Nonetheless, short-term inequity does not appear to disrupt cooperative relationships. Given that many interactions do not typically result in complete equality, this flexibility may clear the way for cooperation to persist despite modest inequality, while long-term inequity can be used as a cue that a particular cooperative relationship is no longer beneficial. Moreover, there is a correlation between the degree to which individuals of a species cooperate with non-kin and the presence of a behavioral response to inequitable outcomes. Although these data are correlational, rather than causal, they provide important evidence regarding the function of the inequity response in decision-making, as well as emphasizing the power of phylogenetic methods in illuminating evolutionary function. Despite the difficulties inherent in collecting such data, it is possible to design behavioral studies which can be run across multiple species. These phylogenetic comparisons can be extremely useful in investigating the evolution of decision-making and other behaviors.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank Ralph Bergmüller for stimulating discussion which led to the ideas regarding punishment and interactions between individuals at different life stages. I also thank Ralph Bergmüller as well as Lydia Hopper, Lucie Salwiczek, and Frans de Waal for very helpful comments on an earlier draft of this manuscript. Funding was provided to the author by a National Science Foundation Human and Social Dynamics Grant (SES 0729244) and an NSF CAREER Award (SES 0847351).
References
- Aghion P., Caroli E., Garcia-Penalosaand C. (1999). Inequality and economic growth: the perspective of the new growth theories. J. Econ. Lit. 37, 1615–1660 [Google Scholar]
- Bekoff M. (2001). Social play behavior: cooperation, fairness, trust, and the evolution of morality. J. Conscious. Stud. 8, 81–90 [Google Scholar]
- Bekoff M. (2004). “Wild justice, cooperation, and fair play: Minding manners, being nice, and feeling good,” in The Origins and Nature of Sociality, eds Sussman R., Chapman A. (Chicago: Aldine; ), 53–79 [Google Scholar]
- Bekoff M., Pierce J. (2010). Wild Justice: The Moral Lives of Animals. Chicago: University of Chicago Press [Google Scholar]
- Beran M. J. (2002). Maintenance of self-imposed delay of gratification by four chimpanzees (Pan troglodytes) and an orangutan (Pongo pygmaeus). J. Gen. Psychol. 129, 49–66 [DOI] [PubMed] [Google Scholar]
- Bergmüller R., Taborsky M. (2005). Experimental manipulation of helping in a cooperative breeder: helpers ‘pay-to-stay’ by pre-emptive appeasement. Anim. Behav. 69, 18–28 [Google Scholar]
- Biro D., Inoue-Nakamura N., Tonooka R., Yamakoshi G., Sousa C., Matsuzawa T. (2003). Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim. Cogn. 6, 213–223 [DOI] [PubMed] [Google Scholar]
- Blount S. (1995). When social outcomes aren't fair: the effect of causal attributions on preferences. Organ. Behav. Hum. Decis. Process 63, 131–144 [Google Scholar]
- Boinski S. (1987). Mating patterns in squirrel monkeys (Saimiri oerstedi): implications for sexual dimorphism. Behav. Ecol. Sociobiol. 21, 13–21 10.1007/BF00324430 [DOI] [Google Scholar]
- Bolton G. E., Zwick R. (1995). Anonymity versus punishment in ultimatum game bargaining. Games Econ. Behav. 10, 95–121 [Google Scholar]
- Bräuer J., Call J., Tomasello M. (2006). Are apes really inequity averse? Proc. R. Soc. Lond. B Biol. Sci. 273, 3123–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuer J., Call J., Tomasello M. (2009). Are apes inequity averse? New data on the token-exchange paradigm. Am. J. Primatol. 7, 175–181 10.1002/ajp.20639 [DOI] [PubMed] [Google Scholar]
- Brosnan S. F. (2006). Nonhuman species’ reactions to inequity and their implications for fairness. Soc. Justice Res. 19, 153–185 10.1007/s11211-006-0002-z [DOI] [Google Scholar]
- Brosnan S. F. (2010). “What do capuchin monkeys tell us about cooperation?,” in For the Greater Good of all: Perspectives on Individualism, Society, and Leadership (Vol. Jepson Studies in Leadership Series), eds Forsyth D. R., Hoyt C. L. (Hampshire: Palgrave Macmillan Publishers; ), 11–28 [Google Scholar]
- Brosnan S. F., de Waal F. B. M. (2002). A proximate perspective on reciprocal altruism. Hum. Nat. 13, 129–152 [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., de Waal F. B. M. (2003). Monkeys reject unequal pay. Nature 425, 297–299 10.1038/nature01963 [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., Freeman C., de Waal F. B. M. (2006). Partner's behavior, not reward distribution, determines success in an unequal cooperative task in capuchin monkeys. Am. J. Primatol. 68, 713–724 10.1002/ajp.20261 [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., Newton-Fisher N. E., van Vugt M. (2009). A melding of the minds: when primatology meets social psychology. Pers. Soc. Psychol. Rev. 13, 129–147 [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., Parrish A., Beran M. J., Flemming T. E., Heimbauer L., Talbot C. F., Lambeth S. P., Schapiro S. J., Wilson B. J. (2011). Responses to the assurance game in monkeys, apes, and humans using equivalent procedures. Proc. Natl. Acad. Sci. U.S.A. [Epub ahead of print]. 10.1073/pnas.1016269108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan S. F., Schiff H. C., de Waal F. B. M. (2005). Tolerance for inequity may increase with social closeness in chimpanzees. Proc. R. Soc. Lond. B Biol. Sci. 1560, 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan S. F., Talbot C., Ahlgren M., Lambeth S. P., Schapiro S. J. (2010a). Mechanisms underlying responses to inequitable outcomes in chimpanzees, Pan troglodytes. Anim. Behav. 79, 1229–1237 10.1016/j.anbehav.2010.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan S. F., Houser D., Leimgruber K., Xiao E., Chen T., de Waal F. B. M. (2010b). Competing demands of prosociality and equity in monkeys. Evol. Hum. Behav. 41, 279–288 10.1016/j.evolhumbehav.2010.02.003 [DOI] [Google Scholar]
- Brosnan S. F., Salwiczek L., Bshary R. (2010c). The interplay of cognition and cooperation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2699–2710 10.1098/rstb.2010.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R., Bergmuller R. (2008). Distinguishing four fundamental approaches to the evolution of helping. J. Evol. Biol. 21, 405–420 [DOI] [PubMed] [Google Scholar]
- Call J., Hare B., Carpenter M., Tomasello M. (2004). ‘Unwilling’ versus ‘unable’: chimpanzees understanding of human intentional action. Dev. Sci. 7, 488–498 [DOI] [PubMed] [Google Scholar]
- Camerer C. (2003). Behavioral Game Theory: Experiments in Strategic Interaction. Princeton, NJ: Russell Sage Foundation, Princeton University Press [Google Scholar]
- Carder B., Berkowitz K. (1970). Rats’ preference for earned in comparison with free food. Science 167, 1273–1274 10.1126/science.167.3922.1273 [DOI] [PubMed] [Google Scholar]
- Chalmeau R., Lardeux K., Brandibas P., Gallo A. (1997). Cooperative problem solving by orangutans (Pongo pygmaeus). Int. J. Primatol. 18, 23–32 10.1023/A:1026337006136 [DOI] [Google Scholar]
- Clark M. S., Grote N. K. (2003). “Close relationships,” in Handbook of Psychology: Personality and Social Psychology, Vol. 5, eds Millon T., Lerner M. J. (New York: John Wiley & Sons; ), 447–461 [Google Scholar]
- Clutton-Brock T. (2002). Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–75 10.1126/science.296.5565.69 [DOI] [PubMed] [Google Scholar]
- Creel S., Creel N. M. (1995). Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim. Behav. 50, 1325–1339 10.1016/0003-3472(95)80048-4 [DOI] [Google Scholar]
- Cronin K. A., Kurian A. V., Snowdon C. T. (2005). Cooperative problem solving in a cooperatively breeding primate. Anim. Behav. 69, 133–142 10.1016/j.anbehav.2004.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin K. A., Snowdon C. T. (2008). The effects of unequal reward distributions on cooperative problem solving by cotton-top tamarins (Saguinus oedipus). Anim. Behav. 75, 245–257 10.1016/j.anbehav.2007.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal F. B. M., Berger M. L. (2000). Payment for labour in monkeys. Nature 404, 563. [DOI] [PubMed] [Google Scholar]
- de Waal F. B. M., Davis J. M. (2002). Capuchin cognitive ecology: cooperation based on projected returns. Neuropsychologia 1492, 1–8 [DOI] [PubMed] [Google Scholar]
- de Waal F. B. M., Leimgruber K., Greenberg A. (2008). Giving is self-rewarding for monkeys. Proc. Natl. Acad. Sci. U.S.A. 105, 13685–13689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal F. B. M., Suchak M. (2010). Prosocial primates: selfish and unselfish motivations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2711–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo M., De Waal F. B. M. (2006). Partner effects on food consumption in brown capuchin monkeys. Am. J. Primatol. 69, 1–6 [DOI] [PubMed] [Google Scholar]
- Drea C. M., Frank L. G. (2003). “The social complexity of spotted hyenas,” in Animal Social Complexity: Intelligence, Culture, and Individualized Societies, eds de Waal F. B. M., Tyack P. (Cambridge, MA: Harvard University Press; ), 121–148 [Google Scholar]
- Dubreuil D., Gentile M. S., Visalberghi E. (2006). Are capuchin monkeys (Cebus apella) inequity averse? Proc. R. Soc. Lond. B Biol. Sci. 273, 1223–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour V., Pelé M., Neumann M., Thierry B., Call J. (2008). Calculated reciprocity after all: computation behind token transfers in orangutans. Biol. Lett. 5, 172–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour V., Sterck E. H. M., Pele M., Theirry B. (2007). Chimpanzee (Pan troglodytes) anticipation of food return: coping with waiting time in an exchange task. J. Comp. Psychol. 121, 145–155 [DOI] [PubMed] [Google Scholar]
- Dugatkin L. A., Bekoff M. (2003). Play and the evolution of fairness: a game theory model. Behav. Processes 60, 209–214 10.1016/S0376-6357(02)00120-1 [DOI] [PubMed] [Google Scholar]
- Edwards S. D., Snowdon C. (1980). Social behavior of captive, group-living orangutans. Int. J. Primatol. 1, 39–62 10.1007/BF02692257 [DOI] [Google Scholar]
- Evans T. A., Beran M. J. (2007a). Chimpanzees use self-distraction to cope with impulsivity. Biol. Lett. 3, 599–602 10.1098/rsbl.2007.0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. A., Beran M. J. (2007b). Delay maintenance by rhesus macaques (Macaca mulatta). J. Gen. Psychol. 134, 199–216 10.3200/GENP.134.2.199-216 [DOI] [PubMed] [Google Scholar]
- Evans T. A., Westergaard G. C. (2006). Self-control and tool use in tufted capuchin monkeys (Cebus apella). J. Comp. Psychol. 120, 163–166 [DOI] [PubMed] [Google Scholar]
- Fehr E., Schmidt K. M. (1999). A theory of fairness, competition, and cooperation. Q. J. Econ. 114, 817–868 [Google Scholar]
- Fletcher G. E. (2008). Attending to the outcome of others: disadvantageous inequity aversion in male capuchin monkeys (Cebus apella). Am. J. Primatol. 70, 901–905 10.1002/ajp.20576 [DOI] [PubMed] [Google Scholar]
- Fontenot M. B., Watson S. L., Roberts K. A., Miller R. W. (2007). Effects of food preferences on token exchange and behavioural responses to inequality in tufted capuchin monkeys, Cebus apella. Anim. Behav. 74, 487–496 10.1016/j.anbehav.2007.01.015 [DOI] [Google Scholar]
- Fragaszy D. M., Visalberghi E., Fedigan L. M. (2004). The Complete Capuchin: The Biology of the Genus Cebus. Cambridge: Cambridge University Press [Google Scholar]
- Frank R. H. (1988). Passions Within Reason: The Strategic Role of the Emotions. New York: W. W. Norton & Company [Google Scholar]
- Frank R. H. (2001). “Cooperation through emotional commitment,” in Evolution and the Capacity for Commitment, ed. Nesse R. M. (New York: Russell Sage Foundation; ), 57–76 [Google Scholar]
- Frank R. H. (2004). What Price the Moral High Ground? Ethical Dilemmas in Competitive Environments. Princeton, NJ: Princeton University Press [Google Scholar]
- Friedan E., Cuello M. I., Kacelnik A. (2009). Successive negative contrast in a bird: starlings’ behaviour after unpredictable negative changes in food quality. Anim. Behav. 77, 857–865 10.1016/j.anbehav.2008.12.010 [DOI] [Google Scholar]
- Fruth B., Hohmann G. (2002). “How bonobos handle hunts and harvests: food sharing,” in Behavioural Diversity in Chimpanzees and Bonobos, eds Boesch C., Hohmann G., Marchant L. F. (Cambridge: Cambridge University Press; ), 231–243 [Google Scholar]
- Guth W., Schmittberger R., Schwartze B. (1982). An experimental analysis of ultimatum bargaining. J. Econ. Behav. Organ. 3, 367–388 [Google Scholar]
- Hare B., Melis A. P., Woods V., Hastings S., Wrangham R. (2007). Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619–623 [DOI] [PubMed] [Google Scholar]
- Heidary F., Vaeze Mahdavi M. R., Momeni F., Minaii B., Rogani M., Fallah N., Heidary R., Gharebaghi R. (2008). Food inequality negatively impacts cardiac health in rabbits. PLoS ONE 3, e3705. 10.1371/journal.pone.0003705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes C. M., Galef B. C. (eds). (1996). Social Learning in Animals: The Roots of Culture. San Diego: Academic Press [Google Scholar]
- Hoffman E., McCabe K., Shachat K., Smith V. (1994). Preferences, property rights and anonymity in bargaining games. Games Econ. Behav. 7, 346–380 [Google Scholar]
- Hohmann G., Fruth B. (2000). Use and function of genital contacts among female bonobos. Anim. Behav. 60, 107–120 10.1006/anbe.2000.1451 [DOI] [PubMed] [Google Scholar]
- Hopper L. M. (2010). ‘Ghost’ experiments and the dissection of social learning in humans and animals. Biol. Rev. 85, 685–701 [DOI] [PubMed] [Google Scholar]
- Horner V., Proctor D., Bonnie K., Whiten A., de Waal F. B. M. (2010). Prestige affects cultural learning in chimpanzees. PLoS ONE 5, e10625. 10.1371/journal.pone.0010625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy S. B. (2009). Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Harvard University Press [Google Scholar]
- Jaeggi A. V., Burkart J. M., Van Schaik C. P. (2010). On the psychology of cooperation in humans and other primates: combining the natural history and experimental evidence of prosociality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2723–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. (2010). Punishment and spite, the dark side of cooperation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2635–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K., Call J., Tomasello M. (2007). Chimpanzees are vengeful but not spiteful. Proc. Natl. Acad. Sci. U.S.A. 104, 13046–13050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K., Hare B., Call J., Tomasello M. (2006). What's in it for me? Self-regard precludes altruism and spite in chimpanzees. Proc. R. Soc. Lond. B Biol. Sci. 273, 1013–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavanich C., Atema J. (1998). Individual recognition and memory in lobster dominance. Anim. Behav. 56, 1553–1560 10.1006/anbe.1998.0914 [DOI] [PubMed] [Google Scholar]
- Knoch D., Pascual-Leone A., Meyer K., Treyer V., Fehr E. (2006). Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science 314, 829–832 10.1126/science.1129156 [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan V., Santos L. R. (2008). Capuchin monkeys are sensitive to others’ welfare. Curr. Biol. 18, R999–R1000 [DOI] [PubMed] [Google Scholar]
- Langergraber K. E., Mitani J. C., Vigilant L. (2007). The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl. Acad. Sci. U.S.A. 104, 7786–7790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. P., Hare B., Tomasello M. (2006a). Engineering cooperation in chimpanzees: tolerance constraints on cooperation. Anim. Behav. 72, 275–286 10.1016/j.anbehav.2005.09.018 [DOI] [Google Scholar]
- Melis A. P., Hare B., Tomasello M. (2006b). Chimpanzees recruit the best collaborators. Science 311, 1297–1300 10.1126/science.1123007 [DOI] [PubMed] [Google Scholar]
- Mitani J. C. (2006). “Reciprocal exchange in chimpanzees and other primates,” in Cooperation in Primates and Humans: Evolution and Mechanisms, eds Kapeller P., van Schaik C. P. (Berlin: Springer; ), 101–11316402199 [Google Scholar]
- Neiworth J. J., Johnson E. T., Whillock K., Greenberg J., Brown V. (2009). Is a sense of inequity an ancestral primate trait? Testing social inequity in cotton top tamarins (Saguinus oedipus). J. Comp. Psychol. 123, 10–17 [DOI] [PubMed] [Google Scholar]
- Parrish A. R. (1996). Female relationships in bonobos (Pan paniscus): evidence for bonding, cooperation, and female dominance in a male-philopatric species. Hum. Nat. 7, 61–96 [DOI] [PubMed] [Google Scholar]
- Perry S. (2009). Conformism in the food processing techniques of white-faced capuchin monkeys (Cebus capucinus). Anim. Cogn. 12, 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raihani N. J., Grutter A. S., Bshary R. (2010). Punishers benefit from third-party punishment in fish. Science 327, 171. [DOI] [PubMed] [Google Scholar]
- Range F., Horn L., Viranyi Z., Huber L. (2008). The absence of reward induces inequity aversion in dogs. Proc. Natl. Acad. Sci. U.S.A. 106, 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J. K., Insel T. (1999). The primate noecortex in comparative perspective using magnetic resonance imaging. J. Hum. Evol. 16, 191–233 [DOI] [PubMed] [Google Scholar]
- Roberts G. (2005). Cooperation through interdependence. Anim. Behav. 70, 901–908 10.1016/j.anbehav.2005.02.006 [DOI] [Google Scholar]
- Roma P. G., Silberberg A., Ruggiero A. M., Suomi S. J. (2006). Capuchin monkeys, inequity aversion, and the frustration effect. J. Comp. Psychol. 120, 67–73 [DOI] [PubMed] [Google Scholar]
- Russon A. E. (1998). The nature and evolution of intelligence in orangutans (Pongo pygmaeus). Primates 39, 485–503 10.1007/BF02557571 [DOI] [Google Scholar]
- Schino G., Aureli F. (2010). Primate reciprocity and its cognitive requirements. Evol. Anthropol. 19, 130–135 [Google Scholar]
- Shumaker R. W., Palkovich A. M., Beck B. B., Guagnano G. A., Morowitz H. (2001). Spontaneous use of magnitude discrimination and ordination by the orangutan (Pongo pygmaeus). J. Comp. Psychol. 115, 385–391 [PubMed] [Google Scholar]
- Silberberg A., Crescimbene L., Addessi E., Anderson J. R., Visalberghi E. (2009). Does inequity aversion depend on a frustration effect? A test with capuchin monkeys (Cebus apella). Anim. Cogn. 12, 505–509 [DOI] [PubMed] [Google Scholar]
- Silk J. B., Brosnan S. F., Vonk J., Henrich J., Povinelli D. J., Richardson A. S., Lambeth S., Mascaro J., Schapiro S. (2005). Chimpanzees are indifferent to the welfare of unrelated group members. Nature 437, 1357–1359 10.1038/nature04243 [DOI] [PubMed] [Google Scholar]
- Singleton I., van Schaik C. P. (2002). The social organisation of a population of Sumatran orangutans. Folia Primatol. 73, 1–20 10.1159/000060415 [DOI] [PubMed] [Google Scholar]
- Steiger S., Ragna F., Eggert A.-K., Müller J. K. (2008). The Coolidge effect, individual recognition and selection for distinctive cuticular signatures in a burying beetle. Proc. R. Soc. Lond. B Biol. Sci. 275, 1831–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. R., Hallinan E. V., Hauser M. D. (2005). The ecology and evolution of patience in two new world monkeys. Biol. Lett. 1, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney W., Kendal J., Capon H., Brown C., Laland K. N. (2001). Familiarity facilitates social learning of foraging behaviour in the guppy. Anim. Behav. 62, 591–598 10.1006/anbe.2001.1788 [DOI] [Google Scholar]
- Talbot C., Williams L. E., Brosnan S. F. (in press). Squirrel monkeys’ response to inequitable outcomes indicates evolutionary convergence within the primates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbets E. A. (2002). Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. Lond. B Biol. Sci. 269, 1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinklepaugh O. L. (1928). An experimental study of representative factors in monkeys. J. Comp. Psychol. 8, 197–236 [Google Scholar]
- van Leeuwen E., Zimmermann E., Davila Ross M. (2010). Responding to inequities: gorillas try to maintain their competitive advantage during play fights. Biol. Lett. [Epub ahead of print]. 10.1098/rsbl.2010.0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaik C. P., Burkart J. (2010). “Mind the gap: cooperative breeding and the evolution of our unique features,” in Mind the Gap: Tracing the Origins of Human Universals, eds Kappeler P. M., Silk J. B. (Heidelberg: Springer; ), 477–497 [Google Scholar]
- van Schaik C. P., Marshall A. J., Wich S. A. (2009). “Geographic variation in orangutan behavior and biology,” in Orangutans: Geographic Variation in Behavioral Ecology and Conservation, eds Wich S. A., Utami Atmoko S. S., Mitra Setia T., van Schaik C. P. (New York: Oxford University Press; ), 351–361 [Google Scholar]
- van Schaik C. P., van Hooff J. A. R. A. M. (1996). “Towards an understanding of the orangutan's social system,” in Great Ape Societies, eds McGrew W. C., Marchant L. F., Nishida T. (Cambridge: Cambridge University Press; ), 3–15 [Google Scholar]
- van Wolkenten M., Brosnan S. F., de Waal F. B. M. (2007). Inequity responses in monkeys modified by effort. Proc. Natl. Acad. Sci. U.S.A. 104, 18854–18859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk J., Brosnan S. F., Silk J. B., Henrich J., Richardson A. S., Lambeth S., Schapiro S., Povinelli D. J. (2008). Chimpanzees do not take advantage of very low cost opportunities to deliver food to unrelated group members. Anim. Behav. 75, 1757–1770 10.1016/j.anbehav.2007.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warneken F., Hare B., Melis A. P., Hanus D., Tomasello M. (2007). Spontaneous altruism by chimpanzees and young children. PLoS Biol. 5, e184. 10.1371/journal.pbio.0050184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warneken F., Tomasello M. (2006). Altruistic helping in human infants and young chimpanzees. Science 311, 1301–1303 10.1126/science.1121448 [DOI] [PubMed] [Google Scholar]
- Xiao E., Houser D. (2005). Emotional expression in human punishment behavior. Proc. Natl. Acad. Sci. U.S.A. 102, 7398–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T., Horita Y., Takagishi H., Shinada M., Tanida S., Cook K. S. (2009). The private rejection of unfair offers and emotional commitment. Proc. Natl. Acad. Sci. U.S.A. 106, 11520–11523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall T. R., Galef B. C. (eds). (1988). Social Learning: Psychological and Biological Perspectives. Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]