Abstract
Background
Catheter-related bloodstream infections (BSI) account for the majority of hemodialysis-related infections. There are no published data on the efficacy of the chlorhexidine-impregnated foam dressing at reducing catheter-related BSI in hemodialysis patients.
Design
Prospective non-blinded cross-over intervention trial to determine the efficacy of a chlorhexidine-impregnated foam dressing (Biopatch®) to reduce catheter-related BSI in hemodialysis patients.
Setting
Two outpatient dialysis centers
Patients
A total of 121 patients who were dialyzed through tunneled central venous catheters received the intervention during the trial.
Methods
The primary outcome of interest was the incidence of catheter-related bloodstream infections. A nested cohort study of all patients who received the Biopatch® Antimicrobial Dressing was also conducted. Backward stepwise logistic regression analysis was used to determine independent risk factors for development of BSI.
Results
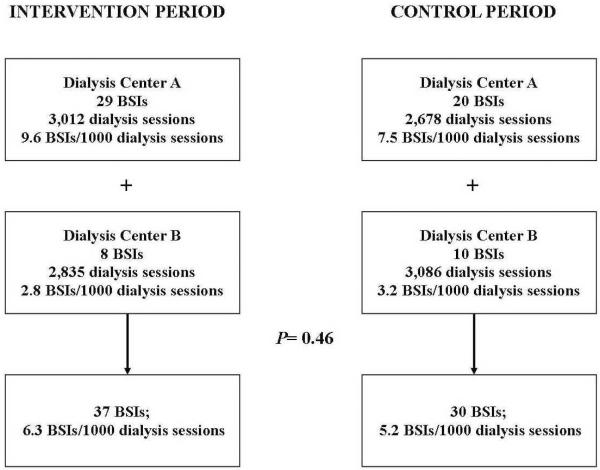
37 bloodstream infections occurred in the intervention group for a rate of 6.3 BSIs/1000 dialysis sessions and 30 bloodstream infections in the control group for a rate of 5.2 BSIs/1000 dialysis sessions and [RR 1.22, CI (0.76, 1.97); P=0.46]. The Biopatch® Antimicrobial Dressing was well-tolerated with only two patients (<2%) experiencing dermatitis that led to its discontinuation. The only independent risk factor for development of BSI was dialysis treatment at one dialysis center [aOR 4.4 (1.77, 13.65); P=0.002]. Age ≥ 60 years [aOR 0.28 (0.09, 0.82); P=0.02] was associated with lower risk for BSI.
Conclusion
The use of a chlorhexidine-impregnated foam dressing (Biopatch®) did not decrease catheter-related BSIs among hemodialysis patients with tunneled central venous catheters.
Keywords: chlorhexidine-impregnated dressing, hemodialysis, bloodstream infection, tunneled catheter
Introduction
Infections account for 16–36% of all deaths among adults with end-stage renal disease [1, 2]. The risk of bloodstream infections among hemodialysis patients is significantly higher among patients undergoing hemodialysis through a central venous catheter compared to arteriovenous (AV) fistulae or grafts [3–5]. Maki et al reported that a silver impregnated cuff placed on the catheter hub decreased non-tunneled catheter-related bacteremia almost fourfold[6]. Routine application of povidone-iodine ointment to temporary hemodialysis catheters was also shown to be effective in reducing catheter-related infections [7].
The BIOPATCH® Antimicrobial Dressing (Johnson & Johnson Wound Management, a division of Ethicon, Inc.; Somerville NJ) is a novel catheter dressing impregnated with chlorhexidine gluconate. It is used in conjunction with the standard catheter dressing to prevent catheter-related bloodstream infections. The chlorhexidine-impregnated dressing was shown to be effective in reducing microbial colonization of epidural catheters [8]. A randomized clinical trial done on neonates with central venous catheters showed that the BIOPATCH® Antimicrobial Dressing, compared to povidone-iodine rub, significantly decreased the rate of colonization of the central venous catheter but not the incidence of bloodstream infections [9]. Among neutropenic patients, a randomized controlled trial showed that use of a chlorhexidine-impregnated dressing (BIOPATCH® Antimicrobial Dressing) with tunneled central venous catheters resulted in fewer documented exit site infections but there was no difference in the catheter removal rate between the intervention and control groups [10]. Timsit et al recently published a randomized controlled trial showing the efficacy of the BIOPATCH® Antimicrobial Dressing at reducing catheter-related bloodstream infections among critically ill patients with non-tunneled central venous catheters [11].
There are currently no published studies on the efficacy of the BIOPATCH® Antimicrobial Dressing in hemodialysis patients. We recently conducted a crossover intervention trial studying the efficacy of the BIOPATCH® Antimicrobial Dressing to reduce catheter-related bloodstream infections among hemodialysis patients in an outpatient setting.
Methods and Study Population
We conducted a prospective crossover intervention trial at two outpatient hemodialysis centers affiliated with Washington University School of Medicine and Barnes-Jewish Hospital in Saint Louis, Missouri between April 1, 2005 and March 31, 2006. Informed consent was obtained from patients before using the BIOPATCH® Antimicrobial Dressing. Any patient who received hemodialysis through a tunneled central venous catheter received the intervention if the dialysis center where he/she was dialyzed were in the intervention arm of the study. The intervention was continued in every patient who was dialyzed through a central venous catheter until the intervention period was over, the patient transferred his/her care to a different facility, a central venous catheter was no longer necessary (i.e., an AV graft or fistula is in place), or if the patient was intolerant of the intervention. The only exclusion criterion was a reported allergy to chlorhexidine gluconate. This study was approved by the Washington University Human Research Protection Office.
Sample Size Estimate
In the six-month period prior to the study, both dialysis centers had 7.05 bloodstream infections/1000 dialysis sessions or 40 distinct bloodstream infections after 5766 dialysis sessions. Based on the study by Maki et al, using the BIOPATCH® Antimicrobial Dressing decreased the bloodstream infection rate by 62% (unpublished data). This suggested that if this intervention worked on dialysis catheters, the number of bloodstream infections in the intervention group should decrease to 16 distinct bloodstream infections in 5766 dialysis sessions (2.28 bloodstream infections/1000 dialysis sessions). Based on the number of dialysis sessions that occurred in the baseline period at both dialysis centers, we would have accumulated an adequate number of dialysis sessions within a year. This difference would have been statistically significant with a P-value of < 0.001.
Protocols for routine catheter care prior to the study included the use of a sodium hypochlorite solution (0.114% by volume) for skin/catheter antisepsis prior to each dialysis session. The catheter exit site was dressed with a transparent dressing every seven days unless there was visible blood, soiling, or if the dressing came off. The same dressing change schedule was continued when the intervention began. A new dialysis catheter care protocol incorporating the use of the BIOPATCH® Antimicrobial Dressing was instituted and standardized at both hemodialysis centers at the start of the intervention. The only difference between the catheter care protocols of the hemodialysis centers during the study was the use of the BIOPATCH® Antimicrobial Dressing. Both dialysis centers had the same nurse to patient ratio, shared the same infection prevention specialist, and although each had its own medical director, both were in the renal division of the affiliated medical school. The intervention was initiated in dialysis center A during the first six month period while dialysis center B patients served as the control group. After six months, the BIOPATCH® Antimicrobial Dressing use was discontinued at dialysis center A and its use was instituted in dialysis center B. A nested cohort study including only patients in whom the BIOPATCH® Antimicrobial Dressing (intervention group) was applied was also performed to determine risk factors for development of bloodstream infections in this select population. Catheter-related bloodstream infections were monitored by the infection control practitioner and the dialysis center staff. All blood cultures of all hemodialysis patients at the two dialysis centers were reviewed, as well as all their hospital admissions to identify catheter-associated bloodstream infections. Demographic information and all clinical data were collected from Cyberren® Database (Cybernius Medical, Ltd., Alberta, Canada), a clinical data management system for nephrology utilized by both dialysis centers.
Definitions
Definitions for infections were based on the Centers for Disease Control and Prevention (CDC) National Nosocomial Infections Surveillance System (NNIS) guidelines [12] with some modifications. A catheter related bloodstream infection was defined as having a positive blood culture at the time the catheter was in place or within 48 hours of catheter removal along with clinical signs and symptoms of sepsis [fever (temperature >38.0°C) or hypotension (systolic blood pressure < 90mmHg)] and no other documented primary site of infection. An infection was considered new only if the patient had not received any treatment for a catheter-related infection in the 21 days prior to the current infection. Assessment of the primary outcome was performed by the infection prevention specialist (A.M.R.) while adverse events were assessed by the principal investigator (B.C.C.). Both investigators were not blinded to the period in which the outcome occurred.
Outcomes
The primary outcome for this study was the incidence of bloodstream infections in the two groups measured in the number of bloodstream infections/1000 dialysis sessions. A secondary outcome studied was the tolerability of prolonged used of the BIOPATCH® Antimicrobial Dressing. Infection rates between the control and intervention groups were compared using chi-square analysis. For the nested cohort study, a patient who developed a bloodstream infection was only counted as a case once even if they had multiple episodes of bloodstream infection for the purposes of determining risk factors. Thirty-one patients accounted for 67 distinct episodes of bloodstream infection over the study period. Bivariate analysis of categorical variables in the cohort study was performed using Mantel Hansel chi-square or Fisher's Exact Test. Continuous variables were compared using the student's t test or the Mann Whitney U test. A two-sided P-value of ≤0.05 was considered significant. Multivariate analysis using backward stepwise logistic regression was performed to determine independent risk factors for development of a bloodstream infection. The final model was determined using the Hosmer-Lemeshow goodness of fit test. Interactions between variables were also tested but no significant interactions were demonstrated. Variables that were found to have a P value of ≤0.1 on bivariate analysis were included in the multivariate analysis logistic regression model. Data analysis was performed using SPSS version 14 (SPSS, Chicago, Il).
Results
One hundred twenty-one patients with tunneled central venous catheters were treated with the chlorhexidine-impregnated dressing at both dialysis centers over the one-year period. Two patients withdrew consent after just two dialysis sessions so use of the BIOPATCH® Antimicrobial Dressing was discontinued on these patients. Baseline patient characteristics are listed in Table 1. The intent to treat analysis included 5847 dialysis sessions in the intervention period and 5764 dialysis sessions in the control period. There were 37 bloodstream infections during the intervention period for a rate of 6.3 BSIs/1000 dialysis sessions and 30 bloodstream infections during the control period for a rate of 5.2 BSIs/1000 dialysis sessions [Relative Risk (RR) 1.22 95% Confidence Interval (CI) (0.75,1.97); P=0.46; see Table 2]. In two patients (<2%) the use of the BIOPATCH© Antimicrobial Dressing was discontinued because of adverse events. Both patients were thought to have developed dermatitis but one patient concomittantly received antimicrobial therapy for an exit site infection since it was difficult to ascertain if the erythema was from contact dermatitis or infection.
Table 1.
Characteristics of patients who received the Biopatch™ intervention
| Total (N=121) | Dialysis Center A (n=55) | Dialysis Center B (n=66) | P-value | |
|---|---|---|---|---|
| n(%) | n(%) | n(%) | ||
| Gender | ||||
| Male | 52 (43%) | 25 (45%) | 27 (41%) | 0.75 |
| Female | 69 (57%) | 31 (55%) | 39 (59%) | |
| Median Age (range in years) | 56 (19–88) | 57 (26–87) | 56 (19–88) | 0.93 |
| Race | ||||
| African-American | 97 (80%) | 42 (76%) | 55 (83%) | 0.46 |
| Caucasian | 23 (19%) | 13 (24%) | 10 (15%) | |
| Other | 1 (1%) | 1 (2%) | ||
| Median BMI (range) | 27.1 (14.7–71.6) | 25.9 (16.3–53.6) | 27.8 (14.8–71.6) | 0.31 |
Table 2.
Bivariate Analysis Comparing Patients with and without Bloodstream Infection
| Variable | BSI Cases (n=31) | Control (n=90) | P-value |
|---|---|---|---|
| Male | 17 (55%) | 35 (39%) | |
| Female | 14 (45%) | 55 (61%) | 0.12 |
| African-American | 24 (77%) | 73 (81%) | 0.72 |
| Caucasian | 7 (23%) | 16(18%) | |
| Age >60 | 7 (23%) | 41 (47%) | 0.02 |
| Median Age | 53 years | 57 years | 0.01 |
| Dialysis Center | |||
| A | 22 (71%) | 33 (37%) | 0.001 |
| B | 9 (9%) | 57 (63%) | |
| Hypertension | 31 (100%) | 83 (97%) | 0.29 |
| CHF | 2 (7%) | 20 (23%) | 0.04 * |
| Diabetes | 18 (58%) | 41 (48%) | 0.32 |
| Obesity | 11 (36%) | 17 (20%) | 0.08 |
| Peripheral Vascular Disease | 9 (29%) | 19 (22%) | 0.44 |
| Immunosuppressants | 1 (3%) | 5 (6%) | 0.39 |
| COPD/Asthma | 5 (16%) | 9 (11%) | 0.41 |
| Hepatitis B or C | 3 (10%) | 5 (6%) | 0.47 |
| CVA History | 7 (23%) | 15 (17%) | 0.53 |
| HIV | 1 (3%) | 1 (1%) | 0.45 |
| Tobacco Use | 3 (10%) | 17 (20%) | 0.20 |
| Substance Abuse | 5 (16%) | 1 (1%) | 0.005 * |
| Hx of Surgery in Past 30 days | 3 (10%) | 14 (16%) | 0.37 |
| Admission in Past 30 days | 5 (16%) | 29 (34%) | 0.06 |
| Previous BSI in Past 90 days | 4 (13%) | 9(11%) | 0.71 |
| Previous Abx in the Past 30 days | 3 (10%) | 18(21%) | 0.19 |
| Dressing Changes | |||
| Once a week | 11(33%) | 53 (62%) | 0.01 |
| > Once a week | 20 (67%) | 33 (38%) |
Fisher's Exact Test
Variables studied for development of catheter-related bloodstream infection on bivariate analysis are shown in Table 2. Receipt of hemodialysis at dialysis center A, a history of substance abuse, and frequent dressing changes (>once/week) were significant risk factors for development of bloodstream infection on bivariate analysis. A history of congestive heart failure and age>60 years were associated with decreased risk of developing a bloodstream infection. Two variables almost met statistical significance on bivariate analysis with p-values <0.1—obesity (increased risk) and admission to the hospital in the previous 30 days (decreased risk). Demographic variables such as age, gender, and race along with obesity, hospital admission in the past 30 days, frequency of dressing changes, and dialysis center were included in the multivariate analysis model. The only independent predictor for development of catheter-related bloodstream infection was dialysis treatment at dialysis center A. Age >60 years was associated with decreased risk of developing a bloodstream infection (see Table 2).
Discussion
This is the first intervention trial using a chlorhexidine-impregnated foam dressing to reduce the risk of catheter-related bloodstream infections in outpatient hemodialysis patients. Other investigators have studied other methods of reducing bloodstream infection among hemodialysis patients. Lok et al randomized 169 patients receiving hemodialysis through a central venous catheter to either receiving polysporin triple antibiotic ointment or placebo over a six-month period. Less infections were observed in the treatment group (12% versus 34%; P = 0.0013). The number of bacteremias per1000 catheter days was also lower in the treatment group. (0.63 versus 2.48; P = 0.0004) [13]. Johnson and colleagues also enrolled 50 patients in an open-label randomized trial comparing the application of mupirocin (n=27) thrice weekly around tunneled cuffed hemodilaysis catheter exit sites versus standard of care. Mupirocin-treated patients experienced significantly fewer catheter-related bacteremias (7% vs. 35%, P<0.01). The mupirocin intervention also resulted in a delay in the occurrence of bacteremia (108 days vs. 55 days; P<0.01) [14]. The same group of investigators conducted a similar study but this time they randomly assigned patients to either thrice weekly application of honey versus mupirocin at the catheter exit site to reduce the risk of bloodstream infections. A total of 101 patients were enrolled in this open label trial (51 honey-treated; 50 mupirocin-treated). The two interventions produced similar rates of bloodstream infections. Although the authors did not report an increase in mupirocin resistance, they did conclude that the use of honey is associated with a lower risk for development of resistance [15].
Aside from the application of honey around the exit-site to prevent catheter-related bloodstream infections, all the above interventions have the potential for the development of antimicrobial resistance that may render the intervention ineffective. The use of a chlorhexidine-impregnated foam dressing to prevent catheter-related bloodstream infections would have a decreased potential for the development of resistance, but in our study this intervention did not decrease the incidence of catheter-related bloodstream infections among hemodialysis patients with tunneled central venous catheters. We speculate that the catheter exit site may have a reduced role in the pathogenesis of bloodstream infections in tunneled central venous catheters. The catheter hub may play a larger role in the pathogenesis of catheter-related bloodstream infections in this patient population. The risk of bloodstream infection due to catheter hub bacterial colonization would not be affected by application of the chlorhexidine-impregnated foam dressing.
A unique aspect of this study is the cohort study that provides new information on potentially modifiable risk factors for the development of bloodstream infection among patients undergoing hemodialysis through central venous catheters. Previous studies have shown that the optimal vehicle for hemodialysis is an AV fistula or graft since it poses less risk for bloodstream infections [16, 17]. Central venous catheters in this cohort were used only as a temporizing vascular access until an AV fistula or graft was available or as last resorts. Most of the studies on risk factors for bacteremia among hemodialysis patients were based on large data sets containing only ICD-9 codes. Risk factors for bloodstream infections reported in the literature include older age, female gender, and African-American race [18, 19]. Potentially modifiable risk factors include hemodialysis versus peritoneal dialysis, temporary catheter versus permanent catheter use, low serum albumin levels, and dialyzer reuse. Although diabetes mellitus can be controlled through insulin therapy and diet, it is unknown if strict control of blood glucose levels leads to a decreased risk of bloodstream infection among hemodialysis patients. Data on the contribution of diabetes to the development of bloodstream infections have been conflicting [17–19]. In this study, we discovered that one dialysis center had a higher risk for bloodstream infections. The potential differences between the two centers include more frequent dressing changes and more patients with a history of substance abuse received hemodialysis at dialysis center A [54% vs. 39% for more frequent dressing changes (P=0.1); 9% vs. 2 % for substance abuse; P= 0.1]. More frequent dressing changes were found to be associated with bloodstream infections on bivariate analysis but eliminated from the logistic regression model because treatment at dialysis center A was a stronger predictor for bloodstream infections. Frequent dressing changes may lead to higher risk for contamination. On the other hand, they may also be a surrogate marker for frequent bleeding, more perspiration, or poor personal hygiene. A history of substance abuse was also an independent risk factor for bloodstream infection. This requires further investigation to determine if this association is due to relative immunosuppression related to substance abuse, catheter manipulation, personal hygiene differences, Staphylococci colonization differences, and other factors.
Age> 60 years was also associated with decreased risk of bloodstream infection which is inconsistent with previous studies [18, 19]. The true reason for this finding is unknown however, obesity and frequent dressing changes in this cohort (both had P-value <0.1 as risk factors on bivariate analysis) were associated with younger patients (mean age 51.2 years vs. 67.4 years; P=0.01). The underlying reason that older age (>60 years) was associated with decreased risk of bloodstream infections may have been that these patients were less likely to be obese and were more likely to adhere to the weekly dressing changes. The small sample size may have contributed to these individual risk factors (obesity and frequent dressing changes) not being independent risk factors for development of bloodstream infection in the logistic regression model.
This study has a few limitations. This study was not a randomized controlled trial. Randomization would have allowed for stratification on the type of catheter and dialysis center to remove potential biases. However this would have required a much bigger study population and inclusion of multiple dialysis centers which would have been very costly. Despite careful standardization of the catheter care protocol and other processes of care, there were still differences in the dialysis center populations that may have affected the results of the study. These differences can be effectively accounted for by a randomized controlled trial. The power analysis was based on previous efficacy data which showed a 62% decrease in bloodstream infection rates among patients with non-tunneled central venous catheters. Our study may not have had sufficient power to show a small difference in bloodstream infection rates between the intervention and control groups. Finally, there was no “washout” period before the crossover took place. It is unlikely that this would have had an impact on the results of the study since the first BSI after crossover occurred more than three weeks after the date of the crossover.
Although not essential to evaluate the primary outcomes of this study, microbiological cultures of the catheter hubs or catheter exit sites were not done and so we were not able to compare these with the bacterial isolates obtained from blood cultures collected from the patients in the trial. Information on organisms colonizing the skin and the catheter hubs could have helped elucidate the mechanisms behind the development of catheter-related bloodstream infections in these hemodialysis patients. Nevertheless, this is the second largest intervention trial on patients dialyzed through central venous catheters to date. The crossover intervention trial design also has increased validity over a before and after intervention trial.
Conclusions
In a cross-over intervention trial, the BIOPATCH® Antimicrobial Dressing did not significantly decrease bloodstream infections among hemodialysis patients with tunneled central venous catheters. Application of antibiotics such as mupirocin or polysporin have been shown in previous studies to be effective interventions and so these interventions should be considered first to reduce the incidence of catheter-related bloodstream infections in hemodialysis patients. The development of resistance to these antibiotics may still limit their use over long periods of time. Older age (> 60 years) was associated with decreased risk for bloodstream infections in our patient population.
Figure 1.
Table 3.
Independent Risk Factors for Development of Bloodstream Infection
| Variable | Adjusted Odds Ratio (95% Confidence Interval) | P-value |
|---|---|---|
| Dialysis Center A | 4.9 (1.77, 13.7) | 0.002 |
| Obesity | 2.4 (0.89, 6.63) | 0.08 |
| Age ≥ 60 years | 0.28 (0.09, 0.82) | 0.02 |
Acknowledgments
This study was partially supported through a research grant from Johnson & Johnson Wound Management, a division of Ethicon, Inc. Salary support was also provided by the Barnes-Jewish Hospital Foundation, the National Institutes of Health (UL1RR024992; K12 HD052194; 1K24AI06779401), and the Centers for Disease Control and Prevention (1U01CI000333-01).
Footnotes
Potential conflict of interests: BCC was on the Speaker's Bureau for Ethicon, Inc. All other authors have no conflicts of interest relevant to this article.
This study was also presented at the 17th Annual Meeting of The Society for Healthcare Epidemiology of America; Baltimore, MD, April 14–17, 2007 (Abstract 299).
References
- 1.Causes of death USRDS. United States Renal Data System. AmJKidney Dis. 1997;30(2 Suppl 1):S107–S17. [PubMed] [Google Scholar]
- 2.Mailloux LU, Bellucci AG, Wilkes BM, et al. Mortality in dialysis patients: analysis of the causes of death. AmJKidney Dis. 1991;18(3):326–35. doi: 10.1016/s0272-6386(12)80091-6. [DOI] [PubMed] [Google Scholar]
- 3.Taylor G, Gravel D, Johnston L, Embil J, Holton D, Paton S. Prospective surveillance for primary bloodstream infections occurring in Canadian hemodialysis units. InfectControl HospEpidemiol. 2002;23(12):716–20. doi: 10.1086/501999. [DOI] [PubMed] [Google Scholar]
- 4.Saeed Abdulrahman I, Al-Mueilo SH, Bokhary HA, Ladipo GOA, Al-Rubaish A. A prospective study of hemodialysis access-related bacterial infections. J Infect Chemother. 2002 Sep;8(3):242–6. doi: 10.1007/s10156-002-0184-8. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson KB, Adcox MJ, Mallea MC, Narasimhan N, Wagnild JP. Standardized surveillance of hemodialysis vascular access infections: 18-month experience at an outpatient, multifacility hemodialysis center. InfectControl HospEpidemiol. 2000;21(3):200–3. doi: 10.1086/501744. [DOI] [PubMed] [Google Scholar]
- 6.Maki DG, Cobb L, Garman JK, Shapiro JM, Ringer M, Helgerson RB. An attachable silver-impregnated cuff for prevention of infection with central venous catheters: a prospective randomized multicenter trial. AmJMed. 1988;85(3):307–14. doi: 10.1016/0002-9343(88)90579-7. [DOI] [PubMed] [Google Scholar]
- 7.Levin A, Mason AJ, Jindal KK, Fong IW, Goldstein MB. Prevention of hemodialysis subclavian vein catheter infections by topical povidone-iodine. Kidney Int. 1991;40(5):934–8. doi: 10.1038/ki.1991.297. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro JM, Bond EL, Garman JK. Use of a chlorhexidine dressing to reduce microbial colonization of epidural catheters. Anesthesiology. 1990;73(4):625–31. doi: 10.1097/00000542-199010000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Garland JS, Alex CP, Mueller CD, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics. 2001;107(6):1431–6. doi: 10.1542/peds.107.6.1431. [DOI] [PubMed] [Google Scholar]
- 10.Chambers ST, Sanders J, Patton WN, et al. Reduction of exit-site infections of tunnelled intravascular catheters among neutropenic patients by sustained-release chlorhexidine dressings: results from a prospective randomized controlled trial. JHospInfect. 2005;61(1):53–61. doi: 10.1016/j.jhin.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009 Mar 25;301(12):1231–41. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 12.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. AmJInfectControl. 1988;16(3):128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 13.Lok CE, Stanley KE, Hux JE, Richardson R, Tobe SW, Conly J. Hemodialysis infection prevention with polysporin ointment. J Am Soc Nephrol. 2003 Jan;14(1):169–79. doi: 10.1097/01.asn.0000038688.76195.a4. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DW, MacGinley R, Kay TD, et al. A randomized controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. NephrolDialTransplant. 2002;17(10):1802–7. doi: 10.1093/ndt/17.10.1802. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DW, van EC, Mudge DW, et al. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. JAmSocNephrol. 2005;16(5):1456–62. doi: 10.1681/ASN.2004110997. [DOI] [PubMed] [Google Scholar]
- 16.Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE. Late creation of vascular access for hemodialysis and increased risk of sepsis. JAmSocNephrol. 2004;15(7):1936–42. doi: 10.1097/01.asn.0000131524.52012.f8. [DOI] [PubMed] [Google Scholar]
- 17.Abbott KC, Agodoa LY. Etiology of bacterial septicemia in chronic dialysis patients in the United States. ClinNephrol. 2001;56(2):124–31. [PubMed] [Google Scholar]
- 18.Powe NR, Jaar B, Furth SL, Hermann J, Briggs W. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int. 1999;55(3):1081–90. doi: 10.1046/j.1523-1755.1999.0550031081.x. [DOI] [PubMed] [Google Scholar]
- 19.Jaar BG, Hermann JA, Furth SL, Briggs W, Powe NR. Septicemia in diabetic hemodialysis patients: comparison of incidence, risk factors, and mortality with nondiabetic hemodialysis patients. AmJKidney Dis. 2000;35(2):282–92. doi: 10.1016/s0272-6386(00)70338-6. [DOI] [PubMed] [Google Scholar]