Abstract
Recent evidence suggests that inflammation in the spontaneously hypertensive rat (SHR) is associated with an uncontrolled matrix metalloproteinase (MMP) activity. We hypothesize that the transcription factor nuclear factor kappa B (NF–κB) is overexpressed in the SHR, enhancing its MMP activity and enzymatic cleavage of the beta-2 adrenergic receptor (β2AR), thereby diminishing catecholamine-mediated arteriolar vasodilation. NF-κB expression level and translocation were compared between Wistar Kyoto rat (WKY) and SHR kidney, heart and brain. The animals were treated with a NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC), for ten weeks and correlations between NF-κB and MMP activity were determined. Immunohistochemistry showed that NF-κB expression is increased in untreated SHR kidney (~ 14%) and brain hypothalamus (~ 22%) compared to that in WKY (p <0.05), but not in myocardium and cerebral cortex. After PDTC treatment, the SHR systolic blood pressure was reduced close to WKY levels. NF-κB expression level in treated-SHR was also decreased in kidney and hypothalamus compared to non-treated animals (p <0.05). Furthermore, MMP-2 and -9 activities in SHR plasma were significantly reduced (~41%) by PDTC treatment. Additionally, zymographic analyses and in situ zymography showed decreased MMP-2 activity in kidney homogenates and decreased MMP-1,-9 activities in brain. The level of the β2AR extracellular, but not intracellular, domain density was found reduced in kidney showing a receptor cleavage process that can be blocked by PDTC treatment. These results suggest NF-κB is an important transcription factor in the SHR and may be involved in the enhanced MMP activity and consequently receptor cleavage.
Keywords: Microcirculation, matrix metalloproteinases, beta-2 adrenergic receptor, receptor cleavage, NF-κB inhibitor, pyrrolidine dithiocarbamate
INTRODUCTION
Chronic hypertension is associated with enhanced risk for cardiovascular disease, including atherosclerosis, stroke and renal failure 1–4. There is increasing evidence suggesting a strong association between hypertension and inflammation as well as end-organ damage3–7, e.g. expression of inducible nitric oxide synthase (iNOS) and inflammatory markers, elevated levels of activated leukocytes in the circulation, enhanced leukocytes cytotoxicity, oxidative stress, and apoptosis5–12.
We recently obtained evidence that members of the matrix metalloproteinase (MMP) family13, known to degrade the extracellular matrix (ECM) and connective tissue proteins in different physiological and pathological conditions14–16, have elevated levels in hypertension. In the spontaneously hypertensive rat (SHR), elevated levels of MMP-2, -9 and -7 cause direct damage to cells by cleavage of the extracellular domain of several key receptors, which results in diverse cell dysfunctions12. For example, proteolytic cleavage of the vasodilatory beta-2 adrenergic receptor (β2AR) causes arteriolar constriction and blood pressure elevation17, cleavage of the extracellular domain of the insulin receptor produces insulin resistance18, and cleavage of the vascular endothelial growth factor receptor-2 (VEGFR-2) leads to endothelial cell apoptosis and capillary rarefaction in the SHR 19.
β2AR is a cell surface receptor associated with sympathetic nervous system pathways and involved in mediating smooth muscle vasodilation to balance vascular tone and blood pressure homeostatis20, 21. Overexpression of β2AR gene in the endothelium of the carotid artery serves to restore vasorelaxation in SHR22. Several studies have also shown a genetic susceptibility of β2AR in hypertension, especially the Arg16 allele polymorphism23–26.
This evidence raises the question what induces the expression of MMPs in the SHR? MMPs can be influenced by a variety of agents and cytokines, such as interleukin-1 (IL-1)27, 28 and transcription factors, like activating protein-1 (AP-1), signal transducer and activator of transcription (STAT), and nuclear factor kappa B (NF-κB), that bind to specific elements on MMP gene promoters13. There is evidence that NF-κB activation is prominent in damaged kidneys or dysfunctional hearts with upregulation of p65 mRNA, increased NF-κB binding activity, and elevated inhibitor of kappa kinase (IKK) activity4, 29, 30.
NF-κB is a transcription protein with regions of DNA-binding and dimerization domains, nuclear translocation signal (NLS), and binding site for the inhibitor of kappa B (IκB)31 which is involved in regulating genes of the inflammatory cascade32, 33. Upon activation by extracellular stimuli, NF-κB translocates from the cytoplasm into the nucleus and regulates specific gene expression34.
The SHR has elevated levels of NF-κB expression18 that may possibly be due to elevated free radical production induced by excess nitric oxide (NO) production and oxidative stress which both have been suggested to have association with hypertension9, 10. Thus we hypothesize, that NF-κB induces MMP expression in the SHR, which in turn causes receptor cleavage induced by MMP, such as the β2AR and consequently the elevated arteriolar contraction and arterial blood pressure.
In the present study, we explored the possibility of chronic NF-κB inhibition on MMP expression and receptor cleavage in the SHR as compared to its normotensive control, the Wistar Kyoto rat (WKY). Among several NF-κB inhibitors we used pyrrolidine dithiocarbamate (PDTC) as an effective inhibitor that can be administered over several weeks29, 32, 35. PDTC attenuates hypertrophy, end-organ damage, and suppresses inducible nitric-oxide synthase as well as superoxide anion formation in the SHR7, 29, 36–39. Even though PDTC may have several biological effects that are not fully understood, it most likely stabilizes IκB to prevent the release of NF-κB rather than acting at the level of cytoplasmic NF-κB/IκB complex or interfering with DNA binding29, 32. We determine NF-κB levels in SHR kidney, heart, and brain by immunohistochemistry, MMP-2 and MMP-9 activities in plasma and MMP-2 and MMP-1,-9 activities in heart, kidney and brain by zymography, together with the level of β2AR proteolytic cleavage as a mechanism that is involved in the arterial pressure elevation.
MATERIALS AND METHODS
Male SHRs at 13–15 weeks of age and their normotensive controls, the Wistar Kyoto rats (WKY), were treated with the NF-κB inhibitor PDTC (150 mg/kg/day) in drinking water for a period of 10 weeks. Untreated controls received standard chow and water ad libitum. The systolic blood pressure of two animals in each group was measured with the tail-cuff method every week by the same person and at the same time of day.
At the end of the treatment period the rats were given general anesthesia. (pentobarbital, 50 mg/kg). Selected tissues (brain, heart and kidney) were removed and frozen sections (10 µm thickness for brain, and 5µm for kidney and heart) were fixed and labeled with anti-NF-κB p65 rabbit polyclonal antibody (sc-109, Santa Cruz Biotechnology, Santa Cruz, CA). The antibody recognizes both the inactive form of p65 subunit, bound to p50 and IκB in the cytoplasm, and the active monomeric form in the nucleus.
To evaluate potential enzymatic cleavage of the β2AR, purified rabbit polyclonal antibodies against the extracellular domain of the β2AR (against a peptide at the N-terminus, NLS2662, Novus Biologicals) and antibodies against the intracellular domain of the β2AR (against a peptide at the C-terminus, M-20, sc-570, Santa Cruz Biotechnology) were used on sequential sections. The primary antibodies were visualized by binding of secondary antibody conjugated to peroxidase activity with diaminobenzidine (DAB) substrate. Buffer alone or nonspecific purified rabbit immunoglobulin G (IgG) served as controls. Selected tissue sections were counterstained for location of cell nuclei (DAPI).
Gelatin zymography with sodium dodecyl sulfate polyacrylamide gels was used to determine plasma protease activity. MMP-2 activity was determined in homogenized tissues using a MMP-2-specific fluorescently quenched substrate. MMP-1 and 9 activities on frozen tissue sections were determined by in-situ zymography using a substrate that is cleaved by both MMPs. Lysis of the substrate was assessed by examination under a fluorescence microscope. Immunolabeling intensities and fluorescence light intensity in the zymography were measured on digital images based on pixel intensity divided by tissue area and presented as mean ± standard deviation. Comparisons of mean values between animal groups were carried out by two-tailed Student’s t-test. p< 0.05 was considered significant.
RESULTS
NF-κB Expression Level
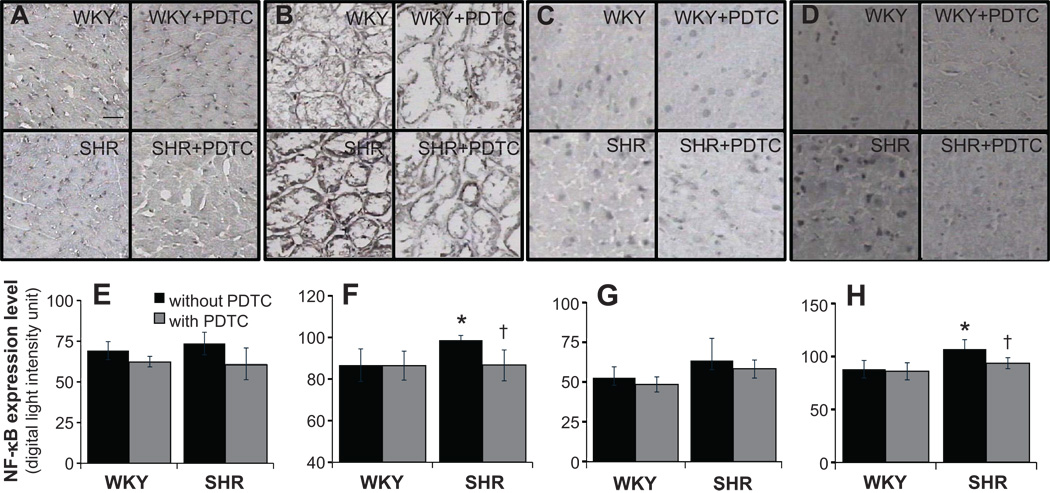
The SHR had higher NF-κB expression (p <0.05) in renal glomerular and tubulointerstitial areas on average by 14% (Fig.1B,F) and in hypothalamus region of brain by 22% (Fig.1D,H) compared with WKY (p < 0.05). The NF-κB expression levels were not significantly elevated (p > 0.05) in SHR myocardium (Fig.1A,E) and cerebral cortex (Fig. 1C, G).
Figure 1.
Micrographs of frozen immunohistochemical sections with primary antibody against NF-κB p65 subunit expression as seen by DAB substrate and light absorption measurements over whole tissue expressed as digital units in myocardium (A, E), renal glomerular and tubulointerstitial areas (B, F), cerebral cortex (C, G), and hypothalamus (D, H). *p <0.05 compared to WKY, †p <0.05 compared to non-treated SHR in student’s t-test. N=4 for non-treated group and N=5 for pyrrolidine dithiocarbamate (PDTC) treated group. Scale bar = 100 µm
Translocation of NF-κB
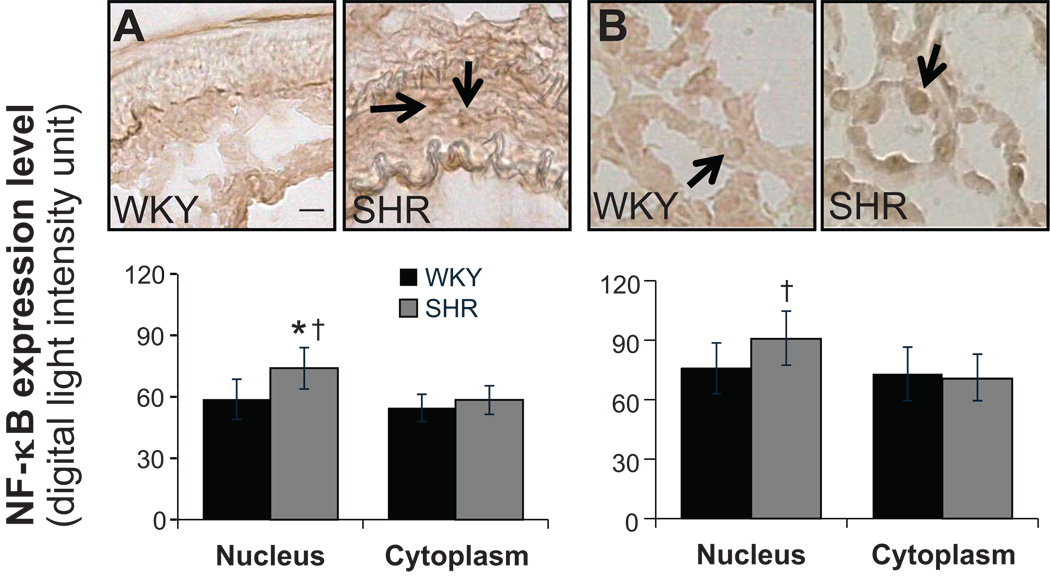
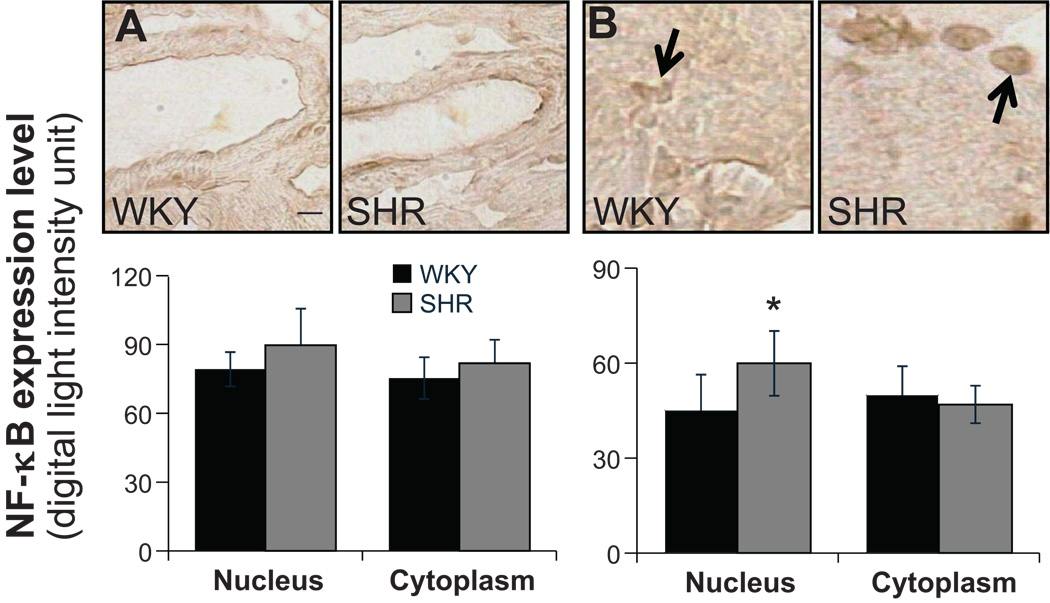
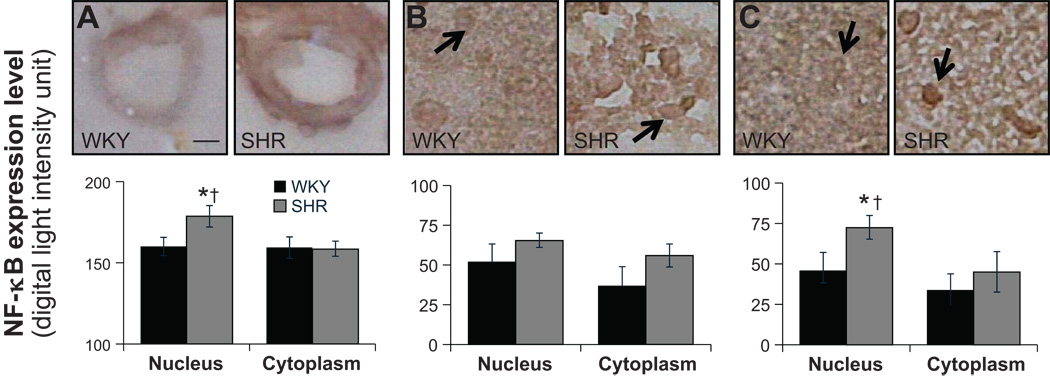
NF-κB label density was measured in both the nucleus and the cell cytoplasm. In renal tubular cells, SHR has a significantly higher NF-κB label density in the nucleus, and there were no differences in cytoplasm between WKY and SHR (Fig.2B). There were no differences of NF-κB density in cytoplasm between WKY and SHR in cells of renal blood vessel wall. But SHR had significantly elevated label density in the nucleus than in cytoplasm (Fig.2A). In cardiac muscle cells, SHR showed significantly increased label density in the nucleus than in cytoplasm (Fig.3B) while WKY and SHR had similar levels of label density in cytoplasm. No differences were found between either WKY and SHR or nucleus and cytoplasm in blood vessels of myocardium (Fig.3A). In the SHR brain, significantly higher expression in the nucleus was detected in hypothalamus and cells of its blood vessel walls but not in cerebral cortex (Fig.4).
Figure 2.
(Top panels) Micrographs of immunohistochemical sections and (bottom bar graphs) NF-κB label expression levels in the nucleus (arrows) and the cell cytoplasm determined by optical density measurements (see detailed methods on-line) in arterioles of the kidney (A) and in renal tubular cells (B). *p <0.05 compared to WKY nucleus, †p<0.05 compared to SHR cytoplasm in student’s t-test. N=5 rats for each group. Scale bar = 25 µm.
Figure 3.
(Top panels) Micrographs of immunohistochemical sections and (bottom bar graphs) NF-κB label expression levels in the nucleus (arrows) and the cell cytoplasm determined by optical density measurements (see detailed methods on-line) in arterioles of the heart (A) and in cardiac muscle cells (B). *p <0.05 compared to SHR cytoplasm in student’s t-test. N=5 rats for each group. Scale bar = 25 µm.
Figure 4.
(Top panels) Micrographs of immunohistochemical sections. (Bottom bar graphs) NF-κB label expression levels in the nucleus (arrows) and the cell cytoplasm determined by optical density measurements in arterioles of brain (A) and in cerebral cortex (B) and in tissue parenchyma of hypothalamus (C) *p<0.05 compared to WKY nucleus, †p < 0.05 compared to SHR cytoplasm in student’s t-test. N=5 rats for each group. Scale bar = 25 µm.
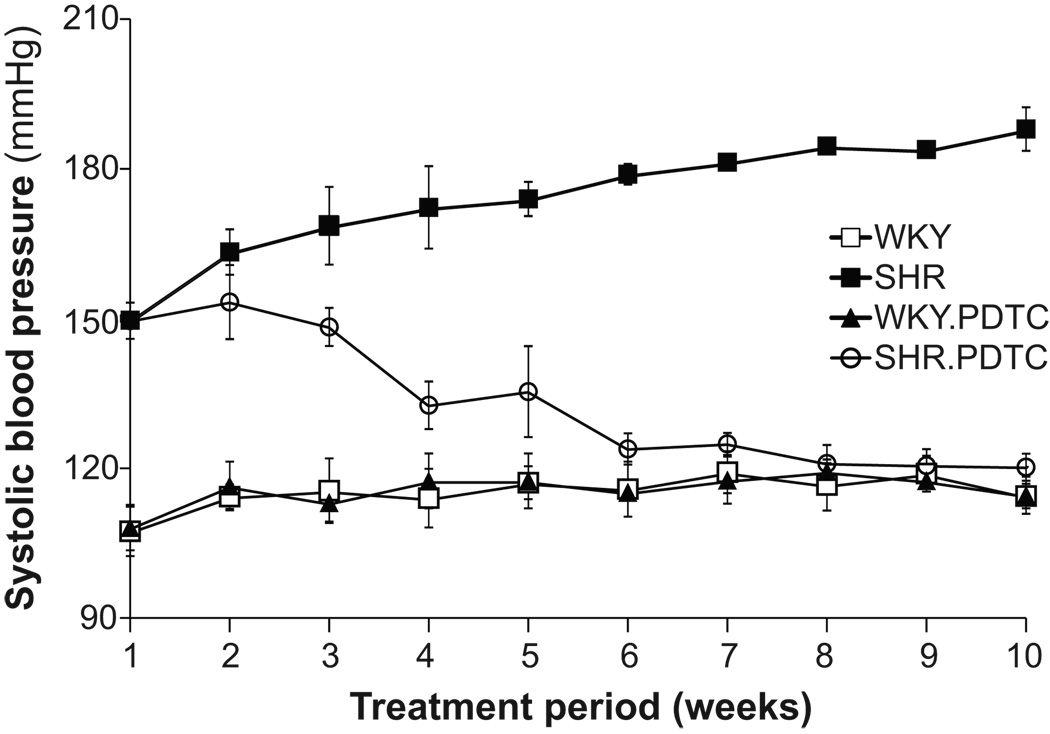
Suppression of Blood Pressure by PDTC Treatment
Systolic blood pressure in SHRs started to decrease in the second week of PDTC treatment compared with non-treated SHR (153 ±7 and 163 ±5 mmHg, respectively, mean ± standard deviation). The time course of blood pressures in SHR during the treatment also showed a difference compared to the non-treated SHR (Fig.5). The mean ± standard deviation of the systolic blood pressure at the end of the treatment were 120 ±3 mmHg in PDTC-treated SHRs and 188 ±4 mmHg in non-treated SHRs, whereas the mean ± standard deviation of the systolic blood pressure of the PDTC-treated and non-treated WKY rats was 115 ±4 mmHg and 114 ±2 mmHg, respectively.
Figure 5.
Systolic blood pressure in conscious age-matched SHR and WKY with and without treatment with pyrrolidine dithiocarbamate (PDTC). Data are presented as mean ± standard deviations. N=2 rats per group.
NF-κB Expression Level after PDTC treatment
The NF-κB expression level after PDTC treatment was significantly decreased by 12 % compared with non-treated SHR in renal glomerular and tubulointerstitial areas (Fig. 1B, F) and brain hypothalamus (Fig. 1D, H), whereas no significant difference were detected between PDTC-treated WKY and non-treated WKY.
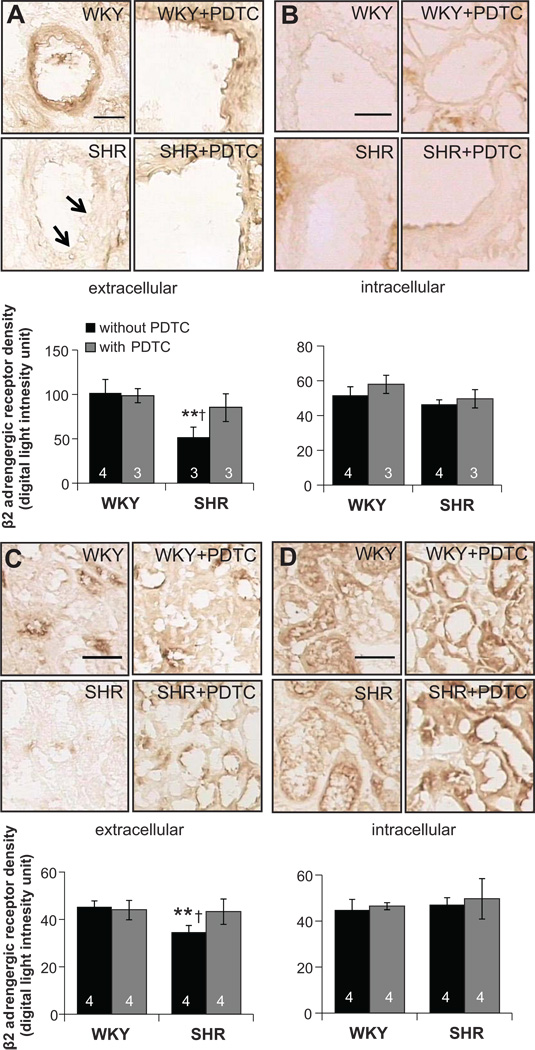
Receptor Cleavage of β2AR in the SHR
Cleavage of the extracellular domain of β2AR was detected by using different antibodies against its intra- and its extracellular domains. In microvessels of the kidney, SHR had a significantly reduced expression of the extracellular domain of the β2ARs (p<0.01; Fig. 6 A,B). A similar trend was found in renal glomerular and tubulointerstitial areas (Fig. 6 C,D). Additionally, the vessels in the myocardium of SHRs had lower expression levels of the extracellular domain of β2AR (p<0.05; please see http://hyper.ahajournals.org Figure S1). The expression levels of the extracellular domain in the brain were not significantly different in SHR compared to WKY, including hypothalamus (please see http://hyper.ahajournals.org Figure S2), cerebral cortex (data not shown), and in blood vessels (please see http://hyper.ahajournals.org Figure S2).
Figure 6.
(Top panels) Micrographs of immunohistochemical sections and in vertical alignment (bottom bar graphs) optical density measurements of the extracellular (A) and intracellular (B) domains of the β2AR in the vessels of the kidney and extracellular (C) and intracellular (D) domains in the renal tubular cells. **p<0.01 compared to WKY, †p<0.05 compared to treated-SHR in student’s t-test. Number of rats is indicated in the bar. Scale bar= 100 µm
Attenuation of MMP Activity in Plasma by PDTC Treatment
Among the MMPs, we here measured the MMP-2, MMP-1 and 9 activities after PDTC treatment based on previous evidence showing increased MMP-2 and MMP-9 activities in SHR18, 19.
The MMP-2 activity in plasma, determined by gelatin gel zymography was not different between WKY and SHR. But the MMP-2 activity was suppressed in both WKY and SHR after PDTC treatment by 14% and 8%, respectively. The pro-MMP-2 activity was suppressed by PDTC only in SHR but not in WKY (http://hyper.ahajournals.org Figure S3).
The SHR has elevated MMP-9 activity compared with WKY rats. During PDTC treatment, the MMP-9 activity was found to be suppressed in SHR but not in WKY rats, and the activity was also significantly lower than treated-WKY rats (http://hyper.ahajournals.org Figure S3). As control, both MMP-2 and MMP-9 activity were blocked in-vitro by metal chelation with ethylenediaminetetraacetic acid (EDTA).
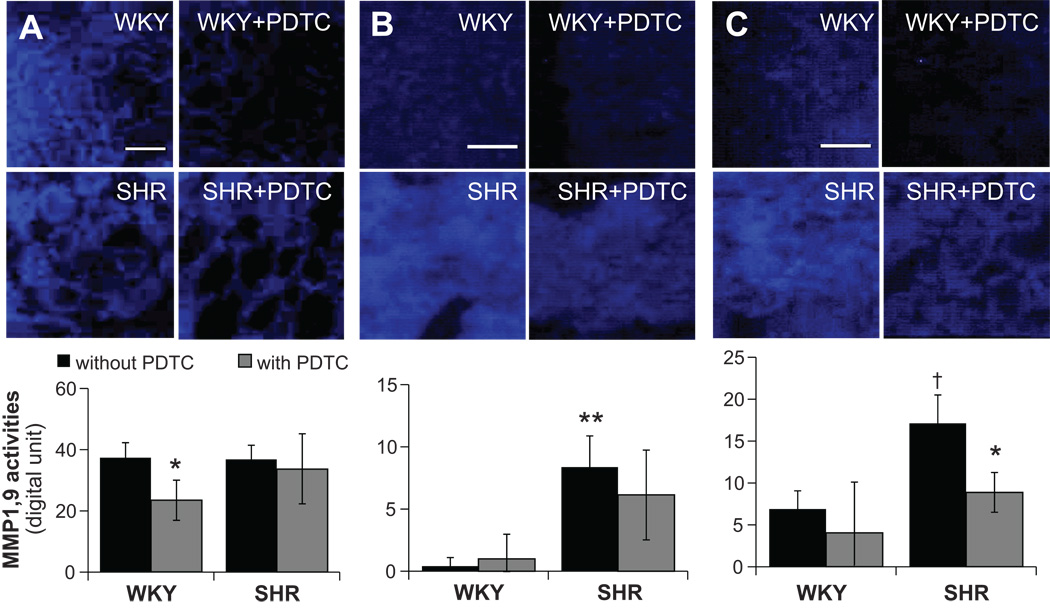
Tissue MMP Activity
In kidney homogenates, no difference in MMP-2 activity, determined with a specific fluorescent substrate, was found between WKY and SHR. However, after treatment both WKY and SHR showed decreased MMP-2 activity by 41% (please see http://hyper.ahajournals.org Figure S4A). The MMP-2 activity was not different between WKY and SHR in both heart and brain homogenates and the PDTC treatment had no effect on MMP-2 activity in either organ (http://hyper.ahajournals.org Figure S4B, C). In an alternative approach, the MMP-2 activities in brain, heart and kidney homogenates were confirmed by gel zymography and similar results were found (http://hyper.ahajournals.org Figure S4D, E). The MMP-2 activity is blocked in vitro by EDTA (data not shown).
In the kidney no difference in MMP-1,9 activities, measured by in-situ zymography, was found between WKY and SHR (Fig. 7 A). In contrast, SHR heart and brain exhibited significantly higher activities than WKY (p<0.05; Fig. 7B, C). The brain of SHR with PDTC treatment and the kidney of WKY with PDTC have decreased MMP-1, 9 activities. However, the PDTC treatment did not have an effect on MMP-1, 9 activities in the heart (Fig. 7B).
Figure 7.
(Top panels) Micrographs of in situ zymography sections using MMP-1, 9 substrate, and (bottom bar graphs) measurements of fluorescence density in kidney (A) heart (B) and brain (C). *p<0.05 compared to non-treated group, **p<0.01 compared to WKY, †p<0.05 compared to WKY in student’s t-test. N=4 for each group. Scale bar=100 µm
Chronic NF-κB Inhibition Attenuated β2AR Cleavage in the SHR
After inhibiting NF-κB with PDTC treatment, the extracellular domain density of the β2AR was increased by 20% in renal glomerular and tubulointerstitial areas and by 39% in kidney vessels in SHR (Fig. 6). In the cerebral cortex (data not shown) and hypothalamus, SHR with PDTC treatment also exhibit significantly elevated β2AR levels (please see http://hyper.ahajournals.org Figure S2). No significant differences between these labels before and after PDTC treatment were found in blood vessels of the myocardium (p>0.05; please see http://hyper.ahajournals.org Figure S1).
DISCUSSION
The current observations indicate an increased expression of the nuclear transcription factor NF-κB in SHR (Fig. 1F, H). Blockade of the transcription factor with PDTC serves to reduce the expression levels and the translocation into the cell nucleus with a reduced MMP activity in kidney and brain (please see http://hyper.ahajournals.org Figure S4) and a reduction of blood pressure (Fig. 5) and cleavage of the extracellular domain of the β2AR (Fig. 6, and http://hyper.ahajournals.org Figures S1, S2).
The expression level of NF-κB in this study was examined by immunohistochemistry. This approach permits detection of the transcription factor in different tissue regions (e.g. endothelial cells, versus mast cells or parenchymal cells), which in alternative approaches (e.g. Western analysis) may not be possible at the microvascular level due to the need to homogenize the tissue. Separate measurements of the immunolabel density in the nucleus and in the cytoplasm can also be used to quantify nuclear translocation.
The nuclear translocation was prominent in the vessel walls of kidney and brain (Fig. 2, 4) showing a possibility that NF-κB activation in vessels in these tissues may be associated with endothelial dysfunction and further organ damage, such as renal failure and stroke. The endothelium of SHR microvessels exhibits enhanced apoptosis8 together with enhanced oxygen free radical formation, and enzymes that synthesize them, together with enhanced MMP expression18.
The elevated NF-κB expression and activation found in glomerular and tubulointerstitial areas of the SHR are in agreement with previous studies in a renal model of hypertension40. The activation of NF-κB in myocardium(Fig. 3B) also supports the association between myocardial infarction, hypertrophy and hypertension41, 42. But these results may have to be looked at from multiple perspectives, especially if apoptosis is present as has been observed in several models of hypertension8, 10. The lack of a higher NF-κB expression level in myocardium (Fig.1A, E), as seen by immunohistochemistry, may be the consequence of extensive apoptosis. Cells that undergo apoptosis lose the ability to synthesize new proteins and therefore the group of cells that are being analyzed in our experiments consists merely of those cells still capable of protein synthesis. Significant NF-κB expression in the SHR hypothalamus region but not in the cerebral cortex(Fig. 1C, D, G, H) may suggest that hypertensive pathology is related to dysfunction of the hypothalamus since the output from the hypothalamus influences nervous system and endocrine system, which in turn is associated with blood pressure control43.
Blockade of NF-κB by PDTC treatment facilitates a number of improvements in different hypertensive models, including reduction of blood pressure, cardiac hypertrophy and inflammatory cell infiltration into heart and kidney tissue 9, 29, 37. In the current study, the systolic blood pressure was already lower in PDTC-treated SHR compared to non-treated SHR after two weeks of treatment (Fig. 5). The decreased blood pressure by PDTC inhibition in this study is also consistent with the fact that suppression of acute inflammation may lower the blood pressure in the Lyon hypertensive model 44. The dose we used in this study (150 mg per day) has been applied in other studies without indication for toxic effect29.
PDTC treatment also suppressed NF-κB expression level (Fig. 1) and serves to reduce the cleavage of β2AR in the SHR (Fig. 6). In our previous study, we identified a role of MMPs cleaving β2AR in SHR and its attenuation after MMPs inhibition17. Therefore, we hypothesize that PDTC may have a similar effect on suppression of receptor cleavage due to inhibition of NF-κB induced MMPs expression. In the current study, the enhanced MMP-2 and MMP-9 activity in the SHR plasma was significantly decreased after the ten-week PDTC treatment (http://hyper.ahajournals.org Figures S3A, B). This evidence is in line with the fact that blockade of NF-κB almost completely inhibits expression of MMP-9 and with the presence of NF-κB binding sites in the promoter region of MMP-945. Furthermore, among different organs we found a mixed picture of NF-κB expression. For example, we did not see significantly decreased NF-κB expression in the myocardium after PDTC treatment. PDTC may possibly affect other transcription factors that can suppress blood pressure without changing the total NF-κB protein level.
Other off-target effects in addition to NF-κB expression of PDTC may also be involved in suppression of the receptor cleavage. Administration of PDTC in different rat models reduces angiotension-II induced inflammation and iNOS expression46, 47. The antioxidant properties of PDTC have been shown to play an important role in several biological reactions32. Barki-Harrington et al. provided evidence for a direct physiological interaction between angiotension and beta adrenergic receptors, both of which are G protein-couple receptors48; therefore, it is possible that the inhibition of the angiotension receptor signaling pathway may have an effect on beta adrenergic receptors as a potential feedback reaction. Since PDTC may have multiple activities, more studies using other inhibitors are required in order to distinguish its off-target effects from its ability to inhibit NF-κB.
Compared to WKY rats, SHRs have elevated level of MMP-2, MMP-1 and -9 mRNA, protein level 18, 49, 50 in line with the current results. Furthermore, our evidence suggests that MMPs may cause cleavage of the extracellular domain of β2AR in the kidney and the heart, which is consistent with previous studies showing reduced expression of this receptor as well as enhanced agonist-mediated desensitization in hypertension51, 52. Even though we did not observe significantly higher MMP-2 or MMP-1 and -9 in the kidney, it is possible that other MMP activities could cause β2AR cleavage and requires further examination.
One of the important issues associated with the proteolytic activity in the SHR is that it may cause not only cleavage of the extracellular domain of the β2AR, but also cleave a variety of other surface receptors. Cleavage of the insulin receptor compromises the ability of insulin to signal intracellular glucose transport (“insulin resistance” 18), cleavage of the vascular endothelial receptor causes endothelial apoptosis and capillary rarefaction19, cleavage of CD18 is associated with a reduced ability to mediate leukocyte adhesion to the endothelium in the SHR (“immune suppression” 18). Therefore treatment targeting the proteolytic activity, be it by intervention against their expression levels or be it by suppression of their activity with pharmacological means, may serve as an approach to attenuate multiple form of cell dysfunctions and organ pathologies at the same time.
With exception of the vessels in the brain, we saw a significant reduction of β2AR in the SHR which was consistent with our previous study17. The cleavage of this receptor contributes to endothelial dysfunction and vasoconstriction in arterioles and elevated central blood pressure. In addition, Rodrigues et al. demonstrated an attenuated response to β2AR agonist and antagonist in SHR also indicating the abnormal activity of this specific receptor compared to WKY control.
Perspectives
The current evidence suggests a role of NF-κB in triggering upregulation of MMP activity, which may causes cleavage of β2AR as a mechanism that is involved in arteriolar/arterial constriction and pressure elevation. Chronic attenuation of NF-κB expression by PDTC serves to prevent β2AR cleavage, restore the vasodilatory response in arteries/arterioles and lower central blood pressure in the SHR. Based on the effect of PDTC on MMP expression and its antioxidant potential53, it may serve to reduce a variety of cell dysfunctions and attenuate end organ injury in hypertension.
Supplementary Material
Acknowledgments
SOURCE OF FUNDING: Supported by NIH Grant HL-10881.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST/DISCLOSURE: The authors declare no conflict of interest.
References
- 1.Alexander RW. Hypertension and the pathogenesis of atherosclerosis : Oxidative stress and the mediation of arterial inflammatory response: A new perspective. Hypertension. 1995;25:155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 2.Johansson BB. Hypertension mechanisms causing stroke. Clin Exp Pharmacol Physiol. 26:563–565. doi: 10.1046/j.1440-1681.1999.03081.x. [DOI] [PubMed] [Google Scholar]
- 3.Li JJ, Fang CH, Hui RT. Is hypertension an inflammatory disease? Med Hypotheses. 2005;64:236–240. doi: 10.1016/j.mehy.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Luft FC, Mervaala E, Muller DN, Gross V, Schmidt F, Park JK, Schmitz C, Lippoldt A, Breu V, Dechend R, Dragun D, Schneider W, Ganten D, Haller H. Hypertension-induced end-organ damage : A new transgenic approach to an old problem. Hypertension. 1999;33:212–218. doi: 10.1161/01.hyp.33.1.212. [DOI] [PubMed] [Google Scholar]
- 5.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: Epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 6.Schmid-Schönbein GW, Seiffge D, DeLano FA, Shen K, Zweifach BW. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension. 1991;17:323–330. doi: 10.1161/01.hyp.17.3.323. [DOI] [PubMed] [Google Scholar]
- 7.Hong HJ, Wu CC, Yen MH. Pyrrolidine dithiocarbamate improves the septic shock syndromes in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1998;25:600–606. doi: 10.1111/j.1440-1681.1998.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 8.Lim H, DeLano F, Schmid-Schönbein G. Life and death cell labeling in the microcirculation of the spontaneously hypertensive rat. J Vasc Res. 2001;38:228–236. doi: 10.1159/000051051. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of nuclear factor-kb prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2005;315:51–57. doi: 10.1124/jpet.105.088062. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi N, DeLano FA, Schmid-Schönbein GW. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2005;25:2114–2121. doi: 10.1161/01.ATV.0000178993.13222.f2. [DOI] [PubMed] [Google Scholar]
- 11.Shen K, DeLano FA, Zweifach BW, Schmid-Schönbein GW. Circulating leukocyte counts, activation, and degranulation in dahl hypertensive rats. Circu Res. 1995;76:276–283. doi: 10.1161/01.res.76.2.276. [DOI] [PubMed] [Google Scholar]
- 12.DeLano FA, Forrest MJ, Schmid-Schönbein GW. Attenuation of oxygen free radical formation and tissue injury during experimental inflammation by p-selectin blockade. Microcirculation. 1997;4:349–357. doi: 10.3109/10739689709146799. [DOI] [PubMed] [Google Scholar]
- 13.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circu Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 15.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 16.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues SF, Tran ED, Fortes ZB, Schmid-Schönbein GW. Matrix metalloproteinases cleave the {beta}2 -adrenergic receptor in the spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H25–H35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLano FA, Schmid-Schönbein GW. Proteinase activity and receptor cleavage: Mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension. 2008;52:415–423. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran ED, DeLano FA, Schmid-Schönbein GW. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: Vegfr-2 cleavage, endothelial apoptosis, and capillary rarefaction. Journal of Vascular Research. 2010;47:423–431. doi: 10.1159/000281582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liggett SB. Pharmacogenetics of beta-1- and beta-2-adrenergic receptors. Pharmacology. 2000;61:167–173. doi: 10.1159/000028397. [DOI] [PubMed] [Google Scholar]
- 21.Monopoli A, Conti A, Forlani A, Ongini E. [beta]1 and [beta]2 adrenoceptors are involved in mediating vasodilation in the human coronary artery. Pharmacological Research. 1993;27:273–280. doi: 10.1006/phrs.1993.1026. [DOI] [PubMed] [Google Scholar]
- 22.Iaccarino G, Cipolletta E, Fiorillo A, Annecchiarico M, Ciccarelli M, Cimini V, Koch WJ, Trimarco B. {beta}2-adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation. 2002;106:349–355. doi: 10.1161/01.cir.0000022690.55143.56. [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson K, Orho-Melander M, Melander O, Lindblad U, Ranstam J, Rastam L, Groop L. {beta}2-adrenergic receptor gene variation and hypertension in subjects with type 2 diabetes. Hypertension. 2001;37:1303–1308. doi: 10.1161/01.hyp.37.5.1303. [DOI] [PubMed] [Google Scholar]
- 24.Bray MS, Krushkal J, Li L, Ferrell R, Kardia S, Sing CF, Turner ST, Boerwinkle E. Positional genomic analysis identifies the {beta}2-adrenergic receptor gene as a susceptibility locus for human hypertension. Circulation. 2000:r9–r15. doi: 10.1161/01.cir.101.25.2877. [DOI] [PubMed] [Google Scholar]
- 25.Dungan JR, Conley YP, Langaee TY, Johnson JA, Kneipp SM, Hess PJ, Yucha CB. Altered beta-2 adrenergic receptor gene expression in human clinical hypertension. Biol Res Nurs. 2009;11:17–26. doi: 10.1177/1099800409332538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato N, Sugiyama T, Morita H, Kurihara H, Sato T, Yamori Y, Yazaki Y. Association analysis of {beta}2-adrenergic receptor polymorphisms with hypertension in japanese. Hypertension. 2001;37:286–292. doi: 10.1161/01.hyp.37.2.286. [DOI] [PubMed] [Google Scholar]
- 27.Fabunmi RP, Baker AH, Murray EJ, Booth RF, Newby AC. Divergent regulation by growth factors and cytokines of 95 kda and 72 kda gelatinases and tissue inhibitors or metalloproteinases-1, -2, and -3 in rabbit aortic smooth muscle cells. Biochem J. 1996;315:335–342. doi: 10.1042/bj3150335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, Kamoi K, Suda T. Regulation of matrix metalloproteinases (mmp-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: Association of mmp induction with bone resorption. Endocrinology. 1998;139:1338–1345. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Young D, Sen S. Inhibition of nf-{kappa}b induces regression of cardiac hypertrophy, independent of blood pressure control, in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H20–H29. doi: 10.1152/ajpheart.00082.2005. [DOI] [PubMed] [Google Scholar]
- 30.Maulik N, Sato M, Price BD, Das DK. An essential role of nf[kappa]b in tyrosine kinase signaling of p38 map kinase regulation of myocardial adaptation to ischemia. FEBS letters. 1998;429:365–369. doi: 10.1016/s0014-5793(98)00632-2. [DOI] [PubMed] [Google Scholar]
- 31.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/nf-kappa b/i kappa b family: Intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 32.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa b activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayo MW, Wang C-Y, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, Baldwin AS., Jr Requirement of nf-{kappa}b activation to suppress p53-independent apoptosis induced by oncogenic ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 34.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-{kappa}b, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 35.Pinkus R, Weiner LM, Daniel V. Role of oxidants and antioxidants in the induction of ap-1, nf-kappa b, and glutathione s-transferase gene expression. J Biol Chem. 1996;271:13422–13429. doi: 10.1074/jbc.271.23.13422. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 37.Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. Nf-kappab inhibition ameliorates angiotensin ii-induced inflammatory damage in rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 38.Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol. 2000;131:631–637. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Antwerp D, Mercurio F, Lee K, Verma I. Severe liver degeneration in mice lacking the ib kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 40.Quiroz Y, Bravo J, Herrera-Acosta J, Johnson RJ, Rodriguez-Iturbe B. Apoptosis and nf[kgr]b activation are simultaneously induced in renal tubulointerstitium in experimental hypertension. Kidney Int. 2003;64:S27–S32. doi: 10.1046/j.1523-1755.64.s86.6.x. [DOI] [PubMed] [Google Scholar]
- 41.Madhavan S, Ooi WL, Cohen H, Alderman MH. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension. 1994;23:395–401. doi: 10.1161/01.hyp.23.3.395. [DOI] [PubMed] [Google Scholar]
- 42.Chanutin A, Barksdale E. Experimental renal insuffciency produced by partial nephrectomy: Ii. Relationship of left ventricular hypertrophy, the width of the cardiac muscle fiber and hypertension in the rat. Arch Intern Med. 1933;52:739–751. [Google Scholar]
- 43.Silverthorn DU, Ober WC, Garrison CW, Silverthorn AC, Johnson BR. Human physiology: An integrated approach. San Francisco, CA: Daryl Fox; 2004. [Google Scholar]
- 44.Bataillard A, Renaudin C, Sassard J. Silica attenuates hypertension in lyon hypertensive rats. J Hypertens. 1995;13:1581–1584. [PubMed] [Google Scholar]
- 45.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor nf-kappa b reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50:556–565. doi: 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 46.Muller DN, Dechend R, Mervaala EMA, Park J-K, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. Nf-{kappa}b inhibition ameliorates angiotensin ii-induced inflammatory damage in rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 47.Liu SF, Ye X, Malik AB. In vivo inhibition of nuclear factor-kappa b activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997;159:3976–3983. [PubMed] [Google Scholar]
- 48.Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of {beta}-adrenergic and angiotensin ii receptors by a single antagonist: A functional role for receptor-receptor interaction in vivo. Circulation. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- 49.Camp TM, Smiley LM, Hayden MR, Tyagi SC. Mechanism of matrix accumulation and glomerulosclerosis in spontaneously hypertensive rats. J Hypertens. 2003;21:1719–1727. doi: 10.1097/00004872-200309000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Hultstrom M, Leh S, Skogstrand T, Iversen BM. Upregulation of tissue inhibitor of metalloproteases-1 (timp-1) and procollagen-n-peptidase in hypertension-induced renal damage. Nephrol Dial Transplant. 2007;2007:1–8. doi: 10.1093/ndt/gfm710. [DOI] [PubMed] [Google Scholar]
- 51.Dishy V, Sofowora GG, Xie H-G, Kim RB, Byrne DW, Stein CM, Wood AJJ. The effect of common polymorphisms of the {beta}2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 52.Wang G, Owens SA, Yu P, Asico LD, Hopfer U, Jose PA, Xu J. Endogenous beta-2 adrenergic receptor involved in abnormal proximal tubule sodium reabsorption in shr. American Journal of Hypertension. 2005;18:A136–A137. [Google Scholar]
- 53.Aoki M, Nata T, Morishita R, Matsushita H, Nakagami H, Yamamoto K, Yamazaki K, Nakabayashi M, Ogihara T, Kaneda Y. Endothelial apoptosis induced by oxidative stress through activation of nf-{kappa}b: Antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension. 2001;38:48–55. doi: 10.1161/01.hyp.38.1.48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.