Abstract
Recent case reports suggest that the short-acting benzodiazepine-like hypnotic zolpidem may have abuse potential among individuals who have no personal history of abusing drugs or alcohol, particularly at doses higher than those recommended for treating insomnia. The present study recruited drug-naïve volunteers to assess the subjective effects of multiple doses of zolpidem (0, 5, 10, or 20 mg) administered in a within-subject double-blind design. Participants (n=11) answered computerized questionnaires (Addiction Research Center Inventory, visual analog scales, and a hypothetical Drug vs. Money Choice) to address the hypothesis that a supra-therapeutic dose (20 mg) would increase ratings of abuse-related subjective effects, while lower therapeutic doses (5 and 10 mg) would not. Although participants rated some effects as negative at 10 and 20 mg, the highest dose engendered predominantly positive abuse-like effects such as “High”, “Like”, and “Good Effects”. However, no dose of zolpidem was chosen over money ($0.35 – $10) when participants made hypothetical choices between them. Results suggest that although individuals without a drug abuse history are not inclined to choose zolpidem when presented with an alternative reinforcer such as money, it may possess moderate abuse potential that limits its clinical utility.
Keywords: zolpidem, subjective effects, abuse, human volunteer, benzodiazepine, hypnotic, self-report, choice
Introduction
Zolpidem (Ambien®) is a short-acting hypnotic that enhances GABAergic neurotransmission via the benzodiazepine binding site on specific GABAA receptors. Zolpidem was approved for the short-term treatment of insomnia almost twenty years ago, and a number of studies were undertaken shortly thereafter to determine its potential for abuse. In agreement with findings documenting that drug history is a major determinant of the reinforcing effects of benzodiazepines (Woods et al., 1992; Griffiths and Weerts, 1997), laboratory studies demonstrated that human volunteers who had extensive drug histories reported more “drug liking” when zolpidem was compared to other hypnotics (Evans et al., 1990; Rush et al., 1999), while drug-naïve individuals did not experience abuse-related effects (Rush and Griffiths, 1996; Rush et al. 1998). A more recent study in healthy normal female participants reiterated those observations, and implied that a therapeutic dose of zolpidem may have aversive-like effects in the absence of drug experience (Licata et al., 2008).
Despite evidence and subsequent marketing indicating that zolpidem poses little threat to the average person, a number of case reports to the contrary have surfaced in the literature. Abuse and physical dependence accompanied by serious withdrawal symptoms have been reported in individuals who were prescribed a therapeutic regimen of zolpidem (i.e., 5 or 10 mg at bedtime) to treat insomnia. Although patients had no drug abuse histories, they escalated their intake gradually over time to reach supra-therapeutic doses ranging anywhere from 60 mg to 2,000 mg per day (Gilbert and Staats, 1997; Liappas et al., 2003; Krueger et al., 2005; Cubała and Landowski, 2007; Huang et al., 2007; Svitek et al., 2008). In many of these cases patients claimed they consumed ultra-high doses in order to reduce daytime anxiety, although zolpidem purportedly has limited anxiolytic effects compared to benzodiazepines (e.g., Rowlett et al., 2006; but see Bailey et al., 2009).
In light of widespread use of zolpidem among individuals who are likely to experience insomnia due to clock disturbances, such as military personnel (Caldwell and Caldwell, 2005), emergency medicine professionals (McBeth et al., 2009), or jet-lagged travelers (Jamieson et al., 2001), the uncertainty regarding the extent of its abuse potential warrants further study. Therefore, the present study was designed to document the drug-induced subjective effects of multiple doses (0, 5, 10, or 20 mg) of zolpidem in drug-naïve volunteers who were participating in a brain imaging study. It was hypothesized that the supra-therapeutic dose (20 mg) would increase self-reported ratings of abuse-related subjective effects, while the lower therapeutic doses (5 and 10 mg) would not.
Methods
Participants
Eleven healthy male (6) and female (5) non-smoking volunteers completed the assessments. Volunteers were between the ages of 21–35 (average age was 24.2 ± 2.3 years; mean ± SD), and they had an average of 16.6 ± 1.7 years (mean ± SD) of education. They could not report >10 lifetime recreational experiences with drugs of abuse, and they could not meet DSM-IV criteria for drug or alcohol abuse or dependence (participants reported consuming ≤6 alcoholic beverages per week). Additional criteria required that volunteers had no family history of alcoholism, no personal history of diagnosis with a DSM-IV Axis I or neurological disorder, no medications, and they would have had to tolerate the high dose of zolpidem during a separate laboratory visit (i.e., did not vomit or report nausea following acute oral administration of 20 mg zolpidem), in order to be invited to participate in the study. Upon arriving at the laboratory prior to all study visits, participants were screened for drug use (QuickTox® urine screen kits, Branan Medical Corporation; Irvine, CA) and breath alcohol level (AlcoSensor, Intoximeter; Saint Louis, MO). Female participants were administered a QuPID® urine pregnancy test (Stanbio Laboratory; Boerne, TX); pregnancy was a contraindication in this study. Any participant who would have been positive on any screen would have been rescheduled and sent home (although none did). All participants were transported to and from the laboratory via taxicab. This study was reviewed and approved by the McLean Hospital Institutional Review Board. All volunteers provided informed consent and were compensated for their participation.
Study design
Subjective assessments took place over four separate study visits as one component of a double-blind, placebo-controlled, functional magnetic resonance imaging study (imaging results are in preparation to be presented elsewhere). Participants were administered one of four treatments (0, 5, 10, or 20 mg zolpidem, p.o.) 30 min prior to the beginning of a 60-min scanning session. Participants answered a series of computerized questionnaires periodically throughout the experimental session in order to provide information about the drug-induced subjective effects they were experiencing. The shortened version of the Addiction Research Center Inventory (ARCI) is a standardized set of scales consisting of 49 true/false items that have been derived in order to measure stimulant-like (Benzedrine group: BG), amphetamine-like (AMPH), euphoric (Morphine-Benzedrine group: MBG), sedative- or intoxicating-like (Pentobarbital-Chlorpromazine-Alcohol group: PCAG), and psychotomimetic or dysphoric (Lysergic acid diethylamide: LSD) drug effects (Jasinski, 1977). The visual analog scale (VAS) required participants to respond to a list of 15 items (“anxious”, “high”, “sleepy”, “good effects”, “dizzy”, “nauseous”, “loose”, “bad effects”, “confused”, “carefree”, “restless”, “like”, “willing to take again”, “willing to pay for”, and “mentally slow”) by placing a mark on a 100-mm continuum that used “not at all” or “extremely” as anchors to report how they were feeling at that moment. In addition to the abuse-related queries in the VAS regarding like, willingness to take again, and willingness to pay for, a Drug vs. Money Choice questionnaire also was administered. This questionnaire was a loose adaptation of the Multiple-Choice Procedure developed by Griffiths et al. (1993) to assess the abuse-related effects of drugs. Participants made 10 hypothetical choices between the study drug and various amounts of money ($0.35, $0.50, $0.85, $0.95, $2.00, $3.25, $4.75, $6.50, $9.00, $10.00) presented in a pseudo-random order. All questionnaires were administered repeatedly throughout the experimental session: at 0 min (baseline), 15 min (prior to entering the scanning suite), 90 min (at the end of the scanning session), and then every 30 min thereafter until 6 hours post-drug administration. At the end of the experimental session, a final 5-item questionnaire required participants to rate the overall effects of the drug they received. The items were “drug strength”, “drug liking”, “good effects”, “bad effects”, and “would take drug again”, and they were rated using the continuum described above. All computerized questionnaires were administered on Macintosh computers running in-house software (Study Log Master v.79).
Physiological monitoring
Physiologic data was collected on an Atlas™ patient monitor (Welch-Allyn Protocol, Inc.; Chicago, IL), and included blood pressure, heart rate, oxygen saturation, and skin temperature. All measures were monitored continuously throughout the session and recorded at the same time points as the subjective data, as described above.
Data analysis
The ARCI, VAS, and physiological measures were analyzed using two-way repeated measures ANOVAs examining the within-subjects factors of treatment by time. The end-of-day questionnaire was analyzed by one-way ANOVA examining the effect of drug treatment. The Drug vs. Money Choice questionnaire data were analyzed by one-way repeated measures ANOVA examining the effects of each zolpidem dose on overall percentages of drug choices relative to money (irrespective of monetary value) throughout the session. Additional comparisons between zolpidem dose and each monetary value were evaluated by one-way repeated measures ANOVA examining the percentages of drug choice relative to the specific amount of money, throughout the session. All data were analyzed using standard statistical software (SigmaStat 3.1; Systat Software, Inc.; San Jose, CA and SPSS 17.0 for Windows; SPSS Inc.; Chicago, IL) with alpha set at 0.05. When appropriate, ANOVA treatment effects were assessed further post hoc using Bonferroni t-tests for multiple comparisons.
Results
ARCI
Of the five ARCI items, only PCAG and BG were affected by zolpidem. For PCAG, there were significant effects of treatment [F(3,30)= 6.68, p< 0.001], time [F(11,110)= 6.60, p< 0.001], and the interaction between them [F(33,330)= 1.83, p< 0.005]. Post-hoc analyses revealed significant effects of treatment at a number of time points, such that the 5 mg dose increased ratings earlier than the 10 mg dose, and the duration of the increase in PCAG was longest for the highest dose (20 mg; Figure 1, top). Similar results were found for BG, in that not only were there significant effects of treatment [F(3,30)= 4.77, p<0.01], time [F(11,110)= 7.10, p< 0.001], and the significant interaction between them [F(33,330)= 1.52, p< 0.05], but post-hoc analyses revealed significant reductions in BG scores that persisted for a longer duration at the 20 mg dose (Figure 1, bottom). Ratings of MBG, AMPH, and LSD showed no treatment effects (data not shown).
Figure 1.
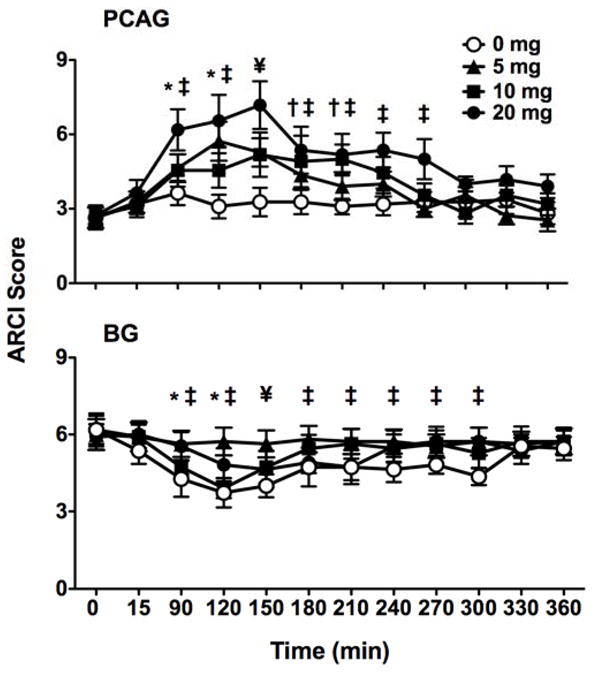
Zolpidem-induced increases in the sedative/intoxicating effects [Pentobarbital, Chlorpromazine, Alcohol Group (PCAG; top)] as well as decreases in the stimulant-like effects [Benzedrine Group (BG; bottom)] as assessed by the ARCI. All values are means ± SEM. Symbols represent significant differences from placebo (p< 0.05) where * is for 5 mg, † is for 10 mg, ‡ is for 20 mg, and ¥ denotes significance for all three doses. Maximum rating scales for PCAG and BG are 15 and 13, respectively; scales have been shortened for illustrative clarity.
VAS
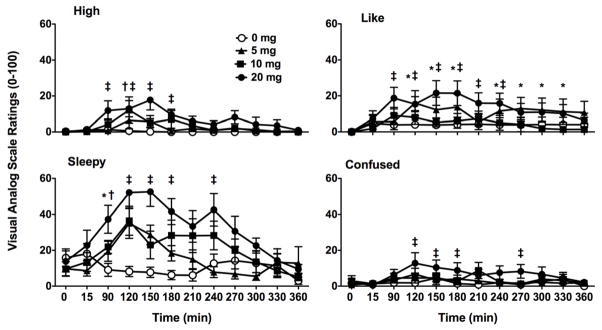
Ratings for most items on the visual analog scale showed an effect of treatment, time, or both. Significant main effects of treatment were observed primarily following the 20 mg dose, such that relative to all other doses (0, 5, and 10 mg), zolpidem increased self-reported ratings of “high” [F(3,330)= 9.95, p< 0.001], “sleepy” [F(3,330)= 12.93, p< 0.001], “good effects” [F(3,330)= 13.53, p< 0.001], “dizzy” [F(3,330)= 8.18, p< 0.001], and “bad effects” [F(3,330)= 6.85, p< 0.001]. Similarly, the highest dose of zolpidem increased ratings of “nauseous” [F(3,330)= 5.06, p< 0.01], “loose” [F(3,330)= 3.58, p< 0.025], “like” [F(3,330)= 3.92, p< 0.02], and “mentally slow” [F(3,330)= 5.10, p< 0.01] relative to placebo. “Confused” [F(3,330)= 5.68, p<0.005] was increased relative to the lowest dose as well as placebo.
Over the course of the experimental session, only “high” [F(33,330)= 1.72, p< 0.01], “sleepy” [F(33, 330)= 2.68, p< 0.001], “like” [F(33,330)= 1.61, p< 0.025], and “confused” [F(33,330)= 1.68, p< 0.02] showed a significant interaction between treatment and time (Figure 2). When participants rated their feelings of “high”, placebo and 5 mg zolpidem had no effect. The 10 mg dose increased feelings of “high” at 120 min, but 20 mg increased those feelings at 90, 120, 150, and 180 min. Ratings of “sleepy” were not affected by placebo, but were increased 120 min after administration by 5 and 10 mg, while 20 mg increased ratings at 90, 120, 150, 180, and 240 min. Placebo and the therapeutic dose of zolpidem had no effect on self-reported ratings of drug liking, while the low dose increased feelings of “like” for much of the experimental session (e.g., 120, 150, 180, 240, 270, 300, and 330 min post-administration). The high dose increased ratings of “like” at similar times, appearing as soon as 90 min, but not persisting beyond the 240 min time point. In contrast to the other items, only the supra-therapeutic dose (20 mg) had an effect on ratings of “confused” that occurred 120 150, 180, and 270 min post-administration.
Figure 2.
Zolpidem-related changes in self-reported subjective effects over the course of the experimental session as assessed with the VAS. All values are means ± SEM. Symbols represent increases in ratings of “high”, “sleepy”, “like”, and “confused” that were significantly different from placebo (p< 0.05), where * is for 5 mg, † is for 10 mg, and ‡ denotes significance for 20 mg. Range of VAS ratings was from 0 to 100; scales have been shortened for illustrative clarity.
Drug vs. Money Choice
The choice questionnaire contained ten hypothetical choices to be made between the drug treatment the participant had received that day and various amounts of money. No dose of zolpidem modulated choice relative to placebo at any individual time point, and participants consistently chose money over zolpidem throughout the experimental session. Given the lack of effect of time, data for each drug vs. money choice were averaged across the entire experimental session. Comparisons between each specific monetary value and the different doses of zolpidem also failed to reveal significant treatment effects, particularly at monetary values greater than $1 (data not shown).
End-of-Day
Four of the five items on the end-of-day questionnaire demonstrated significant treatment effects. Zolpidem was different from placebo when participants rated “drug strength” [F(3,30)= 10.97, p< 0.001], “drug liking” [F(3,30)= 4.76, p<0.001], “good effects” [F(3,30)= 5.02, p<0.01], and “bad effects” [F(3,30)= 3.78, p< 0.025], but there was only a trend toward an effect on “would take drug again” [F(3,30)= 2.82, p= 0.055]. As shown in Figure 3, participants rated all three doses of zolpidem as having more “drug strength” than placebo, while the other effects were a function primarily of the highest dose.
Figure 3.
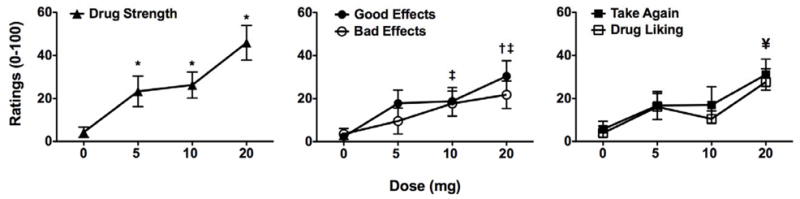
Self-reported ratings of the subjective effects “drug strength”, “good effects”, “bad effects”, “drug liking”, and “would take drug again” on a questionnaire administered at the end of the experimental session. All values are mean ± SEM. Symbols represent significant differences from placebo (p< 0.05), where † represents differences in Good Effects, ‡ is Bad Effects, and ¥ is Drug Liking. Range of ratings was from 0 to 100; scales have been shortened for illustrative clarity.
Physiological Measures
Analyses of the physiological data indicated no treatment effects for measures of heart rate, oxygen saturation, and skin temperature (data not shown). Systolic (but not diastolic) blood pressure exhibited a significant treatment effect [F(3,30)= 3.24, p< 0.05] such that average systolic pressure (mean ± SEM) over the course of the entire experimental session was higher after participants received the 10 mg dose (114.0 ± 0.9) relative to placebo (110.0 ± 0.8), while the 5 mg (111.8 ± 0.5) and 20 mg (112.6 ± 0.9) doses had no effect.
Discussion
The present study documented the subjective effects of multiple doses (0, 5, 10, or 20 mg) of zolpidem across a six-hour experimental session in healthy drug-naïve volunteers. In general, zolpidem increased self-reported measures of sedation, and although abuse-related feelings such as “high”, “like”, and “good effects” were reported (particularly at the highest dose administered), zolpidem had no impact on hypothetical choices between zolpidem and money.
Subjective drug effects were evaluated using self-report instruments such as the ARCI and an investigator-constructed VAS. Consistent with a number of other published studies (Evans et al., 1990; Rush and Griffiths, 1996; Rush et al., 1998; Stoops and Rush, 2003; Licata et al., 2008, 2009), sedative-like effects of zolpidem were revealed by increases in ratings on the sedation/intoxication (PCAG) scale and corresponding decreases on the stimulant-like (BG) scale, following administration of both the therapeutic (5 and 10 mg) and supra-therapeutic (20 mg) doses. Zolpidem also increased ratings of “sleepy”, although the lower doses resulted in only isolated increases in “sleepy”, suggesting that the standardized questionnaire may have superior sensitivity to the VAS when assessing these effects (de Wit and Griffiths, 1991). No zolpidem-induced changes in euphoric (MBG), dysphoric/psychotomimetic (LSD), or amphetamine-like (AMPH) effects were reported using the ARCI, while there were VAS increases in ratings of “confused” as well as “high” and “like”.
Over the course of the session the VAS ratings of abuse-related items were increased to some extent by all three doses of zolpidem. Specifically, the 10 and 20 mg doses increased ratings of “high”, while “like” was increased by 5 and 20 mg. It is not surprising that the 10 mg dose did not engender feelings of “like” since this dose increased ratings of overall “bad effects” on the questionnaire administered at the end of the session, and it produced a number of generally unpleasant feelings in healthy volunteers (Licata et al., 2008, 2009). In this regard, 10 mg may be ideally situated on the dose-response function for therapeutic use. In contrast, even though high doses of zolpidem have been shown to produce nausea and/or emesis (Evans et al., 1990; Balkin et al., 1992; Rush et al., 1998, 1999) and here 20 mg produced overall “bad effects” on the End-of-Day questionnaire, it also increased overall “good effects” and “drug liking”, albeit modestly. Therefore, the supra-therapeutic dose of zolpidem in particular produces some abuse-related subjective effects in drug-naïve healthy individuals.
Despite increased feelings of being high or liking the drug that was administered, End-of-Day ratings for “would take drug again” did not reach significance for any dose of zolpidem in these drug-naïve participants. Moreover, findings from the Drug vs. Money Choice questionnaire revealed that when participants were given a hypothetical choice between the drug administered to them or various sums of money, zolpidem was almost never chosen at any time during the course of the session. In other words, healthy non drug-abusing individuals preferred money in amounts as small as $ 0.35 in lieu of any dose of zolpidem. Although the extent to which a drug high is a positive subjective experience that can influence later use is not known (Schuckit et al., 1997), drug liking has been positively associated with the likelihood of abuse in recreational drug abusers (see reviews by de Wit and Griffiths, 1991; Griffiths and Weerts, 1997). Whether this translates to a healthy drug-naïve population is unclear, but these results suggest that the zolpidem-induced subjective experiences of “high” and/or “like” were insufficiently rewarding to influence choice for drug over money or to precipitate willingness to take the drug again at some point in the future. The present discordant findings are not necessarily unusual; they are consistent with previous studies reporting dissociation between drug liking and the reinforcing effects of a number of drugs from different classes (Lamb et al., 1991; Stoops et al., 2005; Lile et al., 2010; but also see Roache and Griffiths, 1989).
While the present results are in agreement with another study in which drug-naïve participants chose money exclusively relative to zolpidem (Rush and Griffiths, 1996), the choice paradigm used here may have advantages over those reported on previously (e.g., Mumford et al., 1995; Griffiths et al., 1996; Correia and Little, 2006). For instance, since choices were purely hypothetical, they were not confounded by conditioned responses that would be elicited by reinforcement with either drug or money. Both are tangible reinforcers, and money in particular likely has acquired value through complex learned associations that subsequently may motivate responding differently than would a simulated reward (Di Chiara et al., 1999; Bray et al., 2010). Moreover, since hypothetical or imagined outcomes often drive goal-directed behavior in everyday life, presentation of a hypothetical choice in the laboratory may lead the individual to reveal the probable outcome if s/he were to be presented with a similar choice in situ. Thus, hypothetical choice procedures may expose at-risk tendencies among recreational drug users (Petry and Bickel, 1998) and healthy individuals alike. In contrast, when using choice conditions that offer the tangible reinforcement of a monetary reward, it is possible that thoughts would be directed primarily toward how money would be spent (Bray et al., 2010).
In addition to the information that potentially could be gleaned from using a hypothetical scenario, the choice paradigm employed here was designed to examine how chosen outcomes (i.e., money or drug) may change over time, as well as how they may be altered under the influence of drug. Administering the questionnaire repeatedly throughout the session was aimed at providing a unique perspective on the impact of reported subjective effects on any change in drug value since participants made their selections not only while experiencing the effects of zolpidem, but also after the drug effects had subsided. This potential was not realized in the context of the present results, and the choices that were made likely reflect the limited potential of zolpidem for abuse and/or dependence in individuals who have no history of drug or alcohol abuse.
While these findings are encouraging for the practitioner who would be inclined to prescribe zolpidem to treat transient insomnia, its abuse potential still is somewhat ambiguous. Therapeutic doses appeared not to pose any threat, but the highest dose of zolpidem in particular appeared to engender abuse-related effects. Interestingly, the extreme cases of abuse and dependence reported in the literature involved supra-therapeutic doses of zolpidem (Gilbert and Staats, 1997; Liappas et al., 2003; Krueger et al., 2005; Cubała and Landowski, 2007; Huang et al., 2007; Svitek et al., 2008). However, closer examination of the case studies revealed that although those zolpidem-abusing individuals had no histories of abusing drugs or alcohol, most had histories of psychiatric disturbances (Liappas et al., 2003; Cubała and Landowski, 2007; Huang et al., 2007) or chronic pain (Gilbert and Staats, 1997; Krueger et al., 2005), both of which may have led to titrating the dose of zolpidem upward without a physician’s supervision. Psychiatric illness may be considered a risk factor for abusing zolpidem (see review by Hajak et al., 2003) because benzodiazepine-type drugs commonly are prescribed to alleviate some of the symptoms associated with several Axis I disorders (e.g., Karmacharya et al., 2008; Rao and Zisook, 2009; Westenberg, 2009), while pain also may present an opportunity for abusing zolpidem, since antinociceptive effects have been demonstrated at high doses in preclinical pain models (Pick et al., 2005; Munro et al., 2008). Healthy participants in the present study did not report either upon examination during the screening visit, but future studies should investigate further the specific characteristics of a zolpidem-abusing population in order to differentiate who is likely to abuse this drug. Although zolpidem has been shown to be similar to benzodiazepines with respect to behavioral and subjective effects (see review by Rush, 1998), its unique behavioral pharmacology at the GABAA receptor (Benavides et al., 1988; Sanna et al., 2002) as well as its unlikely effects in the treatment of movement disorders (Abe, 2008) and brain injury and/or coma (Shames and Ring, 2008; Whyte and Myers, 2009) together suggest that the extent of how it acts in various populations of people cannot simply be assumed.
In summary, the present investigation demonstrated modest abuse-related effects of zolpidem in healthy drug-naïve participants. Specifically, a supra-therapeutic dose of zolpidem (20 mg) significantly increased abuse-related subjective effects relative to the therapeutic doses (5 and 10 mg) in drug-naïve participants. However, despite having experienced feelings of drug high, drug liking, and a willingness to take zolpidem again (provided it was given to them), those effects appeared insufficient to encourage participants to choose zolpidem over money at any point during the experimental session. One interpretation is that it seems unlikely that healthy individuals (i.e., in the absence of a history of drug abuse, psychiatric disturbance, or chronic pain) would seek out zolpidem on their own for the purpose of abuse. The alternative is that contrary to original beliefs, zolpidem may have modest potential for facilitating abuse and/or dependence.
Acknowledgments
This study was funded by the National Institute on Drug Abuse grants K01 DA023659 (SCL), T32 DA015036 (SEL), and K05 DA000343 (SEL).
Footnotes
Preliminary presentation of this work was made at the 2010 annual meeting of the College on Problems of Drug Dependence.
References
- Abe K. Zolpidem therapy for movement disorders. Recent Pat CNS Drug Discov. 2008;3:55–60. doi: 10.2174/157488908783421519. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Papadopoulos A, Seddon K, Nutt DJ. A comparison of the effects of a subtype selective and non-selective benzodiazepine receptor agonist in two CO(2) models of experimental human anxiety. J Psychopharmacol. 2009;23:117–122. doi: 10.1177/0269881108089603. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, O’Donnell VM, Wesensten N, McCann U, Belenky G. Comparison of the daytime sleep and performance effects of zolpidem versus triazoalm. Psychopharmacology. 1992;107:83–88. doi: 10.1007/BF02244970. [DOI] [PubMed] [Google Scholar]
- Benavides J, Peny B, Dubois A, Perrault G, Morel E, Zivkovic B, Scatton B. In vivo interaction of zolpidem with central benzodiazepine (BZD) binding sites (as labeled by [3H]Ro 15–1788) in the mouse brain. Preferential affinity of zolpidem for the omega 1 (BZD1) subtype. J Pharmacol Exp Ther. 1988;245:1033–1041. [PubMed] [Google Scholar]
- Bray S, Shimojo S, O’Doherty JP. Human medial orbitofrontal cortex is recruited during experience of imagined and real rewards. J Neurophysiol. 2010;103:2506–2512. doi: 10.1152/jn.01030.2009. [DOI] [PubMed] [Google Scholar]
- Caldwell JA, Caldwell JL. Fatigue in military aviation: an overview of US military-approved pharmacological countermeasures. Aviat Space Environ Med. 2005;76 (7 Suppl):C39–C51. [PubMed] [Google Scholar]
- Correia CJ, Little C. Use of a multiple-choice procedure with college student drinkers. Psychol Addict Behav. 2006;20:445–452. doi: 10.1037/0893-164X.20.4.445. [DOI] [PubMed] [Google Scholar]
- Cubała WJ, Landowski J. Seizure following sudden zolpidem withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:539–540. doi: 10.1016/j.pnpbp.2006.07.009. [DOI] [PubMed] [Google Scholar]
- de Wit H, Griffiths RR. Testing the abuse liability of anxiolytic and hypnotic drugs in humans. Drug Alcohol Depend. 1991;28:83–111. doi: 10.1016/0376-8716(91)90054-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, et al. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–1255. [PubMed] [Google Scholar]
- Gilbert DL, Staats PS. Seizure after withdrawal from supratherapeutic doses of zolpidem tartrate, a selective omega I benzodiazepine receptor agonist. J Pain Symptom Manage. 1997;14:118–120. doi: 10.1016/s0885-3924(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals-implications for problems of long-term use and abuse. Psychopharmacology. 1997;134:1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Exp Clin Psychopharmacol. 1996;4:97–106. [Google Scholar]
- Hajak G, Müller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction. 2003;98:1371–1378. doi: 10.1046/j.1360-0443.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- Huang MC, Lin HY, Chen CH. Dependence on zolpidem. Psychiatry Clin Neurosci. 2007;61:207–208. doi: 10.1111/j.1440-1819.2007.01644.x. [DOI] [PubMed] [Google Scholar]
- Jamieson AO, Zammit GK, Rosenberg RS, Davis JR, Walsh JK. Zolpidem reduces the sleep disturbance of jet lag. Sleep Med. 2001;2:423–430. doi: 10.1016/s1389-9457(00)00073-3. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potential of morphine-like drugs (methods used in man) In: Martin WR, editor. Drug Addiction I. 45/1. Heidelberg: Springer-Verlag; 1977. pp. 197–258. [Google Scholar]
- Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109:312–316. doi: 10.1016/j.jad.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Krueger TH, Kropp S, Huber TJ. High-dose zolpidem dependence in a patient with chronic facial pain. Ann Pharmacother. 2005;39:773–774. doi: 10.1345/aph.1E520. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, et al. The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. J Pharmacol Exp Ther. 1991;259:1165–1173. [PubMed] [Google Scholar]
- Liappas IA, Malitas PN, Dimopoulos NP, Gitsa OE, Liappas AI, Nikolaou ChK, Christodoulou GN. Zolpidem dependence case series: possible neurobiological mechanisms and clinical management. J Psychopharmacol. 2003;17:131–135. doi: 10.1177/0269881103017001723. [DOI] [PubMed] [Google Scholar]
- Licata SC, Penetar DM, Dunlap S, Lukas SE. A therapeutic dose of zolpidem has limited abuse-like effects in drug-naïve females: A pilot study. Eur J Pharmacol. 2008;598:64–67. doi: 10.1016/j.ejphar.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Jensen JE, Penetar DM, Prescot AP, Lukas SE, Renshaw PF. A therapeutic dose of zolpidem reduces thalamic GABA in healthy volunteers: a proton MRS study at 4 T. Psychopharmacology. 2009;203:819–829. doi: 10.1007/s00213-008-1431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. The reinforcing, self-reported performance and physiological effects of Delta9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in cannabis users. Behav Pharmacol. 2010;21:29–38. doi: 10.1097/FBP.0b013e32833470d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeth BD, McNamara RM, Ankel FK, Mason EJ, Ling LJ, Flottemesch TJ, Asplin BR. Modafinil and zolpidem use by emergency medicine residents. Acad Emerg Med. 2009;16:1311–1317. doi: 10.1111/j.1553-2712.2009.00586.x. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Evans SM, Fleishaker JC, Griffiths RR. Alprazolam absorption kinetics affects abuse liability. Clin Pharmacol Ther. 1995;57:356–365. doi: 10.1016/0009-9236(95)90162-0. [DOI] [PubMed] [Google Scholar]
- Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EØ, Larsen JS, Ahring PK, et al. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2 carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2008;327:969–981. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK. Polydrug abuse in heroin addicts: a behavioral economic analysis. Addiction. 1998;93:321–335. doi: 10.1046/j.1360-0443.1998.9333212.x. [DOI] [PubMed] [Google Scholar]
- Pick CG, Chernes Y, Rigai T, Rice KC, Schreiber S. The antinociceptive effect of zolpidem and zopiclone in mice. Pharmacol Biochem Behav. 2005;81:417–423. doi: 10.1016/j.pbb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Rao S, Zisook S. Anxious depression: clinical features and treatment. Curr Psychiatry Rep. 2009;11:429–436. doi: 10.1007/s11920-009-0065-2. [DOI] [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Diazepam and triazolam self-administration in sedative abusers: concordance of subject ratings, performance and drug self-administration. Psychopharmacology. 1989;99:309–315. doi: 10.1007/BF00445549. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Tornatzky W, Licata SC. Anti-conflict effects of benzodiazepines in rhesus monkeys: relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology. 2006;184:201–211. doi: 10.1007/s00213-005-0228-8. [DOI] [PubMed] [Google Scholar]
- Rush CR. Behavioral pharmacology of zolpidem relative to benzodiazepines: a review. Pharmacol Biochem Behav. 1998;61:253–269. doi: 10.1016/s0091-3057(98)00102-6. [DOI] [PubMed] [Google Scholar]
- Rush CR, Griffiths RR. Zolpidem, triazolam, and temazepam: behavioral and subject-rated effects in normal volunteers. J Clin Psychopharmacol. 1996;16:146–157. doi: 10.1097/00004714-199604000-00007. [DOI] [PubMed] [Google Scholar]
- Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ. Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol. 1998;18:154–165. doi: 10.1097/00004714-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW, Wright K. Acute behavioral effects and abuse potential of trazodone, zolpidem and triazolam in humans. Psychopharmacology. 1999;144:220–233. doi: 10.1007/s002130050997. [DOI] [PubMed] [Google Scholar]
- Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–110. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J. The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol. 1997;58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Shames JL, Ring H. Transient reversal of anoxic brain injury-related minimally conscious state after zolpidem administration: a case report. Arch Phys Med Rehabil. 2008;89:386–388. doi: 10.1016/j.apmr.2007.08.137. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Differential effects in humans after repeated administrations of zolpidem and triazolam. Am J Drug Alcohol Abuse. 2003;29:281–299. doi: 10.1081/ada-120020513. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of methylpheniate: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;177:349–355. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- Svitek J, Heberlein A, Bleich S, Wiltfang J, Kornhuber J, Hillemacher T. Extensive craving in high dose zolpidem dependency. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:591–592. doi: 10.1016/j.pnpbp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Westenberg HG. Recent advances in understanding and treating social anxiety disorder. CNS Spectr. 2009;14:24–33. doi: 10.1017/s1092852900027267. [DOI] [PubMed] [Google Scholar]
- Whyte J, Myers R. Incidence of clinically significant responses to zolpidem among patients with disorders of consciousness: a preliminary placebo controlled trial. Am J Phys Med Rehabil. 2009;88:410–418. doi: 10.1097/PHM.0b013e3181a0e3a0. [DOI] [PubMed] [Google Scholar]
- Woods JH, Katz JL, Winger G. Benzodiazepines: Use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]