Abstract
Objectives
While highly active antiretroviral therapy (HAART) decreases long-term morbidity and mortality, its short-term effect on hospitalization rates is unknown. The primary objective of this study was to determine hospitalization rates over time in the year after HAART initiation for virological responders and nonresponders.
Methods
Hospitalizations among 1327 HAART-naïve subjects in an urban HIV clinic in 1997–2007 were examined before and after HAART initiation. Hospitalization rates were stratified by virological responders (≥ 1 log10 decrease in HIV-1 RNA within 6 months after HAART initiation) and nonresponders. Causes were determined through International Classification of Diseases, 9th Revision (ICD-9) codes and chart review. Multivariate negative binomial regression was used to assess factors associated with hospitalization.
Results
During the first 45 days after HAART initiation, the hospitalization rate of responders was similar to their pre-HAART baseline rate [75.1 vs. 78.8/100 person-years (PY)] and to the hospitalization rate of nonresponders during the first 45 days (79.4/100 PY). The hospitalization rate of responders fell significantly between 45 and 90 days after HAART initiation and reached a plateau at approximately 45/100 PY from 91 to 365 days after HAART initiation. Significant decreases were seen in hospitalizations for opportunistic and nonopportunistic infections.
Conclusions
The first substantial clinical benefit from HAART may be realized by 90 days after HAART initiation; providers should keep close vigilance at least until this time.
Keywords: AIDS-defining illness, antiretroviral therapy, healthcare utilization, hospitalization, immune reconstitution
Introduction
In the short term after starting highly active antiretroviral therapy (HAART), HIV-infected patients may have an increased risk of serious illness as a result of an immune reconstitution inflammatory syndrome (IRIS), a traditional opportunistic infection (OI), or an adverse drug reaction. While HAART is known to decrease hospitalization rates and mortality in the long term [1–7], the time at which hospitalization risk declines during the weeks to months immediately following HAART initiation is not clear.
In studies in high-income countries conducted since the advent of HAART in 1996, AIDS-defining illnesses (ADIs) and non-ADI infections have been the most frequent reasons for hospital admission [1,4,6,8–11]. The next most common categories of admitting diagnoses have varied among mental illness, gastrointestinal and hepatic disease, and cardiovascular disease. Studies have compared hospitalization rates for these disease categories in the several years prior to the advent of HAART vs. the several years after its advent among cohorts of patients, not all of whom were prescribed HAART [1,4,5,12–17]. These studies did not determine changes in an individual’s risk of serious illness within these disease categories in the weeks to months immediately after initiating HAART.
Our main objective was to measure the rates of all-cause hospitalizations over time in the year after HAART initiation using an urban clinical cohort of HAART-naïve, HIV-infected patients. To assess the effect on hospitalization rates produced by having a significant virological response to HAART, we compared hospitalization rates in virological responders and nonresponders. We examined causes of hospitalization by diagnostic category.
Methods
Population
All patients who engage in HIV continuity care with the Johns Hopkins AIDS service are offered enrolment in the observational Johns Hopkins HIV Clinical Cohort (JHHCC). Fewer than 1% of patients have refused [18]. As part of this study, trained abstractors extract demographic, pharmaceutical and hospitalization data from patient charts at 6-month intervals. Laboratory data are retrieved directly from the hospital laboratory system. The JHHCC is approved by the Institutional Review Board of the Johns Hopkins School of Medicine. All HAART-naïve patients initiating HAART (previous antiretroviral use was allowed) between 1 January 1997 and 31 December 2006 were considered for inclusion in this analysis.
Exposure variables
HAART was defined as any combination of at least three drugs which included at least two classes selected from the nucleoside reverse transcriptase inhibitor (NRTI), nonnucleoside reverse transcriptase inhibitor (NNRTI) and protease inhibitor (PI) classes. CD4 cell count and HIV-1 RNA level at HAART initiation were defined as the clinical measurements on the day of HAART initiation or, if not available, the closest value prior to HAART initiation within 90 days.
For purposes of analysis, race/ethnicity was categorized as African American and non-African American and HIV risk factor as injecting drug use (IDU) and non-IDU. Calendar time for the date of HAART initiation was categorized as 1997–1998, 1999–2002 and 2003–2006, reflecting milestones in antiretroviral development (Food and Drug Administration approval of efavirenz in September 1998 and of atazanavir in June 2003).
Virological and CD4 responses to HAART were determined using the single measurements made between 120 and 180 but closest to 180 days after HAART initiation. Virological responders were defined as having a decrease in HIV-1 RNA of ≥ 1 log10 HIV-1 RNA copies/mL or suppression below the detection limit of the assay. Their HIV-1 RNA levels may subsequently have risen after 180 days. Nonresponders did not achieve a drop in HIV-1 RNA of ≥ 1 log10 copies/mL at 180 days. For analysis purposes, CD4 response was categorized as being above or below the median change in CD4 seen among virological responders. Of the 1685 patients initially considered for inclusion, 300 were excluded because of insufficient virological data.
Outcome variables
Number of hospital admissions per time period was the primary study outcome. Counts of distinct hospital admission dates were obtained, beginning with the period from 180 days prior to HAART initiation to the day of HAART initiation. Patients were then followed for 365 days after HAART initiation, with hospitalization counts assessed in time periods of 1–45, 46–90, 91–180 and 181–365 days after initiation. For persons enrolling < 180 days prior to HAART initiation, the clinic enrolment date was the start of observation. Observation ended at the sooner of (1) 365 days after HAART initiation, (2) death, (3) regimen change (including complete HAART discontinuation or any change from the initial regimen except for dosing changes), or (4) study discontinuation as a result of voluntary withdrawal or loss to follow-up.
The primary reason for each hospitalization was assessed through International Classification of Diseases, Ninth Revision (ICD-9) codes and physician chart abstraction. Hospitalizations related to clinical trials (140) were excluded from all analyses. Using a method that has twice been validated in our cohort with over 95% accuracy compared with chart review [5,15], the first of the top three ICD-9 codes that was neither 042 (AIDS) nor 112.0 (thrush) was defined as the primary diagnosis. Using Clinical Classifications Software (CCS) developed by the Agency for Healthcare Research and Quality [19], the primary ICD-9 code was classified into one of 17 first-level categories: infectious; neoplastic; endocrine, nutritional, metabolic, or immune; haematological; psychiatric (including substance abuse); neurological; cardiovascular; pulmonary; digestive and hepatic; renal (including genitourinary); pregnancy-related; dermatological; musculoskeletal and rheumatological; congenital; perinatal; traumatic; and other. The CCS assigns many infectious conditions to a first-level organ system category rather than to the infectious category. Additional CCS levels were used to reassign the following to the infectious category: central nervous system infection; infection of the eye; otitis media; endocarditis; respiratory infection; intestinal infection; anal and rectal conditions; peritonitis and intestinal abscess; urinary tract infections; inflammatory conditions of the genitals; skin and subcutaneous tissue infections; infective arthritis and osteomyelitis; infection and inflammation of an internal prosthesis; postoperative infection. Finally, a separate category for ADI was generated, and appropriate admissions were reassigned according to individual ICD-9 codes [20]. Any non-first admission for bacterial pneumonia (ICD-9 codes ≥ 481 and < 483) was categorized as an ADI.
IRIS was defined according to established criteria [21,22] as signs or symptoms that were consistent with an inflammatory and/or atypical presentation of an OI or malignancy, were not medication side effects, and occurred in a virological responder within 6 months of HAART initiation. The pathogen or process had to be identified microbiologically or histopathologically. To determine IRIS hospitalizations, chart abstraction specifically for IRIS was undertaken on records of all virological responders admitted within 6 months of HAART initiation. For purposes of analysis, all IRIS cases were considered ADIs.
Analysis
Baseline characteristics of responders and nonresponders were compared using the χ2 or Wilcoxon rank-sum test. Negative binomial regression was used to examine hospitalization rates, which were calculated per 100 person-years (PY) by dividing number of hospital admissions within a time period for each subject by accrued person-time based on the exact day of a subject’s entry or exit into observation. Crude hospitalization rates for responders and nonresponders in various time periods were estimated in a regression model which included response status, time periods before (the 180 days prior) and after initiation (1–45, 46–90, 91–180 and 181–365 days) and the interaction terms between these variables. Each baseline exposure was evaluated with bivariate regression. The final multivariate model included all exposure variables for which the bivariate P was < 0.2. A population-averaged approach employing generalized estimating equations was used to estimate regression coefficients and obtain robust standard errors adjusted for the correlated nature of repeated admissions among patients [23]. P-values < 0.05 were considered statistically significant. stata 10.0 (StataCorp LP, College Station, TX, USA) was used for all analyses [24].
Sensitivity and subgroup analyses
Over 75% of IRIS cases in our cohort occurred in persons with initiation CD4 counts < 100 cells/µL [25]. Therefore, a sensitivity analysis was performed by restricting the analysis to subjects with initiation CD4 counts < 100 cells/µL. A relatively brief period of adherence to HAART may produce a 1 log10 copies/mL drop in HIV-1 RNA level. Therefore, a second sensitivity analysis was performed by defining virological response as a ≥ 2 log10 copies/mL drop in HIV-1 RNA at 6 months after initiation. Because subjects censored for regimen change may have had high hospitalization rates because of drug toxicity, we performed a third sensitivity analysis by excluding subjects thus censored. The 604 subjects reporting IDU as an HIV risk factor make up a significant portion (44%) of our study cohort. We performed a subgroup analysis of hospitalization rates among these subjects.
Results
The analysis was performed on 1385 HAART-naïve patients, almost three-quarters of whom (1010) were responders. Responders tended to be older than nonresponders, with median ages at the time of HAART initiation being 40 and 38 years, respectively (P < 0.01; Table 1). Responders were less likely to be female (34% vs. 40%; P = 0.04) and African American (75% vs. 86%; P < 0.001). A smaller proportion of responders than nonresponders initiated HAART during 1997–1998 (38% vs. 58%; P < 0.001). The median CD4 counts at HAART [interquartile ranges (IQRs)] for patients initiating HAART in 1997–1998, 1999–2002 and 2003–2006 were 156 (41, 331), 133 (30, 266), and 196 (80, 291) cells/µL, respectively. Among subjects with CD4 counts at HAART < 50 cells/µL, responders were more likely than nonresponders to be prescribed Mycobacterium avium prophylaxis (92% vs. 78%; P < 0.001). Median changes in CD4 count at 6 months (IQRs) were increases of 101 cells/µL (39, 173) for responders and 7 cells/µL (−21, 61) for nonresponders.
Table 1.
Baseline characteristics stratified by virological response status
| Responders* (n = 1010) |
Nonresponders* (n = 375) |
P | |
|---|---|---|---|
| Age at HAART initiation | |||
| 18–29 years | 113 (11.2) | 48 (12.8) | 0.02 |
| 30–39 years | 393 (38.9) | 175 (46.7) | |
| 40–49 years | 372 (36.8) | 118 (31.5) | |
| ≥50 years | 132 (13.1) | 34 (9.1) | |
| Median (IQR) age (years) | 40 (34, 45) | 38 (33, 43) | <0.01† |
| Gender | |||
| Female | 342 (33.9) | 149 (39.7) | 0.04 |
| Male | 668 (66.1) | 226 (60.3) | |
| Racial/ethnic category | |||
| African American | 755 (74.8) | 324 (86.4) | <0.001 |
| White | 233 (23.1) | 44 (11.7) | |
| Hispanic (non-African American) | 7 (0.7) | 6 (1.6) | |
| Asian | 4 (0.4) | 0 | |
| Other | 11 (1.1) | 1 (0.3) | |
| HIV risk factors | |||
| IDU | 201 (19.9) | 90 (24.0) | 0.10 |
| IDU-heterosexual | 177 (17.5) | 81 (21.6) | |
| IDU-MSM | 43 (4.3) | 12 (3.2) | |
| Heterosexual | 304 (30.1) | 108 (28.8) | |
| MSM | 221 (21.9) | 63 (16.8) | |
| Unknown/other | 64 (6.3) | 21 (5.6) | |
| CD4 count at HAART initiation | |||
| <50 cells/µL | 257 (25.5) | 115 (30.7) | 0.21 |
| 50–199 cells/µL | 326 (32.3) | 108 (28.8) | |
| 200–349 cells/µL | 250 (24.8) | 94 (25.1) | |
| ≥350 cells/µL | 177 (17.5) | 58 (15.5) | |
| Median (IQR) CD4 count (cells/µL) | 158 (48, 298) | 151 (30, 287) | 0.12† |
| HIV-1 RNA at HAART initiation | |||
| <4 log10 copies/mL | 187 (18.5) | 80 (21.3) | 0.31 |
| 4–5 log10 copies/mL | 391 (38.7) | 150 (40.0) | |
| ≥5 log10 copies/mL | 432 (42.8) | 145 (38.7) | |
| Median (IQR) HIV-1 RNA (log10 copies/mL) | 4.8 (4.3, 5.4) | 4.8 (4.1, 5.3) | 0.08† |
| HAART type | |||
| NNRTI (plus ≥2 NRTIs) | 360 (36.9) | 93 (25.5) | <0.001 |
| PI (plus ≥2 NRTIs) | 503 (51.5) | 231 (63.3) | |
| Both PI and NNRTI (plus ≥1 NRTI) | 114 (11.7) | 41 (11.2) | |
| Calendar era of HAART initiation | |||
| 1997–1998 | 383 (37.9) | 217 (57.9) | <0.001 |
| 1999–2002 | 384 (38.0) | 105 (28.0) | |
| 2003–2006 | 243 (24.1) | 53 (14.1) | |
| PCP prophylaxis at HAART initiation (if CD4 <200 cells/µL) | 0.74 | ||
| Use | 550 (94.3) | 209 (93.7) | |
| Nonuse | 33 (5.7) | 14 (6.3) | |
| MAC prophylaxis at HAART initiation (if CD4 <50 cells/µL) | <0.001 | ||
| Use | 236 (91.8) | 90 (78.3) | |
| Nonuse | 21 (8.2) | 25 (21.7) | |
| Median (IQR) CD4 increase at 6 months after HAART initiation | 101 (39, 173) | 7 (−21, 61) | <0.001 |
Values are n (%) unless otherwise specified.
Statistical comparisons were performed using the χ2 test except where
indicates that the Wilcoxon rank-sum test was used. Significant values are shown in bold.
HAART, highly active antiretroviral therapy; IDU, injecting drug use; IQR, interquartile range; MSM, men who have sex with men; PI, protease inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PCP, Pneumocystis pneumonia; MAC, Mycobacterium avium complex.
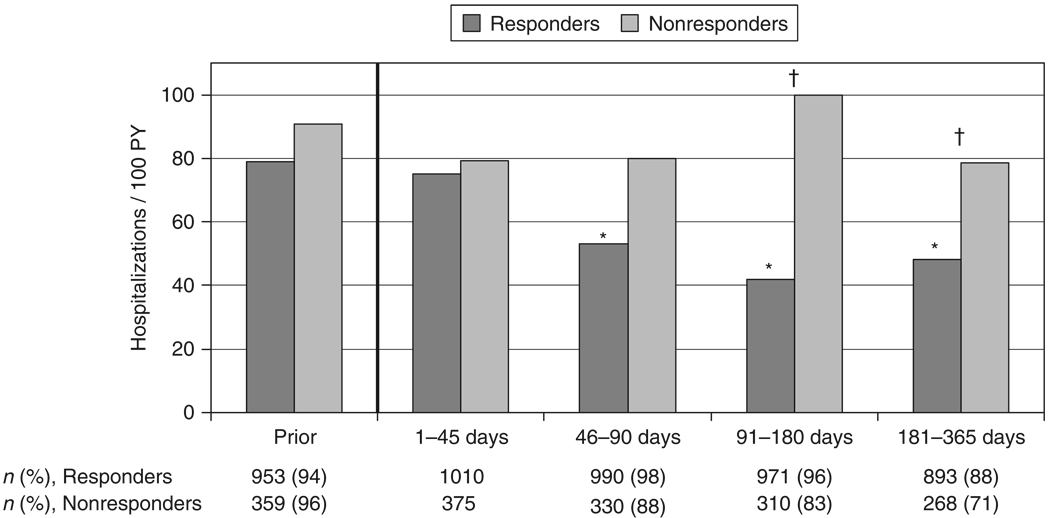
Eighty-eight per cent of responders and 71% of nonresponders were observed > 180 days after HAART initiation and contributed to each post-initiation time period (P < 0.001; Fig. 1). Seventy-nine per cent of responders and 61% of nonresponders were observed for 365 days without censoring. Responders were censored because of regimen change less frequently than nonresponders (13% vs. 34%; P < 0.001). There was no significant difference in censoring because of withdrawal/loss to follow-up (7% of responders and 4% of nonresponders; P = 0.06) or death (1% vs. 2%; P = 0.29). Among the 1385 subjects, there were 23 deaths within 365 days following HAART initiation. There were no significant differences in death rates across time periods or for responders vs. nonresponders within time periods. For the 6-month period prior to HAART initiation, 94% of responders and 96% of nonresponders contributed some observation time; 50% of responders and 68% of nonresponders contributed a full 180 days (P < 0.001).
Fig. 1.
Hospitalization rates for virological responders and nonresponders prior to and over the first year following highly active antiretroviral therapy (HAART) initiation. *Indicates P < 0.05 for the relative rate (RR) vs. days 1–45 for responders; †indicates P < 0.05 for the RR for responders vs. nonresponders within the time period. ‘Prior’ is 180 days preceding HAART initiation. n, number of persons observed within each time period. PY, person-years.
The all-cause hospitalization rate in virological responders during the first 45 days following HAART initiation was 75.1/100 PY [95% confidence interval (CI) 58.2, 96.8/100 PY; Fig. 1]. This was comparable to their pre-HAART rate of 78.8/100 PY (95% CI 66.5, 93.4/100 PY). For responders, the crude hospitalization rate declined statistically significantly during the 46 to 90-day time period, with a relative rate (RR) vs. the first 45 days of 0.71 (95% CI 0.51, 0.98). From 90 days to the end of the year, the hospitalization rate for responders stabilized at near 45/100 PY (RR for days 91–180 vs. the first 45 days, 0.56; 95% CI 0.40, 0.78). For nonresponders, there was no statistically significant change in all-cause hospitalization rates across time periods, with the point estimates ranging from 78.7 to 99.7/100 PY (Fig. 1). Fewer than half of all subjects (34% of responders and 46% of nonresponders; P < 0.001) were ever hospitalized over the entire period beginning 180 days before HAART initiation to 365 days afterwards.
In multivariate analysis (Table 2), responders’ hospitalization rates retained an identical pattern of statistically significant decrease in later time periods vs. earlier periods (RR 0.59; 95% CI 0.42, 0.82 for responders in days 91–180 vs. days 1–45). Having an increase in CD4 count of at least 101 cells/µL (the median increase in CD4 count in virological responders) had a borderline association with a decreased risk of hospitalization (RR 0.83; 95% CI 0.67, 1.03). Additional factors significantly associated with hospitalization included being a nonresponder in the 91–180 day (RR vs. responders 2.14; 95% CI 1.41, 3.25) and 181–365 day (RR vs. responders 1.43; 95% CI 1.00, 2.04) time periods; female gender; African American race; IDU; and lower CD4 cell count at HAART initiation.
Table 2.
Bivariate and multivariate relative all-cause hospitalization rates
| Simple incidence rate ratio (95% CI) | P | Multivariate incidence rate ratio (95% CI) | P | |
|---|---|---|---|---|
| Virological responders over time | ||||
| Prior 6 months | 1.05 (0.80, 1.38) | 0.73 | 1.11 (0.84, 1.47) | 0.44 |
| Days 1–45 after HAART initiation | 1.00 | Ref | 1.00 | Ref |
| Days 46–90 | 0.71 (0.51, 0.98) | 0.04 | 0.74 (0.53, 1.03) | 0.08 |
| Days 91–180 | 0.56 (0.40, 0.78) | 0.001 | 0.59 (0.42, 0.82)† | 0.002 |
| Days 181–365 | 0.64 (0.47, 0.88) | 0.006 | 0.69 (0.49, 0.95)† | 0.02 |
| Nonresponders over time | ||||
| Prior 6 months | 1.14 (0.78, 1.69) | 0.49 | 1.19 (0.78, 1.81) | 0.41 |
| Days 1–45 after HAART initiation | 1.00 | Ref | 1.00 | Ref |
| Days 46–90 | 1.01 (0.60, 1.68) | 0.97 | 1.14 (0.68, 1.90) | 0.62 |
| Days 91–180 | 1.26 (0.79, 2.01) | 0.34 | 1.43 (0.89, 2.31) | 0.14 |
| Days 181–365 | 0.99 (0.63, 1.55) | 0.97 | 1.11 (0.70, 1.76) | 0.65 |
| Age | ||||
| 18–29 years | 1.00 | Ref | ||
| 30–39 years | 1.00 (0.67, 1.49) | 0.99 | ||
| 40–49 years | 1.15 (0.76, 1.74) | 0.52 | ||
| ≥50 years | 1.21 (0.77, 1.92) | 0.41 | ||
| Gender | ||||
| Female | 1.39 (1.13, 1.72) | 0.002 | 1.41 (1.14, 1.74) | 0.001 |
| Male | 1.00 | Ref | 1.00 | Ref |
| Race | ||||
| African American | 1.93 (1.42, 2.63) | <0.001 | 1.46 (1.06, 1.99) | 0.02 |
| Non-African American | 1.00 | Ref | 1.00 | Ref |
| HIV risk factor | ||||
| IDU | 1.55 (1.26, 1.92) | <0.001 | 1.43 (1.17, 1.76) | 0.001 |
| Non-IDU | 1.00 | Ref | 1.00 | Ref |
| CD4 count at HAART initiation | ||||
| <50 cells/µL | 2.63 (2.06, 3.36) | <0.001 | 2.27 (1.65, 3.13) | <0.001 |
| 50–199 cells/µL | 1.46 (1.11, 1.92) | 0.007 | 1.35 (1.01, 1.81) | 0.04 |
| ≥200 cells/µL | 1.00 | Ref | 1.00 | Ref |
| HIV-1 RNA at HAART initiation | ||||
| <4 log10 copies/mL | 1.00 | Ref | 1.00 | Ref |
| 4–5 log10 copies/mL | 1.08 (0.78, 1.50) | 0.64 | 0.94 (0.68, 1.29) | 0.69 |
| ≥5 log10 copies/mL | 1.82 (1.33, 2.47) | <0.001 | 1.26 (0.89, 1.79) | 0.20 |
| HAART type | ||||
| NNRTI (plus ≥2 NRTIs) | 1.00 | Ref | ||
| PI (plus ≥2 NRTIs) | 1.00 (0.80, 1.25) | 0.99 | ||
| Both PI and NNRTI (plus ≥1 NRTI) | 1.07 (0.77, 1.49) | 0.68 | ||
| Calendar era of HAART initiation | ||||
| 1997–1998 | 1.00 | Ref | ||
| 1999–2002 | 1.11 (0.88, 1.40) | 0.37 | ||
| 2003–2006 | 1.11 (0.82, 1.49) | 0.49 | ||
| PCP prophylaxis at HAART initiation* | ||||
| Use | 2.45 (1.90, 3.15) | <0.001 | ||
| Nonuse | 1.00 | Ref | ||
| MAC prophylaxis at HAART initiation* | ||||
| Use | 2.48 (2.02, 3.05) | <0.001 | ||
| Nonuse | 1.00 | Ref | ||
| CD4 count increase at 6 months | ||||
| <101 cells/µL | 1.00 | Ref | 1.00 | Ref |
| ≥101 cells/µL | 0.69 (0.55, 0.86) | 0.001 | 0.83 (0.67, 1.03) | 0.09 |
Significant values are shown in bold.
In a multivariate model (not shown) which included CD4 cell count and use of PCP and MAC prophylaxis variables, relative hospitalization rates over time after HAART initiation were not significantly affected. As lower CD4 cell counts were highly associated with use of prophylaxis, and because CD4 cell count was felt to cause the use of prophylaxis, PCP prophylaxis and MAC prophylaxis were not included in the final multivariate model reported above.
Multivariate P<0.05 for responders vs. nonresponders within the 91–180 and 181–365 day time periods.
CI, confidence interval; HAART, highly active antiretroviral therapy; IDU, injecting drug use; MSM, men who have sex with men; PI, protease inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PCP, Pneumocystis pneumonia; MAC, Mycobacterium avium complex.
Hospitalization rates for the seven diagnostic categories with the highest rates are shown in Fig. 2. Non-ADI infections (the three most frequent individual diagnoses being pneumonia, unspecified organism; lower limb cellulitis; and acute/subacute bacterial endocarditis) and ADIs (pneumocystosis, cryptococcosis and candidal esophagitis) were consistently the most common reasons for admission across all time periods for both responders and nonresponders. Psychiatric illness [major depression, recurrent episode; depressive disorder, not elsewhere classified (NEC); and drug-induced mood disorder] was the third most common category and was followed by gastrointestinal and hepatic disease (acute pancreatitis; chronic pancreatitis; and cirrhosis of the liver, NEC); cardiovascular disease (hypertensive end-stage chronic kidney disease; venous thrombosis, NEC; and cerebral artery occlusion with infarct); endocrine, nutritional, metabolic or immune disease (hypovolaemia, cachexia, and hypercalcaemia); and renal disease (acute renal failure, NEC; chronic renal failure; and lower nephron nephrosis).
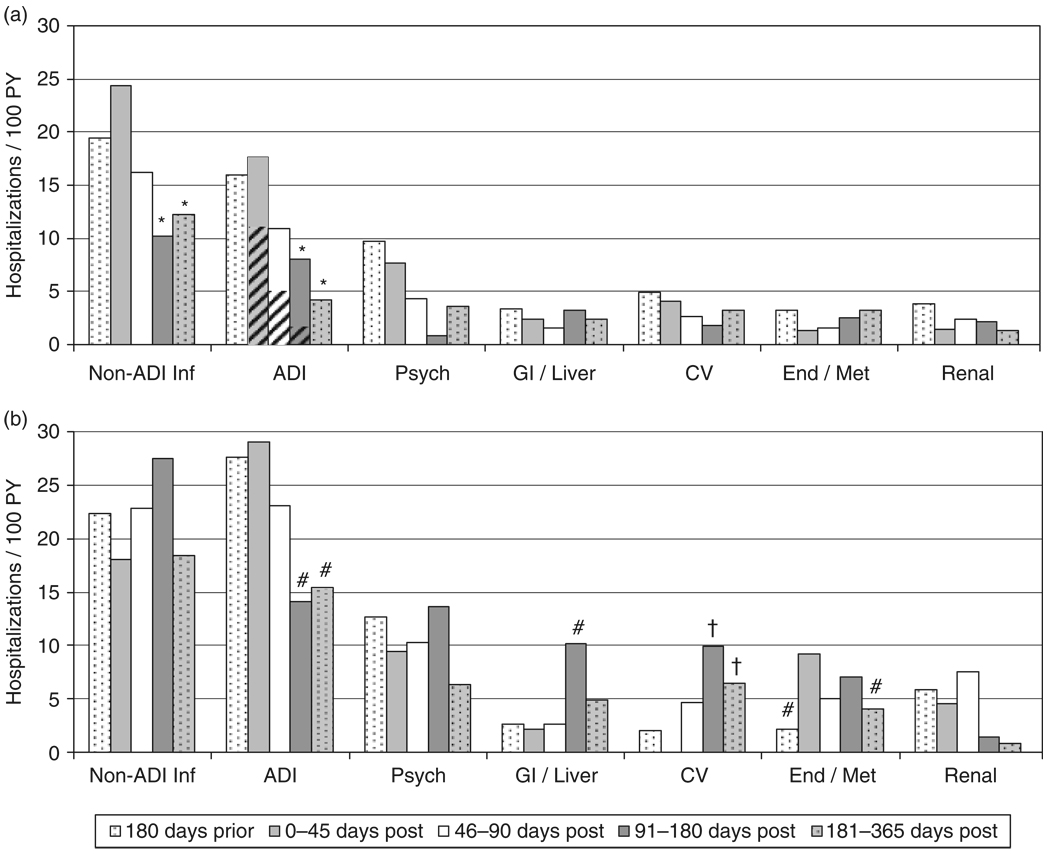
Fig. 2.
Hospitalization rates for the top seven diagnostic categories prior to and over the first year following highly active antiretroviral therapy (HAART) initiation. (a) Virological responders. *Indicates P < 0.05 for the relative rate (RR) vs. days 1–45; hatched areas indicate the portion of AIDS-defining illnesses (ADIs) attributed to immune reconstitution inflammatory syndromes. (b) Nonresponders. #Indicates P between 0.05 and 0.15 for the RR vs. days 1–45; †indicates P < 0.05 for the RR vs. the 180 days prior to HAART initiation, which was used as the referent for cardiovascular admissions among nonresponders as there were no admissions for this group during days 1–45. Inf, infection; Psych, psychiatric; GI, gastrointestinal; CV, cardiovascular; End/Met, endocrine, nutritional, metabolic or immune.
For responders, hospitalizations as a result of ADI and non-ADI infections revealed statistically significant decreases by the period starting 90 days after HAART initiation (Fig. 2a). In the 1–45 day period, IRIS hospitalizations (rate 10.9/100 PY; 95% CI 5.6, 21.1/100 PY) accounted for over half of the admissions in the ADI category and comprised 13% of all admissions among responders. For nonresponders (Fig. 2b), there was no statistically significant decrease compared with day 1–45 rates in any category of admissions, although rates for ADI approached significance in the 91–180- and 181–365-day time periods (P = 0.11 and 0.14, respectively).
In each of four sensitivity/subgroup analyses, the pattern of relative hospitalization rates over time after HAART initiation for responders and nonresponders was identical to the pattern in the primary analysis. The first sensitivity analysis, which was restricted to subjects with HAART initiation CD4 counts < 100 cells/µL, revealed qualitatively higher all-cause hospitalization rates than the primary analysis (responders’ rates ranged from 50.3 to 137.9/100 PY and nonresponders’ from 77.7 to 166.7/100 PY). The other two sensitivity analyses consisted of (1) defining virological response by a ≥ 2 log10 copies/mL drop in HIV-1 RNA at 6 months, and (2) excluding all subjects (13% of responders and 34% of nonresponders) who would have been censored for HAART regimen change. All-cause hospitalization rates in both of these sensitivity analyses were similar to rates in the primary analysis. The subgroup (44%) of subjects reporting IDU as an HIV risk factor had qualitatively higher all-cause hospitalization rates than the full cohort, with responders ranging from 55.4 to 99.7/100 PY and nonresponders from 82.4 to 116.5/100 PY.
Discussion
Our study makes several important findings. First, the hospitalization rate of virological responders appeared stable at near the pre-HAART initiation rate for 45 days and then fell substantially before reaching a plateau after 90 days. This pattern of relative rates remained similar in a multivariate model adjusting for baseline CD4 cell count, CD4 cell count response to HAART, and other potential confounders. Hospitalization rates for ADIs and non-AIDS-defining infections appeared to be the primary reasons for the overall change between 45 and 90 days after HAART initiation.
The overall hospitalization rate, regardless of HAART use or nonuse, for patients in our urban clinical cohort during the years covered by this analysis was approximately 44/100 PY (data not shown). The hospitalization rate of virological responders reached a comparable level around 90 days after HAART initiation. For persons who achieve and maintain complete virological suppression, it is possible that the hospitalization rate would be appreciably lower. Notably, 44/100 PY is consistent with rates seen in several other cohort studies in which all-cause hospitalization rates since 1997 ranged from 11 to 49/100 PY [1,6,8,10,26]. Our high rate may be due to our relatively large proportion of IDUs [6].
In a Vancouver cohort, Fielden et al. [27] found that patients who were ≥ 95% adherent to HAART (and thus may have been virological responders) were half as likely as poorly adherent patients to be hospitalized during 2 years after HAART initiation. Our findings indicate that the difference in hospitalization risk between virological responders and nonresponders starts to occur at 45 days after HAART initiation, may then plateau after 90 days, and is independent of having a large CD4 increase after HAART initiation.
The decreases in hospitalizations as a result specifically of ADIs and non-ADI infections among virological responders indicate that much of the clinical benefit of immune reconstitution may occur between 45 and 90 days after HAART initiation. Furthermore, this benefit may be independent of having a large increase in absolute CD4 cell count after HAART initiation. The initial redistribution of mature CD4 cells from lymphoid tissue to peripheral blood tapers within approximately the same 45- to 90-day time period [28–30]. Clinical benefit may thus appear once an effective repertoire of mature CD4 cells reaches the periphery. Although the number of events was small, the possibility of decreased rates of ADI admissions for nonresponders after 90 days of HAART suggests a possible protective effect even in the absence of a virological response at 6 months.
Studies have indicated possible increases in liver-related and cardiovascular illnesses since the advent of HAART [4–6,31,32]. There have been conflicting results regarding whether cardiovascular risk occurs within a few months or after years of HAART exposure [31,32]. Among virological responders in our study, there was no evidence of increased hepatic or cardiovascular hospitalization rates during the first year after HAART initiation. There was a suggestion that nonresponders (who may have had a brief virological response which then terminated prior to 6 months) had an increased risk of gastrointestinal/liver and cardiovascular illnesses, although numbers of events are too small to be conclusive.
IRIS led to > 13% of all admissions among responders in the first 45 days. Making a diagnosis of IRIS is often complicated and costly as new infections must be considered and ruled out. Previous studies have shown that 20–25% of persons starting HAART will experience an IRIS event, not all of which lead to hospitalization [33,34]. Using the cases within this study, we have previously analysed predictors of IRIS and found boosted PIs, CD4 nadir < 100 cells/µL, and HIV-1 RNA decrease > 2.5 log10 - copies/mL following initiation to be independently associated with IRIS [25].
Calendar era made no appreciable difference to risk of hospitalization during the year following HAART initiation in our analysis. Despite US public health efforts, persons in recent years have enrolled for HIV care at similarly advanced levels of immune compromise as in 1998 and earlier [18,35]. Our results indicate that, until more patients initiate HAART at higher CD4 cell counts, there will continue to be a substantial hospitalization burden in the several weeks after HAART initiation. Hospitalization costs contribute a significant portion of overall societal costs for HIV care [36,37]. There is thus an economic as well as a medical justification for further expanding efforts to promote earlier engagement of HIV-infected persons in medical care.
Consistent with studies examining overall HIV-related hospitalizations, predictors of hospitalization risk in our multivariate analysis included lower CD4 cell count at HAART initiation, female gender, African American race and IDU [1,5,6,9–11,26]. Rates of OI prophylaxis indicated by CD4 cell count criteria (94% and 87%, respectively, for Pneumocystis and M. avium) exceed rates reported in national surveys [38,39] and did not affect the overall pattern of hospitalization rates we found.
There are several potential limitations to this analysis. It is based on data from a single clinic population which has a high proportion of African Americans and IDUs. Although our results may not generalize to all HIV-infected populations, they are likely to be applicable to many urban settings. A previous comparison of hospitalizations captured in our database vs. state-wide hospital insurance claims revealed that 84% of all hospital admissions occur in our hospital [5]. There were no statistically significant differences in hospitalization at our facility vs. outside facilities with regard to gender, HIV risk factor, and race/ethnicity. While our observed hospitalization rates may thus be underestimates, our estimated RRs are probably accurate. Use of ICD-9 codes to ascertain primary reason for admission has obvious limitations compared with prospective event capture. However, our method has been well validated in our cohort against physician chart review. While only a quarter of our cohort were nonresponders, it is surprising that almost two-thirds of these patients did not have a regimen change prior to 1 year after initiation. This does not represent optimal care, and we do not know the reasons why this happened, although we suspect patient preference to keep trying with a prescribed regimen may have been a factor. We do not have data on adherence to HAART and could not include this in our analyses. However, studies evaluating the association between self-reported adherence and plasma HIV-1 RNA levels have shown inconsistent results. Change in HIV-1 RNA level at 6 months is the Food and Drug Administration recommended primary endpoint for drug trials [40].
In sum, our analysis indicates that virological responders continue to have rates of hospitalization similar to their pre-HAART initiation rates for about 45 days after HAART initiation. As a result primarily of a fall in infectious illness, responders’ hospitalization rates then decrease to the clinic population-wide baseline rate by about 90 days after HAART initiation. This pattern occurred independently of CD4 cell count at HAART initiation and independently of having a large increase in CD4 cell count at 6 months. Clinicians should maintain a high degree of vigilance over patients until at least 90 days.
Acknowledgements
Financial support for this study was provided by a National Center for Research Resources clinical scientist training grant (1KL2RR025006-01) and grants from the National Institutes of Aging (R01 AG026250) and Drug Abuse (K24 DA00432 and R01 DA11602).
Footnotes
Potential conflicts of interest: RDM has been a consultant for Bristol-Myers Squibb and GlaxoSmithKline and has received research funding from Merck, Pfizer, and Gilead. KAG has been a consultant for Tibotec and has also received research funding unrelated to this project from Tibotec. Both other authors: no conflicts.
References
- 1.Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22:1345–1354. doi: 10.1097/QAD.0b013e328304b38b. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Walker AS. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 4.Gebo KA, Fleishman JA, Moore RD. Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr. 2005;40:609–616. doi: 10.1097/01.qai.0000171727.55553.78. [DOI] [PubMed] [Google Scholar]
- 5.Gebo KA, Diener-West M, Moore RD. Hospitalization rates differ by hepatitis C status in an urban HIV cohort. J Acquir Immune Defic Syndr. 2003;34:165–173. doi: 10.1097/00126334-200310010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Floris-Moore M, Lo Y, Klein RS, et al. Gender and hospitalization patterns among HIV-infected drug users before and after the availability of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;34:331–337. doi: 10.1097/00126334-200311010-00012. [DOI] [PubMed] [Google Scholar]
- 7.Mocroft A, Monforte A, Kirk O, et al. Changes in hospital admissions across Europe: 1995–2003. Results from the EuroSIDA study. HIV Med. 2004;5:437–447. doi: 10.1111/j.1468-1293.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- 8.Betz ME, Gebo KA, Barber E, et al. Patterns of diagnoses in hospital admissions in a multistate cohort of HIV-positive adults in 2001. Med Care. 2005;43:III3–III14. doi: 10.1097/01.mlr.0000175632.83060.eb. [DOI] [PubMed] [Google Scholar]
- 9.Pulvirenti JJ, Glowacki R, Muppiddi U, et al. Hospitalized HIV-infected patients in the HAART era: a view from the inner city. AIDS Patient Care STDS. 2003;17:565–573. doi: 10.1089/108729103322555953. [DOI] [PubMed] [Google Scholar]
- 10.Paul S, Gilbert HM, Lande L, et al. Impact of antiretroviral therapy on decreasing hospitalization rates of HIV-infected patients in 2001. AIDS Res Hum Retroviruses. 2002;18:501–506. doi: 10.1089/088922202317406646. [DOI] [PubMed] [Google Scholar]
- 11.Fielden SJ, Rusch ML, Levy AR, et al. Predicting hospitalization among HIV-infected antiretroviral naive patients starting HAART: determining clinical markers and exploring social pathways. AIDS Care. 2008;20:297–303. doi: 10.1080/09540120701561296. [DOI] [PubMed] [Google Scholar]
- 12.Paul S, Gilbert HM, Ziecheck W, Jacobs J, Sepkowitz KA. The impact of potent antiretroviral therapy on the characteristics of hospitalized patients with HIV infection. AIDS. 1999;13:415–418. doi: 10.1097/00002030-199902250-00015. [DOI] [PubMed] [Google Scholar]
- 13.Casalino E, Wolff M, Ravaud P, Choquet C, Bruneel F, Regnier B. Impact of HAART advent on admission patterns and survival in HIV-infected patients admitted to an intensive care unit. AIDS. 2004;18:1429–1433. doi: 10.1097/01.aids.0000131301.55204.a7. [DOI] [PubMed] [Google Scholar]
- 14.Nuesch R, Geigy N, Schaedler E, Battegay M. Effect of highly active antiretroviral therapy on hospitalization characteristics of HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2002;21:684–687. doi: 10.1007/s10096-002-0792-3. [DOI] [PubMed] [Google Scholar]
- 15.Gebo KA, Diener-West M, Moore RD. Hospitalization rates in an urban cohort after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:143–152. doi: 10.1097/00126334-200106010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Mocroft A, Barry S, Sabin CA, et al. The changing pattern of admissions to a London hospital of patients with HIV: 1988–1997. Royal Free Centre for HIV Medicine. AIDS. 1999;13:1255–1261. doi: 10.1097/00002030-199907090-00016. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan JH, Moore RD, Keruly JC, Chaisson RE. Effect of antiretroviral therapy on the incidence of bacterial pneumonia in patients with advanced HIV infection. Am J Respir Crit Care Med. 2000;162:64–67. doi: 10.1164/ajrccm.162.1.9904101. [DOI] [PubMed] [Google Scholar]
- 18.Keruly JC, Moore RD. Immune status at presentation to care did not improve among antiretroviral-naive persons from 1990 to 2006. Clin Infect Dis. 2007;45:1369–1374. doi: 10.1086/522759. [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS) Rockville, MD: U.S. Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 20.The Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269:729–730. [PubMed] [Google Scholar]
- 21.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 22.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 24.StataCorp. College Station, TX: StataCorp LP; Stata Statistical Software: Release 10. 2007
- 25.Manabe YC, Campbell JD, Sydnor E, Moore RD. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J Acquir Immune Defic Syndr. 2007;46:456–462. doi: 10.1097/qai.0b013e3181594c8c. [DOI] [PubMed] [Google Scholar]
- 26.Fleishman JA, Gebo KA, Reilly ED, et al. Hospital and outpatient health services utilization among HIVinfected adults in care 2000–2002. Med Care. 2005;43:III40–III52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 27.Fielden SJ, Rusch ML, Yip B, et al. Nonadherence increases the risk of hospitalization among HIV-infected antiretroviral naive patients started on HAART. J Int Assoc Phys AIDS Care (Chicago, IL) 2008;7:238–244. doi: 10.1177/1545109708323132. [DOI] [PubMed] [Google Scholar]
- 28.Bosch RJ, Wang R, Vaida F, Lederman MM, Albrecht MA. Changes in the slope of the CD4 cell count increase after initiation of potent antiretroviral treatment. J Acquir Immune Defic Syndr. 2006;43:433–435. [PubMed] [Google Scholar]
- 29.Bucy RP, Hockett RD, Derdeyn CA, et al. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103:1391–1398. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lederman MM, Connick E, Landay A, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS clinical trials group protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 31.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 32.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 33.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418–427. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 34.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–115. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 35.Jean-Jacques M, Walensky RP, Aaronson WH, Chang Y, Freedberg KA. Late diagnosis of HIV infection at two academic medical centers: 1994–2004. AIDS Care. 2008;20:977–983. doi: 10.1080/09540120701767257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen RY, Accortt NA, Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 37.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 38.Wilson IB, Landon BE, Marsden PV, et al. Correlations among measures of quality in HIV care in the United States: cross sectional study. BMJ. 2007;335:1085–1088. doi: 10.1136/bmj.39364.520278.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000;342:1416–1429. doi: 10.1056/NEJM200005113421907. [DOI] [PubMed] [Google Scholar]
- 40.Rockville, MD: Center for Drug Evaluation and Research, Food and Drug Administration; Guidance for Industry: Antiretroviral Drugs Using Plasma HIV RNA Measurements – Clinical Considerations for Accelerated and Traditional Approval. 2002