Abstract
Background
As those with HIV infection live longer, ‘non-AIDS’ condition associated with immunodeficiency and chronic inflammation are more common. We ask whether ‘non-HIV’ biomarkers improve differentiation of mortality risk among individuals initiating combination antiretroviral therapy (cART).
Methods
Using Poisson models, we analysed data from the Veterans Aging Cohort Study (VACS) on HIV-infected veterans initiating cART between 1 January 1997 and 1 August 2002. Measurements included: HIV biomarkers (CD4 cell count, HIV RNA and AIDS-defining conditions); ‘non-HIV’ biomarkers (haemoglobin, transaminases, platelets, creatinine, and hepatitis B and C serology); substance abuse or dependence (alcohol or drug); and age. Outcome was all cause mortality. We tested the discrimination (C statistics) of each biomarker group alone and in combination in development and validation data sets, over a range of survival intervals, and adjusting for missing data.
Results
Of veterans initiating cART, 9784 (72%) had complete data. Of these, 2566 died. Subjects were middle-aged (median age 45 years), mainly male (98%) and predominantly black (51%). HIV and ‘non-HIV’ markers were associated with each other (P<0.0001) and discriminated mortality (C statistics 0.68–0.73); when combined, discrimination improved (P<0.0001). Discrimination for the VACS Index was greater for shorter survival intervals [30-day C statistic 0.86, 95% confidence interval (CI) 0.80–0.91], but good for intervals of up to 8 years (C statistic 0.73, 95% CI 0.72–0.74). Results were robust to adjustment for missing data.
Conclusions
When added to HIV biomarkers, ‘non-HIV’ biomarkers improve differentiation of mortality. When evaluated over similar intervals, the VACS Index discriminates as well as other established indices. After further validation, the VACS Index may provide a useful, integrated risk assessment for management and research.
Keywords: anaemia, CD4 cell count, hepatitis C coinfection, hepatology, injecting drug use, outcomes, renal/kidney, risk groups, viral load
Introduction
With the advent of combination antiretroviral therapy (cART), people with HIV infection are living longer [1–3] and experiencing fewer AIDS-defining events and more ‘non-AIDS’ events [4]. Further, the majority of deaths occurring among those on treatment are now classified as ‘non-AIDS’ (i.e. not attributable to one or more of the 26 AIDS-defining conditions identified by the Centers for Disease Control and Prevention) [5–8]. Until recently, most considered this the inevitable price of success – people are living long enough on cART to die of other causes.
However, results from the Strategies for Management of Antiretroviral Therapy (SMART) trial [9] suggest that at least some of these ‘non-AIDS’ events are actually associated with immunodeficiency and chronic viral inflammation [9]. The trial compared structured interruption of cART to continuous therapy and made three important observations. First, the differential effects of treatment between the two arms were not fully captured by changes in CD4 cell count or HIV RNA. Secondly, it was found that there were more than twice as many ‘non-AIDS’ events as ‘AIDS’ events and only 8% of the deaths were caused by AIDS conditions [10]. Thirdly, rates of cardiovascular, renal and liver disease and grade IV treatment toxicities were higher in the treatment interruption arm. A combined review of HIV cohort and SMART data [10] demonstrated: (1) that morbidity and mortality among those on cART are dominated by non-AIDS rather than AIDS events; (2) there is a strong positive association between non-AIDS deaths and both low CD4 cell counts and high HIV RNA; and (3) the association with immunodeficiency is consistent across several types of non-AIDS events including liver disease, renal disease and non-AIDS malignancy. The authors concluded that ‘We need to adapt our research priorities to better understand the full role of HIV in causing a wide range of clinical diseases…. Clinicians caring for patients with HIV need to … become aware of the best means to try to prevent and to monitor for early signs of these [non-AIDS] outcomes.’
This goal would be facilitated by an index that combined HIV and ‘non-HIV’ biomarkers associated with immunodeficiency and chronic viral inflammation. The most logical way to weight these factors is according to risk of all cause mortality because all cause mortality avoids assumptions regarding causality. Further, all cause mortality is the outcome of greatest importance to patients. Such an index could be used as a surrogate endpoint for clinical trials and as a guide to clinical therapy.
While excellent weighted all cause mortality indices have been established in HIV infection [3,11–14], these have focused on HIV markers (CD4 cell count, HIV RNA and AIDS-defining conditions). They have largely omitted biomarkers of anaemia [15–18], liver disease [8,19–21], and renal disease [22,23] despite their documented association with both immunodeficiency and survival. In this study we used the Veterans Aging Cohort Study (VACS), a sample of over 13 500 veterans initiating cART within the Veterans Affairs Healthcare System (VA), to develop and initially validate the VACS Index, which combines HIV and ‘non-HIV’ biomarkers.
Methods
Cohorts selected
The VACS includes the Virtual Cohort which has been described in detail elsewhere [24,25]. In brief, the Virtual Cohort consists of over 33 000 veterans with HIV infection treated within the national Veterans Affairs Healthcare System from 1997 to the present. This sample identifies veterans at the point of initiating care for HIV infection and follows them using databases derived from the VA National Electronic Medical Record System. Using chart review, we have determined that 75% of those entering care in the VA for HIV infection initiate their first course of cART after coming to the VA.
Subjects selected and data sources
To ensure adequate follow-up time, we identified subjects who initiated their first course of cART in the VA between 1 January 1997 and 1 August 2002. We used pharmacy data to identify individuals initiating a minimum of three antiretroviral medications and laboratory data to determine that they had received a minimal evaluation (CD4 cell count, HIV-RNA and haemoglobin) within 6 months of initiating cART.
Available data included demographic factors (age, race/ethnicity and gender), administrative diagnostic codes [International Statistical Classification of Diseases and Related Health Problems (ICD)-9 codes], routinely collected clinical laboratory data, pharmacy data and long-term mortality. All laboratory data were collected from the clinical sites through the Immunology Case Registry [26]. Pharmacy data are drawn from the national VA Pharmacy Benefits Management Package [27]. ICD-9 codes were used to determine diagnoses of drug abuse or dependence, alcohol abuse or dependence, and AIDS-defining illnesses. Hepatitis C was defined as a positive antibody, qualitative or quantitative HIV RNA, or ICD-9 codes. Hepatitis B was defined as a positive surface antigen test or ICD-9 codes. In all cases in which ICD-9 codes were used, two out-patient or one in-patient code was required before the condition was considered present. This approach improves the accuracy of ICD-9 codes when compared with chart review [28]. The specific codes used can be found at our website (http://VAcohort.org). All cause mortality data using VA data sources have been demonstrated to be accurate and complete when compared with the National Death Registry [29,30].
Analyses
We ran univariable descriptive statistics and estimated the association between biomarkers using Spearman rank for continuous variables and χ2 for dichotomous markers. We then split the sample. Those who initiated treatment after 31 December 1998 were assigned to the development set and those who initiated treatment on or before this date were reserved for validation. We initially standardized the maximal observation interval for both samples to 6 years, but later conducted sensitivity analyses around this maximal survival window. We chose a nonrandom split based on calendar time to determine the temporal generalizability of our findings [32]. After each model had been fully specified we used the assigned risk estimates from the model to rank patients according to risk from highest to lowest risk of mortality.
Development
We compared Poisson, Weibull and Cox survival models and found that differences in distributional assumptions over the 6-year window did not substantially alter coefficient weights. We present Poisson analyses, as these results are the most directly interpretable.
To facilitate the generalizability of our results, we used a previously validated specification of HIV biomarkers from the Antiretroviral Treatment Cohort Collaboration (ART-CC) model. ART-CC is a carefully validated prognostic model based upon data from cohorts in Europe and North America [3,13,32]. It is focused on markers of HIV disease severity and includes CD4 count (<50, 50–99, 100–199, 200–349 and ≥350 cells/μL), HIV-1 RNA of five log or more and the presence of AIDS-defining illness.
For ‘non-HIV’ biomarkers we considered only: (1) clinical markers that are ordered as part of routine clinical management and (2) markers that have been previously demonstrated to be associated with mortality among patients with HIV infection. We employed previously validated specifications of these markers consistent with major organ system injury. For liver injury, we employed the Fibrosis Index (FIB) 4 [33]. FIB 4 uses aspartate and alanine transaminase (AST and ALT, respectively), platelets and age to estimate likely liver fibrosis [FIB 4: (years of age × AST)/(platelets in 109/L × square root of ALT)]. Two thresholds of FIB 4 are recommended: >3.25, consistent with high risk for fibrosis/cirrhosis; and <1.45, consistent with low risk for fibrosis/cirrhosis. For renal injury, we employed the Modified Diet in Renal Disease (MDRD) estimation which uses age, race, gender and creatinine to estimate creatinine clearance [estimated Glomerular Filtration Rate (eGFR): 186.3 × (serum creatinine – 1.154) × (age – 0.203) × (0.742 for women) × (1.21 if African American)] [34]. Two levels of anaemia were defined: moderate and severe (haemoglobin 10–12 and <10 g/dL, respectively). Finally, we included a combined indicator variable for chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. We created a single indicator because 51% of those with chronic HBV infection also had HCV infection, and coefficients for HBV and HCV infections were similar in preliminary models.
The ART-CC model also adjusts for two demographic factors: age ≥50 years and history of injecting drug use. Because our sample is older [3,13], we adjusted both models for age 50–64 and ≥65 years. We did not have information available in Virtual Cohort on injecting drug use. As a proxy, we adjusted both models for a diagnosis of substance (drug or alcohol) abuse or dependence. We created a single indicator for substance abuse or dependence because 67% of those with a diagnosis of drug abuse or dependence also had a diagnosis of alcohol abuse or dependence [35] and coefficients in preliminary models were similar.
Proportions were compared using the χ2 test. Medians were compared using the rank-sum test. Discriminations were compared using C statistics. The C statistic can be interpreted as the probability that any random pair of uncensored subjects in the data will be ranked correctly by the index with respect to their risk of mortality.
Validation
We fitted the same models in validation data and estimated and compared C statistics. We then combined data sets. Risk quintiles were generated for the HIV biomarker and the combined models. Each Poisson model (HIV, ‘non-HIV’, and combined) was used to generate a risk estimate for each subject. Using each set of model estimates in turn, subjects were ranked from highest to lowest risk and grouped into five quintiles designated by equal numbers of mortality events to ensure similar power to detect differences in risk. Observed mortality rates and 95% CIs were estimated.
To determine the effect of differing survival intervals on its discrimination, we reran the Index in both development and validation samples censoring survival follow-up at 30 days, 6 months, 1 year, 2 years, 4 years, and 6 years in development and validation samples. For each model, we calculated a C statistic and compared this with published C statistics (receiver operator characteristic area estimates) for two commonly used prognostic indices, Acute Physiology and Chronic Health Evaluation (APACHE) [36] and The Charlson Comorbidity Index [37].
Missing data analyses
We fitted a logistic model predicting missing data (0 if no data missing and 1 if at least one variable missing) and including all variables (HIV, ‘non-HIV’, substance abuse or dependence, age, mortality, and year of cART initiation). We used predictions from this model to inversely weigh observations in the development and validation sets and compared these results with those of the complete case analyses.
Results
Of 13 586 HIV-infected veterans initiating cART between 1 January 1997 and 1 August 2002 with laboratory data, 9784 (72%) had complete data (analytic sample). Development and validation sets were clinically similar. Subjects were middle-aged (Table 1; median age 45 years), mainly male (98%), and predominantly black (51%). Over a third had CD4 counts below 200 cells/μL and 18% had HIV RNA above 5 log copies/mL. Diagnoses of alcohol or drug abuse or dependence were common (31%), as were anaemia (21%), HBV infection (12%), and HCV infection (43%). Twelve per cent had likely liver fibrosis (FIB 4>3.25). Two per cent had stage IV renal failure (eGFR<30 mL/min). AIDS diagnoses were relatively uncommon. In pairwise comparisons, CD4 cell count, HIV RNA and AIDS-defining illnesses were strongly associated with haemoglobin, FIB 4, and eGFR <30 mL/min (P<0.0001 for each; data not otherwise shown).
Table 1.
Development and validation samples
| Full cohort (n =9784) | Development (n =4813) | Validation (n =4971) | |
|---|---|---|---|
| Age (years) | |||
| Median (years) | 45 | 46 | 44 |
| <50 years [n (%)] | 6876 (70.3) | 3120 (64.8) | 3756 (75.6) |
| 50–64 years [n (%)] | 2536 (25.9) | 1487 (30.9) | 1049 (21.1) |
| ≥65 years [n (%)] | 372 (3.8) | 206 (4.3) | 166 (3.3) |
| Gender [n (%)] | |||
| Male | 9574 (97.9) | 4710 (97.9) | 4864 (97.9) |
| Race/ethnicity [n (%)] | |||
| Black | 4978 (50.9) | 2387 (49.6) | 2591 (52.1) |
| White | 3158 (32.3) | 1476 (30.7) | 1682 (33.8) |
| Hispanic/other | 1648 (16.8) | 950 (19.7) | 698 (14.0) |
| CD4 count | |||
| Median (cells/μL) | 281 | 243 | 316 |
| <50 cells/μL [n (%)] | 1225 (12.5) | 806 (16.8) | 419 (8.4) |
| 50–99 cells/μL [n (%)] | 732 (7.5) | 440 (9.1) | 292 (5.9) |
| 100–199 cells/μL [n (%)] | 1606 (16.4) | 806 (16.8) | 800 (16.1) |
| 200–349 cells/μL [n (%)] | 2354 (24.1) | 1132 (23.5) | 1222 (24.6) |
| ≥350 cells/μL [n (%)] | 3867 (39.5) | 1629 (33.9) | 2238 (45.0) |
| HIV-1 RNA | |||
| Median (log copies/mL) | 3.6 | 4.2 | 3.1 |
| >5 log copies/mL [n (%)] | 1795 (18.4) | 1219 (25.3) | 576 (11.6) |
| Substance addiction or abuse [n (%)] | |||
| Drugs | 2458 (25.1) | 1188 (24.7) | 1270 (25.6) |
| Alcohol | 2258 (23.1) | 1138 (23.6) | 1120 (22.5) |
| Either one | 3055 (31.2) | 1512 (31.4) | 1543 (31.0) |
| Haemoglobin | |||
| Median (g/dL) | 13.8 | 13.4 | 14 |
| 10–12 g/dL [n (%)] | 1540 (15.7) | 939 (19.5) | 601 (12.1) |
| <10 g/dL [n (%)] | 557 (5.7) | 400 (8.3) | 157 (3.2) |
| Hepatic measures | |||
| Hepatitis B [n (%)] | 1140 (11.7) | 552 (11.5) | 588 (11.8) |
| Hepatitis C [n (%)] | 4159 (42.5) | 1958 (40.7) | 2201 (44.2) |
| Hepatitis B or C [n (%)] | 4675 (47.8) | 2209 (45.9) | 2466 (49.6) |
| AST (U/L; median) | 34 | 34 | 34 |
| ALT (U/L; median) | 35 | 36 | 35 |
| Platelets (103 cells/μL) | 204 | 209 | 200 |
| FIB 4>3.25 [n (%)] | 1186 (12.1) | 606 (12.6) | 580 (11.7) |
| FIB 4<1.45 [n (%)] | 5417 (55.4) | 2624 (54.5) | 2793 (56.2) |
| Renal measures | |||
| Creatinine (mg/dL; median) | 1 | 1 | 1 |
| eGFR (mL/min; median) | 97 | 97 | 97 |
| eGFR<30 [n (%)] | 195 (2.0) | 101 (2.1) | 94 (1.9) |
| AIDS diagnoses [n (%)] | |||
| PJP | 527 (5.4) | 311 (6.5) | 216 (4.4) |
| MAI/TB | 238 (2.4) | 133 (2.8) | 105 (2.1) |
| Bacterial pneumonia | 980 (10.0) | 503 (10.5) | 477 (9.6) |
| Fungal infections | 190 (1.9) | 112 (2.3) | 78 (1.6) |
| AIDS cancers | 322 (3.3) | 164 (3.4) | 158 (3.2) |
| Wasting | 150 (1.5) | 71 (1.5) | 79 (1.6) |
| Dementia | 266 (2.7) | 115 (2.4) | 151 (3.0) |
| Deaths/100 person-years | 5.29 | 5.33 | 5.26 |
| Median person-years of observation | 6.47 | 5.87 | 7.76 |
FIB 4: (years of age × AST)/(platelets in 109/L × square root of ALT).
eGFR: 186.3 × (serum creatinine – 1.154) × (age – 0.203) × (0.742 for women) × (1.21 if African American).
ALT, alanine transaminase; AST, aspartate transaminase; eGFR, estimated Glomerular Filtration Rate; FIB, Fibrosis Index; MAI/TB, Mycobacterium avium intracellulare/tuberculosis; PJP, Pneumocystis jiroveci pneumonia.
In development and validation sets, HIV and ‘non-HIV’ biomarkers were associated with mortality when modelled separately (Table 2). In both sets, ‘non-HIV’ biomarkers, as a group, added discrimination to the HIV model when combined into a single index [C statistic improved from 0.68 to 0.72 in development (P<0.0001) and from 0.71 to 0.77 in validation (P<0.0001)]. In all cases, all biomarkers retained independent associations with mortality after full adjustment.
Table 2.
Adjusted Poisson models: HIV biomarkers, ‘non-HIV’ biomarkers* and combined, for (a) the development set and (b) the validation set
| Development set Initiated cART 1999–2002 (n =4813) |
HIV biomarkers (C statistic = 0.68) |
‘Non-HIV’ biomarkers (C statistic = 0.70) |
Combined (C statistic = 0.72) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | ||||
| (a) | |||||||||
| HIV RNA>5 log copies/mL | 1.28 | 1.12 | 1.45 | 1.19 | 1.04 | 1.35 | |||
| CD4 50–99 cells/μL | 0.79 | 0.65 | 0.96 | 0.76 | 0.63 | 0.93 | |||
| CD4 100–199 cells/μL | 0.70 | 0.59 | 0.84 | 0.73 | 0.61 | 0.87 | |||
| CD4 200–349 cells/μL | 0.57 | 0.48 | 0.68 | 0.65 | 0.54 | 0.77 | |||
| CD4 ≥350 cells/μL | 0.45 | 0.37 | 0.54 | 0.57 | 0.47 | 0.69 | |||
| AIDS-defining diagnosis | 1.55 | 1.37 | 1.76 | 1.44 | 1.27 | 1.63 | |||
| Haemoglobin<10 g/dL | 2.34 | 1.97 | 2.77 | 1.65 | 1.38 | 1.98 | |||
| Haemoglobin 10–12 g/dL | 2.02 | 1.78 | 2.3 | 1.54 | 1.34 | 1.76 | |||
| FIB 4 ≥3.25 | 1.67 | 1.44 | 1.93 | 1.62 | 1.40 | 1.89 | |||
| FIB 4<1.45 | 0.71 | 0.62 | 0.81 | 0.74 | 0.65 | 0.85 | |||
| eGFR<30 mL/min | 1.88 | 1.44 | 2.47 | 2.05 | 1.57 | 2.69 | |||
| Viral hepatitis | 1.31 | 1.16 | 1.48 | 1.37 | 1.21 | 1.55 | |||
| Validation set Initiated cART 1997–1998 (n =4971) |
HIV biomarkers (C statistic = 0.71) |
‘Non-HIV’ biomarkers (C statistic = 0.73) |
Combined (C statistic = 0.77) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | IRR | 95% CI | ||||
| (b) | |||||||||
| HIV RNA>5 log copies/mL | 1.56 | 1.35 | 1.82 | 1.54 | 1.33 | 1.79 | |||
| CD4 50–99 cells/μL | 0.63 | 0.51 | 0.78 | 0.64 | 0.52 | 0.80 | |||
| CD4 100–199 cells/μL | 0.42 | 0.35 | 0.50 | 0.52 | 0.43 | 0.63 | |||
| CD4 200–349 cells/μL | 0.38 | 0.32 | 0.46 | 0.51 | 0.43 | 0.62 | |||
| CD4 ≥350 cells/μL | 0.24 | 0.20 | 0.28 | 0.36 | 0.30 | 0.44 | |||
| AIDS-defining diagnosis | 1.47 | 1.30 | 1.66 | 1.38 | 1.22 | 1.56 | |||
| Haemoglobin<10 g/dL | 3.98 | 3.24 | 4.90 | 2.65 | 2.14 | 3.28 | |||
| Haemoglobin 10–12 g/dL | 2.13 | 1.86 | 2.44 | 1.66 | 1.44 | 1.90 | |||
| FIB 4 ≥3.25 | 2.06 | 1.79 | 2.36 | 1.93 | 1.68 | 2.22 | |||
| FIB 4<1.45 | 0.56 | 0.49 | 0.63 | 0.65 | 0.57 | 0.75 | |||
| eGFR<30 mL/min | 1.85 | 1.42 | 2.43 | 1.97 | 1.50 | 2.58 | |||
| Viral hepatitis | 1.19 | 1.05 | 1.34 | 1.25 | 1.11 | 1.42 | |||
Reported IRRs are from nested Poisson models restricted to those patients with all variables complete.
P<0.00001 for addition of non-HIV biomarkers to HIV biomarkers.
All models also include two age variables (age 50–64 years and age ≥ 65 years) and a combined variable for alcohol or drug abuse and dependence. See Methods section for rationale.
FIB 4: (years of age × AST)/(platelets in 109/L × square root of ALT).
eGFR: 186.3 × (serum creatinine – 1.154) × (age – 0.203) × (0.742 for women) – (1.21 if African American).
ALT, alanine transaminase; AST, aspartate transaminase; cART, combination antiretroviral therapy; CI, confidence interval; eGFR, estimated Glomerular Filtration Rate; FIB, Fibrosis Index; IRR, incident rate ratio.
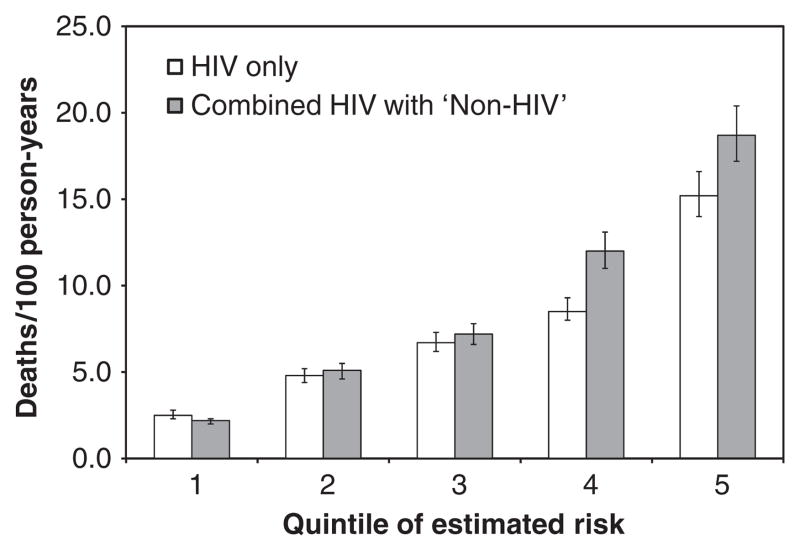
When data sets were combined, and quintiles of risk estimated, the combined index offered improved differentiation of mortality (Fig. 1). This was most pronounced for the highest risk groups: 4th quintile [8.5 (95% CI 8.0–9.3) vs. 12.0 (95% CI 11.0–13.1) deaths/100 person-years (PY)] and 5th quintile [15.2 (95% CI 14.0–16.6) vs. 18.7 (95% CI 17.2–20.4) deaths/100 PY].
Fig. 1.
Observed mortality rate by quintiles of risk estimated using HIV only and combined HIV and ‘non-HIV’ biomarkers.
When biomarkers were characterized by risk quintile as estimated by the three models, the overlapping associations with mortality became apparent (Table 3). Despite omitting all ‘non-HIV’ biomarkers, the HIV model identified a strong gradient for haemoglobin, but a somewhat less pronounced gradient in FIB 4, eGFR or viral hepatitis. Despite omitting all HIV biomarkers, the ‘non-HIV’ model identified a strong gradient for CD4 cell count, HIV RNA and AIDS-defining conditions. Consistent with its improved discrimination, the combined model improved gradients in CD4, HIV RNA and AIDS-defining conditions compared with the ‘non-HIV’ model and gradients in haemoglobin, FIB 4, eGFR and viral hepatitis compared with the HIV model.
Table 3.
Biomarkers characterized by risk quintile for each model
| n | Deaths | Deaths/100 PY | Age ≥65 years (%) | Substance ab/dep (%) | CD4 (cells/μL) | HIV RNA (log copies/mL) | AIDS-defining conditions (%) | HGB (g/dL) | FIB 4> 3.25 (%) | eGFR <30 mL/min (%) | Viral hepatitis (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | |||||||||||||
| HIV and ‘non-HIV’ combined quintiles | |||||||||||||
| 1 | 4379 | 513 | 2.2 | 2.0, 2.3 | 7.0 | 20.1 | 419 | 2.9 | 5.7 | 14.5 | 0.0 | 0.1 | 29.0 |
| 2 | 2011 | 513 | 5.1 | 4.6, 5.5 | 2.9 | 38.2 | 263 | 3.7 | 21.9 | 13.8 | 0.7 | 0.4 | 58.6 |
| 3 | 1510 | 513 | 7.2 | 6.6, 7.8 | 6.2 | 35.2 | 171 | 4.2 | 36.4 | 12.9 | 18.4 | 2.2 | 60.0 |
| 4 | 1057 | 513 | 12.0 | 11.0, 13.1 | 8.6 | 44.2 | 122 | 4.7 | 43.3 | 12.1 | 38.4 | 3.5 | 67.6 |
| 5 | 827 | 514 | 18.7 | 17.2, 20.4 | 12.0 | 49.2 | 48 | 5.1 | 60.7 | 10.8 | 59.0 | 14.0 | 73.4 |
| HIV quintiles | |||||||||||||
| 1 | 3796 | 513 | 2.5 | 2.3, 2.8 | 0.0 | 19.1 | 466 | 2.7 | 3.7 | 14.5 | 5.9 | 1.2 | 42.5 |
| 2 | 2089 | 513 | 4.8 | 4.4, 5.2 | 0.0 | 32.4 | 262 | 3.7 | 11.9 | 13.9 | 11.5 | 1.8 | 50.9 |
| 3 | 1599 | 513 | 6.7 | 6.2, 7.3 | 6.6 | 42.2 | 163 | 4.0 | 25.5 | 13.4 | 16.9 | 2.3 | 54.7 |
| 4 | 1365 | 513 | 8.5 | 8.0, 9.3 | 10.8 | 37.1 | 111 | 4.6 | 52.5 | 12.6 | 17.4 | 2.9 | 47.9 |
| 5 | 935 | 514 | 15.2 | 14.0, 16.6 | 12.7 | 50.5 | 26 | 5.3 | 73.5 | 11.6 | 23.1 | 3.7 | 50.4 |
| ‘Non-HIV’ quintiles | |||||||||||||
| 1 | 4051 | 513 | 2.3 | 2.1, 2.6 | 0.0 | 23.3 | 365 | 3.2 | 13.7 | 14.4 | 0.0 | 0.0 | 29.2 |
| 2 | 2083 | 513 | 4.9 | 4.5, 5.4 | 2.9 | 20.1 | 268 | 3.7 | 21.8 | 14.0 | 0.0 | 0.4 | 43.6 |
| 3 | 1664 | 513 | 6.4 | 5.9, 7.0 | 7.0 | 48.3 | 238 | 3.9 | 31.4 | 12.9 | 4.0 | 0.8 | 65.1 |
| 4 | 1104 | 513 | 11.0 | 10.1, 12.0 | 4.4 | 42.4 | 192 | 4.1 | 29.3 | 12.5 | 52.3 | 3.9 | 78.0 |
| 5 | 882 | 514 | 16.4 | 15.1, 17.9 | 16.6 | 47.6 | 142 | 4.4 | 39.3 | 10.8 | 61.5 | 14.6 | 72.5 |
ab/dep, abuse/dependence; CI, confidence interval; eGFR, estimated Glomerular Filtration Rate; FIB, Fibrosis Index; HGB, haemoglobin; PY, person-years.
FIB 4: (years of age × AST)/(platelets in 109/L × square root of ALT).
eGFR: 186.3 × (serum creatinine – 1.154) × (age – 0.203) × (0.742 for women) × (1.21 if African American).
When observations were inversely weighted by association with missing data, calendar year included in the model, and observations no longer censored at 6 years, results were similar. In combined data, the index that included both HIV and ‘non-HIV’ biomarkers improved the discrimination of HIV biomarkers alone (C statistic improved from 0.69 to 0.74, P<0.0001). While individual coefficient weights varied somewhat from those of the models estimated without inverse weighting by the propensity for missing data, all biomarkers retained strong independent associations of similar magnitude and direction with mortality (P<0.0001).
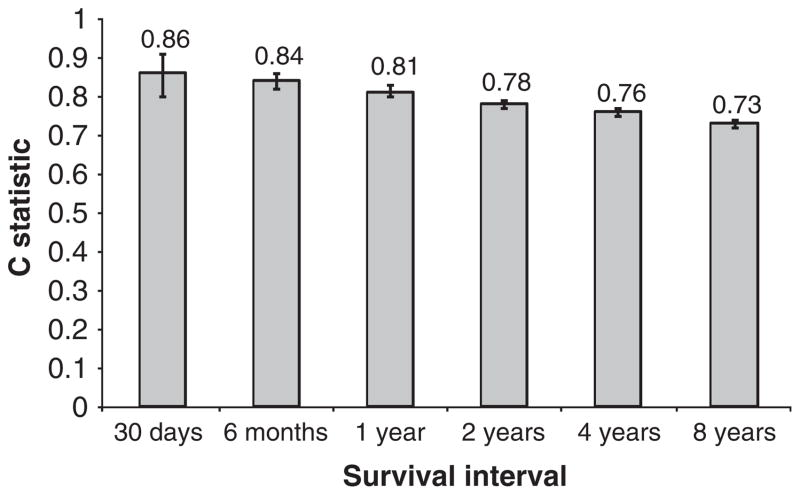
Finally, the discrimination of the index (C statistic) for mortality depended upon the survival interval. Discrimination for the VACS Index was greater for shorter survival intervals (Fig. 2; 30-day C statistic 0.86, 95% CI 0.80–0.91), but good for intervals of up to 8 years (C statistic 0.73, 95% CI 0.72–0.74).
Fig. 2.
Discrimination of Veterans Aging Cohort Study (VACS) Index (C statistic) by survival interval (n =9748).
Discussion
Although associated with death from HIV disease progression, CD4 cell count, HIV RNA, and AIDS-defining conditions fail to capture important effects of HIV and its treatment on morbidity and mortality [38–40]. After accounting for CD4 cell count, HIV RNA and AIDS-defining conditions, the routine clinical biomarkers of anaemia, liver injury, renal injury, and chronic viral hepatitis substantially improve discrimination of mortality among HIV-infected veterans initiating cART. We have validated these results in independent data and demonstrated that they are robust adjusting for missing data and across differing survival intervals. ‘Non-HIV’ biomarkers add independent information to risk estimation of all cause mortality in combination with HIV biomarkers and are independently associated with immunodeficiency (CD4 cell count and AIDS-defining conditions) and HIV RNA. Finally, by combining HIV and ‘non-HIV’ markers into a single weighted index, we can recognize the likely complex effects of HIV and its treatment on HIV and ‘non-HIV’ disease, and provide an improved risk estimation of all cause mortality. This is the first essential step towards an integrated surrogate endpoint for research and a potentially useful risk index for clinical management.
Our study has unique advantages over previously published work. We had sufficient sample size and longitudinal follow-up to analyse all cause mortality among a sample of patients with uniform data sources and methods of data collection and near complete mortality ascertainment [29,30]. We were able to study an older population, ensuring the relevance of this work to the rapidly growing population of older patients with HIV infection [39]. Importantly, we were able to demonstrate that our results generalized to an independent sample before and after accounting for missing data.
Our study also has limitations. The first course of cART within the VA may not be the first course of cART. We conducted an eight-site chart review (n =3250) demonstrating that 75% of veterans are cART naïve at VA entry, but some individuals probably had prior cART exposure. Additionally, there were few women in the sample and we cannot determine whether our findings generalize beyond men. Future work is planned that will explore whether additional clinical data, laboratory data, and time-updated analyses improve the index. Data on smoking, wasting, cancer diagnoses, cardiovascular and cerebral vascular disease, pulmonary disease, microalbumin, anaemia type and short-term response to cART may all further improve the differentiation of mortality risk. Additionally, when more standardized and clinically available, markers of inflammation and immune senescence may prove valuable. It will also be useful to test the discrimination of the index for other important patient outcomes including specific causes of death, functional compromise and hospitalization.
Nevertheless, the VACS Index currently predicts mortality as well as two established prognostic indices when evaluated over comparable survival intervals (a major determinant of prognostic accuracy) [31,39]. For 30-day survival, the index achieved C statistics of 0.86 (95% CI 0.80–0.91), consistent with the range of performance of the APACHE III, a prognostic index for short-term hospital or 30-day intensive care unit survival (C statistics between 0.70 and 0.86) [40–42]. For 1-year survival, the VACS index achieved a C statistic of 0.81 (95% CI 0.80–0.83), which compares favourably to that for the Charlson Index (C statistic 0.70–0.77) [43]. It is important to note that the index discriminated reasonably well over all survival intervals analysed, which suggests that it offers a reasonable risk assessment of both short- and long-term mortality [31].
Of note, some question whether findings among veterans apply to nonveteran populations. While veterans in care generally do experience higher rates of mortality, comorbid disease, and substance use than the general population, these differences are less pronounced among clinical populations of veterans and nonveterans with HIV infection [6,8,44,45]. Finally, while it would be interesting to consider the performance of the index based upon cause of death, we caution that the primary consideration must be all cause mortality. As we have seen from the SMART study, substantial morbidity and mortality previously classified as ‘non-AIDS’ may in fact be caused by HIV disease progression.
Covariance among substance use, anaemia, viral hepatitis and liver injury probably explains why the association between substance abuse and dependence and mortality was mitigated in adjusted models. By adjusting for liver injury, the association between viral hepatitis and mortality was reduced, but not eliminated. This suggests additional mechanisms of injury for viral hepatitis such as chronic inflammation [46]. Of note, we used a diagnosis of substance abuse or dependence. We did not have information on injecting drug use specifically, which has been shown to be associated with mortality [11,32]. As we used the same adjustment for substance use in all models, the comparison between HIV biomarkers and ‘non-HIV’ biomarkers should remain valid.
As expected, HIV and ‘non-HIV’ biomarkers were strongly interrelated. We recommend against over-interpretation of individual weights in the index. Instead, emphasis should be upon the risk estimated by the full index. This estimate of overall risk is less subject to the problems of variation that can undermine the utility of a single biomarker [47]. Finally, while clinicians have been slow to adopt complex prognostic indices, preferring simplified algorithms, simplified systems compromise the power, precision and calibration of prognostic models estimated on large samples [48–50]. The availability of hand-held personal data assistants (PDAs) and the adoption of electronic health systems should overcome data and computational barriers to the use of these more accurate and generalizable models [31].
This study represents an essential step towards the development of a combined index for survival among those in treatment with HIV infection. We have shown that ‘non-HIV’ biomarkers of anaemia, liver disease, renal disease and viral hepatitis add important mortality risk discrimination to HIV markers and are associated with immunodeficiency (CD4 cell count and AIDS-defining illnesses) and HIV RNA. The next steps include testing its performance in nonveteran populations and in women, and its longitudinal response to treatment effects [47,51,52]. We need to determine whether other biomarkers and non-HIV clinical diagnoses associated with immunodeficiency and chronic inflammation improve the calibration and discrimination of the model. It will also be useful to test the discrimination of the index for other important patient outcomes, including specific causes of death, functional compromise and hospitalization. These evaluations will probably suggest additional variables to improve the index. Once more completely validated, the VACS Index may offer a superior prognostic index and integrated surrogate endpoint for clinical management and research.
Acknowledgments
Funding: This study was funded by the National Institute on Alcohol Abuse and Alcoholism (2U10 AA 13566).
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1.Hogg G, Lima V, Sterne JA, et al. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braithwaite RS, Justice AC, Chang CC, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005;118:890–898. doi: 10.1016/j.amjmed.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 3.May MT, Sterne JA, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–458. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 4.d’Arminio Monforte A, Sabin CA, Phillips A, et al. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch Intern Med. 2005;165:416–423. doi: 10.1001/archinte.165.4.416. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Morbid Mortal Wkly Rep. 1987;36:82–94. [Google Scholar]
- 6.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 7.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med. 2005;6:99–106. doi: 10.1111/j.1468-1293.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 8.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV Outpatient Study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 9.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4 count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 10.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chene G, Sterne JA, May M, et al. Prognostic importance of initial response in HIV-1-infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 14.Mocroft AJ, Johnson MA, Sabin CA, et al. Staging system for clinical AIDS patients. Lancet. 1995;346:12–17. doi: 10.1016/s0140-6736(95)92649-6. [DOI] [PubMed] [Google Scholar]
- 15.Moore RD, Forney D. Anemia in HIV-infected patients receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;29:54–57. doi: 10.1097/00042560-200201010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Mocroft A, Kirk O, Barton SE, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA Study Group. AIDS. 1999;13:943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV Disease Surveillance Project. Blood. 1998;91:301–308. [PubMed] [Google Scholar]
- 18.The Antiretroviral Therapy Cohort Collaboration. Prognostic importance of anaemia in HIV type-1-infected patients starting antiretroviral therapy: collaborative analysis of prospective cohort studies. Antivir Ther. 2008;13:959–967. [PMC free article] [PubMed] [Google Scholar]
- 19.Justice AC, Wagner JH, Fusco GP, et al. HIV survival: liver function tests independently predict survival. 14th International AIDS Conference; Barcelona, Spain. July 2002. [Google Scholar]
- 20.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 21.Puoti M, Spinetti A, Ghezzi A, et al. Mortality for liver disease in patients with HIV infection: a cohort study. J Acquir Immune Defic Syndr. 2000;24:211–217. doi: 10.1097/00126334-200007010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Fine DM, Atta MG. Kidney disease in the HIV-infected patient. AIDS Patient Care STDS. 2007;21:813–824. doi: 10.1089/apc.2006.0210. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt CM, Klotman PE. HIV-associated nephropathy in the era of antiretroviral therapy. Am J Med. 2007;120:488–492. doi: 10.1016/j.amjmed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a ‘virtual’ cohort using the National VA Health Information System. Med Care. 2006;44:S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 25.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): overview and description. Med Care. 2006;44:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backus L, Mole L, Chang S, Deyton L. The immunology case registry. J Clin Epidemiol. 2001;54:S12–S15. doi: 10.1016/s0895-4356(01)00442-5. [DOI] [PubMed] [Google Scholar]
- 27.Sales MM, Cunningham FE, Glassman PA, Valentino MA, Good CB. Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manage Care. 2005;11:104–112. [PubMed] [Google Scholar]
- 28.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006;44:S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 29.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 30.Fisher SG, Weber L, Goldberg J, Davis F. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol. 1995;141:242–250. doi: 10.1093/oxfordjournals.aje.a117426. [DOI] [PubMed] [Google Scholar]
- 31.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 32.May M, Royston P, Egger M, Justice AC, Sterne JA. Development and validation of a prognostic model for survival time data: application to prognosis of HIV-positive patients treated with antiretroviral therapy. Stat Med. 2004;23:2375–2398. doi: 10.1002/sim.1825. [DOI] [PubMed] [Google Scholar]
- 33.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 34.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 35.Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9:591–597. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 38.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Justice AC. PhD Dissertation. Ann Arbor, MI: UMI Number 9627942; 1996. The Development, Validation, and Evaluation of Prognostic Systems: An Application to the Acquired Immunodeficiency Syndrome (AIDS) [Google Scholar]
- 40.den Boer S, de Keizer NF, de Jonge E. Performance of prognostic models in critically ill cancer patients – a review. Crit Care. 2005;9:R458–R463. doi: 10.1186/cc3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arabi Y, Al Shirawi N, Memish Z, Venkatesh S, Al-Shimemeri A. Assessment of six mortality prediction models in patients admitted with severe sepsis and septic shock to the intensive care unit: a prospective cohort study. Crit Care. 2003;7:R116–R122. doi: 10.1186/cc2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Render ML, Welsh DE, Kollef M, et al. Automated computerized intensive care unit severity of illness measure in the Department of Veterans Affairs: preliminary results. SISVistA Investigators. Scrutiny of ICU severity Veterans Health Systems technology architecture. Crit Care Med. 2000;28:3540–3546. doi: 10.1097/00003246-200010000-00033. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care. 2005;43:607–615. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 44.Kohli R, Lo Y, Howard AA, et al. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41:864–872. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- 45.Conigliaro J, Justice AC, Gordon AJ, Bryant K. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: a conceptual model to guide the implementation of evidence-based interventions into practice. Med Care. 2006;44:S1–S6. doi: 10.1097/01.mlr.0000223659.36369.cf. [DOI] [PubMed] [Google Scholar]
- 46.Effros RB, Allsopp R, Chiu C-P, et al. Shortened telomeres in the expanded CD28− CD8+cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 47.De Gruttola VG, Clax P, DeMets DL, et al. Considerations in the evaluation of surrogate endpoints in clinical trials. Summary of a National Institutes of Health workshop. Control Clin Trials. 2001;22:485–502. doi: 10.1016/s0197-2456(01)00153-2. [DOI] [PubMed] [Google Scholar]
- 48.Harrell FE, Lee KL, Mark DB. Tutorial in biostatistics. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Lynn J, Teno JM, Harrell FE., Jr Accurate prognostications of death. Opportunities and challenges for clinicians. West J Med. 1995;163:250–257. [PMC free article] [PubMed] [Google Scholar]
- 50.Harrell FE, Lee KL, Matchar DB, Reichert TA. Regression models for prognostic prediction: advantages, problems and suggested solutions. Cancer Treatment Rep. 1985;69:1071–1077. [PubMed] [Google Scholar]
- 51.Fleming TR, Prentice RL, Pepe MS, Glidden D. Surrogate and auxiliary endpoints in clinical trials, with potential applications in cancer and AIDS research. Stat Med. 1994;13:955–968. doi: 10.1002/sim.4780130906. [DOI] [PubMed] [Google Scholar]
- 52.HIV Surrogate Marker Collaborative Group. Human immunodeficiency virus type 1 RNA level and CD4 count as prognostic markers and surrogate end points: a meta-analysis. AIDS Res Hum Retroviruses. 2000;16:1123–1133. doi: 10.1089/088922200414965. [DOI] [PubMed] [Google Scholar]