Abstract
Background
The association between hepatitis C virus (HCV) infection and coronary artery disease (CAD) is controversial. We conducted this study to determine and quantify this association.
Methods
We used an established, national, observational cohort of all HCV-infected veterans receiving care at all Veterans Affairs facilities, the Electronically Retrieved Cohort of HCV Infected Veterans, to identify HCV-infected subjects and HCV-uninfected control subjects. We used the Cox proportional-hazards model to determine the risk of CAD among HCV-infected subjects and control subjects.
Results
We identified 82,083 HCV-infected and 89,582 HCV-uninfected subjects. HCV-infected subjects were less likely to have hypertension, hyperlipidemia, and diabetes but were more likely to abuse alcohol and drugs and to have renal failure and anemia. HCV-infected subjects had lower mean (± standard deviation) total plasma cholesterol (175 ± 40.8 mg/dL vs. 198 ± 41.0 mg/dL), low-density lipoprotein cholesterol (102 ± 36.8 mg/dL vs. 119 ± 38.2 mg/dL), and triglyceride (144 ± 119 mg/dL vs. 179 ± 151 mg/dL) levels, compared with HCV-uninfected subjects. In multivariable analysis, HCV infection was associated with a higher risk of CAD (hazard ratio, 1.25; 95% confidence interval, 1.20–1.30; P < .001 for all comparisons). Traditional risk factors (age, hypertension, chronic obstructive pulmonary disease, diabetes, and hyperlipidemia) were associated with a higher risk of CAD in both groups, whereas minority race and female sex were associated with a lower risk of CAD.
Conclusions
HCV-infected persons are younger and have lower lipid levels and a lower prevalence of hypertension. Despite a favorable risk profile, HCV infection is associated with a higher risk of CAD after adjustment for traditional risk factors.
Several infectious etiologies for coronary artery disease (CAD) have been proposed in recent years on the basis of epidemiological associations, but there is no consensus regarding a causative role [1–3]. The association between hepatitis C virus (HCV) infection and CAD is less clear. A small number of reported studies have shown conflicting results; some have reported no association between HCV infection and CAD [4–7], whereas others have reported an increased risk [8] or an increase in measures of subclinical atherosclerosis (e.g., carotid intima-media thickness) [9–11]. Many of the studies showing no association between HCV infection and CAD used a case-control design in which subjects with known CAD were compared with control subjects without CAD and the prevalence of HCV infection was compared between the 2 groups without adjustment for all CAD risk factors.
Persons with HCV infection are at an increased risk of developing hepatic steatosis, which shares many clinical features with the metabolic syndrome [12, 13]. Hepatic steatosis has also been associated with elevated levels of markers of inflammation and endothelial dysfunction [14]. These factors suggest a biologically plausible mechanism of increased risk of CAD in at least a subset of HCV-infected persons. We set out to determine the association between HCV infection and CAD in a large, national, electronically retrieved cohort of HCV-infected veterans (ERCHIVES). Such large observational studies with carefully identified controls are better suited to identify any true association between HCV infection and cardiovascular disease.
METHODS
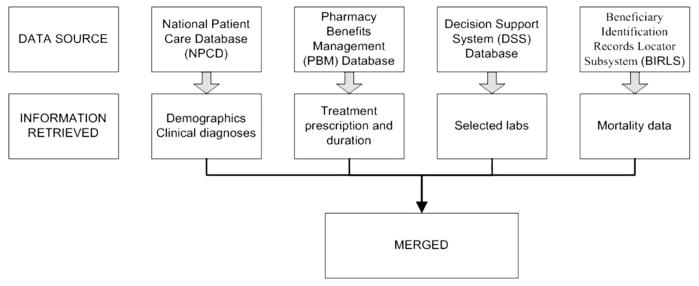
The creation of ERCHIVES has been described elsewhere [15–18]. The current study used an updated cohort of subjects identified from 2001 through 2006. In brief, we assembled a national cohort of HCV-infected veterans from the VA National Patient Care Database, the VA Pharmacy Benefits Management database, and the Decisions Support System database in VA fiscal years 2001–2006 (1 October 2001 through 30 September 2006; the original cohort included subjects identified in 1998–2003) (figure 1). Demographic and clinical data were extracted from the National Patient Care Database. The utility and accuracy of the VA administrative data and VA Pharmacy Benefits Management data have been previously reported by our group and others [17, 19–24]. The National Patient Care Database contains hospitalization records, including discharge diagnoses from 1970 onward. The discharge diagnoses are coded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). For 1997 onward, the National Patient Care Database also contains outpatient visit records, including diagnoses and clinic visits. The validity of ICD-9-CM codes has been tested previously for a range of comorbid conditions, and the sensitivity, specificity, and agreement (κ values) have been found to correlate well with chart abstractions [20, 23, 25, 26].
Figure 1.
Sources of data for the electronically retrieved cohort of hepatitis C virus–infected veterans (ERCHIVES).
The laboratory values were retrieved from the Decisions Support System database, which contains selected laboratory data collected from 2000 onward during routine clinical care of veterans. The laboratory data included measurements of HCV antibody, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride, hemoglobin, alanine and aspartate aminotransferase, serum albumin, bilirubin, international normalization ratio, and glucose levels. To validate the Decisions Support System data, we compared data collected in the Decisions Support System and the Immunology Case Registry for 22,647 human immunodeficiency virus (HIV)–infected veterans with an in-patient or outpatient visit in fiscal year 2002 for 9 laboratory tests. For 6 of the 9 laboratory tests, the Decisions Support System provided laboratory values for more individuals. Overlapping results were nearly perfectly correlated [27].
For our study, HCV infection was defined by the presence of HCV antibody or a positive result of qualitative or quantitative testing for HCV RNA. CAD was defined by the presence of at least 1 inpatient or 2 outpatient ICD-9-CM codes for myocardial infarction or congestive heart failure or any code for coronary artery bypass grafting or percutaneous transluminal coronary angioplasty. Dyslipidemia was defined by the presence of any of the following: (1) total cholesterol level >200 mg/dL on 2 separate occasions, (2) total cholesterol level >200 mg/dL on 1 occasion and LDL-C level >130 mg/dL on 1 occasion, and (3) prescription of cholesterol-lowering medication for >30 days. Subjects were considered to have diabetes if they met any of the following criteria: (1) glucose level ≥200 mg/dL on 2 separate occasions; (2) ICD-9-CM codes (2 outpatient or 1 inpatient) and treatment with an oral hypoglycemic or insulin for ≥30 days; (3) ICD-9-CM codes (2 outpatient or 1 inpatient) and glucose level ≥126 mg/dL on 2 separate occasions; and (4) glucose level ≥200 mg/dL on 1 occasion and treatment with an oral hypoglycemic or insulin for ≥30 days. We compared our definition with the presence of at least 1 inpatient or at least 2 outpatient codes for diabetes. Our definition had a sensitivity of 86.6%, specificity of 97.5%, and agreement of 95.5%, with a κ value of 0.85, suggesting a very high degree of correlation. Renal failure was defined by an estimated glomerular filtration rate <30 mL/min/1.73 m2, as calculated by the simplified modification of diet in renal disease (i.e., MDRD) equation. Liver injury was defined by alanine or aspartate aminotransferase levels above the upper limit of normal. Because the diagnostic codes for smoking have been found to be inaccurate (A. Justice, personal communication), we used the diagnosis of chronic obstructive pulmonary disease (COPD) as a surrogate for heavy smoking. The number of pack years of smoking has been demonstrated to correlate with a diagnosis of COPD in HIV-infected and -uninfected persons among veterans [28].
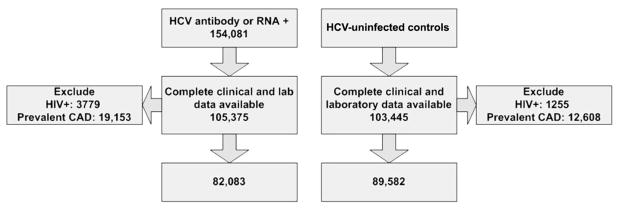
Case patients were all HCV-infected subjects initially identified in the ERCHIVES on the basis of a positive result of an HCV antibody test or a positive result of an HCV RNA test performed during routine clinical care. Control subjects were matched by age (in 5-year increments), sex, race, and year of entry into care in the VA health care system. We retained subjects with complete clinical and laboratory data and excluded subjects who were coinfected with HIV. We also excluded subjects who had a diagnosis of CAD at baseline. We analyzed all subjects with complete data, as well as HCV-infected subjects and their corresponding individually matched HCV-uninfected control subjects with complete data for our analysis (figure 1).
Baseline characteristics were compared using the χ2 test or the t test as appropriate. Predictors of incident CAD were determined in univariable and multivariable Cox regression analyses. Unadjusted and adjusted Kaplan-Meier plots were drawn to plot the hazards of developing CAD over time. We used Stata, version 8.2 (Stata Corp), for statistical analyses.
To determine whether the exclusion of any subjects may have led to a bias in our analysis, we compared the subjects who were excluded with those who were retained in the final analysis. We also compared the characteristics of subjects who were excluded because of a prevalent diagnosis of CAD at entry into the cohort. Finally, we determined the number of inpatient and outpatient visits for HCV-infected and -uninfected subjects, as well as for those with and without CAD, to indirectly analyze any diagnosis bias due to care outside the VA health care system.
RESULTS
Our final analysis set consisted of 171,665 subjects (82,083 HCV-infected and 89,582 HCV-uninfected subjects) (figure 2). HCV-infected subjects were less likely to have hypertension, hyperlipidemia, and diabetes but were more likely to abuse alcohol or drugs and to have renal failure and anemia, compared with HCV-uninfected control subjects (table 1). Mean plasma levels of total cholesterol, LDL-C, and triglycerides were significantly lower in the HCV-infected subjects, compared with HCV-uninfected subjects.
Figure 2.
Flow chart depicting the number of subjects included in the study. CAD, coronary artery disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Table 1.
Baseline characteristics of hepatitis C virus (HCV)–infected subjects and HCV-uninfected control subjects.
| Characteristic | HCV-infected subjects (n = 82,083) | HCV-uninfected subjects (n = 89,582) | P |
|---|---|---|---|
| Demographic | |||
| Age, mean years ± SD | 51.2 ± 7.3 | 51.8 ± 7.8 | <.001 |
| Race | <.001 | ||
| White | 55.4 | 55.8 | |
| Black | 29.5 | 29.5 | |
| Hispanic | 1.9 | 2.2 | |
| Other or unknown | 13.2 | 12.5 | |
| Male sex | 97.1 | 97.0 | .1 |
| Risk factor for CAD | |||
| Hypertension | 41.6 | 50.4 | <.001 |
| COPD | 10.1 | 10.5 | .005 |
| Diabetes | 20.8 | 21.8 | <.001 |
| Hyperlipidemia | 39.4 | 72.2 | <.001 |
| Total cholesterol level, mg/dL | |||
| Median | 172 | 195 | |
| Mean ± SD | 175 ± 40.8 | 198 ± 41.9 | <.001 |
| LDL-C level, mg/dL | |||
| Median | 100 | 118 | |
| Mean ± SD | 102 ± 36.8 | 119 ± 38.2 | <.001 |
| Triglycerides level, mg/dL | |||
| Median | 113 | 138 | |
| Mean ± SD | 144 ± 119 | 179 ± 151 | <.001 |
| HDL-C level, mg/dL | |||
| Median | 43 | 43 | |
| Mean ± SD | 46 ± 16.8 | 46 ± 15.2 | <.001 |
| Renal failure | 2.6 | 1.4 | <.001 |
| Liver injury | 78.1 | 29.0 | <.001 |
| Other factor | |||
| Anemia | 11.1 | 9.8 | <.001 |
| Alcohol abuse or dependence | 38.6 | 19.1 | <.001 |
| Drug abuse or dependence | 31.4 | 11.6 | <.001 |
NOTE. Data are percentage of subjects, unless otherwise indicated. CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HDL-C, high-density lipo-protein cholesterol; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation.
In univariable analysis, factors associated with a higher risk of CAD in the whole group were increasing age, hypertension, COPD, diabetes, hyperlipidemia, renal failure, and anemia. Female sex and Hispanic race were associated with a lower risk of CAD. When we analyzed HCV-infected and -uninfected subjects separately, the same factors were associated with risk of CAD, except that black race was associated with a higher risk in the HCV-infected group, and drug abuse or dependence was associated with a lower risk of CAD in the HCV-uninfected group. The magnitude of effect for hypertension, COPD, and renal failure was slightly greater in the HCV-infected group than in the HCV-uninfected group (table 2).
Table 2.
Predictors of incident coronary artery disease (CAD) in the full sample and in hepatitis C virus (HCV)–infected and HCV-uninfected groups, by univariable Cox proportional-hazards model.
| Characteristic | Hazard ratio (95% CI) | ||
|---|---|---|---|
| Overall | HCV-infected subjects | HCV-uninfected subjects | |
| HCV infection | 1.01 (0.97–1.05) | … | … |
| Demographic | |||
| Age (5-year increments) | 1.20 (1.19–1.22) | 1.21 (1.19–1.22) | 1.20 (1.18–1.22) |
| Race | |||
| White (comparator) | 1.00 | 1.00 | 1.00 |
| Black | 1.04 (1.00–1.08) | 1.10 (1.04–1.16) | 0.97 (0.92–1.03) |
| Hispanic | 0.78 (0.68–0.89) | 0.63 (0.50–0.78) | 0.90 (0.76–1.06) |
| Other or unknown | 0.73 (0.68–0.78) | 0.60 (0.54–0.66) | 0.87 (0.80–0.95) |
| Female sex | 0.58 (0.50–0.66) | 0.61 (0.50–0.73) | 0.54 (0.45–0.66) |
| Risk factor for CAD | |||
| Hypertension | 1.92 (1.85–1.99) | 2.11 (2.00–2.22) | 1.74 (1.65–1.84) |
| COPD | 1.61 (1.53–1.69) | 1.64 (1.53–1.76) | 1.57 (1.46–1.69) |
| Diabetes | 2.38 (2.29–2.47) | 2.40 (2.28–2.53) | 2.36 (2.23–2.49) |
| Hyperlipidemia | 2.13 (2.04–2.22) | 2.28 (2.17–2.40) | 2.41 (2.23–2.61) |
| Renal failure | 4.70 (4.37–5.05) | 5.01 (4.58–5.48) | 4.14 (3.65–4.70) |
| Other factor | |||
| Anemia | 1.88 (1.79–1.97) | 1.98 (1.86–2.12) | 1.75 (1.63–1.89) |
| Alcohol abuse or dependence | 0.95 (0.91–0.99) | 0.98 (0.93–1.03) | 0.89 (0.83–0.96) |
| Drug abuse or dependence | 0.95 (0.91–0.99) | 0.99 (0.94–1.04) | 0.85 (0.78–0.93) |
NOTE. CI, confidence interval; COPD, chronic obstructive pulmonary disease.
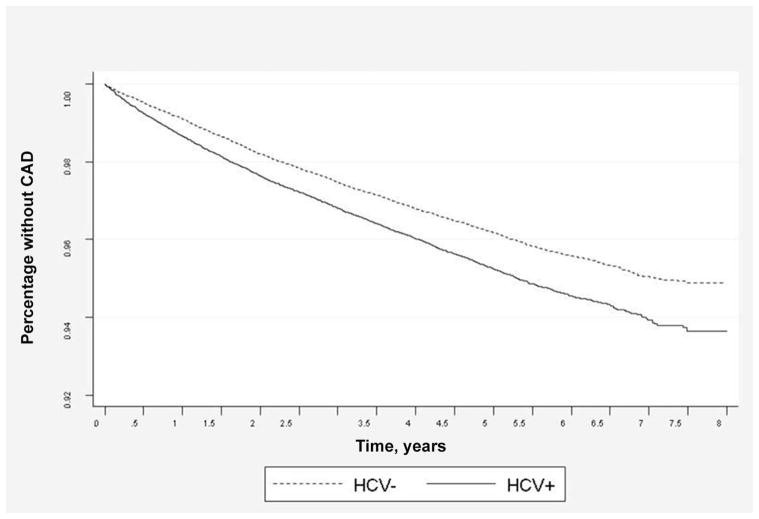
In multivariable Cox regression analysis of the whole group, HCV infection was associated with a higher risk of CAD (hazard ratio, 1.27; 95% confidence interval [CI], 1.22–1.31). As in the univariable model, traditional risk factors (increasing age, hypertension, COPD, diabetes, hyperlipidemia, and renal failure) were associated with a higher risk of CAD, whereas minority race and female sex were associated with a lower risk of CAD. When we analyzed HCV-infected and -uninfected subjects separately, the same factors were associated with the risk of CAD. Drug abuse or dependence was associated with a higher risk of CAD in the HCV-infected group but not in the HCV-un-infected group (table 3). In Kaplan-Meier analysis, HCV-infected subjects had a higher risk of CAD after adjustment for traditional risk factors (age, race, sex, hypertension, diabetes, hyperlipidemia, and COPD) (figure 3).
Table 3.
Predictors of incident coronary artery disease (CAD) in the full sample and in hepatitis C virus (HCV)–infected and HCV-uninfected groups, by multivariable Cox proportional-hazards model.
| Characteristic | Hazard ratio (95% CI) | ||
|---|---|---|---|
| Overall | HCV-infected subjects | HCV-uninfected subjects | |
| HCV infection | 1.27 (1.22–1.31) | … | … |
| Demographic | |||
| Age (5-year increments) | 1.14 (1.13–1.16) | 1.14 (1.12–1.15) | 1.15 (1.13–1.17) |
| Race | |||
| White (comparator) | 1.00 | 1.00 | 1.00 |
| Black | 0.89 (0.86–0.93) | 0.90 (0.85–0.95) | 0.89 (0.84–0.94) |
| Hispanic | 0.71 (0.62–0.81) | 0.58 (0.47–0.73) | 0.81 (0.68–0.96) |
| Other or unknown | 0.75 (0.70–0.80) | 0.64 (0.58–0.70) | 0.87 (0.80–0.95) |
| Female sex | 0.70 (0.62–0.81) | 0.72 (0.60–0.86) | 0.69 (0.56–0.84) |
| Risk factor for CAD | |||
| Hypertension | 1.37 (1.32–1.43) | 1.50 (1.42–1.58) | 1.25 (1.18–1.32) |
| COPD | 1.46 (1.39–1.54) | 1.44 (1.34–1.54) | 1.48 (1.38–1.59) |
| Diabetes | 1.82 (1.75–1.90) | 1.79 (1.69–1.89) | 1.87 (1.76–1.98) |
| Hyperlipidemia | 2.08 (2.00–2.18) | 2.06 (1.95–2.17) | 2.14 (1.97–2.31) |
| Renal failure | 2.78 (2.57–3.00) | 2.82 (2.56–3.11) | 2.57 (2.25–2.94) |
| Other factor | |||
| Anemia | 1.37 (1.30–1.44) | 1.42 (1.32–1.53) | 1.32 (1.22–1.43) |
| Alcohol abuse or dependence | 1.04 (0.99–1.09) | 1.06 (0.99–1.13) | 1.01 (0.93–1.10) |
| Drug abuse or dependence | 1.10 (1.04–1.16) | 1.10 (1.03–1.17) | 1.07 (0.96–1.19) |
NOTE. CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Figure 3.
Risk of coronary artery disease (CAD) in hepatitis C virus (HCV)–infected (HCV+) subjects and HCV-uninfected (HCV−) subjects (P < .0001), adjusted for age, race, sex, hypertension, diabetes, hyperlipidemia, and chronic obstructive pulmonary disease.
We determined the number of inpatient and outpatient visits to any VA facility for subjects with and without a diagnosis of incident CAD, and we compared the number of visits in the years before and the years after the CAD diagnosis to determine whether there may be a bias in seeking care at a VA facility versus outside the VA health care system. For this analysis, we excluded visits to outpatient mental health facilities, group therapy, and substance abuse clinics, as well as laboratory visits and visits related to hemodialysis. The median number of visits before CAD diagnosis among subjects who later developed CAD was 7, compared with 5 visits among subjects who never developed CAD. In years after the CAD diagnosis, the median number of visits was 13.5 among those with CAD and 7 among those without CAD, which suggests that a high proportion of veterans seek care from VA facilities after a diagnosis of coronary events.
To determine the role of liver injury in the risk of CAD among HCV-infected and -uninfected subjects, we computed the risk of liver injury in the hazards of CAD in the Cox proportional-hazards model. The overall hazard ratio was 1.09 (95% CI, 1.05–1.14); for HCV-infected and -uninfected groups, the hazard ratios were 1.05 (95% CI, 0.80–1.06) and 1.15 (95% CI, 1.09–1.22), respectively. We conducted further analyses limited to those subjects who had evidence of liver injury in the absence of a diagnosis of alcohol abuse or dependence. In multivariable Cox proportional-hazards analysis, HCV infection was not associated with a higher risk of CAD (hazard ratio, 0.99; 95% CI, 0.93–1.06). All other associations (age, sex, hypertension, COPD, diabetes, dyslipidemia, renal failure, and anemia) remained significant as in the previous models.
DISCUSSION
To our knowledge, this is the largest study to determine the role of HCV infection in the risk of CAD. We found that HCV-infected subjects were at a significantly higher risk of developing CAD, compared with HCV-uninfected subjects, even after adjustment for traditional risk factors for cardiovascular disease. The reason for the increased risk is unclear, especially because several cardiovascular risk factors were less prevalent in the HCV-infected subjects. For example, HCV-infected subjects were younger; had lower total cholesterol, LDL-C, and triglyceride levels; and had a lower prevalence of hypertension. Because the risk of CAD was higher after adjustment for the traditional risk factors, HCV infection itself or other unknown factors are at least partly responsible for the increased risk.
Recent studies support the role of inflammation in the pathogenesis of CAD [29–32]. According to these studies, a complex balance between proinflammatory and anti-inflammatory cytokines dictates the initiation, propagation, and rupture of atherosclerotic lesions. Some studies have shown that the levels of inflammatory markers (e.g., high sensitivity to C-reactive protein, interleukin 6, and tumor necrosis factor α) are higher in HCV-infected subjects, compared with HCV-uninfected control subjects [33–35]. Since inflammation and thrombosis are critical pathways in the genesis of CAD and since HCV infection is also associated with alterations in inflammatory markers, this might be the common pathway that increases CAD risk. Markers of thrombosis and inflammation have also been associated with more-severe CAD, and the malnutrition inflammation scores are elevated in HCV-infected persons with CAD, compared with those without CAD [36, 37]. Most studies have shown an increased prevalence of diabetes among HCV-infected persons, which is a major cardiovascular risk factor. Although we did not find an increased prevalence of diabetes among the HCV-infected persons in the current study, we did not adjust for body mass index in the 2 groups. In our previous work, we found that HCV-infected persons have a lower body mass index, compared with HCV-uninfected persons (authors’ unpublished data), which could at least partly explain this finding. Another possibility is that HCV-infected persons are seen less frequently for care and are less likely to receive a diagnosis of diabetes. In fact, nonadherence to follow-up visits was the most common reason that HCV-infected people were not prescribed treatment for HCV infection in one study [38]. Although diabetes was not more prevalent among the HCV-infected group in our study, when present, it was associated with a significantly higher risk of CAD in both groups.
The proportion of subjects with evidence of liver injury was higher among the HCV-infected persons. This finding is intuitive and confirms multiple previous studies. Liver injury was associated with an increased risk of CAD, but this risk was contributed to by the HCV-uninfected subjects and was not significant for the HCV-infected subjects. Furthermore, the lack of association between HCV infection and CAD in the subset of persons with liver injury (in the absence of a diagnosis of alcohol abuse or dependence) suggests a role for liver injury in determining the risk of CAD.
Our finding of lower lipid levels in the HCV-infected persons is consistent with previous studies [39–41]. Postulated mechanisms for lower lipid levels in HCV-infected persons include binding of HCV particles to various lipid fractions, impaired hepatocyte assembly of very-low-density lipoprotein because of inhibition of microsomal transfer protein, and entry of HCV into hepatocytes through the LDL-C receptors [39]. Although the lipid levels were lower in the HCV-infected subjects, the risk of CAD associated with hyperlipidemia was similar in the HCV-infected and -uninfected subjects.
Other traditional risk factors (e.g., age, male sex, hypertension, COPD [a surrogate for heavy smoking], and diabetes) were associated with a higher risk of CAD in both HCV-infected and -uninfected groups, as shown in other studies. Unanticipated was the association of black race with a lower risk of CAD. Numerous studies indicate that minority race is associated with a higher risk of cardiovascular risk factors (e.g., hypertension and diabetes), but this did not translate into a higher risk of CAD in our study. Whether the lower risk of CAD found in our study is related to access-to-care issues or other factors needs further study. The finding of a positive association between drug abuse or dependence and CAD in the HCV-infected persons is an interesting finding. Cocaine use has been well established as a risk factor for acute myocardial infarction in persons with and without preexisting coronary disease [42]. Although coronary vasospasm has been implicated as the most likely mechanism, arrhythmias and increased atherosclerosis due to adventitial mast cells have also been proposed to effect such risk [43, 44]. Whether our observation is associated with such behavioral risk factors is unclear at this time.
There are many strengths to our study. We studied a large national population, rather than a geographically limited sample. The VA health care system is a unique population that offers significant advantages for large studies of outcomes. Foremost is the availability of data at centralized centers, from which appropriate clinical, laboratory, pharmacy, and outcome parameters can be retrieved. The VA is the largest single provider of comprehensive health care to HCV-infected persons in the United States. Its extensive electronic medical information–gathering system is linked nationally and provides unparalleled opportunity for longitudinal follow-up of patients. The patients at the VA medical centers are cared for regardless of their ability to pay. Patients may relocate multiple times and will still be cared for by the same system, with health care providers having access to patient information from other sites.
A limitation of our study is the use of ICD-9-CM codes for the diagnosis of CAD. Although it would be ideal to use adjudicated clinical measures for the diagnosis of CAD, it would not be possible in such a large national study. ICD-9-CM codes have been used in other large national studies for cardiovascular outcomes. Another limitation is the lack of inclusion of body mass index and family history of CAD, which are important risk factors for CAD. Although we do not believe that family history of CAD may be different in HCV-infected and -un-infected persons, the former are more likely to have a lower body mass index. If true, the higher risk found in our study is actually an underestimation of the true risk. We used the diagnosis of COPD as a surrogate for heavy smoking status, because it is likely to pick up only a subset of smokers with the most severe consequences of smoking. It may be argued that persons who receive a diagnosis of an acute coronary event may not have presented to a VA facility for care and instead may have been taken to the nearest emergency facility. If true, this is likely to affect both HCV-infected and -uninfected persons equally.
To summarize, the increased risk of CAD in HCV-infected persons may be related to a differential level of cytokines, which are markers of inflammation, thrombosis, and endothelial dysfunction [33–35, 37]; behavioral and social risk profile [42–44]; malnutrition and/or inflammation pathway activation [36]; or liver injury. More likely, a combination of these factors acts in concert to negate the protective effect of a favorable risk profile and increases the overall risk of CAD.
In conclusion, in a comparison of HCV-infected subjects with HCV-uninfected control subjects, HCV infection is associated with a higher risk of CAD, even after adjustment for traditional risk factors. The reason(s) and mechanism(s) of this association need further study.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System and the central data repositories maintained by the VA Information Resource center, including the National Patient Care Database, the Decisions Support System database, and the Pharmacy Benefits Management database.
Financial support. National Institutes of Health, National Institute on Drug Abuse (K23 DA016175–01A1 to A.A.B.).
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–6. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 2.Sheehan J, Kearney PM, Sullivan SO, Mongan C, Kelly E, Perry IJ. Acute coronary syndrome and chronic infection in the Cork coronary care case-control study. Heart. 2005;91:19–22. doi: 10.1136/hrt.2003.031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong IW. Emerging relations between infectious diseases and coronary artery disease and atherosclerosis. CMAJ. 2000;163:49–56. [PMC free article] [PubMed] [Google Scholar]
- 4.Arcari CM, Nelson KE, Netski DM, Nieto FJ, Gaydos CA. No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis. 2006;43:e53–6. doi: 10.1086/507031. [DOI] [PubMed] [Google Scholar]
- 5.Volzke H, Schwahn C, Wolff B, et al. Hepatitis B and C virus infection and the risk of atherosclerosis in a general population. Atherosclerosis. 2004;174:99–103. doi: 10.1016/j.atherosclerosis.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Momiyama Y, Ohmori R, Kato R, Taniguchi H, Nakamura H, Ohsuzu F. Lack of any association between persistent hepatitis B or C virus infection and coronary artery disease. Atherosclerosis. 2005;181:211–3. doi: 10.1016/j.atherosclerosis.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Tong DY, Wang XH, Xu CF, Yang YZ, Xiong SD. Hepatitis B virus infection and coronary atherosclerosis: results from a population with relatively high prevalence of hepatitis B virus. World J Gastroenterol. 2005;11:1292–6. doi: 10.3748/wjg.v11.i9.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassalle C, Masini S, Bianchi F, Zucchelli GC. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565–6. doi: 10.1136/hrt.2003.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishizaka N, Ishizaka Y, Takahashi E, et al. Association between hepatitis C virus seropositivity, carotidartery plaque, and intima-media thickening. Lancet. 2002;359:133–5. doi: 10.1016/s0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 10.Sawayama Y, Okada K, Maeda S, Ohnishi H, Furusyo N, Hayashi J. Both hepatitis C virus and Chlamydia pneumoniae infection are related to the progression of carotid atherosclerosis in patients undergoing lipid lowering therapy. Fukuoka Igaku Zasshi. 2006;97:245–55. [PubMed] [Google Scholar]
- 11.Fukui M, Kitagawa Y, Nakamura N, Yoshikawa T. Hepatitis C virus and atherosclerosis in patients with type 2 diabetes. JAMA. 2003;289:1245–6. doi: 10.1001/jama.289.10.1245-b. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal AJ, Contos MJ, Sterling RK, et al. Nonalcoholic fatty liver disease in patients with hepatitis C is associated with features of the metabolic syndrome. Am J Gastroenterol. 2003;98:2064–71. doi: 10.1111/j.1572-0241.2003.07640.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanyal AJ. Review article: non-alcoholic fatty liver disease and hepatitis C—risk factors and clinical implications. Aliment Pharmacol Ther. 2005;22:48–51. doi: 10.1111/j.1365-2036.2005.02596.x. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men: role of visceral adipose tissue. Diabet Med. 2005;22:1354–8. doi: 10.1111/j.1464-5491.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 15.Butt AA, Justice AC, Skanderson M, Rigsby M, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut. 2007;56:385–9. doi: 10.1136/gut.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt AA, Khan UA, McGinnis KA, Skanderson M, Kwoh CK. Comorbid medical and psychiatric illness and substance abuse in HCV-infected and uninfected veterans. J Viral Hepat. 2007;14:890–6. doi: 10.1111/j.1365-2893.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 17.Butt AA, Justice AC, Skanderson M, Good CB, Kwoh CK. Rates and predictors of HCV treatment in HCV-HIV coinfected persons. Aliment Pharmacol Ther. 2006;24:585–91. doi: 10.1111/j.1365-2036.2006.03020.x. [DOI] [PubMed] [Google Scholar]
- 18.Butt AA, Skanderson M, McGinnis KA, Kwoh CK, Justice AC. Real-life rates of treatment completion for HCV. Program and abstracts of the 58th Annual Meeting of the American Association for the Study of Liver Diseases; Boston. 2007. [Google Scholar]
- 19.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 20.Kramer JR, Giordano TP, Souchek J, El Serag HB. Hepatitis C coinfection increases the risk of fulminant hepatic failure in patients with HIV in the HAART era. J Hepatol. 2005;42:309–14. doi: 10.1016/j.jhep.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100:56–63. doi: 10.1111/j.1572-0241.2005.40670.x. [DOI] [PubMed] [Google Scholar]
- 22.Giordano TP, Kramer JR, Souchek J, Richardson P, El Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992–2001. Arch Intern Med. 2004;164:2349–54. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 23.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(Suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 24.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006;44(Suppl 2):S52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 25.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. The risk of diabetes in HIV infected veterans in the pre- and post-HAART era and the role of hepatitis C virus co-infection. Hepatology. 2004;40:115–9. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 26.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19(Suppl 3):S99–105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 27.McGinnis KA, Skanderson M, Levin F, Brandt C, Erdos J, Justice AC. Comparison of two VA laboratory data repositories indicates that missing data vary despite originating from the same source. Med Care. 2009;47:121–4. doi: 10.1097/MLR.0b013e31817d69c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas M, Crystal S, Justice AC. Increased chronic obstructive pulmonary disease among HIV positive compared to HIV negative veterans. Chest. 2006;130:1326–33. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 29.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 30.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 31.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 32.Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10:63–71. doi: 10.5551/jat.10.63. [DOI] [PubMed] [Google Scholar]
- 33.Nascimento MM, Bruchfeld A, Suliman ME, et al. Effect of hepatitis C serology on C-reactive protein in a cohort of Brazilian hemodialysis patients. Braz J Med Biol Res. 2005;38:783–8. doi: 10.1590/s0100-879x2005000500017. [DOI] [PubMed] [Google Scholar]
- 34.Riordan SM, Skinner NA, Kurtovic J, et al. Toll-like receptor expression in chronic hepatitis C: correlation with pro-inflammatory cytokine levels and liver injury. Inflamm Res. 2006;55:279–85. doi: 10.1007/s00011-006-0082-0. [DOI] [PubMed] [Google Scholar]
- 35.Rios-Olivares E, Vila LM, Reyes JC, et al. Impaired cytokine production and suppressed lymphocyte proliferation activity in HCV-infected cocaine and heroin (“speedball”) users. Drug Alcohol Depend. 2006;85:236–43. doi: 10.1016/j.drugalcdep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Elsurer R, Afsar B, Sezer S, Arat Z, Ozdemir FN, Haberal M. Malnutrition inflammation score is associated with coronary artery disease in hepatitis C virus-infected hemodialysis patients. Eur J Clin Nutr. 2008;62:1449–54. doi: 10.1038/sj.ejcn.1602867. [DOI] [PubMed] [Google Scholar]
- 37.Alyan O, Kacmaz F, Ozdemir O, et al. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J. 2008;72:1960–5. doi: 10.1253/circj.cj-08-0459. [DOI] [PubMed] [Google Scholar]
- 38.Butt AA, Wagener M, Shakil AO, Ahmad J. Reasons for non-treatment of hepatitis C in veterans in care. J Viral Hepat. 2005;12:81–5. doi: 10.1111/j.1365-2893.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 39.Marzouk D, Sass J, Bakr I, et al. Metabolic and cardiovascular risk profiles and hepatitis C virus infection in rural Egypt. Gut. 2007;56:1105–10. doi: 10.1136/gut.2006.091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarmay K, Karacsony G, Nagy A, Schaff Z. Changes in lipid metabolism in chronic hepatitis C. World J Gastroenterol. 2005;11:6422–8. doi: 10.3748/wjg.v11.i41.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siagris D, Christofidou M, Theocharis GJ, et al. Serum lipid pattern in chronic hepatitis C: histological and virological correlations. J Viral Hepat. 2006;13:56–61. doi: 10.1111/j.1365-2893.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 42.Gradman AH. Cardiac effects of cocaine: a review. Yale J Biol Med. 1988;61:137–47. [PMC free article] [PubMed] [Google Scholar]
- 43.Kolodgie FD, Virmani R, Cornhill JF, Herderick EE, Smialek J. Increase in atherosclerosis and adventitial mast cells in cocaine abusers: an alternative mechanism of cocaine-associated coronary vasospasm and thrombosis. J Am Coll Cardiol. 1991;17:1553–60. doi: 10.1016/0735-1097(91)90646-q. [DOI] [PubMed] [Google Scholar]
- 44.Bauman JL, Grawe JJ, Winecoff AP, Hariman RJ. Cocaine-related sudden cardiac death: a hypothesis correlating basic science and clinical observations. J Clin Pharmacol. 1994;34:902–11. doi: 10.1002/j.1552-4604.1994.tb04003.x. [DOI] [PubMed] [Google Scholar]