Abstract
Vascular injury and remodeling are common pathological sequelae of hypertension. Previous studies have suggested that the renin-angiotensin system (RAS) acting through the type I (AT1) angiotensin (AT1)-receptor promotes vascular pathology in hypertension. To study the role of AT1-receptors in this process, we generated mice with cell-specific deletion of AT1-receptors in VSMCs using Cre/Loxp technology. We crossed the SM22α-Cre transgenic mouse line expressing Cre recombinase in smooth muscle cells with a mouse line bearing a conditional allele of the Agtr1a gene (Agtr1a flox), encoding the major murine AT1-receptor isoform (AT1A). In SM22α-Cre+Agtr1a flox/flox (SMKO) mice, AT1A-receptors were efficiently deleted from VSMCs in larger vessels, but not from resistance vessels such as pre-glomerular arterioles. Thus, vasoconstrictor responses to angiotensin II were preserved in SMKOs. To induce hypertensive vascular remodeling, mice were continuously infused with angiotensin II for 4 weeks. During infusion of angiotensin II, blood pressures increased significantly and to a similar extent in SMKOs and controls. In control mice, there was evidence of vascular oxidative stress indicated by enhanced nitrated tyrosine residues in segments of aorta; this was significantly attenuated in SMKOs. Despite these differences in oxidative stress, the extent of aortic medial expansion induced by angiotensin II infusion was virtually identical in both groups. Thus, vascular AT1A-receptors promote oxidative stress in the aortic wall but are not required for remodeling in angiotensin II-dependent hypertension.
Keywords: angiotensin II, hypertrophy, hyperplasia, aorta, smooth muscle, hypertension
Introduction
The renin-angiotensin system (RAS) is a principal regulator of blood pressure homeostasis; dysregulation of this system commonly contributes to human hypertension.1, 2 Accordingly, pharmacological inhibitors of the RAS including ACE inhibitors and ARBs can effectively lower blood pressure in a significant proportion of patients with essential hypertension.3, 4 Moreover, these agents also attenuate end-organ damage, decreasing cardiovascular morbidity and slowing the progression of chronic kidney injury.5, 6 It has been suggested that RAS inhibitors provide protection against complications of hypertension beyond their effects to lower blood pressure, indicating non-hemodynamic, cellular actions of angiotensin II to promote tissue damage.7 However, in some clinical trials, end-organ protection by RAS inhibition has been accompanied by more effective reduction of blood pressure.4, 8, 9 Moreover, studies in animal models have suggested that the anti-hypertensive actions of RAS inhibitors are critical for preventing cardiac hypertrophy 10 and progressive kidney injury.11
The vascular system is a major target of damage in hypertension. Expansion of arteries and arterioles in the kidney, also called nephrosclerosis, is the most common renal pathological lesion accompanying hypertension12 and is an important cause of chronic kidney disease in African Americans.13 Vessel remodeling with changes in compliance is also seen in the aorta and other vascular beds in hypertension14 where the RAS has potent actions to influence vascular structure and function.15 For example, angiotensin II causes systemic vasoconstriction by activation of AT1-receptors in VSMCs.16 Along with their effects on vascular tone, AT1-receptors may also stimulate growth and hypertrophy of vascular smooth muscle cells,17 thereby directly contributing to vascular remodeling in hypertension. It has been suggested that non-hemodynamic actions of AT1-receptors, including enhanced generation of reactive oxygen species (ROS) may promote changes in vascular structure that perpetuate the development of hypertension.14 Furthermore, ARBs reverse vascular remodeling in patients with hypertension suggesting a direct role for vascular AT1-receptors in this process.18
Nonetheless, the precise role of AT1-receptors in individual tissues is difficult to discern through experiments using pharmacological inhibitors or conventional gene targeting, where actions of AT1-receptors are abrogated in all tissues, and changes in blood pressure may further confound interpretations. Accordingly, we generated mice with cell-specific deletion of AT1A-receptors from smooth muscle cells in conduit vessels that are subject to hypertensive remodeling. During angiotensin II-dependent hypertension, we find reduced oxidative stress in vascular segments lacking AT1A-receptors, but vascular remodeling is unaffected.
Methods
Generation of experimental animals
A mouse line with a conditional Agtr1a allele was generated using homologous recombination in embryonic stem cells as described (Gurley et al., submitted and Supplemental Data please see http://hyper.ahajournals.org).19 SM22α-Cre mice were purchased from The Jackson Laboratory (stock number 004746). Mice were bred and maintained in the AAALAC-accredited animal facilities at the Durham VA Medical Center according to NIH guidelines. All of the animal studies were approved by the Durham Veterans’ Affairs Medical Center Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals had free access to standard rodent chow and water unless specified. 8–12 week-old male mice and littermate controls were used for experiments.
Isolation of pre-glomerular vessels for receptor expression analysis
Afferent arterioles and interlobular arteries were isolated from kidneys using a modified iron oxide-sieving technique according to Chatziantoniou et al.20, 21 The enriched preparation of pre-glomerular arteries and arterioles was transferred to a tube containing RNAlater and stored at 4°C for 24 hours, then at −80°C.
Measurement of AT1A mRNA levels and RT-PCR
Relative levels of mRNA for the AT1A-receptor in various tissues were determined by real time RT-PCR with the ABI Prism 7700 sequence detection system as described.22 Tissues were harvested and total RNA was isolated using TRI Reagent (Sigma-Aldrich) per the manufacturer’s instructions. The number of copies of the PCR template in the starting sample is calculated using the Sequence Detector Software (SDS) incorporated in the ABI Prism 7700 Sequence Detector System. For each experimental sample, the amounts of the target and of the endogenous control were determined from the appropriate standard curves or ΔΔCT
Isometric force measurements in aortic and mesenteric rings
Aortic and mesenteric artery rings were mounted in a wire myograph as described previously.23 Dose-response curves were generated for phenylephrine and Angiotensin II. Forces are expressed as a percentage of the maximal response to phenylephrine, this was equivalent between groups.
Assessment of acute vasoconstrictor responses
Our previous studies showed that vasoconstrictor responses to acute administration of angiotensin II are almost completely extinguished in mice with complete AT1A receptor-deficiency.24 Therefore, to determine the veracity of the deletion of vascular AT1A receptors, we examined acute pressor responses to angiotensin II as described previously.24 At 5-min intervals, increasing doses (0.1, 1 and 10 μg/kg) of Angiotensin II (Sigma Aldrich) or 10 μg/kg of epinephrine (Sigma Aldrich) were injected intravenously while intra-arterial pressures were continuously monitored.
Blood pressure measurements in conscious mice
Blood pressures were measured in 8–12 week old male conscious SMKO and control mice using radiotelemetry, as described previously.25 During the measurement period, mice were housed in a monitoring room where quiet is maintained and no other experiments are performed. Arterial blood pressures were collected, stored, and analyzed using Dataquest A. R. T. software (version 4.0, Transoma Medical). Measurements were recorded over a ten-second interval every five minutes at baseline and during twenty-one days while Ang II was infused chronically (1000ng/kg/min) by osmotic mini-pump (Alzet).
Analysis of oxidized amino acids in aortae
Following 28 days of angiotensin II infusion, thoracic aortae were dissected from control and SMKO mice and immediately place in antioxidant buffer (100 μM diethylene tetramino pentaacetic acid (metal chelator), 50 μM butylated hydroxytoluene (lipid soluble antioxidant), 10μL/ml protease inhibitor (Halt™ Protease Inhibitor Cocktail, Pierce, Rockford, IL) in 50 mM sodium phosphate buffer, pH 7.4) at −80C. Amino acids were isolated from the acid hydrolysate using a solid-phase column (Supelclean ENVI ChromP column, Supelco Inc., Bellefonte, PA) as described.26 Oxidized amino acids were quantified by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) using multiple reaction monitoring (MRM) mode. Under these chromatography conditions, authentic compounds and isotopically labeled standards were base line-separated and exhibited retention times identical to those of analytes derived from tissue samples. 3-nitrotyrosine and dityrosine were detected by characteristic LC retention time and specific ion transitions in the MRM mode. The ratio of the peak areas of the analyte with corresponding 13C internal standard were utilized to quantify levels of oxidized amino acids in tissue. Results are normalized to protein content of tyrosine, the precursor of 3-nitrotyrosine and dityrosine.
Measurement of hydrogen peroxide production in aortae
Hydrogen Peroxide (H2O2) of freshly prepared thoracic aorta from both SMKO and controls (n=3 for both) were measured with the Amplex Red H2O2 Assay Kit (Molecular Probes). Thoracic aortae were harvested and adventitial tissue was dissected free in ice cold Krebs-Henseleit buffer. After opening the aorta with scissors and washing out the blood, the aorta was incubated in the reaction mixture for 60 minutes in the dark at 37°C. The supernatant was then read in a fluorescent spectrophotometery according to the protocol provided by the manufacturer. The fluorescent values were normalized to the protein content measured in each sample (BioRad).
Measurement of medial thickness, medial area and luminal area of aortae
The extent of vascular pathology was assessed by measuring medial thickness of descending thoracic aorta. 2 cm of descending aorta was dissected in animals that were fixed and perfused with 4% paraformaldehyde and placed in 10% formalin overnight. 10μm sections were obtained after paraffin embedding. Sections were stained with hematoxalin and eosin and photographs were taken at 40× and 10X (Zeiss Axio Imager, QImaging MicroPublisher 5.0 MP colour camera). Medial thickness, medial area and luminal area of the aorta using 4 random sections throughout the specimen and was quantified using MetaMorph in a blinded fashion.
Statistical analysis
The values for each parameter within a group are expressed as mean ± SEM. For comparisons between control and SMKO groups, statistical significance was assessed using an unpaired 2-tailed Student’s t test. A paired 2-tailed Student’s t test was used for comparisons within groups. P values less than 0.05 were considered significant. For blood pressure tracings measured over multiple days, two-way AVOVA was performed followed by Bonferroni correction.
Results
Generation of mice with deletion of AT1-receptors from smooth muscle
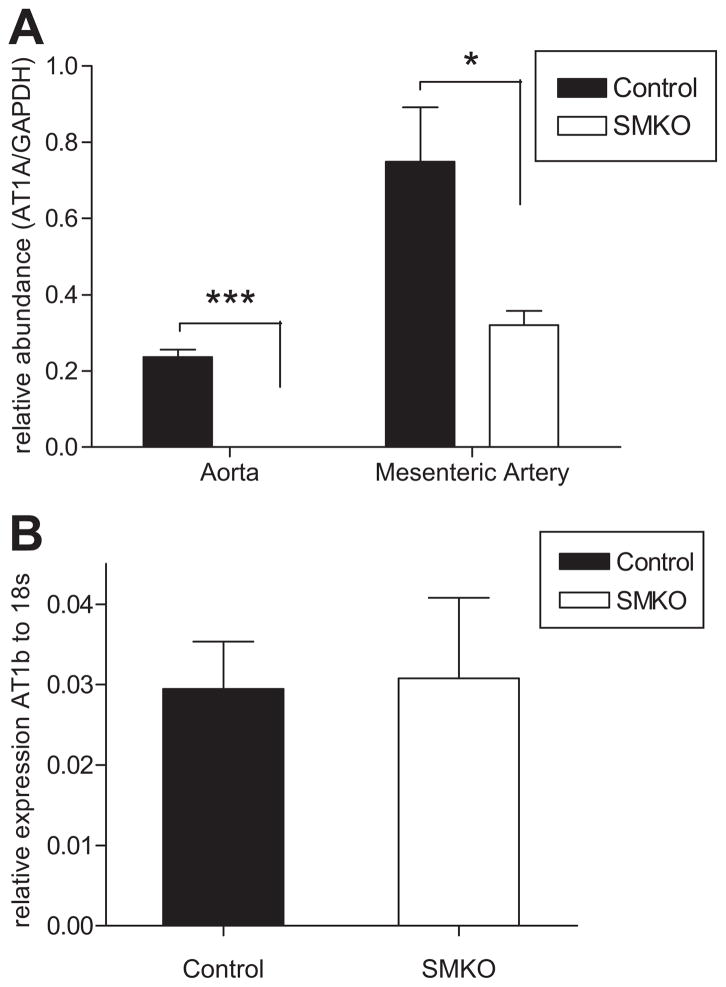
We carried out successive intercrosses between the SM22α-Cre line and mice homozygous for the conditional “floxed” Agtr1a allele (Agtr1aflox/flox) to generate SM22α-Cre+-Agtr1aflox/flox (SMKO) and SM22α-Cre−-Agtr1aflox/flox (Control) mice for experiments. To confirm elimination of AT1A-receptors from various vascular beds, levels of expression for AT1A-receptor mRNA were measured by real-time RT-PCR. Segments of aorta were isolated from SMKO and control mice, and the adventitia and endothelium were removed by dissection. As shown in Figure 1A, mRNA for the AT1A-receptor was easily detected in aortae from control mice, but not from SMKOs (P<0.0005). Similarly, AT1A mRNA expression in mesenteric arteries, with intact endothelium and adventitia, was decreased by 60% in SMKO mice compared to controls (P<0.05; Figure 1A). Thus, in SMKOs, AT1A-receptors are efficiently eliminated from VSMCs in conduit vessels. Furthermore, no difference was seen in the relatively low levels of AT1B-receptor expression in aorta between the groups (Figure 1B).
Figure 1. The AT1A-receptor is deleted from conduit vessels.
(A) Real time PCR performed on aorta stripped of adventitia and endothelium showed that mRNA for the AT1A-receptor was easily detected in aortae from control mice (black bars), but not from SMKOs (white bars)(n ≥ 3, ***P<0.0005 vs. control). AT1A mRNA expression in mesenteric arteries, with intact endothelium and adventitia, was decreased by 60% in SMKO mice compared to controls (n=5,*P<0.05). (B) No difference was seen in AT1B-receptor expression in the aorta between SMKOs and controls (n=3, P=NS).
Vasoconstrictor responses measured ex vivo
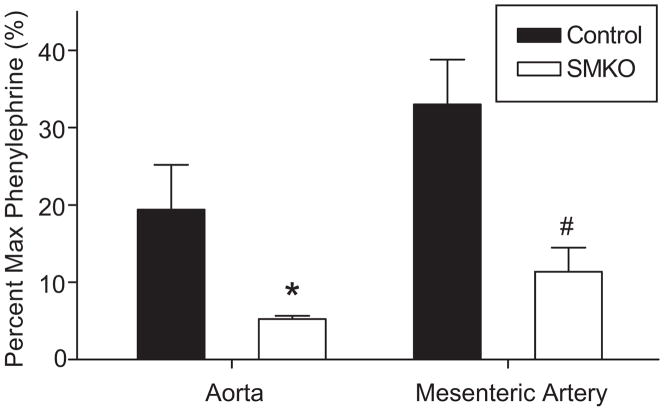
In order to functionally verify the efficiency of deletion of the AT1A-receptor from VSMCs, the contractile response of isolated vessels was assessed ex vivo. Isometric force was first measured after exposure to phenylephrine and then was independently measured to angiotensin II. Forces are expressed as a percentage of the maximal response to phenylephrine, which was equivalent between groups. As shown in Figure 2, the contractile response to Ang II was significantly reduced by ≈75% in aortae from the SMKOs compared to controls (P≤0.0005) consistent with the absence of AT1A-receptor mRNA depicted in Figure 1A above. Similarly, there was a corresponding reduction of ≈65% in the mesenteric artery segments from SMKO mice compared to controls (P≤0.05; Figure 2).
Figure 2. VSMC-specific AT1A-receptor deletion leads to diminished acute vascular angiotensin II responses in conduit vessels ex vivo.
Isometric force was measured in abdominal aorta and mesenteric artery ex vivo in response to increasing doses of phenylephrine, followed by 100 nM angiotensin II. Forces are shown as a percentage of the maximal response to phenylephrine, which was equal between groups. The contractile response to angiotensin II was significantly reduced in both the abdominal aorta (*P<0.05 SMKO, n=5 vs. control, n=5) and mesenteric artery (#P<0.01 SMKO, n=6 vs. control, n=5).
AT1A mRNA expression in resistance arteries
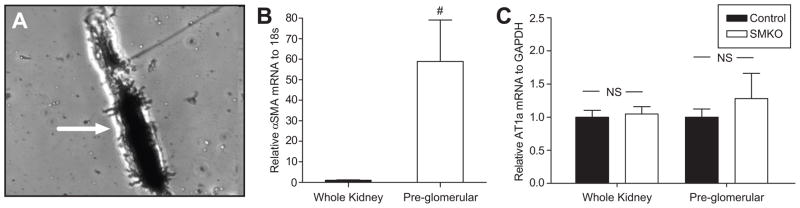
To examine AT1A-receptor expression in a preparation of resistance arteries, pre-glomerular arterioles were isolated from kidneys using the iron oxide sieving technique 21. Based on microscopic examination (Figure 3A) and augmented mRNA expression of VSMC markers such as smooth muscle actin (Figure 3B), there was marked enrichment for pre-glomerular vessels using this approach. However, unlike results with the aorta or mesenteric arteries, expression of AT1A mRNA in pre-glomerular arterioles isolated from SMKOs was preserved at levels that were not significantly different from controls indicating lack of efficient excision of the floxed Agtr1a gene in these segments (P=NS; Figure 3C).
Figure 3. Microscopic examination and gene expression analysis of an enriched preparations of pre-glomerular vessels.
(A) Photomicrograph (40X) showing a representative pre-glomerular arteriole (Arrow) purified using the iron oxide-sieving technique. (B) Real time PCR confirmed a significant enrichment of α-smooth muscle actin (SMA) mRNA in the pre-glomerular vessel isolation as compared to whole kidney (#P<0.005, n=8). (C) There were no differences in AT1A receptor mRNA levels in either the whole kidney (n=4) or pre-glomerular vessel preparation (n=3) between SMKOs and controls (P=NS).
Acute responses to vasoconstrictors
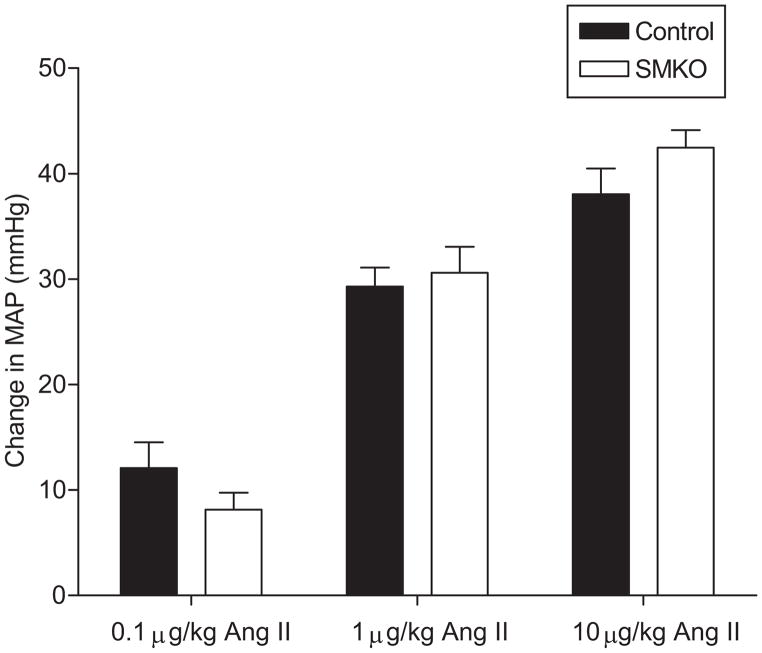
We next compared acute vasoconstrictor responses in SMKOs and controls in vivo. As shown in Figure 4, we observed robust acute vasoconstriction in the controls in response to escalating doses of angiotensin II from 0.1–10 μg/kg. The magnitude of vasoconstriction was dose-proportional and virtually identical in SMKOs and controls. Preservation of a normal vasoconstrictor response to angiotensin II in the SMKOs is consistent with their unmodified expression of AT1A-receptors in resistance vessels (Figure 3C).
Figure 4. Intact response to acute vascular angiotensin II response in vivo.
Acute vasoconstrictor responses to angiotensin II and epinephrine were measured in anesthetized mice. Blood pressure was measured continuously while increasing doses of angiotensin II (0.1–10 μg/kg) were administered at 5–10 minute intervals. No difference was seen in peak pressor response at any dose between SMKOs and controls (P=NS at 0.1μg/kg, 1μg/kg and 10μg/kg doses, n=6 for both groups).
Blood pressure homeostasis is not affected in SMKOs
Radiotelemetry units were implanted into 8–12 week old male SMKO and control mice in order to measure blood pressure in the conscious, unrestrained state. Mean arterial pressures measured over 5 days were virtually identical between SMKO and control mice fed a normal-salt (0.4% NaCl) diet (116±2 vs. 116±1 mmHg; n=11 in each group). Moreover, diurnal variation of blood pressure was not affected in SMKOs (data not shown).
Blood pressure responses in angiotensin II-dependent hypertension
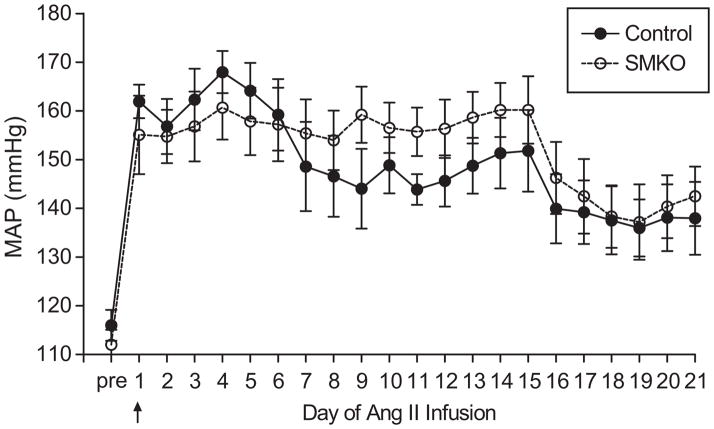
The preceding studies suggest that AT1A-receptors are effectively deleted from VSMCs in the aorta in SMKOs but their blood pressure responses to angiotensin II are preserved. Thus, we reasoned that the SMKOs would be a useful model for separating the relative contributions of hypertension from direct actions of AT1A-receptors in VSMCs to aortic remodeling. In order to induce vascular remodeling, osmotic minipumps were implanted subcutaneously to infuse angiotensin II at 1000 ng/kg/min, while blood pressures were continuously monitored.27, 28 As shown in Figure 5, the blood pressure responses to chronic angiotensin II infusion were very similar in the SMKOs and controls. MAP increased significantly by ≈30 mm Hg in both groups and remained elevated to an equivalent extent throughout the period of the infusion. Further, there was no difference in MAP between the groups averaged for the duration of angiotensin II administration (157±6 mm Hg vs. 153±6 mm Hg).
Figure 5. VSMC-specific AT1A-receptor deletion does not affect blood pressure response to chronic angiotensin II administration.
Blood pressures were measured in groups of conscious, unrestrained SMKO (open circles and dashed line) and control (closed circles, solid line) mice using radiotelemetry before and during chronic infusion of angiotensin II. No differences were seen in MAP between SMKOs and controls at baseline or during chronic angiotensin II administration (1000ng/kg/min). There were a total of 11 animals in each experimental group (control and SMKO). In the first experiment (n=7 per group), blood pressures were measured for 14 days of angiotensin II infusion. In the second experiment (n=4 per group), technically suitable blood pressure tracings were available for 21 days of angiotensin II infusion.
Vascular Oxidative Stress
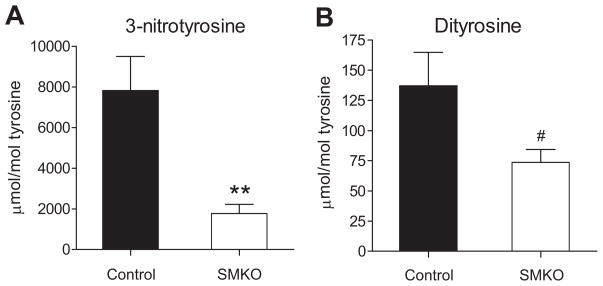
To examine levels of oxidative stress specifically in the vasculature, we assessed oxidation of proteins in the vascular wall. To this end, we isolated vascular wall proteins from thoracic aortic segments of control and SMKO mice. After hydrolyzing the proteins with acid, amino acids were isolated as described previously.26, 29–31 We then determined the content of the two oxidized amino acids, 3-nitrotyrosine and dityrosine, “molecular signatures” characteristic of peroxynitrite-mediated oxidation, by isotope dilution liquid chromatography tandem mass spectrometry (LC/ESI/MS/MS). As shown in Figures 6A and B, increased levels of both 3-nitrotyrosine and dityrosine were detected in aortic segments from control mice after angiotensin II infusion. This increase was significantly attenuated in SMKOs by ≈50% for dityrosine (137±27 vs. 74±10μmol/mol tyrosine; P<0.05) by ≈80% for 3-nitrotyrosine (7819±1676 vs. 1768±450 μmol/mol tyrosine; P<0.005). To examine ROS generation in the vasculature, we measured local production of hydrogen peroxide using amplex red in freshly prepared thoracic aortae from control and SMKO mice at baseline. Hydrogen peroxide generation was significantly lower in aortic segments from SMKO mice (0.59±0.4 μM/mg protein) than in controls (4.723±1.1 μM/mg protein; P<0.05). Taken together, these data indicate that oxidative stress in the aortic wall is significantly attenuated in the SMKOs.
Figure 6. VSMC-specific AT1A-receptor mice have reduced local oxidative stress in the vascular wall after 4 weeks of angiotensin II-dependent hypertension.
Oxidized amino acids in freshly dissected thoracic aorta were quantified by liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) using multiple reaction monitoring (MRM) mode in SMKO (n=11) and control (n=13) mice after 4-weeks of angiotensin II administration. Increased levels of both 3-nitrotyrosine (A) and dityrosine (B) were detected in aortic segments from control mice after angiotensin II infusion. These increases were significantly attenuated in SMKOs by almost 80% for 3-nitrotyrosine (7819±1676 vs. 1768±450 μmol/mol tyrosine; **P<0.005) and by almost 50% for dityrosine (137±27 vs. 74±10μmol/mol tyrosine; #P<0.05).
Vascular responses in angiotensin II-dependent hypertension
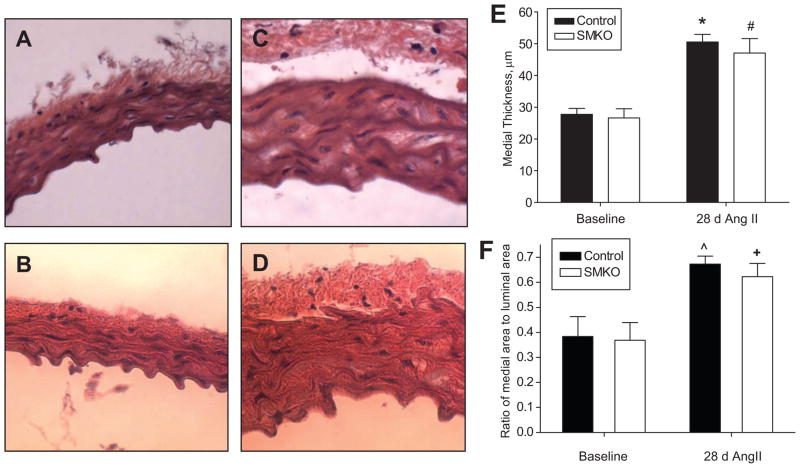
To determine the extent of vascular remodeling associated with the angiotensin II infusion, medial thickness and medial to luminal area ratio of thoracic aortic sections was measured by morphometry. As shown in Figures 7, there were no differences between controls and SMKOs at baseline, suggesting that the absence of AT1A-receptors in VSMCs does not significantly impact normal development and structure of the aorta. Following 4 weeks of angiotensin II infusion, there was significant remodeling of the aorta in control mice reflected by an increase in medial thickness from 27.7±1.9 μm at baseline to 50.5±2.4 μm (Figure 7E; P<0.0005) and an increase in medial-to-lumen ratio from 0.38±0.08 at baseline to 0.67±0.03 (Figure 7F; P<0.05). Similar increases in medial expansion (26.6±2.9 μm vs. 47±4.6 μm; p<0.005) and medial-to-lumen ratio (0.36±0.07 vs. 0.62±0.05;P<0.05) were seen in the SMKOs with angiotensin II infusion, such that after angiotensin II infusion, these parameters were virtually identical in SMKOs and controls (Figures 7C, D, E, and F).
Figure 7. Vascular remodeling of aorta is unaffected by VSMC-specific AT1A-receptor deletion after angiotensin II-induced hypertension.
Representative H & E-stained sections taken at 40X of thoracic aortae from control, n=3 (A, C) and SMKO, n=6 (B, D) mice before (A, B) and after (C, D) 4 weeks of angiotensin II infusion (n=9 control, n=8 SMKO). (E–F) Aortic medial thickness was quantified using morphometry. (E) There were no differences in medial thickness at baseline between the groups; medial thickness increased significantly and to a similar extent in controls and SMKOs during angiotensin II infusion. (F) Ratios of medial to lumen areas were also similar in the two groups before and after 4 weeks of angiotensin II infusion. (*P<0.005 vs. Control Baseline; #P<0.005 vs. SMKO Baseline; ^P<0.05 vs. Control Baseline; +P<0.05 vs. SMKO Baseline).
Discussion
Vascular remodeling and injury are typical features of end-organ damage from hypertension, contributing to clinical morbidity and mortality.14 In the kidney, vascular lesions, interstitial fibrosis and arteriosclerosis are the defining characteristics of hypertensive nephrosclerosis, a common cause of chronic kidney disease.12 In larger vessels such as the aorta, vascular remodeling in hypertension produces changes in compliance resulting in increased pulse pressure, which has been associated with enhanced cardiovascular risk.32, 33 Medial thickening of the carotid artery has been similarly associated with increased cardiovascular risk and is commonly used as a surrogate marker for vascular outcomes in clinical trials.34
The renin-angiotensin system (RAS) is a major determinant of vascular function and pathology.35 For example, angiotensin II acting through the AT1-angiotensin receptor causes potent vasoconstriction.16 This vasoconstrictor response is mediated by activation of AT1-receptors in VSMCs, triggering increased intra-cellular calcium leading to myosin phosphorylation.36 Along with these physiological effects, activation of the RAS also promotes vascular remodeling in patients with hypertension.18 In smaller vessels, these structural changes can have hemodynamic consequences.18, 37 Further, activation of the RAS may also impact the development of atherosclerosis38 and aortic aneurysms.39 Many of the vascular consequences of AT1-receptor activation seem to emanate from direct effects in VSMCs but these have largely been characterized in cultured cell systems.40, 41 The precise contribution of AT1-receptors in VSMCs to vascular physiology and pathology in vivo has been difficult to define since pharmacological antagonists or conventional gene knockouts lower blood pressure and produce broad inhibition of AT1-receptors across all tissues including VSMCs. Accordingly, in order to examine their actions in isolation in the intact animal, we developed a mouse model to eliminate expression of AT1A-receptors specifically in smooth muscle using Cre-loxp technology.
To excise the floxed Agtr1a allele from smooth muscle, we used the Sm22α-Cre transgenic mouse line expressing Cre recombinase under control of the promoter of the Sm22α gene.42 We found that AT1A-receptors were efficiently deleted from aorta and early branches of the mesenteric arteries in SMKOs (Figure 1). However, there was little or no excision from resistance vessels, reflected by preserved acute pressor responses to angiotensin II (Figure 4) and normal levels of AT1A-receptor mRNA expression in pre-glomerular vessels isolated from SMKO mice (Figure 3C). Furthermore, the extent of excision from small resistance arteries could not be appreciably enhanced when the Sm22α-Cre transgene was crossed onto an Agtr1aflox/null background (data not shown). While previous studies have suggested that the Sm22α promoter drives expression in most smooth muscle lineages including VSMCs,43–45 the levels of expression in small resistance vessels have not been clearly documented. Our study indicates that deletion of a floxed allele from peripheral resistance vessels cannot be efficiently accomplished using this Cre transgene. Nonetheless, since AT1A-receptors were efficiently eliminated from VSMC in the aorta but were preserved in resistance arteries, we reasoned that this SMKO model could be useful for separating the consequences of elevated blood pressure from direct actions of AT1-receptors in VSMCs on aortic remodeling during hypertension. Indeed, the extent of blood pressure elevation with angiotensin II infusion was very similar in the SMKOs and controls (Figure 5).
One of the key pathways linked to AT1-receptors in VSMCs is the generation of reactive oxygen species (ROS).46 In this regard, AT1-receptors activate NADPH oxidases (Nox1, Nox4) in VSMCs47, 48 generating ROS such as superoxide anion and H2O2, which may contribute to the pathogenesis of hypertension.41, 49 Furthermore, stimulation of ROS production by AT1-receptors in cultured VSMCs has been associated with cellular hypertrophy.50 To examine the capacity of AT1A-receptors to promote oxidative stress in vivo, we compared indices of oxidative stress between SMKOs and controls before and during chronic angiotensin II infusion. At baseline, we found reduced levels of hydrogen peroxide production by isolated aortic segments from SMKOs compared to controls. In addition, we found evidence for marked reductions in the levels of oxidized tyrosine residues (Figure 6). Excess O2•− produced by NOX enzymes or uncoupled eNOS can form hydrogen peroxide or react with NO• to form the highly reactive oxidant peroxynitrite.26, 51, 52
Along with contributing to oxidative stress, this reaction extinguishes the beneficial actions of NO in the vasculature. Moreover, peroxynitrite can oxidize tyrosine residues in the vascular wall and this covalent alteration provides a footprint to assess the extent of oxidative stress over time.53 Our findings of decreased 3-nitrotyrosine and dityrosine in SMKOs after angiotensin II administration are consistent with decreased peroxynitirite formation by either of these mechanisms.30, 26 Taken together, our data indicate a key role for AT1-receptors in VSMCs to promote oxidative stress as evidenced by the profound reduction in 3-nitrotyrosine and dityrosine in hypertension, independent of any concomitant effects of elevated blood pressure per se.
As discussed above, there exists ample evidence suggesting that generation of ROS by AT1-receptor activation in VSMCs may have direct consequences on vascular structure and function. In this regard, a number of studies have shown that administration of potent anti-oxidant agents can attenuate angiotensin II-dependent hypertension.54, 55 Our studies suggest that reduced oxidative stress in large conduit arteries alone is not sufficient to lower blood pressure. Instead, this may require more robust ROS inhibition in resistance arteries, the CNS,56 and/or the kidney.10 Moreover, the absence of AT1A-receptors and the dramatic diminution of oxidative stress in the aortic wall of the SMKOs did not result in detectable attenuation of medial hypertrophy compared to controls (Figure 7). This indicates that direct actions of AT1A-receptors in VSMCs including generation of oxidative stress are not required to induce aortic remodeling in this setting and is consistent with previous reports describing dissociations between levels of reactive oxygen species generation and cardiac or aortic remodeling.57–59 Since the severity of hypertension was similar in SMKOs and controls, we suggest that elevated blood pressure is the major mechanism driving medial expansion in this setting. This is in line with previous studies by our group and others documenting the dominant actions of blood pressure to drive end-organ damage in the heart and vasculature.10, 60, 61
Perspectives.
Other factors such as elevated blood pressure may play a dominant role in hypertensive vascular remodeling since the extent of hypertension was very similar between the groups in our study. Alternatively, AT1-receptor actions, including ROS generation, in other cell lineages such as endothelium or circulating inflammatory cells may have significant roles in hypertensive vascular remodeling. Furthermore, our studies do not rule out the possibility that pathways linked to AT1-receptors in VSMCs may contribute to the pathogenesis of more complex vascular lesions such as atherosclerosis or aneurysms, where oxidative stress has also been implicated.62
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by National Institutes of Health Grant (HL56122) (TC), by funding from the Medical Research Service of the Veterans Administration (TC), the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research (TC), Emerging Leaders in Hypertension Research Fellowship Grant from the Forest Research Institute (MS), and NRSA Research Fellowship (HL086181) (KP). SP gratefully acknowledges pilot and feasibility grant support from the George O’Brien Kidney Center (DK081943). Mass Spectrometry experiments were conducted in Molecular Phenotyping Core, Michigan Nutrition and Obesity Center (DK089503).
Footnotes
Disclosure Statement
None
References
- 1.Husain AaG R. Drugs, enzymes and receptors of the renin-angiotensin system: Celebrating a century of discovery. Sidney: Hardwood Academic; 2000. [Google Scholar]
- 2.Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Buhler FR. Essential hypertension: Renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- 3.Low-dose captopril for the treatment of mild to moderate hypertension. I. Results of a 14-week trial. Veterans administration cooperative study group on antihypertensive agents. Arch Intern Med. 1984;144:1947–1953. doi: 10.1001/archinte.144.10.1947. [DOI] [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 5.Berl T. Review: Renal protection by inhibition of the renin-angiotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. 2009;10:1–8. doi: 10.1177/1470320309102747. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (life): A randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 8.Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM. Effects of blood pressure level on progression of diabetic nephropathy: Results from the renaal study. Arch Intern Med. 2003;163:1555–1565. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 10.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin ii causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bidani AK, Griffin KA, Bakris G, Picken MM. Lack of evidence of blood pressure-independent protection by renin-angiotensin system blockade after renal ablation. Kidney Int. 2000;57:1651–1661. doi: 10.1046/j.1523-1755.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 12.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R. Accuracy of the diagnosis of hypertensive nephrosclerosis in african americans: A report from the african american study of kidney disease (aask) trial. Aask pilot study investigators. Kidney Int. 1997;51:244–252. doi: 10.1038/ki.1997.29. [DOI] [PubMed] [Google Scholar]
- 13.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the aask trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 14.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 15.Diep QN, Li JS, Schiffrin EL. In vivo study of at(1) and at(2) angiotensin receptors in apoptosis in rat blood vessels. Hypertension. 1999;34:617–624. doi: 10.1161/01.hyp.34.4.617. [DOI] [PubMed] [Google Scholar]
- 16.Timmermans P, Chiu A, Herblin W, Wong P, Smith R. Angiotensin ii receptor subtypes. Am J Hypertens. 1992;5:406–410. doi: 10.1093/ajh/5.6.406. [DOI] [PubMed] [Google Scholar]
- 17.Geisterfer AA, Peach MJ, Owens GK. Angiotensin ii induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 18.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–1659. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988;16:8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatziantoniou C, Arendshorst WJ. Angiotensin receptor sites in renal vasculature of rats developing genetic hypertension. Am J Physiol. 1993;265:F853–862. doi: 10.1152/ajprenal.1993.265.6.F853. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhari A, Kirschenbaum MA. A rapid method for isolating rabbit renal microvessels. Am J Physiol. 1988;254:F291–296. doi: 10.1152/ajprenal.1988.254.2.F291. [DOI] [PubMed] [Google Scholar]
- 22.Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, Pisetsky DS, Barisoni L, Prieto-Carrasquero MC, Jeansson M, Foster MH, Coffman TM. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest. 2009;119:943–953. doi: 10.1172/JCI34862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol. 2003;140:701–706. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM. Angiotensin ii responses in at1a receptor-deficient mice: A role for at1b receptors in blood pressure regulation. Am J Physiol. 1997;272:F515–520. doi: 10.1152/ajprenal.1997.272.4.F515. [DOI] [PubMed] [Google Scholar]
- 25.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O’Brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 27.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin ii-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 28.Qin Z. Newly developed angiotensin ii-infused experimental models in vascular biology. Regul Pept. 2008;150:1–6. doi: 10.1016/j.regpep.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: The role of oxldl/lox-1. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: A potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem. 2005;280:22706–22714. doi: 10.1074/jbc.M500839200. [DOI] [PubMed] [Google Scholar]
- 31.Shu L, Park JL, Byun J, Pennathur S, Kollmeyer J, Shayman JA. Decreased nitric oxide bioavailability in a mouse model of fabry disease. J Am Soc Nephrol. 2009;20:1975–1985. doi: 10.1681/ASN.2008111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- 33.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 34.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: The rotterdam study. Circulation. 2004;109:1089–1094. doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases: Expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 36.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin ii: Modulated by g proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 37.Park JB, Intengan HD, Schiffrin EL. Reduction of resistance artery stiffness by treatment with the at(1)-receptor antagonist losartan in essential hypertension. J Renin Angiotensin Aldosterone Syst. 2000;1:40–45. doi: 10.3317/jraas.2000.009. [DOI] [PubMed] [Google Scholar]
- 38.Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–1266. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 39.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. Ang ii infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 mapk and mapkapk-2 in angiotensin ii-induced akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C494–499. doi: 10.1152/ajpcell.00439.2003. [DOI] [PubMed] [Google Scholar]
- 41.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of nadh/nadph oxidase-derived h2o2 in angiotensin ii-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 42.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. Lrp: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn M, Volker K, Schwarz K, Carbajo-Lozoya J, Flogel U, Jacoby C, Stypmann J, van Eickels M, Gambaryan S, Hartmann M, Werner M, Wieland T, Schrader J, Baba HA. The natriuretic peptide/guanylyl cyclase--a system functions as a stress-responsive regulator of angiogenesis in mice. J Clin Invest. 2009;119:2019–2030. doi: 10.1172/JCI37430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative bmp-2/ppargamma/apoe axis in human and murine smcs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodwin JE, Zhang J, Geller DS. A critical role for vascular smooth muscle in acute glucocorticoid-induced hypertension. J Am Soc Nephrol. 2008;19:1291–1299. doi: 10.1681/ASN.2007080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin ii signaling. Regul Pept. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 47.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin ii stimulates nadh and nadph oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 48.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin ii infusion on the expression and function of nad(p)h oxidase and components of nitric oxide/cgmp signaling. Circ Res. 2002;90:E58–65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 49.Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. P22phox is a critical component of the superoxide-generating nadh/nadph oxidase system and regulates angiotensin ii-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 50.Garrido AM, Griendling KK. Nadph oxidases and angiotensin ii receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: Critical for nitric oxide bioavailability and role in angiotensin ii uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pennathur S, Wagner JD, Leeuwenburgh C, Litwak KN, Heinecke JW. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J Clin Invest. 2001;107:853–860. doi: 10.1172/JCI11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin ii-induced increases in blood pressure and endothelin. Hypertension. 2001;38:655–659. doi: 10.1161/01.hyp.38.3.655. [DOI] [PubMed] [Google Scholar]
- 55.Delbosc S, Cristol JP, Descomps B, Mimran A, Jover B. Simvastatin prevents angiotensin ii-induced cardiac alteration and oxidative stress. Hypertension. 2002;40:142–147. doi: 10.1161/01.hyp.0000024348.87637.6f. [DOI] [PubMed] [Google Scholar]
- 56.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin ii infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 57.Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin ii. Hypertension. 2003;42:945–951. doi: 10.1161/01.HYP.0000094220.06020.C8. [DOI] [PubMed] [Google Scholar]
- 58.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, Reudelhuber TL. Angiotensin ii-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing nadph oxidase. Hypertension. 2005;45:530–537. doi: 10.1161/01.HYP.0000158845.49943.5e. [DOI] [PubMed] [Google Scholar]
- 59.Wang HD, Johns DG, Xu S, Cohen RA. Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin ii. Am J Physiol Heart Circ Physiol. 2002;282:H1697–1702. doi: 10.1152/ajpheart.00914.2001. [DOI] [PubMed] [Google Scholar]
- 60.Polichnowski AJ, Cowley AW., Jr Pressure-induced renal injury in angiotensin ii versus norepinephrine-induced hypertensive rats. Hypertension. 2009;54:1269–1277. doi: 10.1161/HYPERTENSIONAHA.109.139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reudelhuber TL, Bernstein KE, Delafontaine P. Is angiotensin ii a direct mediator of left ventricular hypertrophy? Time for another look. Hypertension. 2007;49:1196–1201. doi: 10.1161/HYPERTENSIONAHA.106.075085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part ii: Animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.