Abstract
Cancer gender disparity in incidence, disease aggressiveness and prognosis has been observed in a variety of cancers. Thyroid cancer is one of the fastest growing cancer diagnoses worldwide. It is 2.9-times more common in women than men. The less aggressive histologic subtypes of thyroid cancer are more common in women, whereas the more aggressive histologic subtypes have similar gender distribution. The gender disparity in incidence, aggressiveness and prognosis is well established for thyroid cancer but the cause of the disparity is poorly understood. The aim of this article is to evaluate the current evidence on the cause of thyroid cancer gender disparity. Dietary and environmental factors do not appear to have a significant role in thyroid cancer gender disparity. Common somatic mutations in BRAF, rearranged in transformation/papillary thyroid carcinomas (RET/PTC) and neurotrophin receptor-tyrosine kinase (NTRK) also do not account for the gender disparity in thyroid cancer. While reproductive factors would seem a logical hypothesis to account for the gender disparity, there appears to be no conclusive effect on the risk of developing thyroid cancer. Recent studies on estrogen receptor status in thyroid cancer show a difference in the receptor subtypes expressed based on the histology of thyroid cancer. Moreover, the response to estrogen is dependent on the specific estrogen receptor expressed in thyroid cancer cells. However, what determines the tumor-specific sex hormone receptor expression is unclear. No established molecular factors appear to explain gender differences in thyroid cancer. Therefore, the application of high-throughput genomic and proteomic approaches to the study of thyroid cancer gender disparity could be helpful for better understanding the molecular basis for gender differences in thyroid and other cancers.
Keywords: disparity, gender, genetics, molecular basis, papillary thyroid cancer, risk factors, thyroid cancer
Cancer gender disparity in incidence, disease aggressiveness and prognosis has been observed for a variety of cancers. In sex-specific organs, such as the uterus, prostate, ovary and breast, this disparity can easily be explained. However, some sex-shared sites, such as the thyroid, lung and liver, also show clear gender differences in incidence and/or survival [1–4]. Although the incidence of lung cancer in men is on the decline, rates are increasing in women with a projected equalization in rate over the next decade [1,4]. In lung cancer, women are more likely to have adenocarcinoma, present at an earlier stage of disease, and are diagnosed at a younger age [1]. These differences may be due to medical access to care, but women with non-small-cell lung cancer respond better to neoadjuvant chemotherapy, suggesting a difference in tumor biology [1]. In liver cancer, there is also a gender disparity. Men are three- to five-times more likely to develop hepatocellular carcinoma [3,4]. Studies in hepatocellular carcinoma have identified IL-6 as a possible candidate gene with higher levels in patients with hepatocellular carcinoma relative to unaffected individuals [5]. In an animal model, estrogen inhibits IL-6 secretion, decreasing and preventing chemically induced liver carcinogenesis [3]. Thus, the effects of endogenous sex hormones could account for the gender disparity in hepatocellular carcinoma and other cancers with a gender disparity.
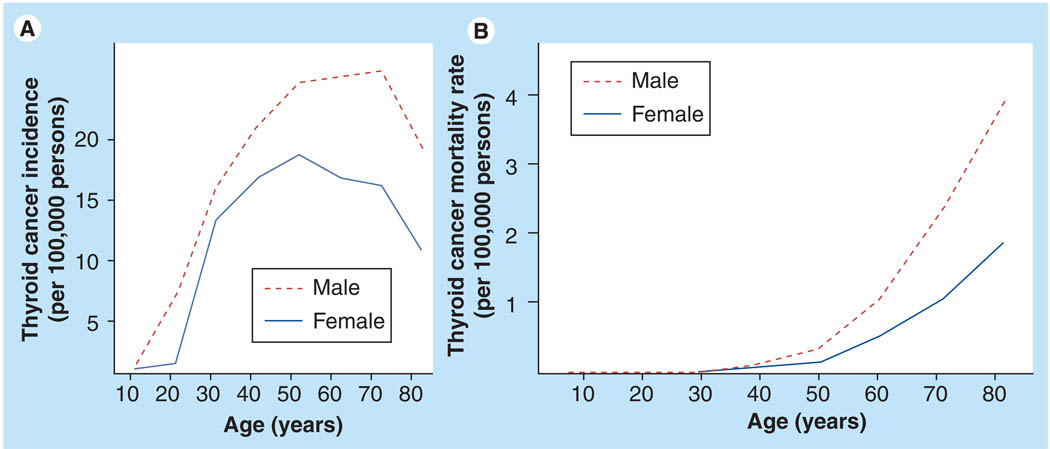
Thyroid cancer is the most common malignancy of the endocrine system and the seventh most common malignancy in women, but it is not among the most common 15 cancers in men [4]. It is one of the fastest growing cancer diagnoses in the USA, with a 6.2% annual percentage change from 1997 to 2006 [4]. Thyroid cancer had an age-adjusted incidence rate of 11 per 100,000 persons in 2006, with a 2.9-times higher rate in women (female:male ratio 16.3:5.7) [4]. The gender disparity in thyroid cancer is also specific to the histologic subtype of thyroid cancer. The more aggressive types of thyroid cancer, anaplastic thyroid cancer and medullary thyroid cancer have similar rates of incidence in men and women. Meanwhile, differentiated thyroid cancer of follicular cell origin, such as follicular thyroid cancer and papillary thyroid cancer, are more common in woman. Papillary thyroid cancer is the most common type of thyroid cancer, accounting for approximately 80% of the cases. Its incidence has nearly doubled over the last 30 years and is thought to be due in part to earlier diagnosis of subclinical disease [4,6]. Not much is known about the factors responsible for the dramatic increase in papillary thyroid cancer, and established risk factors such as radiation exposure and a family history of thyroid cancer do not appear to account for the increasing incidence [7]. The rate of papillary thyroid cancer among woman is nearly three-times higher than men. In females, the age-specific incidence rate rises sharply at the beginning of the reproductive years, with increasing age peaking at 40–49 years, while in men the peak is at 60–69 years (Figure 1). The incidence rates equalize by 85 years of age [2,4]. Women have an earlier age of onset but men tend to have more aggressive disease at diagnosis. Moreover, in most studies, male gender is associated with a lower disease-free survival and higher mortality [2,8]. As the incidence of thyroid cancer increases, in particular papillary thyroid cancer, the gender differences observed are likely to be even more dramatic.
Figure 1. Thyroid cancer incidence and mortality.
(A) Thyroid cancer incidence by age and gender. (B) Thyroid cancer mortality rates by age and gender. Rates per 100,000 persons from 2003 to 2007.
Data from the Survellance, Epidemiology and End Results (SEER) Program using 12 SEER areas (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose–Monterey, Los Angeles and Alaska) [101].
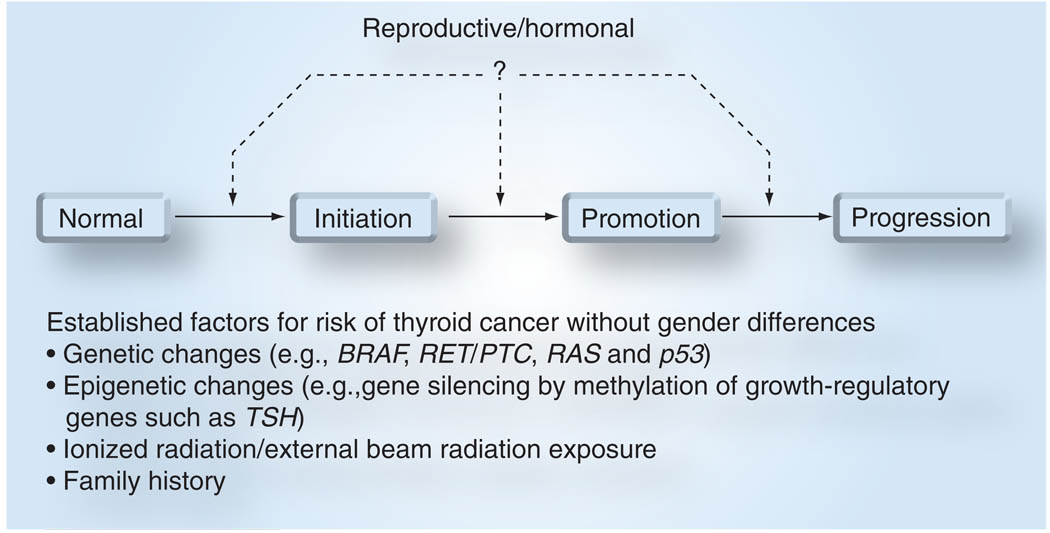
It has been hypothesized that reproductive, menstrual and environmental factors may account for this disparity, but very little is known about what molecular factors account for this difference (Figure 2). We reviewed the current literature on gender disparity in thyroid cancer focusing on the most common histologic subtype, papillary thyroid cancer.
Figure 2. Thyroid carcinogenesis model.
Genetic changes that are involved in thyroid carcinogenesis, such as BRAF, RET/PTC and RAS mutations that occur in benign and malignant thyroid neoplasm of follicular cell origin, as well as epigenetic changes that result in gene silencing of thyroid follicular cell growth-regulatory hormones are risk factors for thyroid cancer. Two established risk factors that are associated with higher risk of thyroid cancer and benign neoplasm are radiation exposure and family history of thyroid cancer. However, none of these factors are associated with gender difference in thyroid cancer of follicular cell origin.
Review criteria
Information for this review was compiled by searching the PubMed database for articles published in English. Search terms included the words ‘thyroid cancer’ and the following terms: ‘gender’, ‘reproductive’, ‘menstrual’, ‘environment’, ‘radiation’, ‘diet’, ‘nutrition’, ‘gene’, ‘oncogene’, ‘RET/PTC’, ‘chromosomal rearrangement’, ‘NTRK’, ‘TRK’, ‘BRAF’, ‘estrogen’, ‘testosterone’ and ‘sex hormone’. Full articles were obtained and references were checked for additional source material as appropriate. Primary sources have been quoted where possible. References were chosen on the basis of the best experimental and clinical evidence, especially if the results had been supported by other published papers.
Radiation exposure
Exposure to external-beam radiation and, more recently, ionized radiation, particularly during childhood, has been well established as a risk factor for thyroid cancer. The experience from atomic bomb survivors in Japan and individuals exposed to the Chernobyl reactor meltdown demonstrate that almost all radiation-associated thyroid cancers are of the papillary histologic subtype, with children being most susceptible [9,10]. A pooled analysis of seven studies showed that women had a nearly twofold increase in thyroid cancer risk, but no individual study showed a statistical difference between men and women [11]. Post-Chernobyl epidemiologic studies have showed that, although the incidence of papillary thyroid cancer increased in the exposed population, there was a decrease in the female to male ratio [9]. Based on the current evidence, ionizing radiation does not appear to play a role in the gender disparity of papillary thyroid cancer, with both genders being at equal risk of developing thyroid cancer after exposure.
Dietary & nutritional factors
Dietary and nutritional factors have been implicated as risk factors for differentiated thyroid cancer of follicular cell origin. Iodine deficiency is a well-established risk factor for developing follicular thyroid cancer, and iodine supplementation has been implemented in most regions with endemic goiter [12]. By contrast, iodine excess has been associated with an increased risk of papillary thyroid cancer. Chronic iodine deficiency may have a protective effect in females [13], but there are no equivalent studies looking at male gender. In the San Francisco Bay Area Thyroid Cancer study group, a protective effect of dietary iodine and phytoestrogens was found in women, but the study did not have any male subjects [13,14]. The role of fish (high in iodine) and cruciferous vegetables on thyroid cancers have been debated. However, there are no studies that show a gender difference in the effects of fish and cruciferous vegetables in thyroid cancer [15,16]. The wide range of food items investigated combined with the variable pattern of consumption in different epidemiologic groups, which have different malignancy rates, lends itself to much speculation. These same variables also make it extremely difficult to compare the data on nutritional factors and come to a clear consensus. There is no strong evidence for dietary factors having a role in the gender disparity seen in thyroid cancer.
Common somatic genetic changes associated with thyroid cancer
Our understanding of the molecular biology of thyroid cancer initiation and progression has improved over the last two decades. The three most common types of somatic mutations observed in papillary thyroid cancer are rearranged in transformation/papillary thyroid carcinomas (RET/PTC) and neurotrophin receptor-tyrosine kinase (NTRK)1 rearrangements, and an activation mutation in the mitogen signaling protein BRAF. These genetic alterations are present in approximately two-thirds of papillary thyroid cancer and are specific to papillary thyroid cancer. The RET/PTC and NTRK mutations are chromosomal rearrangements involving tyrosine kinase receptors [17,18]. The RET/PTC rearrangement is found in 20–40% of adult sporadic papillary thyroid cancer, with the RET/PTC3 being the most common followed by RET/PTC1 [17]. The NTRK rearrangement is less common and it is found in up to 13% of papillary thyroid cancer [17,18]. BRAF is a serine/threonine kinase that mediates cellular response to growth and differentiation signaling. The BRAF V600E point mutation accounts for over 97% of the BRAF mutations in papillary thyroid cancer. The BRAF mutation is present in approximately 40% of papillary thyroid cancer [17,18].
BRAF
There have been several studies that have demonstrated that the presence of BRAF V600E mutation is associated with an aggressive tumor phenotype in papillary thyroid cancer, including a larger tumor size, extrathyroidal invasion, poorly differentiated histology, lymph node and distant metastases, and it is also associated with older age, higher recurrence rates and gender [19]. Of the many studies on BRAF V600E mutation in papillary thyroid cancer, we found 12 studies that evaluated the relationship between gender and BRAF mutation status [20–31]. Two studies showed a significantly higher rate of BRAF V600E mutation in men [29,31]. Xu and colleagues observed that BRAF V600E mutation in men with papillary thyroid cancer was 64% compared with 27% in women [31]. In another study of 203 patients with papillary thyroid cancer, BRAF V600E mutation was present in 91% of men (n = 35) compared with 70% of women (n = 168) [29]. The remaining ten tudies did not show a difference in the prevalence of BRAF V600E mutation in papillary thyroid cancer by gender [20–28]. A study from Japan showed a trend in men having a higher rate of BRAF V600E with ten out of 11 male patients (91%) compared with 16 out of 29 female patients (55%) having the mutation [30]. A meta-analysis of twelve studies with a total of 1168 patients (male:female ratio 259:909) did not show a significant difference in mutation rate by gender, with 50% of the males and 49% of the females with papillary thyroid cancer having the BRAF V600E mutation [24]. In one of the larger studies of 500 consecutive patients with papillary thyroid cancer, there was no significant difference in BRAF V600E status in men (46%) versus women (36%), controlling for extent of disease [27]. Although some studies suggest BRAF V600E mutations are more common in men with papillary thyroid cancer, this finding has not been uniformly observed and may be due to the more aggressive disease present in men at diagnosis. Therefore, the association between BRAF V600E mutation and gender in papillary thyroid cancer is, at best, a weak association.
RET/PTC & NTRK rearrangement
The RET/PTC rearrangement was one of the first frequent genetic changes associated with papillary thyroid cancer, now with over 15 different rearrangements described to date [17,18,32]. Multiple studies have looked at the association of clinicopathological features of papillary thyroid cancer with the presence of this rearrangement [26,31,33–40]. Although the association of RET/PTC rearrangements with radiation is well established, there is limited evidence supporting any association with tumor phenotype and gender [26,31,33–40]. The only study to report a higher rate of RET/PTC rearrangements in papillary thyroid cancer by gender was in a small cohort study (n = 39) in children exposed to low-dose external-beam irradiation [33]. Therefore, RET/PTC rearrangement in papillary thyroid cancer does not appear to be different by gender.
To our knowledge, only one study analyzed the presence of an NTRK rearrangement and gender. This study from Poland evaluated the presence of an NTRK rearrangement in 33 patients with papillary thyroid cancer and found 12% prevalence. This rearrangement was present in 12.5% of men (one out of eight) and 12% of women (three out of 25) with papillary thyroid cancer [27]. Overall, there appears to be limited evidence that supports specific somatic mutations being associated with papillary thyroid cancer is different by gender, and thus is unlikely to account for the gender disparity in papillary thyroid cancer observed [41].
Reproductive factors
An obvious difference between men and women is in sex hormones and their influence on the various systems in the body. The fluctuation of sex hormones during a woman’s menstrual cycle and pregnancy has been hypothesized as the reason for the gender disparity in papillary thyroid cancer. In particular, the peak incidence of papillary thyroid cancer has been observed in women aged 40–49 years, this being the age group at which most women approach or enter menopause [2,4,42]. There have been several studies looking at the association of papillary thyroid cancer with reproductive factors such as age of menarche, menopause, number of pregnancies and other parameters [42–47]. However, the evidence for hormonal, menstrual or reproductive roles in thyroid cancer in women is inconclusive. A comprehensive pooled analysis of previous reports by Negri and colleagues found no strong association for reproductive factors and risk of thyroid cancer [42]. Late age of menarche, late age of first child birth, artificial menopause and miscarriage for the first pregnancy were significant but weak risk factors with odds ratios of 1.04, 1.3, 1.8 and 1.8, respectively [42]. The investigators hypothesized that the effects of most menstrual and reproductive factors occur at a younger age and these effects appear to level off after the exposure to hormonal fluctuations has stopped [42]. Thus, they suggest a weak association of reproductive factors related with thyroid cancer risk. Since these studies, several investigators have revisited this issue with epidemiologic cohort studies from various geographic regions, which focused on differentiated thyroid cancer of follicular cell origin, in particular papillary thyroid cancer. The Japanese Collaborative Cohort Study followed 37,986 women between the ages of 40 and 79 years [45]. A total of 86 patients developed thyroid cancer during follow-up, with 70% of the cases being papillary thyroid cancer. No differences were found in terms of BMI, tobacco smoking status or diabetes between subjects who did and did not develop thyroid cancer. The age of menarche, age at menopause, number of pregnancies or oral contraceptive use were not associated with a higher risk of developing thyroid cancer. The incidence of thyroid cancer, however, is considerably lower in the Japanese population (3.8 per 100,000 in women) than in the US population (14.2 per 100,000 in women), suggesting that Japanese woman may be exposed to fewer hormonal fluctuations or that the risk factors may be different [45].
Two separate groups have focused on high-risk populations in the South Pacific and French Polynesia [43,47]. In the French Polynesian study, the only positive association was seen in the woman with menopausal status, but the age at menopause was not associated with an increased risk of thyroid cancer [43]. There was no association observed with irregular menstrual cycles, history of miscarriage or induced abortion, time since last birth, age and outcome of first pregnancy or breastfeeding. In this study, 201 differentiated thyroid cancer cases were observed with 79% (158 out of 201) of the cases being papillary thyroid cancer. The other study was a case–control study focusing on the population in New Caledonia (South Pacific) [47]. Unlike the French Polynesian study, the New Caledonian population did not show any association with menopausal status. It did show an association with a history of irregular cycles, history of miscarriage, induced abortion and number of pregnancies greater than or equal to eight. Approximately 15% of patients enrolled had eight or more live births. In summary, these studies in high-risk populations found a weak association between menstrual and pregnancy history, and thyroid cancer risk.
In the USA, two studies have evaluated reproductive factors and the risk of thyroid cancer in cohorts from California. One study was a matched case–control study of thyroid cancer patients in Los Angeles County [9]. The histological distribution of thyroid cancer in this study was 82% papillary thyroid cancer, 12% follicular thyroid cancer and 5% other thyroid cancers. Patients with a prior history of radiation to the head and neck, and history of multinodular goiter were not excluded but a multivariate analysis was performed to account for these confounding factors. This study did not show a significant difference in risk of thyroid cancer associated with age of menarche, menstrual irregularity, number of pregnancies and other reproductive factors. A significant statistical increase in risk was observed in women who had a hysterectomy with bilateral oophrectomy. The San Francisco Bay Area Thyroid Cancer Study was also a matched case–control study, but focused on patients with papillary thyroid cancer [46]. No association was observed with age of menopause or menopausal status and papillary thyroid cancer risk. Age of menarche less than 12 years was a weak but significant risk factor. Furthermore, in subgroup analysis of women older than 45 years of age, menarche at less than 12 years was also a weak but significant risk factor. However, in the same group, menarche after 15 years of age was also significant for risk of developing papillary thyroid cancer. Pregnancy history was less of a clear risk factor, as having two live births was a risk factor but having more than three children was not a significant risk factor. Among women who had ever used oral contraceptives, a protective effect with an odds ratio of 0.73 was found. Approximately 30% of the women in the study were born in an Asian country, but adjusting for place of birth in or out of the USA did not change the findings of the study.
In summary, there have been several studies looking at reproductive and hormonal factors and the risk of thyroid cancer with inconclusive results. The interpretation of these population-based studies is hampered by the fact that the rate of thyroid cancer varies dramatically among populations. The suggestion that the rates of thyroid cancer may differ due to reproductive and menstrual factors in these populations would support the hypothesis that these factors influence rates of thyroid cancer. However, based on clinical and epidemiology studies, there is no conclusive association between menstrual, reproductive or hormonal history with thyroid cancer risk (Table 1).
Table 1.
Summary of studies evaluating the relationship between thyroid cancer and reproductive factors.
| Study | Age at menarche (OR) |
Age at menopause (OR) |
Irregular cycles (OR) |
Artificial menopause (OR) |
Parity (OR) | Miscarriage (OR) |
Induced abortion (OR) |
Oral contraceptive (OR) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Japan (Pham et al.) | NS | NS | NR | NR | NS | NR | NR | NS | [45] |
| French Polynesia (Brindel et al.) | NS | NS | NS | 4.5 | NS | NS | NS | NR | [43] |
| South Pacific (Truong et al.) | NS | NS | 1.9 | NS | 2.2 (>8 births) | 2.2 | 3.1 | NS | [47] |
| Los Angeles (Mack et al.) | NS | NS | NS | 6.5 | NS | NS | NR | NS | [44] |
| San Francisco (Sakoda et al.) | 1.5 (<12 years) | NS | NR | NS | NS | NR | NR | 0.73 | [46] |
| San Francisco (Sakoda et al.) | 2.1 (<12 years) | NS | NR | NS | 1.7 (2 births) | NR | NR | NS | [46] |
| Subgroup analysis (age >45 years) | 3.0 (>15 years) | ||||||||
Significant odds ratios of p < 0.05 presented.
NR: Not reported; NS: No statistically significant association; OR: Odds ratio.
Sex hormones
The important role of sex hormones in cancer is well documented for breast and prostate cancers. Sex hormone effects are mediated by hormone-specific nuclear receptors that regulate gene expression and tumor cell biology [48]. The α- and β-estrogen receptors mediate the effect of estrogen and are expressed in papillary thyroid cancer [49]. It has been hypothesized that the polymorphism in estrogen receptors could be a risk factor for thyroid cancer [50]. In 1993, Inoue and colleagues demonstrated that although papillary thyroid cancer cells have low levels of estrogen receptor-α, with physiologic estrogen stimulation the receptor level is significantly upregulated and cell proliferation is promoted [51]. Furthermore, estrogen can dramatically increase the rate of cell proliferation in thyroid cancer cell lines compared with male sex hormones [49,52]. It also alters the expression of estrogen receptor subtypes in thyroid cancer cell lines [49,52,53]. The effects of estrogen on thyroid cancer cell lines are dependent on the type of thyroid cancer, and estrogen dramatically increases estrogen receptor-α levels in papillary thyroid cancer, whereas in anaplastic thyroid cancer and follicular thyroid cancer the receptor levels are not significantly altered [52,53]. The expression of estrogen receptor-β in papillary thyroid cancer cells was not found to be affected by estrogen but was increased in anaplastic thyroid cancer cell lines. Furthermore, estrogen treatment promoted the growth of papillary thyroid cancer cells in culture but inhibited growth in anaplastic thyroid cancer. Rajoria and colleagues also documented that estrogen is associated with increased adherence, invasion and migration in thyroid cancer cell lines [54]. These effects were shown to be reversed with the addition of an estrogen antagonist, suggesting that the nuclear receptor status in the cells was an important determinant of the effects of estrogen [53,54]. Based on these in vitro studies, in papillary thyroid cancer, estrogen receptor status is affected by sex hormones. This may play a role in the gender disparity observed in papillary thyroid cancer, and can serve as a potential target for the treatment of advanced papillary thyroid cancer.
Similar molecular observations have been made during pregnancy. In particular, chorionic gonadotropin, which has been demonstrated to have close homology with thyroid-stimulating hormone, has been shown to cause rapid growth of thyroid tumors (benign or malignant) during pregnancy [55,56]. In addition, as previously noted, estrogen significantly increases the rate of proliferation, migration and invasive properties in thyroid cancer cell lines [54]. The increase in serum estrogen has been associated with increased size of pre-existing thyroid nodules and the possible development of new benign thyroid nodules, but the same relationship has not been shown for malignant lesions [57]. Vannucchi and colleagues retrospectively followed 123 patients with differentiated thyroid cancer at different intervals during pregnancy, and found that patients with thyroid cancer detected during pregnancy were more likely to develop persistent and recurrent disease. They also observed that up to 87.5% of patients who developed thyroid cancer during pregnancy had an estrogen receptor-α-positive tumor [58].
Some investigators have suggested that a detection bias may account for the higher rate of thyroid cancer incidence in women. This is because thyroid nodules are more common in women and thus more likely to undergo diagnostic work-up for thyroid cancer, leading to higher rates in women. Nonetheless, given the in vitro studies of estrogen effect in thyroid cancer cells and the findings associated with pregnancy, estrogen probably has a growth-promoting effect in both benign and malignant thyroid tumors.
Conclusion
The gender disparity in thyroid cancer incidence, aggressiveness and prognosis are well established, but our understanding of the molecular factors that mediate this difference is poorly understood. Given the increasing incidence of thyroid cancer, this disparity is likely to be even more significant. The fact that the rates of thyroid cancer have been increasing combined with the increase in the gender disparity lends itself to much hypothesizing, but there are very few studies looking at the causes of the disparity. The rates of thyroid cancer differ across populations, yet the gender differences are uniformly observed among different regions and continents. Dietary and environmental factors do not appear to have a significant role in thyroid cancer gender disparity. Common somatic mutation in BRAF, RET/PTC and NTRK also do not account for the gender disparity in thyroid cancer. While reproductive factors would appear to be a logical hypothesis to account for the gender disparity, there is no conclusive evidence that they increase the risk of developing thyroid cancer.
Recent estrogen receptor-status studies in thyroid cancer cells demonstrate a difference in the receptor subtypes expressed based on the histology of thyroid cancer. Moreover, the response to estrogen is dependent on the specific estrogen receptor expressed in thyroid cancer cells. However, what determines the tumor-specific sex hormone receptor expression is unclear. We believe the application of high-throughput genomic and proteomic approaches to the study of gender cancer disparity may provide useful information that could lead to a better understanding of the molecular factors that have an important role in tumor cell biology and account for the gender disparity observed for thyroid and other cancers.
Future perspective
Our understanding of the environmental and genetic susceptibility for thyroid cancer will improve and can serve to help identify factors influencing thyroid cancer gender disparity. High-throughput approaches, such as genome-wide single-nucleotide polymorphism analyses, may help identify new susceptibility loci for thyroid cancer, which could serve to explain the gender disparity found in thyroid cancer.
Application of genomic and proteomic approaches to the study of gender cancer disparity may:
-
•
Provide new insights to the molecular basis of cancer disparity;
-
•
Identify secondary effectors of sex hormone in thyroid cancer initiation and progression.
Executive summary
-
•
The incidence of thyroid cancer is increasing worldwide.
-
•
Women of a reproductive age are at a threefold higher risk of developing thyroid cancer.
-
•
The gender disparity in thyroid cancer incidence is age dependent.
-
•
Although thyroid cancer is less common in men, they have a worse survival and more aggressive disease at presentation.
-
•
Clinical and epidemiologic studies show no consistent evidence in reproductive factors contributing to thyroid cancer gender disparity.
-
•
Well-established environmental risk factors for thyroid cancer and common somatic genetic alterations do not appear to account for thyroid cancer gender disparity.
-
•
Recent studies suggest estrogen and estrogen hormone receptor status in thyroid cancer cells may have a role in thyroid cancer progression.
Acknowledgments
Electron Kebebew received funding from the NIH (grant ZIA BC 011275)
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Cerfolio RJ, Bryant AS, Scott E, et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130:1796–1802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- 2.Kilfoy BA, Devesa SS, Ward MH, et al. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol. Biomarkers Prev. 2009;18:1092–1100. doi: 10.1158/1055-9965.EPI-08-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 4.Ortega J, Sala C, Flor B, Lledo S. Efficacy and cost–effectiveness of the UltraCision harmonic scalpel in thyroid surgery: an analysis of 200 cases in a randomized trial. J. Laparoendosc. Adv. Surg. Tech. A. 2004;14:9–12. doi: 10.1089/109264204322862289. [DOI] [PubMed] [Google Scholar]
- 5.Soresi M, Giannitrapani L, D’Antona F, et al. Interleukin-6 and its soluble receptor in patients with liver cirrhosis and hepatocellular carcinoma. World J. Gastroenterol. 2006;12:2563–2568. doi: 10.3748/wjg.v12.i16.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 7.Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 8.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Moysich KB, Menezes RJ, Michalek AM. Chernobyl-related ionising radiation exposure and cancer risk: an epidemiological review. Lancet Oncol. 2002;3:269–279. doi: 10.1016/s1470-2045(02)00727-1. [DOI] [PubMed] [Google Scholar]
- 10.Cardis E, Kesminiene A, Ivanov V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J. Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 11.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat. Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 12.Delange F, Lecomte P. Iodine supplementation: benefits outweigh risks. Drug Saf. 2000;22:89–95. doi: 10.2165/00002018-200022020-00001. [DOI] [PubMed] [Google Scholar]
- 13.Horn-Ross PL, Morris JS, Lee M, et al. Iodine and thyroid cancer risk among women in a multiethnic population: the Bay Area Thyroid Cancer Study. Cancer Epidemiol. Biomarkers Prev. 2001;10:979–985. [PubMed] [Google Scholar]
- 14.Horn-Ross PL, Hoggatt KJ, Lee MM. Phytoestrogens and thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiol. Biomarkers Prev. 2002;11:43–49. [PubMed] [Google Scholar]
- 15.Bosetti C, Kolonel L, Negri E, et al. A pooled analysis of case–control studies of thyroid cancer. VI. Fish and shellfish consumption. Cancer Causes Control. 2001;12:375–382. doi: 10.1023/a:1011267123398. [DOI] [PubMed] [Google Scholar]
- 16.Bosetti C, Negri E, Kolonel L, et al. A pooled analysis of case–control studies of thyroid cancer. VII. Cruciferous and other vegetables (International) Cancer Causes Control. 2002;13:765–775. doi: 10.1023/a:1020243527152. [DOI] [PubMed] [Google Scholar]
- 17.Shibru D, Chung KW, Kebebew E. Recent developments in the clinical application of thyroid cancer biomarkers. Curr. Opin. Oncol. 2008;20:13–18. doi: 10.1097/CCO.0b013e3282f27e49. [DOI] [PubMed] [Google Scholar]
- 18.Trovisco V, Soares P, Preto A, Castro P, Maximo V, Sobrinho-Simoes M. Molecular genetics of papillary thyroid carcinoma: great expectations. Arq. Bras. Endocrinol. Metabol. 2007;51:643–653. doi: 10.1590/s0004-27302007000500002. [DOI] [PubMed] [Google Scholar]
- 19.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Brzezianska E, Pastuszak-Lewandoska D, Wojciechowska K, et al. Investigation of V600E BRAF mutation in papillary thyroid carcinoma in the Polish population. Neuro. Endocrinol. Lett. 2007;28:351–359. [PubMed] [Google Scholar]
- 21.Frasca F, Nucera C, Pellegriti G, et al. BRAF (V600E) mutation and the biology of papillary thyroid cancer. Endocr. Relat. Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 22.Fugazzola L, Mannavola D, Cirello V, et al. BRAF mutations in an Italian cohort of thyroid cancers. Clin. Endocrinol. (Oxf.) 2004;61:239–243. doi: 10.1111/j.1365-2265.2004.02089.x. [DOI] [PubMed] [Google Scholar]
- 23.Fugazzola L, Puxeddu E, Avenia N, et al. Correlation between B-RAF V600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr. Relat. Cancer. 2006;13:455–464. doi: 10.1677/erc.1.01086. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer. 2007;110:38–46. doi: 10.1002/cncr.22754. [DOI] [PubMed] [Google Scholar]
- 25.Lee X, Gao M, Ji Y, et al. Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann. Surg. Oncol. 2009;16:240–245. doi: 10.1245/s10434-008-0233-3. [DOI] [PubMed] [Google Scholar]
- 26.Liu RT, Chen YJ, Chou FF, et al. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin. Endocrinol. (Oxf.) 2005;63:461–466. doi: 10.1111/j.1365-2265.2005.02367.x. [DOI] [PubMed] [Google Scholar]
- 27.Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2007;92:4085–4090. doi: 10.1210/jc.2007-1179. [DOI] [PubMed] [Google Scholar]
- 28.Trovisco V, Soares P, Preto A, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch. 2005;446:589–595. doi: 10.1007/s00428-005-1236-0. [DOI] [PubMed] [Google Scholar]
- 29.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin. Endocrinol. (Oxf.) 2006;65:364–368. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama H, Yoshida A, Nakamura Y, et al. Clinical significance of BRAF (V600E) mutation and Ki-67 labeling index in papillary thyroid carcinomas. Anticancer Res. 2007;27:3645–3649. [PubMed] [Google Scholar]
- 31.Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. 2003;63:4561–4567. [PubMed] [Google Scholar]
- 32.Fagin JA. Genetics of papillary thyroid cancer initiation: implications for therapy. Trans. Am. Clin. Climatol. Assoc. 2005;116:259–269. discussion 269–271. [PMC free article] [PubMed] [Google Scholar]
- 33.Sadetzki S, Calderon-Margalit R, Modan B, Srivastava S, Tuttle RM. Ret/PTC activation in benign and malignant thyroid tumors arising in a population exposed to low-dose external-beam irradiation in childhood. J. Clin. Endocrinol. Metab. 2004;89:2281–2289. doi: 10.1210/jc.2003-030481. [DOI] [PubMed] [Google Scholar]
- 34.Basolo F, Molinaro E, Agate L, et al. RET protein expression has no prognostic impact on the long-term outcome of papillary thyroid carcinoma. Eur. J. Endocrinol. 2001;145:599–604. doi: 10.1530/eje.0.1450599. [DOI] [PubMed] [Google Scholar]
- 35.Brzezianska E, Karbownik M, Migdalska-Sek M, Pastuszak-Lewandoska D, Wloch J, Lewinski A. Molecular analysis of the RET and NTRK1 gene rearrangements in papillary thyroid carcinoma in the Polish population. Mutat. Res. 2006;599:26–35. doi: 10.1016/j.mrfmmm.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Puxeddu E, Moretti S, Giannico A, et al. Ret/PTC activation does not influence clinical and pathological features of adult papillary thyroid carcinomas. Eur. J. Endocrinol. 2003;148:505–513. doi: 10.1530/eje.0.1480505. [DOI] [PubMed] [Google Scholar]
- 37.Collins BJ, Chiappetta G, Schneider AB, et al. RET expression in papillary thyroid cancer from patients irradiated in childhood for benign conditions. J. Clin. Endocrinol. Metab. 2002;87:3941–3946. doi: 10.1210/jcem.87.8.8748. [DOI] [PubMed] [Google Scholar]
- 38.Elisei R, Romei C, Vorontsova T, et al. RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J. Clin. Endocrinol. Metab. 2001;86:3211–3216. doi: 10.1210/jcem.86.7.7678. [DOI] [PubMed] [Google Scholar]
- 39.Rabes HM, Demidchik EP, Sidorow JD, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-Chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin. Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 40.Tuttle RM, Lukes Y, Onstad L, et al. Ret/PTC activation is not associated with individual radiation dose estimates in a pilot study of neoplastic thyroid nodules arising in Russian children and adults exposed to Chernobyl fallout. Thyroid. 2008;18:839–846. doi: 10.1089/thy.2008.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moses W, Weng J, Khanafshar E, Duh QY, Clark OH, Kebebew E. Multiple genetic alterations in papillary thyroid cancer are associated with younger age at presentation. J. Surg. Res. 2010;160:179–183. doi: 10.1016/j.jss.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 42.Negri E, Dal Maso L, Ron E, et al. A pooled analysis of case–control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control. 1999;10:143–155. doi: 10.1023/a:1008880429862. [DOI] [PubMed] [Google Scholar]
- 43.Brindel P, Doyon F, Rachedi F, et al. Menstrual and reproductive factors in the risk of differentiated thyroid carcinoma in native women in French Polynesia: a population-based case–control study. Am. J. Epidemiol. 2008;167:219–229. doi: 10.1093/aje/kwm288. [DOI] [PubMed] [Google Scholar]
- 44.Mack WJ, Preston-Martin S, Bernstein L, Qian D, Xiang M. Reproductive and hormonal risk factors for thyroid cancer in Los Angeles County females. Cancer Epidemiol. Biomarkers Prev. 1999;8:991–997. [PubMed] [Google Scholar]
- 45.Pham TM, Fujino Y, Mikami H, et al. Reproductive and menstrual factors and thyroid cancer among Japanese women: the Japan Collaborative Cohort Study. J. Womens Health (Larchmt) 2009;18:331–335. doi: 10.1089/jwh.2008.1038. [DOI] [PubMed] [Google Scholar]
- 46.Sakoda LC, Horn-Ross PL. Reproductive and menstrual history and papillary thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiol. Biomarkers Prev. 2002;11:51–57. [PubMed] [Google Scholar]
- 47.Truong T, Orsi L, Dubourdieu D, Rougier Y, Hemon D, Guenel P. Role of goiter and of menstrual and reproductive factors in thyroid cancer: a population-based case–control study in New Caledonia (South Pacific), a very high incidence area. Am. J. Epidemiol. 2005;161:1056–1065. doi: 10.1093/aje/kwi136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 49.Lee ML, Chen GG, Vlantis AC, Tse GM, Leung BC, van Hasselt CA. Induction of thyroid papillary carcinoma cell proliferation by estrogen is associated with an altered expression of Bcl-xL. Cancer J. 2005;11:113–121. doi: 10.1097/00130404-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Rebai M, Kallel I, Charfeddine S, Hamza F, Guermazi F, Rebai A. Association of polymorphisms in estrogen and thyroid hormone receptors with thyroid cancer risk. J. Recept. Signal Transduct. Res. 2009;29:113–118. doi: 10.1080/10799890902845682. [DOI] [PubMed] [Google Scholar]
- 51.Inoue H, Oshimo K, Miki H, Kawano M, Monden Y. Immunohistochemical study of estrogen receptors and the responsiveness to estrogen in papillary thyroid carcinoma. Cancer. 1993;72:1364–1368. doi: 10.1002/1097-0142(19930815)72:4<1364::aid-cncr2820720435>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Zeng Q, Chen GG, Vlantis AC, van Hasselt CA. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell Prolif. 2007;40:921–935. doi: 10.1111/j.1365-2184.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng Q, Chen G, Vlantis A, Tse G, van Hasselt C. The contributions of oestrogen receptor isoforms to the development of papillary and anaplastic thyroid carcinomas. J. Pathol. 2008;214:425–433. doi: 10.1002/path.2297. [DOI] [PubMed] [Google Scholar]
- 54.Rajoria S, Suriano R, Shanmugam A, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20:33–41. doi: 10.1089/thy.2009.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimura M, Hershman JM. Thyrotropic action of human chorionic gonadotropin. Thyroid. 1995;5:425–434. doi: 10.1089/thy.1995.5.425. [DOI] [PubMed] [Google Scholar]
- 56.Knudsen N, Laurberg P, Perrild H, Bulow I, Ovesen L, Jorgensen T. Risk factors for goiter and thyroid nodules. Thyroid. 2002;12:879–888. doi: 10.1089/105072502761016502. [DOI] [PubMed] [Google Scholar]
- 57.Kung AW, Chau MT, Lao TT, Tam SC, Low LC. The effect of pregnancy on thyroid nodule formation. J. Clin. Endocrinol. Metab. 2002;87:1010–1014. doi: 10.1210/jcem.87.3.8285. [DOI] [PubMed] [Google Scholar]
- 58.Vannucchi G, Perrino M, Rossi S, et al. Clinical and molecular features of differentiated thyroid cancer diagnosed during pregnancy. Eur. J. Endocrinol. 2010;162:145–151. doi: 10.1530/EJE-09-0761. [DOI] [PubMed] [Google Scholar]
Website
- 101.National Cancer Institute: Surveillance, Epidemiology and End Results (SEER) Program. www.seer.cancer.gov.