Abstract
Progressive Multifocal Leukoencephalopathy (PML) is a demyelinating disease of the brain caused by the polyomavirus JC (JCV) in immunosuppressed people. There is no cure for PML but one-year survival has increased from 10% to 50% in HIV-infected individuals treated with highly active antiretroviral therapy (HAART). We describe herein the clinical outcome of 24 PML patients whose survival exceeded 5 years, with a mean follow-up of 94.2 months (range 60–188 months). Of all patients, only 2 were females including one who had non-Hodgkin’s lymphoma and was HIV-negative. All 23 HIV-positive patients received HAART, and additional experimental therapies were not associated with a better clinical outcome.
Marked neurological improvement occurred in 4/24(17%) of patients, while 11/24 (46%) had partial improvement and 9/24(37%) remained stable. By the end of the period of observation, 8/24(33%) of patients had no significant disability despite persistent symptoms (modified Rankin disability scale (MRDS) =1), 6/24(25%) had slight disability and were living independently (MRDS=2), 5/24(21%) were moderately disabled, requiring some help during activities of daily living (MRDS=3) and 5/24(21%) had moderately severe disability, requiring constant help or institutionalization (MRDS=4). Patients with cerebellar lesions tended to have a worse clinical outcome.
MRI showed leukomalacia with ventricular enlargement secondary to destruction of the white matter at the site of previous PML lesions, and focal areas of subcortical atrophy with preservation of the cortical ribbon.
Of 20 patients tested, 19(95%) had detectable CD8+ cytotoxic T-lymphocytes against JCV in their blood. In absence of a specific treatment, immunotherapies aiming at boosting the cellular immune response against JCV may improve the prognosis of PML.
Introduction
Progressive Multifocal Leukoencephalopathy (PML) is a rare demyelinating disease of the CNS caused by reactivation of JC virus (JCV)1. Primary infection occurs in childhood and the virus remains latent in the kidney or lymphoid organs thereafter. In the setting of cellular immunosuppression, the virus may spread to the central nervous system, leading to a lytic infection of oligodendrocytes and subsequent demyelination. Classically, PML was observed in patients with advanced HIV infection, lymphoproliferative disorders and transplant recipients. Hovewer, the use of new selective immunomodulatory or immunossupressive medications —such as natalizumab, efalizumab and rituximab—has recently altered the epidemiology of PML, which has now also been diagnosed in patients with psoriasis, rheumatoid arthritis, multiple sclerosis or Crohn’s disease2.
There is no specific treatment for PML, but the survival in HIV-infected PML patients has increased substantially during the last decade. Before the introduction of highly active antiretroviral therapy (HAART), only 10% patients with PML lived for more than a year and patients usually survived only weeks to months after the diagnosis was made3. In contrast, recent studies have shown at least 50% one-year survival of HIV- infected PML patients4 5. However, the prognosis of PML associated with other immunosuppressive conditions remains poor.
Since until recently, long-term PML survivors were extremely rare, the clinical outcome of this group of patients has not been characterized in detail. Herein, we describe the clinical features and disability profile of PML patients whose survival has exceeded five years.
Methods
We reviewed the records of PML patients followed in clinical studies at the HIV/Neurology Center of the Beth Israel Deaconess Medical Center, the Massachusetts General Hospital and the Washington University School of Medicine between January 1992 and December 2006. We included a total of 24 patients with diagnosis of PML confirmed by biopsy (n=2) or the detection of JCV DNA in the cerebrospinal fluid (CSF) by polymerase chain reaction (PCR) (n=16). We also included patients with clinical and radiological features and evolution typical of PML but negative JCV CSF PCR, based on consensus terminology criteria (n=6) 6. These patients had a negative work-up for other CNS infections or tumors. Long-term survivors were defined as survival exceeding 5 years (sixty months) from onset of PML symptoms by 12/31/06. The degree of disability was measured using the Modified Rankin disability scale (MRDS)7. The cellular immune response against JCV was measured using 51Cr release or tetramer staining assays as previously described 8.
Results
We identified 24 PML patients who survived more than 5 years from disease onset. Among them, 22 (92%) were men and 2 (8%) women. The mean age at onset of PML was 38 years (range 31–54 years). The predisposing condition in all patients was HIV infection, except one who had non-Hodgkin’s lymphoma (Patient #6, table 1). The mean length of follow up was 94.2 months (range 60–188 months).
Table 1.
clinical characteristics and disability status of long term PML survivors
| Patient | Age at onset |
Treatment | Duration of disease (months) |
Disability at time of PML diagnosis |
Outcome (Modified Rankin Scale) |
|---|---|---|---|---|---|
| 1 | 43 | HAART | 90 | Bilateral pyramidal syndrome and impulsiveness | Stable (1) |
| 2 | 38 | HAART | 113 | Decreased visual acuity and executive functions | Partial improvement (1) |
| 3 | 33 | HAART + 5HT2A antagonist | 75 | Expressive aphasia and right upper extremity apraxia | Partial improvement (2) |
| 4 | 45 | HAART after four months of PML onset | 78 | Expressive aphasia, cognitive dysfunction, right pyramidal syndrome and sensory loss | Partial improvement (2) |
| 5 | 44 | HAART | 84 | Cognitive dysfunction, cerebellar syndrome | Marked improvement but developed seizures (1) |
| 6* | 54 | ARA-C | 188 | Bilateral cerebellar syndrome | Partial improvement (3) |
| 7 | 36 | HAART + ARA-C + peptide T | 149 | Bilateral cerebellar syndrome | Stable (3) |
| 8 | 35 | HAART | 162 | Dysarthria and truncal ataxia | Partial improvement (2) |
| 9 | 49 | HAART | 80 | Bilateral cerebellar syndrome | Stable but developed seizures (4) |
| 10 | 33 | HAART | 180 | Bilateral cortical blindness | Stable but developed seizures (4) |
| 11 | 40 | HAART + 5HT2A antagonist | 75 | Abulia, transcortical aphasia, right homonymous hemianopsia and right pyramidal syndrome | Partial improvement but developed seizures (4) |
| 12 | 33 | HAART | 83 | Right pyramidal syndrome | Stable (2) |
| 13 | 39 | HAART + IFNα | 79 | Left pyramidal and cerebellar syndrome | Partial improvement (4) |
| 14 | 34 | HAART | 74 | Left pyramidal syndrome | Partial improvement (2) |
| 15 | 37 | HAART | 150 | Expressive aphasia | Marked improvement (1) |
| 16 | 31 | HAART | 68 | Seizures, left pyramidal syndrome, cognitive dysfunction | Marked improvement of cognitive and motor dysfunction, but developed seizures (1) |
| 17 | 33 | HAART | 99 | Cognitive dysfunction and a right sided sensory loss | Stable (1) |
| 18 | 31 | HAART+ IFNα | 96 | Expressive aphasia and cognitive dysfunction | Marked improvement of aphasia (1) |
| 19 | 39 | HAART+ IFNα | 114 | Cognitive dysfunction | Stable (3) |
| 20 | 39 | HAART | 92 | Right pyramidal syndrome and sensory loss | Stable (1) |
| 21 | 41 | HAART | 105 | Right cerebellar syndrome and dysarthria | Partial improvement (3) |
| 22 | 36 | HAART | 162 | Expressive aphasia and right pyramidal syndrome | Partial improvement of the aphasia (3) |
| 23 | 31 | HAART | 162 | Left cerebellar syndrome and dysarthria | Partial improvement of the cerebellar syndrome (2) |
| 24 | 33 | HAART + IFNα | 186 | Expressive aphasia and right pyramidal syndrome | Stable (4) |
HAART: highly active antiretroviral therapy, INFα: interferon α 5HT2A: serotonin receptor 2A, ARA-C: cytarabine,
HIV negative
Clinical features at diagnosis and treatment are described in Table 1. All HIV-infected individuals received HAART. Two patients received cytosine arabinoside (ARA-C). Four patients were treated with α interferon (IFN- α), while two patients were treated with the 5HT2a serotonin receptor antagonist mirtazapine. Marked improvement of neurological function was observed in 4/24 (17%) of patients, while 11/24 (46%) had partial improvement and 9/24 (37%) remained stable. At the end of the period of observation, 8/24 (33%) of patients had no significant disability despite persistent symptoms (MRDS =1), 6/24 (25%) had slight disability and were living independently (MRDS=2), 5/24 (21%) were moderately disabled, requiring some help during activities of daily living (MRDS=3) and 5/24 (21%) had moderately severe disability, requiring constant help or institutionalization (MRDS=4). There was a trend towards a worse long-term disability (MRDS 3 and 4) in patients who presented with cerebellar features (5/7; 71%) when compared with patients who developed other neurological syndromes (5/17; 29%) (p= 0.08).
None of the patients enjoyed a complete recovery. Conversely, no patient became bedridden. However, 5/24 (21%) developed seizures, including generalized tonic-clonic in three patients and focal motor seizures in two, which were successfully treated with levetiracetam monotherapy or in combination with gabapentin or topiramate.
Late radiological aspects of PML lesions in long-term survivors included leukomalacia with subsequent ventricular enlargement secondary to destruction of the white matter, as well as focal areas of subcortical atrophy. Despite extensive damage in the affected areas, there was preservation of the cortical ribbon. Three representative cases are shown in Figure 1.
Figure 1.
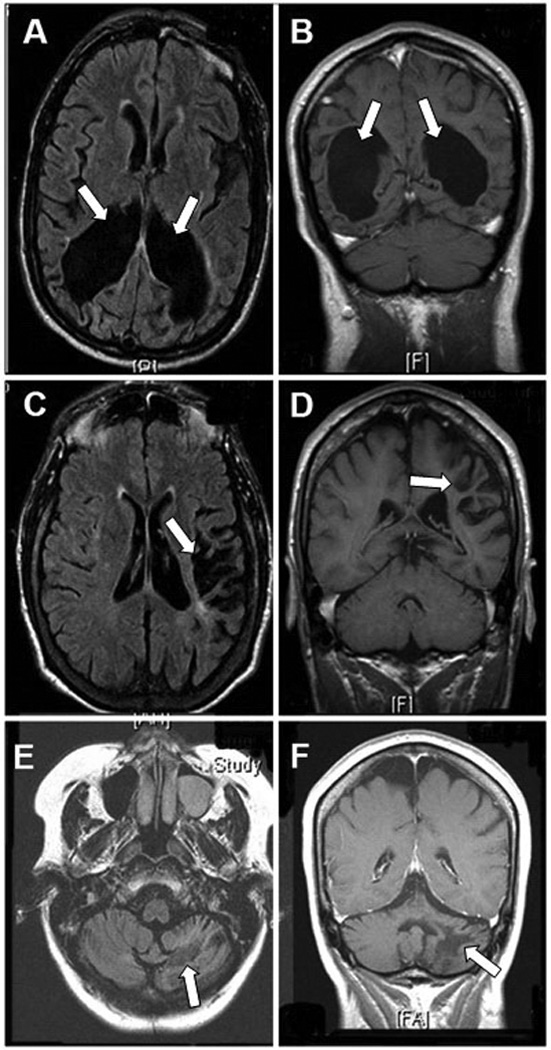
Magnetic resonance imaging of chronic aspects of long survivors of PML: axial FLAIR (A) and coronal T1-weighted images (B) showing marked ventricular enlargement secondary to white matter destruction in the occipital lobes of patient # 10 (arrows) 11 years from onset of PML symptoms with preservation of the occipital gray matter. In patient # 24, there is focal left fronto-parietal subcortical atrophy in axial FLAIR (C) and coronal T1-weigted images (D) (arrows) with preservation of the cortical ribbon after 13 years of evolution. Patient # 6 has extensive destruction of the left cerebellar white matter 10 years from PML diagnosis with atrophy in axial FLAIR (E) and coronal T1-weighted images (F) (arrows). None of these chronic lesions enhanced after administration of gadolinium (not shown).
The median CD4 count of the 23 HIV+ PML patients at the end of the period of observation was 389/µl (range 127–984) and 19/23 (83%) had undetectable HIV RNA in the plasma. Finally, we measured the cellular immune response against JCV in 20 subjects, and JCV-specific CD8+ cytotoxic T-lymphocytes (CTL) were detectable in the blood in 19 of them (95%).
Discussion
PML is an evolving disease and important changes in epidemiology have been observed recently. Indeed, immune recovery associated with HAART has resulted in a better prognosis for HIV-infected PML patients. In fact, PML is becoming a chronic disease—rather than a fatal disease—in a growing number of HIV-infected patients. For this reason, it is important to understand the clinical outcome of long-term survivors of PML in HIV-infected patients. In contrast, HIV-negative patients who develop PML in the setting of hematologic malignancies, treatment for auto-immune diseases or organ transplantation, more rarely achieve a meaningful restoration of their immune system supporting survival. While such patients account for 20% of PML cases9, only 1/24 (4%) of long term-survivors in our cohort had a predisposing disease other than HIV. Interestingly, the overwhelming majority of tested subjects had a detectable cellular immune response against JCV, which confirms previous studies on the role of T lymphocytes in PML survival8 10.
Our study shows that some patients with PML may achieve an extended survival and, although none recovered entirely, one third of them were left with no significant functional disability. The prompt institution of HAART in HIV infected PML patients is the most effective therapeutic approach in increasing survival in this group. Several studies have shown that PML survival increased from 10 to 50% in the last decade4 11. However, data on long-term neurological sequelae in PML survivors is still scarce. In our study, we observed that 66.6% of survivors improved or were stable in their deficits after five years from onset of symptoms. This finding is in accordance with a recent Danish study that reported improvement or stabilization in 83% of patients after three years of follow-up12. Another study reported improvement in 33 of 75 patients (44%)13. In our study, severe neurologic impairment was observed in almost one third of survivors. A similar picture was described in a recent study where 39% of PML patients surviving 12 months had a MRSD ≥ 4 5.
These data indicate that in some cases, PML may indeed become inactive or “burnt out”, leaving patients with permanent neurological deficits reflecting the functional brain region lesioned by the infection. Interestingly, lesions affecting the cerebellum tended to be more disabling, associated with gait ataxia and incoordination, precluding independent living.
Over the years, several drugs such as cidofovir 5, topotecan14, and interferon α(IFN- α)15 have been investigated for the treatment of PML, with disappointing results. A retrospective analysis showed stabilization of PML in one-third of HIV-negative patients with leukemia or lymphoma who developed PML after intravenous administration of cytarabine (ara-C), but hematological toxicity remains a limiting factor with this drug16. A randomized controlled trial in HIV-infected patients with PML failed to show any benefit of cytarabine. Recently, it has been shown that JC virus entry in astroglial cells in vitro is mediated in part through the 5HTA2 receptors17. Mirtazapine, an antidepressive drug which blocks the 5HTA2 receptor has been used empirically in an attempt to limit virus spread within the brain and delay progression of disease. However, except for HAART, none of the medications taken by the patients in the present study appeared to be associated with a better clinical outcome, although there is limited power to detect an effect because of the small sample size. In absence of a specific treatment for JCV, immunotherapies aimed at boosting the cellular immune response against this virus may well improve the prognosis of PML. In a recent study, JCV-peptide loaded dendritic cells from PML patients, HIV-infected individuals and healthy control subjects could elicit a strong cellular immune response mediated by CD8+ cytotoxic T lymphocytes cell response in vitro18, which suggests that autologous dendritic cell-based immunotherapy could be a potential therapeutic option for PML.
Since a cure for PML is currently not available, re-establishing latency of this infection is critical to survival. Our observations suggest that this is now possible, allowing modest functional recovery. Remarkably, the immune reconstitution achieving latency has been persistent, and none of the patients described in our study experienced another clinical reactivation of JC virus after their first episode of PML.
Acknowledgements
This work was supported in part by Public Health Service Grants R01 NS/AI 041198 NS 047029, and K24 NS 060950 to IJK.
References
- 1.Koralnik IJ. Progressive multifocal leukoencephalopathy revisited: Has the disease outgrown its name? Ann Neurol. 2006;60(2):162–173. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- 2.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab--unforeseen consequences. N Engl J Med. 2005;353(4):414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 3.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4(1):59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- 4.Falco V, Olmo M, del Saz SV, Guelar A, Santos JR, Gutierrez M, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr. 2008;49(1):26–31. doi: 10.1097/QAI.0b013e31817bec64. [DOI] [PubMed] [Google Scholar]
- 5.De Luca A, Ammassari A, Pezzotti P, Cinque P, Gasnault J, Berenguer J, et al. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. Aids. 2008;22(14):1759–1767. doi: 10.1097/QAD.0b013e32830a5043. [DOI] [PubMed] [Google Scholar]
- 6.Cinque P, Koralnik IJ, Clifford DB. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J Neurovirol. 2003;9 Suppl 1:88–92. doi: 10.1080/13550280390195298. [DOI] [PubMed] [Google Scholar]
- 7.Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1989;20(6):828. doi: 10.1161/01.str.20.6.828. [DOI] [PubMed] [Google Scholar]
- 8.Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127(Pt 9):1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- 9.Koralnik IJ, Schellingerhout D, Frosch MP. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 14-2004. A 66-year-old man with progressive neurologic deficits. N Engl J Med. 2004;350(18):1882–1893. doi: 10.1056/NEJMcpc030038. [DOI] [PubMed] [Google Scholar]
- 10.Gasnault J, Kahraman M, de Goer de Herve MG, Durali D, Delfraissy JF, Taoufik Y. Critical role of JC virus-specific CD4 T-cell responses in preventing progressive multifocal leukoencephalopathy. Aids. 2003;17(10):1443–1449. doi: 10.1097/00002030-200307040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Antinori A, Cingolani A, Lorenzini P, Giancola ML, Uccella I, Bossolasco S, et al. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA) J Neurovirol. 2003;9 Suppl 1:47–53. doi: 10.1080/13550280390195388. [DOI] [PubMed] [Google Scholar]
- 12.Engsig FN, Hansen AB, Omland LH, Kronborg G, Gerstoft J, Laursen AL, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis. 2009;199(1):77–83. doi: 10.1086/595299. [DOI] [PubMed] [Google Scholar]
- 13.Berenguer J, Miralles P, Arrizabalaga J, Ribera E, Dronda F, Baraia-Etxaburu J, et al. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clin Infect Dis. 2003;36(8):1047–1052. doi: 10.1086/374048. [DOI] [PubMed] [Google Scholar]
- 14.Royal W, 3rd, Dupont B, McGuire D, Chang L, Goodkin K, Ernst T, et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. 2003;9(3):411–419. doi: 10.1080/13550280390201740. [DOI] [PubMed] [Google Scholar]
- 15.Counihan T, Venna N, Craven D, Sabin TD. Alpha Interferon in AIDS-Related Progressive Multifocal Leukoencephalopathy. J NeuroAIDS. 1996;1(4):79–88. doi: 10.1300/j128v01n04_08. [DOI] [PubMed] [Google Scholar]
- 16.Aksamit AJ. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirol. 2001;7(4):386–390. doi: 10.1080/13550280152537292. [DOI] [PubMed] [Google Scholar]
- 17.Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306(5700):1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 18.Marzocchetti A, Lima M, Tompkins T, Kavanagh DG, Gandhi RT, O'Neill DW, et al. Efficient in vitro expansion of JC virus-specific CD8(+) T-cell responses by JCV peptide-stimulated dendritic cells from patients with progressive multifocal leukoencephalopathy. Virology. 2009;383(2):173–177. doi: 10.1016/j.virol.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]


