Abstract
In the central nervous system, chemokines are primarily mediators of inflammatory processes. Their receptors, in particular, CXCR4 and CCR5, serve as co-factors along with CD4 that permit Human immunodeficiency virus-1 (HIV) infection. Moreover, experimental evidence has shown that CXCR4 and CCR5 mediate the neurotoxic effects of the HIV envelope protein gp120, suggesting that these receptors could also promote the neuropathogenesis observed in HIV-positive individuals. Therefore, a better understanding of the molecular mechanisms governing the expression of chemokine receptors in the brain may lead to improved therapies that reduce HIV neurotoxicity. This study presents evidence that the expression of chemokine receptors in the brain is modulated by two neurotrophins in an area-specific manner. This new evidence suggests that the neurotrophins may be an adjunct therapy to reduce HIV-mediated neuronal injury evoked by chemokine receptor activation.
Keywords: NGF, NT-3, BDNF, CXCR4, CCR5
Introduction
Chemokine receptors play a vital role in the organization and control of the hematopoietic/lymphopoietic system, where they orchestrate the migration of leukocytes and their precursors. However, experimental data have shown that CXCR4 and CCR5 mediate human immunodeficiency virus 1 (HIV) infection of microglia cells (Albright et al. 1999; He et al. 1997). Moreover, CXCR4 or CCR5 either alone (Martin-Garcia et al. 2002) or in combination (Westmoreland et al. 2002) is expressed by several neuronal populations. Excessive activation of these receptors by HIV (Bachis et al. 2009) or HIV envelope glycoprotein gp120 reduces neuronal survival (Hesselgesser et al. 1998; Kaul et al. 2007; Meucci et al. 1998). Thus, inhibitors of the expression of these receptors may be useful to limit HIV-associated neuronal degeneration (Fox et al. 1997; Subbiah et al. 1996).
A promising compound that modulates CXCR4 expression/function is brain-derived neurotrophic factor (BDNF). Indeed, pharmacological levels of BDNF have been shown to reduce the neuronal expression of CXCR4 and to limit gp120 toxicity in vitro and in vivo (Bachis et al. 2003; Nosheny et al. 2007). Conversely, the reduction of BDNF in BDNF heterozygous (het) mice increases CXCR4 levels (Ahmed et al. 2008) and potentiates the toxic effect of gp120 in the striatum when compared to wild-type (WT) littermates (Nosheny et al. 2004). However, CXCR4 expression does not change in the cerebellum and hypothalamus in BDNF het mice (Ahmed et al. 2008), suggesting that BDNF may not be suitable to reduce CXCR4 in these brain areas. BDNF is a member of the neurotrophic factor family of the neurotrophins, which include nerve growth factor (NGF), neurotrophin-3 (NT-3), and NT-4. The neurotrophins have distinct as well as overlapping functions. Moreover, NT-3 can also bind to the BDNF receptor TrkB (Bothwell, 1995), and neurons may undergo a switch in neurotrophin dependence (Coppola et al. 2001). Therefore, it is appropriate to consider whether the expression of chemokine receptors in the brain is equally controlled by the other neurotrophins. In this work we used the same experimental approach of het mice employed to examine the effect of BDNF on chemokine receptors in vivo, to establish whether NGF and NT-3 modulate CXCR4 and CCR5 expression.
Results
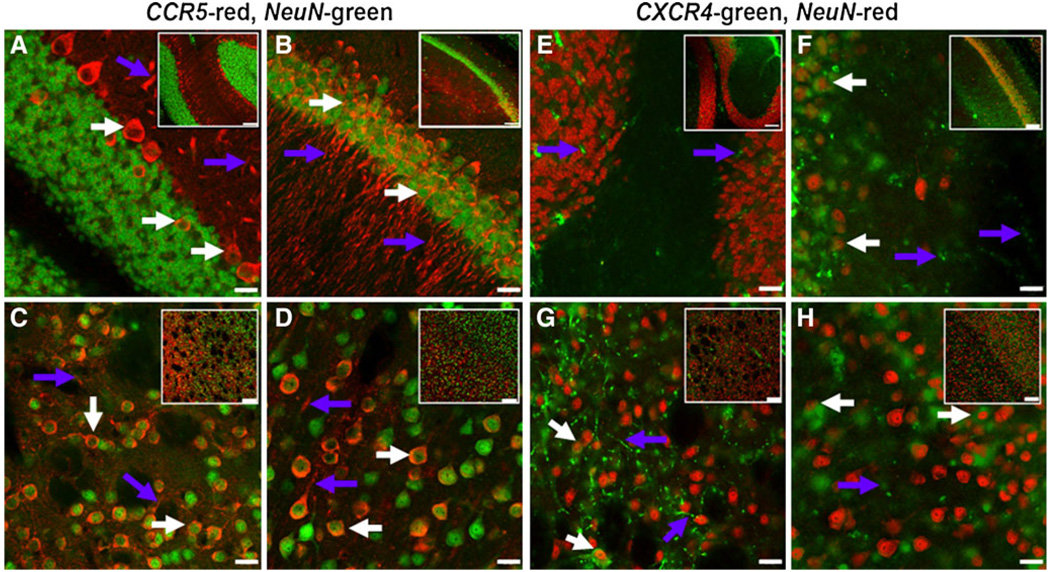
The cerebral cortex, hippocampus, and cerebellum are brain areas sensitive to the action of the neurotrophins. To provide an estimate of which neuronal population may be targeted by NGF and NT-3, we first examined, by histological analysis, CCR5 and CXCR4 immunoreactivity (IR) in adult mice. The majority of CCR5 IR colocalized with the neuronal marker NeuN. CCR5 was always localized in the cytoplasm of neuronal perikaryon and processes (Fig. 1). The best examples were observed in the Purkinje cells of the cerebellum (Fig. 1a) and neurons of the pyramidal cell layer of hippocampus (Fig. 1b). In addition, some populations of striatal (Fig. 1c) and cortical (Fig. 1d) neurons were also positive for CCR5, supporting previous findings obtained in rhesus macaque (Westmoreland et al. 2002).
Fig. 1.
Immunohistochemical analysis of CCR5 and CXCR4 immunoreactivity in mouse brain. a–d, sections were stained with CCR5 (red) and NeuN (green) antibodies. e–h, sections were stained with CXCR4 (green) and NeuN (red) antibodies as described in the Materials and Methods section. Orange denotes co-localization. a and e, cerebellar cortex; b and f, hippocampus; c and g, striatum; d and h, cerebral cortex. White arrows indicate neuronal cell bodies, and purple arrows indicate fibers. Images are higher magnifications (60×) of inserts. Bar = 20 µm; bar in inserts are 100 µm
The pattern of expression of CXCR4 was different. Indeed, with the exception of some neurons of the hippocampus (Fig. 1f) and some neurons in the cortex (Fig. 1h), CXCR4 IR was localized mainly in fibers. This was most apparent for the striatum, which contained numerous CXCR4-positive fibers (Fig. 1g). This is not surprising because previous findings have shown that CXCR4 is highly expressed in nigro-striatal terminals in rats (Banisadr et al. 2002; Trecki et al. 2010) and humans (Shimoji et al. 2009; van der Meer et al. 2000). Overall, CXCR4 and CCR5 expression varies by brain region and cell type.
To examine whether lower levels of NGF and NT-3 promote a change in the expression patterns of CXCR4 and CCR5, we have used NGF and NT-3 het mice, which exhibit 50% of the expression of NGF or NT-3 than compared to WT mice (Tessarollo et al. 1994). To provide a relative quantitation of CXCR4 and CCR5 expression, we have analyzed mRNA levels by quantitative real-time PCR (qRT-PCR). In WT animals, CCR5 mRNA expression was higher than CXCR4 in several brain regions (Fig. 2). Analysis of NGF het (Fig. 2a) and NT-3 het (Fig. 2b) mice revealed a brain area-dependent increase in CCR5 and CXCR4 mRNAs when compared to WT animals. In fact, CCR5 mRNAwas affected throughout all regions examined in NGF het (Fig. 2a), but not in NT-3 het mice (Fig. 2b). However, CXCR4 mRNA expression was higher in the cerebellum and hypothalamus in both NGF and NT-3 het animals (Fig. 2). This is not uncommon, as the populations of NGF and NT-3 sensitive cells in the CNS are not identical. Nevertheless, further studies are needed to identify the molecular mechanisms underlying the region- and cell-specific regulation of chemokine receptors by the neurotrophins.
Fig. 2.
NGF and NT-3 heterozygous mice exhibit higher levels of CXCR4 mRNA than wild type. The mRNA levels for CCR5 and CXCR4 were determined in 4-month-old C57BL/6 mice using qRT-PCR as described in the Materials and Methods section. a NGF het mice, b NT-3 het mice. Data were normalized to β-actin mRNA and expressed as arbitrary units (AU, mean±SEM of five independent samples). *p<0.05, **p<0.005 vs wild type using a one-way ANOVA and Student’s t-test
Discussion
The data presented here and elsewhere (Ahmed et al. 2008) show that the expression of CXCR4 and CCR5 is modulated by the neurotrophins in a region-specific manner. In NT-3 and NGF het mice, the levels of CXCR4 mRNA increased in the cerebellum and hypothalamus only, whereas in BDNF het mice, CXCR4 increases in other brain areas such as the cerebral cortex, striatum, and hippocampus (Ahmed et al. 2008). CCR5 mRNA levels are also differentially modulated by the neurotrophins. In fact, het mice with reduced levels of NGF and BDNF (Ahmed et al. 2008), but not NT-3, exhibit increases in CCR5 mRNA. These data suggest that each neurotrophin exerts region-specific effects on the expression of chemokine receptors that may be dependent upon the abundance and type of neurotrophin signaling. It is noteworthy that in the hippocampus, cerebral cortex and striatum BDNF is more abundant than NGF. Nevertheless, some sensory neurons co-express more than one Trk receptor (Huang et al. 1999), and BDNF and NT-3 are functionally redundant in promoting the survival of these neurons (Coppola et al. 2001). Thus, we cannot rule out the possibility that a given neurotrophin may substitute another one in the modulation of chemokine receptors in selected brain areas.
CXCR4 and CCR5 are crucial for HIV infection and neurotoxicity. Therefore, inhibitors of the expression/signaling of these receptors may have an important therapeutic property against HIV-mediated neurological impairment caused by CXCR4/CCR5 activation. Previous data have shown that BDNF exerts a neurotrophic activity against T-trophic gp120 by reducing the expression of CXCR4 (Bachis et al. 2003; Nosheny et al. 2007). The neuro-protective effect of BDNF occurs through the high affinity TrkB receptor (Mocchetti and Bachis 2004). Conversely, BDNF does not prevent gp120 toxicity in neurons that do not express TrkB (Ahmed et al. 2008). Similarly, NGF does not prevent gp120 toxicity in TrkA-negative neurons (Mocchetti and Bachis 2004). These data suggest that each neurotrophin may have a different pharmacological profile as potential inhibitors of CXCR4 and CCR5 expression in the adult brain. Nevertheless, our findings allow the speculation that the neurotrophins could be used to reduce the ability of HIV/gp120 to promote a chemokine receptor-mediated apoptotic pathway.
In addition to neurotoxicity, CXCR4 is also implicated in neuroblastoma progression (Carlisle et al. 2009) and adult neurogenesis (Tran and Miller 2005). The latter appears essential for specific cognitive functions that decline in HIV-positive subjects (Venkatesan et al. 2007). The neurotrophins (Frielingsdorf et al. 2007; Li et al. 2008) also promote adult neurogenesis but inhibit neuroblastoma growth (Kaplan et al. 1993). Thus, our data allow the speculation that the neurotrophins by down-regulating CXCR4 may control abnormal proliferation of progenitor cells. More studies are needed to prove this hypothesis.
A reduction of BDNF expression occurs in the brain of gp120-injected rats (Nosheny et al. 2004). Likewise, alteration of the NGF pro-survival pathway by another HIV protein, Tat, has been suggested to play a role in neuronal apoptosis evoked by HIV (Darbinian et al. 2008). Moreover, neurodegeneration in HIV subjects is also associated with a decrease in the expression of other growth factors such as acidic fibroblast growth factor (Everall et al. 2001). From the standpoint of understanding the multistep process leading to HIV-associated neuronal degeneration, we can propose a scenario in which the virus (or viral proteins) causes a reduction of trophic factors. Such reduction leads to an up-regulation of chemokine receptors that are crucial mediators of HIV neurotoxicity. This hypothesis would suggest that neurotrophic factors may be useful to diminish chemokine receptor-mediated neuronal dysfunction.
Materials and methods
Animals
Four-month-old NGF and NT-3 het mice and WT littermates were generated in the C57BL/6 genetic back-ground as previously described (Lyons et al. 1999) and were back-crossed for 10–12 generations to a C57BL/6 genetic background, a strategy that resulted in greatly reducing the genetic heterogeneity present in the original 129 Sv-C57BL/6 mixed background. Mice were group-housed under standard conditions with food and water available ad libitum and were maintained on a 12-h light/dark cycle. Both male and female mice were used for the analyses.
Quantitative real-time PCR
Total RNA was extracted from mouse brain using RNeasy Plus Mini Kit and reverse transcribed using QuantiTect Reverse Transcription Kit according to the manufacturer’s specifications (Qiagen, Valencia, CA). The qPCR mix contained Platinum SYBR Green qPCR SuperMix (Invitrogen, Carlsbad, CA), cDNA template or negative controls, and primers. Reaction conditions for amplification were as follows: 50°C, 2 min; 95°C, 2 min; 40 cycles of three steps (95°C, 15 s; 60°C, 1 min; and 72°C, 30 s) on the 7900HT Fast Real-Time PCR System (Applied Biosystems, Inc., Carlsbad, CA). Primers for CXCR4 were 5′-ACTCTGAAGAAGTGGGTTC-3′ (forward) and 5′-CGACTATGCCAGTCAAGAA-3′ (reverse); for CCR5, 5′-TTTTCCAGCAAGACAATCCT-3′ (forward) and 5′-TCCTACTCCCAAGCTGCATAG-3′ (reverse); for β-actin, 5′-AAGAGCTATGAGCTGCCTGA-3′ (forward) and 5′-TACGGATGTCAACGTCACAC-3′ (reverse). Dissociation curve analysis was run at the end of 40 cycles to verify PCR product identity. All primer pairs were selected that displayed linear amplification. Standard curves for each set of primers were plotted using serial dilution of cDNA to verify equal amplification efficiency. β-Actin transcript levels were used as normalization control to obtain arbitrary units. To exclude genomic DNA contamination, samples without reverse transcriptase were used as negative control.
Immunohistochemistry
Mice were anesthetized with 2-2-2-tribromoethanol (125 mg/kg, Sigma-Aldrich, St. Louis, MO) and perfused transcardially with ice-cold PBS (pH 7.4). The brain was removed and post-fixed in 4% paraformaldehyde and then transferred into 30% sucrose, and serial sections (40 µm) were prepared using a sliding microtome. Floating sections were incubated overnight at 4°C in primary polyclonal antibodies (CXCR4, 1:200, Santa Cruz Biotech, Inc, Santa Cruz, CA; CCR5, 1:100, Abcam, San Francisco, CA) followed by biotinylated secondary antibodies for 1 h at room temperature. Reaction was visualized with TSA Cy-3 amplification kit for CCR5 and TSA Fluor for CXCR4 (Perkin Elmer, Waltham, MA) according to manufacturer’s instructions. Mouse anti-NeuN, clone A60, followed by Cy-3 and FITC goat antimouse antibodies (1:250 and 1:100, respectively, Jackson Lab, West Grove, PA) were used to visualize neurons. Reactions were imaged using an FV300 laser confocal scanning system attached to an Olympus IX-70 upright microscope.
Acknowledgments
This work was supported by the Public Health Service grants DA026174, NS040670 (IM), and T32 DA007291 (LAC) and by the Intramural Research Program of the NIH, National Cancer Institute (JB and LT).
Contributor Information
Valeriya Avdoshina, Department of Neuroscience, Georgetown University Medical Center, Washington, DC, USA.
Jody Becker, Neural Development Group, Mouse Cancer Genetics Program, Center for Cancer Research, National Cancer Institute, Frederick, MD, USA.
Lee A. Campbell, Department of Neuroscience, Georgetown University Medical Center, Washington, DC, USA
Maia Parsadanian, Department of Neuroscience, Georgetown University Medical Center, Washington, DC, USA.
Timothy Mhyre, Department of Neuroscience, Georgetown University Medical Center, Washington, DC, USA.
Lino Tessarollo, Neural Development Group, Mouse Cancer Genetics Program, Center for Cancer Research, National Cancer Institute, Frederick, MD, USA.
Italo Mocchetti, Email: moccheti@georgetown.edu, Department of Neuroscience, Georgetown University Medical Center, Washington, DC, USA.
References
- Ahmed F, Tessarollo L, Thiele C, Mocchetti I. Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain. Brain Res. 2008;1227:1–11. doi: 10.1016/j.brainres.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O’Connor MJ, Doms RW, Gonzalez-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Biggio F, Major EO, Mocchetti I. M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol. 2009;4:150–160. doi: 10.1007/s11481-008-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Parsadaniantz SM. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Carlisle A, Lyttle C, Carlisle R, Maris J. CXCR4 expression heterogeneity in neuroblastoma cells due to ligand-independent regulation. Molecular Cancer. 2009;8:126. doi: 10.1186/1476-4598-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola V, Kucera J, Palko ME, Martinez-De Velasco J, Lyons WE, Fritzsch B, Tessarollo L. Dissection of NT3 functions in vivo by gene replacement strategy. Development. 2001;128:4315–4327. doi: 10.1242/dev.128.21.4315. [DOI] [PubMed] [Google Scholar]
- Darbinian N, Darbinyan A, Czernik M, Peruzzi F, Khalili K, Reiss K, Gordon J, Amini S. HIV-1 Tat inhibits NGF-induced Egr-1 transcriptional activity and consequent p35 expression in neural cells. J Cell Physiol. 2008;216:128–134. doi: 10.1002/jcp.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Trillo-Pazos G, Bell C, Mallory M, Sanders V, Masliah E. Amelioration of neurotoxic effects of HIV envelope protein gp120 by fibroblast growth factor: a strategy for neuroprotection. J Neuropathol Exp Neurol. 2001;60:293–301. doi: 10.1093/jnen/60.3.293. [DOI] [PubMed] [Google Scholar]
- Fox L, Alford M, Achim C, Mallory M, Masliah E. Neuro-degeneration of somatostatin-immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1997;56:360–368. doi: 10.1097/00005072-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiology of Disease. 2007;26:47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Wilkinson GA, Farinas I, Backus C, Zang K, Wong SL, Reichardt LF. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development. 1999;126:2191–2203. doi: 10.1242/dev.126.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ. Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Neuron. 1993;11:321–331. doi: 10.1016/0896-6273(93)90187-v. [DOI] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon C-H, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Garcia J, Kolson DL, Gonzalez-Scarano F. Chemokine receptors in the brain: their role in HIV infection and pathogenesis. Aids. 2002;16:1709–1730. doi: 10.1097/00002030-200209060-00003. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A. Brain-derived neurotrophic factor activation of TrkB protects neurons from HIV-1/gp120-induced cell death. Crit Rev Neurobiol. 2004;16:51–57. doi: 10.1615/critrevneurobiol.v16.i12.50. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Amhed F, Yakovlev AG, Meyer EM, Ren K, Tessarollo L, Mocchetti I. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Shimoji M, Pagan F, Healton EB, Mocchetti I. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson’s disease. Neurotox Res. 2009;16:318–328. doi: 10.1007/s12640-009-9076-3. [DOI] [PubMed] [Google Scholar]
- Subbiah P, Mouton P, Fedor H, McArthur JC, Glass JD. Stereological analysis of cerebral atrophy in human immunodeficiency virus-associated dementia. J Neuropathol Exp Neurol. 1996;55:1032–1037. [PubMed] [Google Scholar]
- Tessarollo L, Vogel KS, Palko ME, Reid SW, Parada LF. Targeted mutation in the neurotrophin-3 gene results in loss of muscle sensory neurons. Proc Natl Acad Sci U S A. 1994;91:11844–11848. doi: 10.1073/pnas.91.25.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. HIV-1, chemokines and neurogenesis. Neurotox Res. 2005;8:149–158. doi: 10.1007/BF03033826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecki J, Brailoiu GC, Unterwald EM. Localization of CXCR4 in the forebrain of the adult rat. Brain Res. 2010;1315:53–62. doi: 10.1016/j.brainres.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer P, Ulrich AM, Gonzalez-Scarano F, Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol. 2000;69:192–201. doi: 10.1006/exmp.2000.2336. [DOI] [PubMed] [Google Scholar]
- Venkatesan A, Nath A, Gl M, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64:2120–2132. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland SV, Alvarez X, deBakker C, Aye P, Wilson ML, Williams KC, Lackner AA. Developmental expression patterns of CCR5 and CXCR4 in the rhesus macaque brain. J Neuroimmunol. 2002;122:146–158. doi: 10.1016/s0165-5728(01)00457-x. [DOI] [PubMed] [Google Scholar]