Abstract
There is a fundamental gap in understanding how brain structural and functional network connectivity are interrelated, how they change with age, and how such changes contribute to older adults’ sensorimotor deficits. Recent neuroimaging approaches including resting state functional connectivity MRI (fcMRI) and diffusion tensor imaging (DTI) have been used to assess brain functional (fcMRI) and structural (DTI) network connectivity, allowing for more integrative assessments of distributed neural systems than in the past. Declines in corpus callosum size and microstructure with advancing age have been well documented, but their contributions to age deficits in unimanual and bimanual function are not well defined. Our recent work implicates age-related declines in callosal size and integrity as a key contributor to unimanual and bimanual control deficits. Moreover, our data provide evidence for a fundamental shift in the balance of excitatory and inhibitory interhemispheric processes that occurs with age, resulting in age differences in the relationship between functional and structural network connectivity. Training studies suggest that the balance of interhemispheric interactions can be shifted with experience, making this a viable target for future interventions.
Keywords: interhemispheric, inhibition, aging, motor control
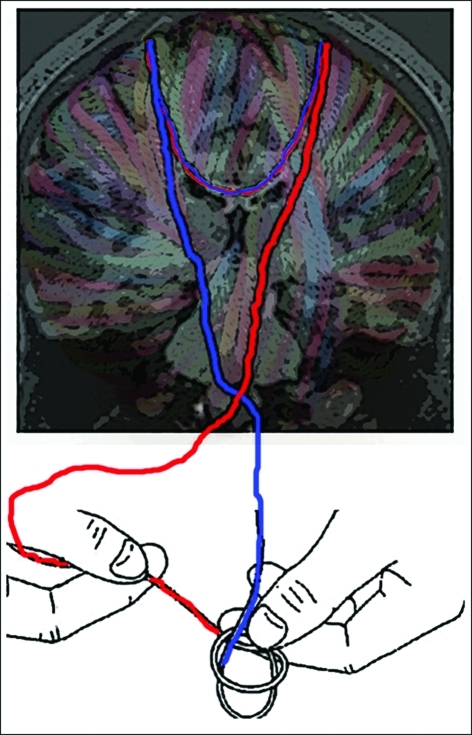
The brain is organized with certain specialized functions lateralized to each hemisphere. For example, the left hemisphere is preferentially involved in verbal processing (Ivry and Robertson, 1998) and motor control (Serrien et al., 2006), while the right hemisphere plays a stronger role in spatial cognition (Ivry and Robertson, 1998). Interhemispheric transfer via the corpus callosum plays a key role in the production of coherently integrated behavior, assuring a complex balance of excitatory and inhibitory processes. For specific motor behaviors, interhemispheric inhibition is required to prevent interference of control processes between the two hemispheres. Consider tying your shoes – each hand moves independently to accomplish a unified goal. While each primary motor cortex has dense projections to the muscles of the contralateral hand, the two are also highly interconnected via the corpus callosum, allowing for interhemispheric transfer of information (Figure 1). It is known that individuals with callosal damage or those who have undergone callosal section to alleviate severe epilepsy have particular difficulty with coordinating the two hands when each follows its own independent spatio-temporal path (Mark and Sperry, 1968; Kennerley et al., 2002), likely due to reduced interhemispheric inhibition. Indeed it has been shown using transcranial magnetic stimulation (TMS) that interhemispheric inhibition levels increase during bimanual tasks when the two hands move out of phase with each other (Giovannelli et al., 2009). This is thought to help alleviate interference that arises due to “motor overflow,” or interhemispheric excitation resulting in too much sharing of information between the two motor cortices.
Figure 1.
The brain's white matter is conceptualized as yarn in this diagram. The descending strands represent corticospinal control of each hand by its respective contralateral motor cortex. The callosal strands represent the capacity for interhemispheric interactions. In an asynchronous bimanual task such as shoe tying, interhemispheric inhibition is relied upon to prevent interference between control processes for the two hands. At the same time, interhemispheric facilitation may be relied upon to integrate actions of the two hands, allowing them to achieve a single unified goal.
Here, we review the literature addressing the behavioral relevance of interhemispheric interactions between the sensorimotor cortices, including consideration of the changes that occur with advanced age. We advance the hypothesis that there is a fundamental shift with age in the relationships between callosal structure, callosal neurophysiological function, and motor performance. Numerous studies have identified age differences in brain structure, function, and biochemistry that are associated with deficits in motor and cognitive performance (for review see Seidler et al., 2010). For example, the corpus callosum undergoes significant degeneration with age both in terms of white matter quantity and quality (Fling et al., 2010; Sullivan et al., 2010). Additionally, work from our lab has documented age deficits in both unimanual (Seidler et al., 2002; Langan et al., 2010) and bimanual (Bangert et al., 2010; Fling et al., 2010) control. Recent research approaches using techniques to measure brain structural and functional network connectivity have begun to elucidate the detailed mechanisms by which corpus callosum pathways contribute to unimanual and bimanual motor behavior, as well as the functional consequences of age-related degeneration of interhemispheric connections. Investigating brain functional and structural network integrity has the potential to make a significant impact on the field of neuromotor control because it allows for assessment of distributed brain networks as opposed to focusing on individual brain structures. Indeed, the use of these techniques allows for evaluation of Geschwind's theory that white matter disruptions can be as detrimental to function as gray matter damage (Geschwind, 1965a,b).
Age Differences in Brain Activation Patterns
The hemispheric asymmetry reduction in older adults (HAROLD) model, describing the finding that older adults (65+ years of age) demonstrate increased bilateral prefrontal cortex recruitment across different memory and cognitive tasks, was originally proposed by Cabeza (2001) a decade ago. In the interim, the effects of age on brain activation patterns and their relation to cognitive function have been studied extensively (cf. Li et al., 2001; Raz et al., 2007; Park and Reuter-Lorenz, 2009). Initial reports have suggested that over-recruitment patterns in older adults are reflective of either (i) compensatory activation where additional brain recruitment is positively associated with task performance (Cabeza, 2002; Reuter-Lorenz and Lustig, 2005; Heuninckx et al., 2008) or (ii) dedifferentiation suggesting that brain structure-function relationships become less distinctive with age and thus the increased brain activity has either no or negative behavioral consequences (Logan et al., 2002; Park et al., 2004; Langan et al., 2010). A recent model from Reuter-Lorenz and Cappell (2008), the compensation-related utilization of neural circuits hypothesis (CRUNCH) predicts that the distinctiveness of neural representations should be increased in older adults (relative to young adults) at low levels of task demand but reduced at high levels of demand. Consistent with the CRUNCH model, recent work from Carp et al. (2010) demonstrates both dedifferentiation- and compensation-like brain activation patterns in the same participants for the same task, arguing that comprehensive theories of cognitive aging must incorporate both interpretations of over-activation to fully explain complex patterns of age-related neuro-cognitive change.
While the literature on age-related differences in the neural control of movement has not developed as quickly, numerous studies have begun to elucidate the neural underpinnings associated with healthy aging and motor control (cf. Seidler et al., 2010). As proposed in the HAROLD model, older adults exhibit cortical over-activation relative to young adults when performing motor tasks. More symmetrical activation of the motor system (i.e., bilateral engagement of the motor cortices) in older adults is predominantly associated with increased engagement of the motor cortex ipsilateral to the moving hand (Calautti et al., 2001; Mattay et al., 2002; Ward and Frackowiak, 2003; Heuninckx et al., 2005, 2008; Langan et al., 2010). In our recent work, we reported that increased ipsilateral motor cortex activity was associated with poorer unimanual motor performance in older adults (Langan et al., 2010) indicating that the cognitive and motor systems may demonstrate distinct patterns of age-related change. Additional recent studies have provided insight into the role of interhemispheric interactions in the production of bilateral motor cortical activation in older adults.
Age Differences in Resting State Interhemispheric Functional Connectivity
Resting state functional connectivity (fcMRI) studies have shown that remote but functionally related gray matter regions with known anatomical connections exhibit strong correlations in the low frequency (<0.1 Hz) blood oxygen level dependent (BOLD) signal when individuals are at rest (Biswal et al., 1995; Fox and Raichle, 2007; Rogers et al., 2007; Vincent et al., 2007). These correlations are highly spatially structured following anatomical networks and are therefore thought to reflect functional connectivity of the human brain. It is important to note, however, that fcMRI can provide insight beyond that of known anatomical connections. For example, a recent case study in an individual with a complete section of the corpus callosum revealed a striking loss of interhemispheric BOLD correlations, with the possible exception of the somatomotor system, hippocampal formation, and the thalamus (Johnston et al., 2008). Likewise Uddin et al. (2008) reported normal functional connectivity between interhemispheric middle occipital and cingulate gyri in an individual with complete commissurotomy. Because functional correlations can arise from multisynaptic pathways, fcMRI networks are not necessarily restricted to occur between structures with direct anatomical connections.
Three networks of relevance for motor control have been identified with fcMRI: (i) motor cortical, including interhemispheric primary motor, premotor, parietal, and supplementary motor cortex connectivity (Biswal et al., 1995; Peltier et al., 2005; Langan et al., 2010); (ii) striatal thalamo-cortical (Di Martino et al., 2008; Kelly et al., 2009; Kwak et al., 2010); and (iii) cerebellar thalamo-cortical (Krienen and Buckner, 2009). Along with others, we have made significant inroads into identifying the spatial structure and functional relevance of these networks in healthy young adults (Peltier et al., 2005; Biswal et al., 2010), older adults (Langan et al., 2010), and disease-affected individuals (Jelsone-Swain et al., 2010; Kwak et al., 2010).
Resting state functional network correlations have behavioral relevance. For example, default mode network connectivity is altered following visual (Lewis et al., 2009) or motor (Albert et al., 2009) learning. Additionally, medication-related changes in the frequency content of resting state striatal seed regions correlates with cognitive function in patients with Parkinson's disease (Kwak et al., 2010). Moreover, greater resting state motor interhemispheric connectivity in older adults is correlated with recruitment of the ipsilateral motor cortex during a unimanual task (motor overflow, Langan et al., 2010). In other words, while most patient groups exhibit disrupted (reduced) functional network connectivity, our recent data provide compelling evidence that older adults demonstrate increased connectivity of interhemispheric motor cortical networks compared to young adults (Langan et al., 2010). We propose that this reflects a release from the normally predominantly inhibitory interhemispheric projections, shifting the overall balance of interhemispheric interactions toward excitatory processes.
Age Differences in Corpus Callosum Macro- and Microstructure
Age differences in brain activation patterns are often posited to be the result of changes in brain structure. Transcallosal fibers connect largely homologous cortical regions of the right and left hemispheres; thus, the corpus callosum mediates the transfer and integration of lateralized cognitive, motor, and sensory information between cortices (Aboitiz, 1992). Structural magnetic resonance imaging (MRI) has demonstrated that there is substantial interindividual variability in callosal size and morphology for both young and older adults (Stancak et al., 2003; Fling et al., 2010; Raz et al., 2010; Sullivan et al., 2010). Interestingly, less lateralized task processing during both cognitive (Muller-Oehring et al., 2007) and motor (Langan et al., 2010) tasks has been shown to be associated with reductions in callosal cross-sectional area in older adults. Therefore, structural differences of the corpus callosum appear to impact cortical activity both at rest and during task performance, with significant implications for behavior.
An emerging body of literature indicates that not only is the quantity of white matter reduced in older adults, but the quality of remaining white matter is compromised as well (reviewed in Seidler et al., 2010). The use of conventional MRI allows for measurements of regional brain volume, while diffusion tensor imaging (DTI) allows assessment of white matter microstructure. Thus, DTI may be more sensitive to subtler age-related white matter changes than conventional volumetric MRI. For example, some MRI investigations suggest that the corpus callosum does not undergo extensive volumetric declines with age (Raz et al., 2001), whereas several studies have shown declining callosal microstructure with age (Fling et al., 2010; Sullivan et al., 2010). Numerous studies have identified associations between callosal macrostructure or microstructure and behavior (Stancak et al., 2003; Fling et al., 2010; Sullivan et al., 2010). Furthermore, recent studies utilizing fiber tractography have demonstrated relationships between interhemispheric motor fiber tract microstructure and task performance in both healthy participants (Johansen-Berg et al., 2007) and in those with white matter dysfunction (Bartels et al., 2008; Bonzano et al., 2008; Kern et al., 2010). While individual differences in callosal quantity and quality have shown to be behaviorally relevant in both young and older adults, it appears there may be a fundamental shift with age in these relationships.
Interhemispheric communication can have either net facilitatory or inhibitory effects on the cortex (Chen et al., 2003). Multiple lines of research indicate that interhemispheric connections between the sensorimotor cortical regions have primarily inhibitory effects (Netz, 1999; De Gennaro et al., 2004; Lenzi et al., 2007). This interhemispheric inhibition is presumably to prevent interference from the opposite hemisphere, or motor overflow (cf. Hoy et al., 2004). This is thought to allow for simultaneous but independent control of the two hands, such as required for shoe tying. Intriguingly, we have recently reported that young adults with increased microstructural quality of callosal regions connecting sensorimotor cortical areas perform with more variability on unimanual and asynchronous bimanual motor tasks (Fling et al., 2010). Conversely, larger size and better microstructure of these same callosal regions was associated with better performance in older adults. Moreover, we recently reported that older adults exhibit greater recruitment of ipsilateral primary motor cortex during unimanual motor task performance, which was associated with longer reaction times (Langan et al., 2010). Additionally, greater recruitment of ipsilateral primary motor cortex in older adults was correlated with reduced resting state interhemispheric connectivity and a larger corpus callosum. We posit that reduced interhemispheric motor connectivity may be associated with a loss of the ability to inhibit the ipsilateral hemisphere during unimanual motor task performance for older adults, which has a negative impact on response time. These data provide evidence for a link between callosal structural and physiological changes with age, lending credence to the dedifferentiation hypothesis. Specifically, interhemispheric interactions require a balance between excitatory and inhibitory processes; taken together the aforementioned studies suggest that this overall balance is likely shifted in the aging brain.
Evidence for Age Differences in Interhemispheric Neurophysiological Function
Experiments conducted with individuals with callosal pathology (e.g., partial callosotomy or multiple sclerosis) demonstrate that while the total number of callosal fibers connecting the two primary motor cortices is relatively few in number, communication between these homologous areas has the capability to strongly influence motor behavior (Eliassen et al., 1999, 2000; Kennerley et al., 2002; Lenzi et al., 2007; Bonzano et al., 2008). The fiber tracts that comprise the corpus callosum are integral for inhibiting the ipsilateral motor cortex during both unimanual and bimanual control (Netz, 1999; Perez and Cohen, 2008; Vercauteren et al., 2008). Monosynaptic connections between primary motor cortices (Porter and Lemon, 1993), along with densely transcallosally connected secondary motor areas, have been shown to significantly influence interhemispheric inhibition and neuromotor control. Although full characterization of the transcallosal inhibitory sensorimotor network is still lacking, neuroimaging data suggest that it includes the supplementary and pre-supplementary motor areas (Serrien et al., 2002; Grefkes et al., 2008), the dorsal premotor cortices (Giovannelli et al., 2006; van den Berg et al., 2010), and the somatosensory cortices (Ni et al., 2009).
Callosally mediated interhemispheric inhibition is a complex process that has traditionally been measured in humans using paired-pulse TMS to each primary motor cortex at an interstimulus interval of (i) 8–12 ms (short-interval interhemispheric inhibition, SIHI) or (ii) ∼40 ms (long-interval interhemispheric inhibition, LIHI; cf. Chen, 2004). Paired-pulse TMS utilizes two magnetic stimulators to investigate the effect of a supra-threshold conditioning stimulus over one primary motor cortex (M1) on the size of a test motor evoked potential elicited by stimulation of the opposite M1. Although both SIHI and LIHI are reflective of transcallosal inhibition, they do not appear to represent the same phenomenon, nor are the two values correlated with each other across individuals (Chen et al., 2003). Although both measures reflect inhibition of synchronized activation of the corticospinal system induced by the conditioning stimulus (the first stimulus applied), a pharmacological experiment suggests that LIHI is likely mediated by postsynaptic gamma-aminobutyric acid type B (GABA)B receptors, whereas SIHI is potentially mediated by GABAA receptors, although this remains to be fully elucidated (Irlbacher et al., 2007).
In line with our hypothesis of reductions in interhemispheric inhibitory interactions with age, accumulating evidence demonstrates reduced inhibition within the nervous system of older adults, both at the cortical (Talelli et al., 2008a,b) and spinal levels (Kido et al., 2004). A discussion of age-related changes in inhibition at the spinal level is beyond the scope of the current review, and thus we will focus on cortical inhibition. Paired-pulse TMS studies have shown that healthy older adults display decreased excitability of intracortical inhibitory circuits within the motor cortex while at rest (Peinemann et al., 2001). Task-related increases in LIHI also diminish with advancing age and are correlated with the degree of ipsilateral primary motor cortex recruitment during unimanual motor tasks (Talelli et al., 2008a,b). In agreement with Talelli and colleagues, Fujiyama et al. (2009) found that older adults have a reduced ability to modulate inhibitory function in a task-dependent manner in comparison to young adults. Specifically, older adults exhibited a reduced ability to increase inhibition during coordination of the arm and leg on the same side of the body. These age-related declines in interhemispheric inhibition and in the ability to modulate inhibitory function to meet task demands may be associated with the bimanual movement deficits observed in older adults.
Considering that the unimanual and bimanual motor tasks included in Fling et al. (2010) and Langan et al. (2010) rely heavily on timing, some insight may be provided from research in expert musicians, individuals with exquisite temporal control. Surprisingly, both inter- and intra-hemispheric inhibition are reduced in musicians (Ridding et al., 2000; Nordstrom and Butler, 2002). It is interesting that cortical inhibition is less effective in musicians, who have extraordinary control of independent finger movements. However, it is unclear whether these effects represent an adaptive change related to exceptional control of finger movements or a maladaptive change brought about by overuse of the hand from extensive training (Nordstrom and Butler, 2002).
Training experiments may shed some light on the counterintuitive finding that musicians with superior bimanual control and larger callosal size have reduced interhemispheric inhibition. Shim et al. (2005) were the first to investigate the effects of practice on callosal physiology; following just 2 days of practice of a novel bimanual force production task, participants demonstrated task-selective reductions in interhemispheric inhibition. In accord with this finding, Hortobagyi et al. (2010) reported a significant decrease in interhemispheric inhibition and a concomitant increase in ipsilateral primary motor cortex excitability following 20 sessions of unimanual submaximal force production. These studies, taken together with those in trained musicians, suggest that interhemispheric inhibitory projections can show plastic changes that favor the execution of a practiced task, likely through a cooperative action of the two hemispheres (Shim et al., 2005). In other words, although interhemispheric inhibition is integral for the performance of coordinated bimanual tasks, it does not appear that high levels of inhibition are beneficial to task performance.
Synthesis and Future Directions
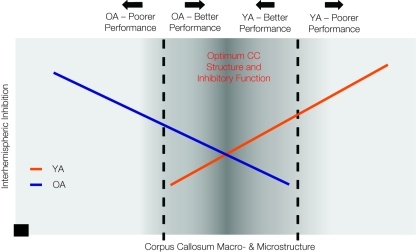
If decreased interhemispheric inhibition favors the performance of bimanual tasks, how can we reconcile decreased interhemispheric inhibition in older adults and decreased bimanual task performance? Recent work suggests that the microstructure of transcallosal motor fibers, assessed with DTI, reflects the capacity for interhemispheric inhibition between the primary motor cortices. Wahl et al. (2007) report a positive relationship between microstructural integrity of transcallosal motor fibers and strength of SIHI in young adults. No data is currently available regarding the nature of this relationship in older adults. Based upon our recent findings (Fling et al., 2010; Langan et al., 2010) it seems likely that there is a fundamental shift in the relationship between callosal size, microstructure, and interhemispheric physiologic function from young to older adults. Figure 2 presents a graphic representation of our hypothesis. This figure portrays the relationship between these neural measures of the callosum and performance of motor tasks requiring interhemispheric interactions for older and younger adults (see Figure 2). Based upon our recent findings (Fling et al., 2010; Langan et al., 2010), we suggest that in young adults, increased interhemispheric inhibitory function is associated with larger callosal size and increased fiber tract microstructure, whereas the inverse relationship is true in older adults. Furthermore, a parsimonious and testable hypothesis that builds upon our recent findings is that performance on bimanual tasks falls along a continuum which demonstrates a shared optimal region of callosal microstructure and interhemispheric inhibition for young and older adults. That is, young and older adults exhibit maximal performance for a comparable range of callosal microstructure and interhemispheric inhibition values. However, structure-physiological function-performance relationships differentially diverge from this range for the two age groups.
Figure 2.
This diagram provides a graphic representation of our hypothesis that performance on bimanual tasks falls along a continuum which demonstrates a shared optimal region of callosal microstructure and interhemispheric inhibition for young and older adults (darker gray central zone). Structure-physiological function-performance relationships differentially diverge from this range for the two age groups, with greater interhemispheric inhibition and reduced callosal structure associated with poorer performance in older adults and greater interhemispheric inhibition and greater callosal structure associated with poorer performance in young adults. The black square data point at x, y = 0 indicates that there is no capacity for interhemispheric inhibition in the complete absence of the corpus callosum.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by National Institutes of Health [grant numbers AG024106 (Rachael D. Seidler), T32-AG000114], and the UM National Institutes of Health Claude D. Pepper Center Human Subjects and Assessment Core (grant number AG024824). Rachael D. Seidler is a member of the LIFE program (The LIFE Course: Evolutionary and Ontogenetic Dynamics).
Key Concept
- Interhemispheric inhibition
Suppression of the activity of a brain region by its contralateral homolog, transmitted via the corpus callosum.
- Motor overflow
The term typically refers to involuntary movements which may accompany the production of voluntary movements. Here we use the term to refer to inadvertent activation of a motor structure but at a level that is insufficient to result in movement.
- Interhemispheric excitation
Facilitation of the activity of a brain region by its contralateral homolog, transmitted via the corpus callosum.
- Resting state functional connectivity
Correlations in the low frequency blood oxygen dependent fMRI signal between brain regions. Typically, remote but functionally related gray matter regions with known anatomical connections exhibit strong correlations when individuals are at rest.
- Diffusion tensor imaging
Neuroimaging technique that uses the diffusion of water molecules to provide information about brain microstructure. This technique is particularly beneficial for investigating white matter due to the dense axonal organization found within large fiber bundles such as the corpus callosum.
Biography
 Rachael D. Seidler utilizes behavioral and brain imaging approaches to determine the neural and cognitive underpinnings of motor control in healthy individuals and those with movement disorders. She studied exercise science and biology at the University of Oregon, and then went on for doctoral study in motor control at Arizona State University and postdoctoral training in neuroscience at the University of Minnesota. She currently holds a joint appointment in the Department of Psychology and the School of Kinesiology at the University of Michigan, where she directs the Neuromotor Behavior Laboratory.
Rachael D. Seidler utilizes behavioral and brain imaging approaches to determine the neural and cognitive underpinnings of motor control in healthy individuals and those with movement disorders. She studied exercise science and biology at the University of Oregon, and then went on for doctoral study in motor control at Arizona State University and postdoctoral training in neuroscience at the University of Minnesota. She currently holds a joint appointment in the Department of Psychology and the School of Kinesiology at the University of Michigan, where she directs the Neuromotor Behavior Laboratory.
References
- Aboitiz F. (1992). Brain connections: interhemispheric fiber systems and anatomical brain asymmetries in humans. Biol. Res. 25, 51–61 [PubMed] [Google Scholar]
- Albert N. B., Robertson E. M., Miall R. C. (2009). The resting human brain and motor learning. Curr. Biol. 19, 1023–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert A. S., Reuter-Lorenz P. A., Walsh C. M., Schachter A. B., Seidler R. D. (2010). Bimanual coordination and aging: neurobehavioral implications. Neuropsychologia 48, 1165–1170 10.1016/j.neuropsychologia.2009.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C., Mertens N., Hofer S., Merboldt K. D., Dietrich J., Frahm J., Ehrenreich H. (2008). Callosal dysfunction in amyotrophic lateral sclerosis correlates with diffusion tensor imaging of the central motor system. Neuromuscul. Disord. 18, 398–407 [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M., Hyde J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 [DOI] [PubMed] [Google Scholar]
- Biswal B. B., Mennes M., Zuo X. N., Gohel S., Kelly C., Smith S. M., Beckmann C. F., Adelstein J. S., Buckner R. L., Colcombe S., Dogonowski A. M., Ernst M., Fair D., Hampson M., Hoptman M. J., Hyde J. S., Kiviniemi V. J., Kotter R., Li S. J., Lin C. P., Lowe M. J., Mackay C., Madden D. J., Madsen K. H., Margulies D. S., Mayberg H. S., McMahon K., Monk C. S., Mostofsky S. H., Nagel B. J., Pekar J. J., Peltier S. J., Petersen S. E., Riedi V., Rombouts S. A., Rypma B., Schlaggar B. L., Schmidt S., Seidler R. D., Siegle G. J., Sorg C., Teng G. J., Veijola J., Villringer A., Walter M., Wang L., Weng X. C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y. F., Zhang H. Y., Castellanos F. X., Milham M. P. (2010). Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U.S.A. 107, 4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzano L., Tacchino A., Roccatagliata L., Abbruzzese G., Mancardi G. L., Bove M. (2008). Callosal contributions to simultaneous bimanual finger movements. J. Neurosci. 28, 3227–3233 10.1523/JNEUROSCI.4076-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. (2001). Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand. J. Psychol. 42, 277–286 [DOI] [PubMed] [Google Scholar]
- Cabeza R. (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging 17, 85–100 [DOI] [PubMed] [Google Scholar]
- Calautti C., Serrati C., Baron J. C. (2001). Effects of age on brain activation during auditory-cued thumb-to-index opposition: a positron emission tomography study. Stroke 32, 139–146 10.1161/hs1101.097401 [DOI] [PubMed] [Google Scholar]
- Carp J., Gmeindl L., Reuter-Lorenz P. A. (2010). Age differences in the neural representation of working memory revealed by multi-voxel pattern analysis. Front. Hum. Neurosci. 4:217. 10.3389/fnhum.2010.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. (2004). Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp. Brain Res. 154, 1–10 10.1007/s00221-003-1684-1 [DOI] [PubMed] [Google Scholar]
- Chen R., Yung D., Li J. Y. (2003). Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J. Neurophysiol. 89, 1256–1264 10.1152/jn.00950.2002 [DOI] [PubMed] [Google Scholar]
- De Gennaro L., Bertini M., Pauri F., Cristiani R., Curcio G., Ferrara M., Rossini P. M. (2004). Callosal effects of transcranial magnetic stimulation (TMS): the influence of gender and stimulus parameters. Neurosci. Res. 48, 129–137 [DOI] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D. S., Kelly A. M., Uddin L. Q., Shehzad Z., Biswal B., Walters J. R., Castellanos F. X., Milham M. P. (2008). Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex 18, 2735–2747 10.1093/cercor/bhn041 [DOI] [PubMed] [Google Scholar]
- Eliassen J. C., Baynes K., Gazzaniga M. S. (1999). Direction information coordinated via the posterior third of the corpus callosum during bimanual movements. Exp. Brain Res. 128, 573–577 10.1007/s002210050884 [DOI] [PubMed] [Google Scholar]
- Eliassen J. C., Baynes K., Gazzaniga M. S. (2000). Anterior and posterior callosal contributions to simultaneous bimanual movements of the hands and fingers. Brain 123(Pt 12), 2501–2511 10.1093/brain/123.12.2501 [DOI] [PubMed] [Google Scholar]
- Fling B. W., Walsh C. M., Bangert A. S., Reuter-Lorenz P. A., Welsh R. C., Seidler R. D. (2010). Differential callosal contributions to bimanual control in young and older adults. J. Cogn. Neurosci. [Epub ahead of print]. 10.1162/jocn.2010.21600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D., Raichle M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 [DOI] [PubMed] [Google Scholar]
- Fujiyama H., Garry M. I., Levin O., Swinnen S. P., Summers J. J. (2009). Age-related differences in inhibitory processes during interlimb coordination. Brain Res. 1262, 38–47 10.1016/j.brainres.2009.01.023 [DOI] [PubMed] [Google Scholar]
- Geschwind N. (1965a). Disconnexion syndromes in animals and man. I. Brain 88, 237–294 10.1093/brain/88.2.237 [DOI] [PubMed] [Google Scholar]
- Geschwind N. (1965b). Disconnexion syndromes in animals and man. II. Brain 88, 585–644 10.1093/brain/88.3.585 [DOI] [PubMed] [Google Scholar]
- Giovannelli F., Borgheresi A., Balestrieri F., Ragazzoni A., Zaccara G., Cincotta M., Ziemann U. (2006). Role of the right dorsal premotor cortex in “physiological” mirror EMG activity. Exp. Brain Res. 175, 633–640 10.1007/s00221-006-0581-9 [DOI] [PubMed] [Google Scholar]
- Giovannelli F., Borgheresi A., Balestrieri F., Zaccara G., Viggiano M. P., Cincotta M., Ziemann U. (2009). Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J. Physiol. 587(Pt 22), 5393–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C., Eickhoff S. B., Nowak D. A., Dafotakis M., Fink G. R. (2008). Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage 41, 1382–1394 10.1016/j.neuroimage.2008.03.048 [DOI] [PubMed] [Google Scholar]
- Heuninckx S., Wenderoth N., Debaere F., Peeters R., Swinnen S. P. (2005). Neural basis of aging: the penetration of cognition into action control. J. Neurosci. 25, 6787–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S., Wenderoth N., Swinnen S. P. (2008). Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J. Neurosci. 28, 91–99 10.1523/JNEUROSCI.3300-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi T., Richardson S. P., Lomarev M., Shamim E., Meunier S., Russman H., Dang N., Hallett M. (2010). Interhemispheric plasticity in humans. Med. Sci. Sports Exerc. [Epub ahead of print]. 10.1249/MSS.0b013e31820a94b8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy K. E., Fitzgerald P. B., Bradshaw J. L., Armatas C. A., Georgiou-Karistianis N. (2004). Investigating the cortical origins of motor overflow. Brain Res. Brain Res. Rev. 46, 315–327 [DOI] [PubMed] [Google Scholar]
- Irlbacher K., Brocke J., Mechow J. V., Brandt S. A. (2007). Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin. Neurophysiol. 118, 308–316 [DOI] [PubMed] [Google Scholar]
- Ivry R. B., Robertson L. C. (1998). The Two Sides of Perception. Cambridge, MA: The Massachusetts Institute of Technology [Google Scholar]
- Jelsone-Swain L. M., Fling B. W., Seidler R. D., Hovatter R., Gruis K., Welsh R. C. (2010). Reduced interhemispheric functional connectivity in the motor cortex during rest in limb-onset amyotrophic lateral sclerosis. Front. Syst. Neurosci. 4:158. 10.3389/fnsys.2010.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H., Della-Maggiore V., Behrens T. E., Smith S. M., Paus T. (2007). Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage 36(Suppl. 2), T16–T21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. M., Vaishnavi S. N., Smyth M. D., Zhang D., He B. J., Zempel J. M., Shimony J. S., Snyder A. Z., Raichle M. E. (2008). Loss of interhemispheric functional connectivity after complete section of the corpus callosum. J. Neurosci. 25, 6453–6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., de Zubicaray G., Di Martino A., Coplands D. A., Reiss P. T., Klein D. F., Castellanos F. X., Milham M. P., McMahon K. (2009). L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J. Neurosci. 29, 7364–7678 10.1523/JNEUROSCI.0810-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley S. W., Diedrichsen J., Hazeltine E., Semjen A., Ivry R. B. (2002). Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat. Neurosci. 5, 376–381 [DOI] [PubMed] [Google Scholar]
- Kern K. C., Sarcona J., Montag M., Giesser B. S., Sicotte N. L. (2010). Corpus callosal diffusivity predicts motor impairment in relapsing-remitting multiple sclerosis: a TBSS tractography study. Neuroimage 55, 1169–1177 10.1016/j.neuroimage.2010.10.077 [DOI] [PubMed] [Google Scholar]
- Kido A., Tanaka N., Stein R. B. (2004). Spinal excitation and inhibition decrease as humans age. Can. J. Physiol. Pharmacol. 82, 238–248 [DOI] [PubMed] [Google Scholar]
- Krienen F. M., Buckner R. L. (2009). Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex 19, 2485–2497 10.1093/cercor/bhp135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y., Peltier S., Bohnen N. I., Muller M. L., Dayalu P., Seidler R. D. (2010). Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front. Syst. Neurosci. 4:143. 10.3389/fnsys.2010.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J., Peltier S. J., Bo J., Fling B. W., Welsh R. C., Seidler R. D. (2010). Functional implications of age differences in motor system connectivity. Front. Syst. Neurosci. 4:17. 10.3389/fnsys.2010.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D., Conte A., Mainero C., Frasca V., Fubelli F., Totaro P., Caramia F., Inghilleri M., Pozzilli C., Pantano P. (2007). Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum. Brain Mapp. 28, 636–644 10.1002/hbm.20305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. M., Baldassarre A., Committeri G., Romani G. L., Corbetta M. (2009). Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl. Acad. Sci. U.S.A. 106, 17558–17563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. C., Lindenberger U., Sikstrom S. (2001). Aging cognition: from neuromodulation to representation. Trends Cogn. Sci. 5, 479–486 [DOI] [PubMed] [Google Scholar]
- Logan J. M., Sanders A. L., Snyder A. Z., Morris J. C., Buckner R. L. (2002). Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron 33, 827–840 10.1016/S0896-6273(02)00612-8 [DOI] [PubMed] [Google Scholar]
- Mark R. F., Sperry R. W. (1968). Bimanual coordination in monkeys. Exp. Neurol. 21, 92–104 [DOI] [PubMed] [Google Scholar]
- Mattay V. S., Fera F., Tessitore A., Hariri A. R., Das S., Callicott J. H., Weinberger D. R. (2002). Neurophysiological correlates of age-related changes in human motor function. Neurology 58, 630–635 [DOI] [PubMed] [Google Scholar]
- Muller-Oehring E. M., Schulte T., Raassi C., Pfefferbaum A., Sullivan E. V. (2007). Local-global interference is modulated by age, sex and anterior corpus callosum size. Brain Res. 1142, 189–205 10.1016/j.brainres.2007.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J. (1999). Asymmetry in transcallosal inhibition. Electroencephalogr. Clin. Neurophysiol. Suppl. 51, 137–144 [PubMed] [Google Scholar]
- Ni Z., Gunraj C., Nelson A. J., Yeh I. J., Castillo G., Hoque T., Chen R. (2009). Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb. Cortex 19, 1654–1665 10.1093/cercor/bhn201 [DOI] [PubMed] [Google Scholar]
- Nordstrom M. A., Butler S. L. (2002). Reduced intracortical inhibition and facilitation of corticospinal neurons in musicians. Exp. Brain Res. 144, 336–342 10.1007/s00221-002-1051-7 [DOI] [PubMed] [Google Scholar]
- Park D. C., Polk T. A., Park R., Minear M., Savage A., Smith M. R. (2004). Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci. U.S.A. 101, 13091–13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. C., Reuter-Lorenz P. (2009). The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol. 60, 173–196 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinemann A., Lehner C., Conrad B., Siebner H. R. (2001). Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci. Lett. 313, 33–36 [DOI] [PubMed] [Google Scholar]
- Peltier S. J., LaConte S. M., Niyazov D. M., Liu J. Z., Sahgal V., Yue G. H., Hu X. P. (2005). Reductions in interhemispheric motor cortex functional connectivity after muscle fatigue. Brain Res. 1057, 10–16 10.1016/j.brainres.2005.06.078 [DOI] [PubMed] [Google Scholar]
- Perez M. A., Cohen L. G. (2008). Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J. Neurosci. 28, 5631–5640 10.1523/JNEUROSCI.0093-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R., Lemon R. (1993). Corticospinal Function and Voluntary Movement. Oxford: Clarendon Press [Google Scholar]
- Raz N., Ghisletta P., Rodrigue K. M., Kennedy K. M., Lindenberger U. (2010). Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage 51, 501–511 10.1016/j.neuroimage.2010.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Gunning-Dixon F., Head D., Williamson A., Acker J. D. (2001). Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am. J. Neuroradiol. 22, 1161–1167 [PMC free article] [PubMed] [Google Scholar]
- Raz N., Rodrigue K. M., Haacke E. M. (2007). Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann. N. Y. Acad. Sci. 1097, 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., Cappell K. A. (2008). Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 17, 177–182 [Google Scholar]
- Reuter-Lorenz P. A., Lustig C. (2005). Brain aging: reorganizing discoveries about the aging mind. Curr. Opin. Neurobiol. 15, 245–251 [DOI] [PubMed] [Google Scholar]
- Ridding M. C., Brouwer B., Nordstrom M. A. (2000). Reduced interhemispheric inhibition in musicians. Exp. Brain Res. 133, 249–253 10.1007/s002210000428 [DOI] [PubMed] [Google Scholar]
- Rogers B. P., Morgan V. L., Newton A. T., Gore J. C. (2007). Assessing functional connectivity in the human brain by fMRI. Magn. Reson. Imaging 25, 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. D., Alberts J. L., Stelmach G. E. (2002). Changes in multi-joint performance with age. Motor Control 6, 19–31 [DOI] [PubMed] [Google Scholar]
- Seidler R. D., Bernard J. A., Burutolu T. B., Fling B. W., Gordon M. T., Gwin J. T., Kwak Y., Lipps D. B. (2010). Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 34, 721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien D. J., Ivry R. B., Swinnen S. P. (2006). Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci. 7, 160–166 [DOI] [PubMed] [Google Scholar]
- Serrien D. J., Strens L. H., Oliviero A., Brown P. (2002). Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neurosci. Lett. 328, 89–92 [DOI] [PubMed] [Google Scholar]
- Shim J. K., Kim S. W., Oh S. J., Kang N., Zatsiorsky V. M., Latash M. L. (2005). Plastic changes in interhemispheric inhibition with practice of a two-hand force production task: a transcranial magnetic stimulation study. Neurosci. Lett. 374, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancak A., Cohen E. R., Seidler R. D., Duong T. Q., Kim S. G. (2003). The size of corpus callosum correlates with functional activation of medial motor cortical areas in bimanual and unimanual movements. Cereb. Cortex 13, 475–485 10.1093/cercor/13.5.475 [DOI] [PubMed] [Google Scholar]
- Sullivan E. V., Rohlfing T., Pfefferbaum A. (2010). Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol. Aging 31, 464–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P., Ewas A., Waddingham W., Rothwell J. C., Ward N. S. (2008a). Neural correlates of age-related changes in cortical neurophysiology. Neuroimage 40, 1772–1781 10.1016/j.neuroimage.2008.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P., Waddingham W., Ewas A., Rothwell J. C., Ward N. S. (2008b). The effect of age on task-related modulation of interhemispheric balance. Exp. Brain Res. 186, 59–66 10.1007/s00221-007-1205-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L. Q., Mooshagian E., Zaidel E., Scheres A., Margulies D. S., Clare Kelly A. M., Shehzad Z., Adelstein J. S., Castellanos F. X., Biswal B. B., Milham M. P. (2008). Residual functional connectivity in the split-brain revealed with resting-state functional MRI. Neuroreport 19, 703–709 10.1097/WNR.0b013e3282fb8203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg F. E., Swinnen S. P., Wenderoth N. (2010). Hemispheric asymmetries of the premotor cortex are task specific as revealed by disruptive TMS during bimanual versus unimanual movements. Cereb. Cortex 20, 2842–2851 10.1093/cercor/bhq034 [DOI] [PubMed] [Google Scholar]
- Vercauteren K., Pleysier T., Van Belle L., Swinnen S. P., Wenderoth N. (2008). Unimanual muscle activation increases interhemispheric inhibition from the active to the resting hemisphere. Neurosci. Lett. 445, 209–213 [DOI] [PubMed] [Google Scholar]
- Vincent J. L., Patel G. H., Fox M. D., Snyder A. Z., Baker J. T., Van Essen D. C., Zempel J. M., Snyder L. H., Corbetta M., Raichle M. E. (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86 10.1038/nature05758 [DOI] [PubMed] [Google Scholar]
- Wahl M., Lauterbach-Soon B., Hattingen E., Jung P., Singer O., Volz S., Klein J. C., Steinmetz H., Ziemann U. (2007). Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J. Neurosci. 27, 12132–12138 10.1523/JNEUROSCI.2320-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N. S., Frackowiak R. S. (2003). Age-related changes in the neural correlates of motor performance. Brain 126(Pt 4), 873–888 10.1093/brain/awg071 [DOI] [PMC free article] [PubMed] [Google Scholar]