Abstract
The striatum is considered to be critical for the control of goal-directed action, with the lateral dorsal striatum (latDS) being implicated in modulation of habits and the nucleus accumbens thought to represent a limbic–motor interface. Although medium spiny neurons from different striatal subregions exhibit many similar properties, differential firing and synaptic plasticity could contribute to the varied behavioral roles across subregions. Here, we examined the contribution of small-conductance calcium-activated potassium channels (SKs) to action potential generation and synaptic plasticity in adult rat latDS and nucleus accumbens shell (NAS) projection neurons in vitro. The SK-selective antagonist apamin exerted a prominent effect on latDS firing, significantly decreasing the interspike interval. Furthermore, prolonged latDS depolarization increased the interspike interval and reduced firing, and this enhancement was reversed by apamin. In contrast, NAS neurons exhibited greater basal firing rates and less regulation of firing by SK inhibition and prolonged depolarization. LatDS neurons also had greater SK currents than NAS neurons under voltage-clamp. Importantly, SK inhibition with apamin facilitated long-term depression (LTD) induction in the latDS but not the NAS, without alterations in glutamate release. In addition, SK activation in the latDS prevented LTD induction. Greater SK function in the latDS than in the NAS was not secondary to differences in sodium or inwardly rectifying potassium channel function, and apamin enhancement of firing did not reflect indirect action through cholinergic interneurons. Thus, these data demonstrate that SKs are potent modulators of action potential generation and LTD in the dorsal striatum, and could represent a fundamental cellular mechanism through which habits are regulated.
Keywords: action potential, plasticity, potassium channel, SK, striatum
Introduction
It has long been recognized that the striatum plays a critical role in the regulation of a number of motivated behaviors, although the exact contribution may depend on the particular demands of the task. The lateral dorsal striatum (latDS) has been associated with habit learning and expression (Graybiel, 1998; Berke & Hyman, 2000; Packard & Knowlton, 2002; Yin et al., 2006) [but see Atallah et al. (2007)], including in relation to addiction (Everitt & Robbins, 2005; Vanderschuren et al., 2005; Fuchs et al., 2006), and the nucleus accumbens (NAcb), a ventral subregion of the striatum, regulates several forms of learning and behavioral control by limbic and motivational inputs (Cardinal et al., 2002; Kalivas & McFarland, 2003; Atallah et al., 2007). The medium spiny neuron (MSN) is the primary striatal projection neuron (Gerfen, 2004), and neurons from different striatal subregions share a number of properties, including spiny morphology, low basal firing rates in vivo, lack of spontaneous activity in vitro, and expression of DARPP-32 (Wilson, 1998; Greengard et al., 1999; Surmeier et al., 2007). However, some differences across regions have been recognized in intrinsic properties (Pennartz et al., 1992; Zahm & Brog, 1992) [but see Kawaguchi et al. (1989) and O'Donnell & Grace (1993)] and synaptic plasticity (Thomas et al., 2000; Gerdeman et al., 2003), and there are different MSN populations in the different regions (Gerfen, 2004; Surmeier et al., 2007).
Action potential (AP) firing is a major mechanism by which neurons process information, and there is considerable interest in improving our understanding of the mechanisms that control generation of firing in striatal neurons in relation to behavioral events (Ghitza et al., 2003; Schultz et al., 2003; Nicola et al., 2004; Barnes et al., 2005; Wheeler & Carelli, 2009). A number of channels are active during repetitive AP generation (Wilson & Kawaguchi, 1996; Hille, 2001). Several channels, including persistent sodium and inwardly rectifying and A-type potassium channels, regulate the ability of glutamate-mediated depolarization to reach a voltage sufficient for AP firing (Calabresi et al., 1987; Cepeda et al., 1995; Nisenbaum & Wilson, 1995; Tkatch et al., 2000; Mahon et al., 2003). At near-threshold voltages, many additional currents are activated, including depolarizing sodium and calcium currents (Calabresi et al., 1987; Hoehn et al., 1993; Bargas et al., 1994; Churchill & Macvicar, 1998; Zhang et al., 1998) and hyperpolarizing potassium currents (Vilchis et al., 2000; Sah & Faber, 2002).
Activation of the latDS and NAcb contribute differently to the generation of behavior (Graybiel, 1998; Cardinal et al., 2002; Ghitza et al., 2003; Schultz et al., 2003; Nicola et al., 2004; Barnes et al., 2005), probably, in part, because of differential connectivity with other brain regions (Gerfen, 2004). However, different AP generation and synaptic plasticity could also contribute to the varied behavioral roles of each subregion. Here, we examined the function of the small-conductance calcium-activated potassium channels (SKs) in the adult rat latDS and NAcb shell (NAS), and observed differential SK regulation of firing and induction of synaptic plasticity in the two regions.
Materials and methods
Slice preparation and electrophysiology
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health, and with approval from the Ernest Gallo Clinic and Research Center's Institute for Animal Care and Use Committee. Eighty-seven postnatal day 55–90 male Long Evans rats (Harlan, Livermore, CA, USA) were deeply sedated with 40 mg/kg intraperitoneal pentobarbital before transcardial perfusion with ~30 mL of chilled Ringer's solution containing 225 mm sucrose, 119 mm NaCl, 2.5 mm KCl, 1.0 mm NaH2PO4, 4.9 mm MgCl2, 0.1 mm CaCl2, 26.2 mm NaHCO3, 1.25 mm glucose, 1 mm ascorbic acid, and 3 mm kynurenic acid. The brain was then rapidly removed, and coronal slices (300 μm) were cut in this same solution and then allowed to recover at 32°C in carbogen-bubbled artificial cerebrospinal fluid (ACSF), containing 126 mm NaCl, 2.5 mm KCl, 1.2 mm NaH2PO4, 1.2 mm MgCl2, 2.4 mm CaCl2, 18 mm NaHCO3, 11 mm glucose (pH 7.2–7.4 and 301–305 mOsm) for 30 min to 5 h. Ascorbic acid (1 mm) was added ~15 min before slices were placed in the recovery chamber. During electrophysiology experiments, slices were submerged and continuously perfused (using a peristaltic pump, ~2.5 mL/min) with carbogen-bubbled ACSF warmed to 31–32°C, and supplemented with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μm, to block α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptors) and picrotoxin (50 μm, to block γ-aminobutyric acid-A receptors). Field potential recordings were performed without CNQX. All reagents were bath applied.
Whole-cell recordings were made from latDS and NAS MSNs, identified as previously described (Hopf et al., 2003), using a potassium methanesulfonate-based internal solution (130 mm KOH, 105 mm methanesulfonic acid, 17 mm hydrochloric acid, 20 mm HEPES, 0.2 mm EGTA, 2.8 mm NaCl, 2.5 mg/mL MgATP, 0.25 mg/mL NaGTP, pH 7.2–7.4, 275–285 mOsm). Patch-clamping was performed using infrared differential interference contrast video-microscopy with 2.5–3.5-MΩ electrodes. Patch-clamp data were collected at 15 kHz and filtered at 2 kHz using a Clampex 9.2 and an Axon 1D or a Multiclamp 700 A patch amplifier in current-clamp mode (Axon Instruments, Union City, CA, USA). Series resistance correction was 15–25 MΩ. The resting membrane potential was determined just after breaking into a neuron, and each neuron was then brought to a resting potential of approximately −90 mV by passage of direct current via the patch amplifier before further firing experiments. The resting membrane potential was not different between latDS and NAS neurons (latDS, −94.6 ± 0.4 mV; NAS, −94.8 ± 0.5 mV; n = 52 and n = 33, respectively; t83 = 0.261; P = 0.795; unpaired t-test).
To generate APs, neurons in current-clamp were depolarized with a series of seven or eight 300-ms current pulses, with 20 pA between each current pulse, the initial current pulse for each neuron being just subthreshold for firing. To analyse changes in firing parameters in the presence of a given ion channel blocker, data were obtained just before addition of drug and at 8–10 min after drug addition. During experiments in which BaCl2 was used, bath application of BaCl2 depolarized the neurons; thus, the resting membrane potential was returned to approximately −90 mV before determination of the input resistance and parameters related to firing. The input–output slope was calculated by fitting a line relating the number of APs generated in the first three suprathreshold current pulses and the last subthreshold current pulse in a given neuron.
AP waveform parameters were determined at the current step where the first AP was fired. AP amplitude was calculated relative to the AP threshold, where the AP threshold was defined as the voltage during the AP upstroke when the rate of rise exceeded 2 V/s, determined using custom software written in Python2.3 (http://www.python.org). Input resistance was determined from the voltage change produced by a 33.3-pA hyperpolarizing pulse from a −90-mV resting membrane potential.
Voltage-clamp experiments were performed using the external ACSF solution and internal pipette solution described above. For SK tail current measures, neurons were held at −70 mV, and depolarizing current steps (400 ms, from −60 to −10 mV in 10-mV steps) were applied, with 1 s between successive steps. In some neurons, only the step to −20 mV was performed. Upon return to −70 mV, a tail current was evident (see Fig. 4A). The peak magnitude of the tail current before and after addition of apamin was determined for the steps to −20 through −50 mV. The rate of inactivation of the SK current was estimated in the tail current evoked after the −20-mV depolarization by fitting a single exponential from ~55 ms after the peak of the tail current to 550 ms after the peak of the tail current (Abel et al., 2004). Analyses of apamin-sensitive peak currents and the decay constant τ of inactivation was determined for the step to −20 mV because of occasional apamin-insensitive rapidly activating and inactivating currents at steps to −10 mV, which probably represent large-conductance calcium-activated potassium channel currents (Sah & Faber, 2002). For inwardly rectifying potassium channel (IRK)/leak currents, neurons were held at −90 mV, and 250-ms voltage pulses, ranging from −70 to −150 mV in 10-mV steps, were applied. The evoked current was determined at the end of the plateau response.
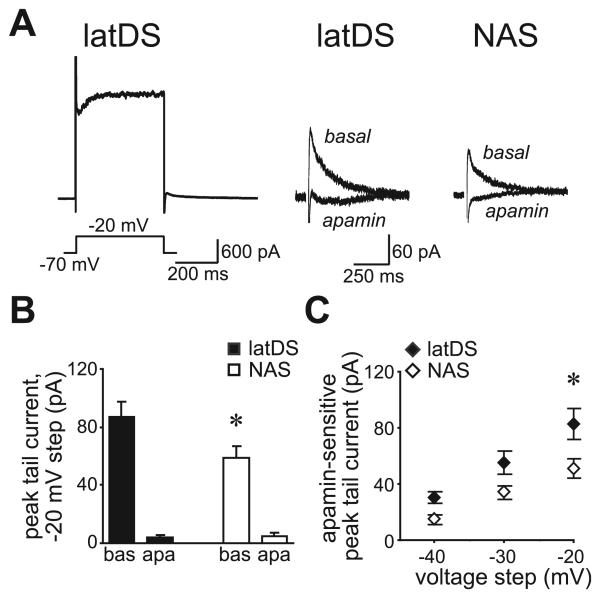
Fig. 4.
Small-conductance calcium-dependent potassium channel function, measured with voltage-clamp, was significantly greater in lateral dorsal striatum (latDS) than in nucleus accumbens shell (NAS) neurons. (A) Depolarizing current steps resulted in a tail current upon return to the −70-mV holding potential. The left trace shows an example from a latDS neuron of the full current response to the −20-mV depolarization. The right traces show examples of the post-depolarization tail current in latDS and NAS neurons, before and after apamin administration. (B) Data for the step to −20 mV showing that the peak of the tail current was significantly greater in latDS than in NAS neurons, and was significantly reduced by apamin in both regions. bas, baseline; apa, apamin. (C) Apamin-sensitive peak tail currents were significantly larger in latDS than in NAS neurons. *P < 0.05.
A low level of the calcium buffer EGTA (200 μm) was included in the pipette solution in order to preserve calcium-dependent potassium currents during whole-cell current-clamp and voltage-clamp recordings, as has been previously done in other studies of SKs and other calcium-dependent currents (Bargas et al., 1999; Wolfart et al., 2001). As we are able to conclusively identify apamin-sensitive SK currents under voltage-clamp (Fig. 4), and the magnitude of these SK currents is greater in latDS than in NAS neurons (Fig. 4), in agreement with analyses of firing (Figs 1 and 2), we believe that our results, taken together, strongly suggest that the low level of EGTA used does not interfere substantially with the calcium-dependent activation of SKs during AP firing elicited by depolarizing current steps. Also, we did not see changes in the interspike interval (ISI) or AP generation across time after breaking into the whole-cell configuration, suggesting that dialysis of MSNs with the intracellular solution did not significantly alter basal firing.
Fig. 1.
Inhibition of the small-conductance calcium-activated potassium channel by apamin (100 nm) significantly enhanced firing in lateral dorsal striatum (latDS) neurons, but had a smaller effect in nucleus accumbens shell (NAS) neurons. (A and B) The interspike interval (ISI) was greater in latDS than in NAS neurons at baseline, and apamin reduction of the ISI was greater in latDS than in NAS neurons. The ISI was determined at the lowest current step that elicited at least two action potentials (APs). bas, baseline; apa, apamin. (C and D) The apamin-dependent increase in AP firing was greater in latDS than in NAS neurons. The number of APs was determined at a current step at which three APs were generated (or four APs, if no current step produced three APs). (E–G) The slope relationship between input current and number of evoked spikes (the input–output slope), determined from the last subthreshold pulse and the first three suprathreshold pulses (E and F), was significantly smaller in latDS than in NAS neurons (G), and the apamin-dependent enhancement of the input–output slope was significantly greater in latDS than in NAS neurons (G). Data in E and F correspond to the traces shown in A. *P < 0.05, **P < 0.01.
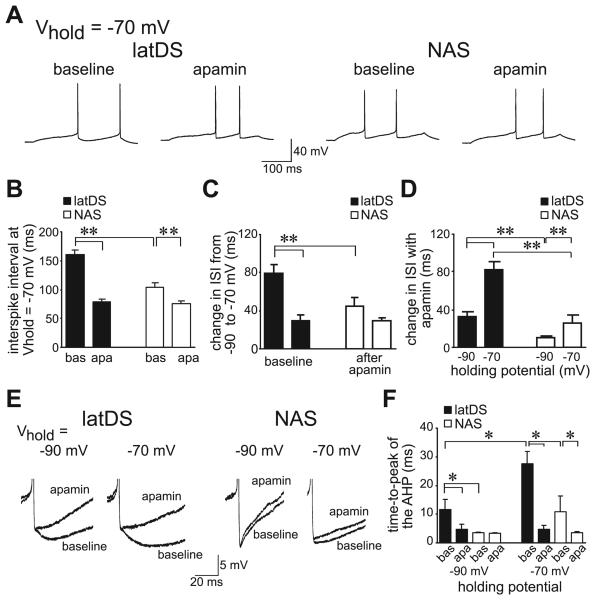
Fig. 2.
Prolonged depolarization to −70 mV dramatically enhanced small-conductance calcium-dependent potassium channel regulation of firing, with a greater effect in lateral dorsal striatum (latDS) than in nucleus accumbens shell (NAS) neurons. (A–C) Depolarization to −70 mV significantly enhanced interspike intervals (ISIs) in both latDS and NAS neurons relative to holding at −90 mV, with a significantly greater effect in the latDS neurons, and with (D) a larger apamin-sensitive portion of the ISI in latDS neurons at −70 mV. bas, baseline; apa, apamin. (E and F) Prolonged depolarization to −70 mV also dramatically enhanced the time-to-peak of the afterhypolarization (AHP), with a significantly greater effect in latDS than in NAS neurons. After apamin administration, there were no differences in the time-to-peak of the AHP. *P < 0.05, **P < 0.01. Vhold, holding potential.
Voltage values were corrected for the liquid junction potential, estimated to be 10 mV using the Junction Null Calculator in clampex 9.2 (Axon Instruments, Union City, CA, USA) and by direct measurement of the potential difference between internal solutions and ACSF present after zeroing the pipette current.
Long-term depression (LTD) methods
Field potentials were recorded using a glass electrode filled with ACSF, and generated using a bipolar stimulating electrode placed ~100 μm dorsal to the recording electrode. Test field potentials were evoked every 15 s, and high-frequency stimulation (HFS) was generated by stimulation at 50 Hz for 2 s, repeated four times, with 15 s between initiation of trains, using a Master 8 (AMPI, Jerusalem, Israel). Fifty hertz was originally chosen because it approximates the maximal firing in the latDS during a procedural task (Barnes et al., 2005), and because it could be subthreshold for LTD generation, as a somewhat similar stimulation protocol, except with 100 Hz, induces LTD in the latDS of young rats (Partridge et al., 2000). Field potentials were recorded and analysed using igor pro (Wavemetrics, Lake Oswego, OR, USA), data were acquired at 5 kHz and filtered at 2 kHz, and the N2 field potential amplitude was analysed in 2-min bins. Striatal N2 field potentials were synaptically evoked, as the N2, but not the N1, component of the field potential was completely blocked by CNQX [data not shown; see also Schotanus et al. (2006)]. However, the N2 field potential may include both postsynaptic glutamate receptor-mediated excitatory postsynaptic potentials and population spikes (PSs) representing APs elicited subsequent to synaptic activation. Thus, we refer to this potential as the field excitatory postsynaptic potential (fEPSP)/PS, following Schotanus et al. (2006). For experiments with the CB1 receptor antagonist AM251, AM251 was applied before the HFS and also throughout the rest of the experiment to remove any possible confounds related to altered fEPSP amplitude in the presence of AM251 in some cells (data not shown). To analyse the statistical significance of changes in LTD induction with apamin, normalized data from the last 5 min of recording from each cell were averaged and then compared across groups.
Spike-timing-dependent LTD was induced using a variation of a previously described spike-timing-dependent protocol (STDP) (Liu et al., 2005) consisting of 20 bouts of excitatory postsynaptic potential (EPSP) spike pairs delivered 5 s apart, measured using the whole-cell patch methods described above. Each bout consisted of a burst of five paired stimuli delivered at 100-ms intervals, with the onset of EPSPs following the peak of the postsynaptic action potential by 15 ms. EPSP amplitudes were set to ~8 mV at baseline. Peak EPSP amplitudes were calculated by taking the mean of a 1–2-ms window around the peak and comparing this with the mean of an 8-ms window immediately before the stimulation artefact. EPSP data were analysed in 2-min bins.
Statistics
Statistical significance between latDS and NAS neurons before and after apamin was determined using a two-way repeated-measures anova with a post hoc Holm–Sidak test. Other statistical tests were utilized as indicated. Statistical analyses were performed using sigmastat (Systat, Point Richmond, CA, USA). All data are shown as means ± standard errors of the mean.
Reagents
Apamin, CNQX and tetrodotoxin (TTX) were prepared as aliquots in water and frozen at −20°C. 1-Ethyl-2-benzimidazolinone (1-EBIO) (Tocris Bioscience, Ellisville, MO, USA) was prepared as aliquots in dimethylsulfoxide and frozen at −20°C. Picrotoxin and BaCl2 were dissolved in water and kept as stock solutions at room temperature. Unless otherwise indicated, all reagents were prepared as 1 : 1000 stock solutions, and were purchased from Sigma (St Louis, MO, USA).
Results
SK regulation of AP firing is significantly greater in latDS than in NAS neurons, and is facilitated by prolonged depolarization
The firing properties of latDS and NAS MSNs, identified as described in Hopf et al. (2003), were examined in brain slices prepared from adult (postnatal day 55–90) rats. Neurons were brought to a resting membrane potential of approximately −90 mV by passage of direct current via the patch amplifier, and a series of 300-ms current pulses was then delivered. A low level of the calcium buffer EGTA (200 μm) was included in the pipette solution in order to preserve calcium-dependent potassium currents during whole-cell recordings (Bargas et al., 1999; Wolfart et al., 2001). SK currents can profoundly retard AP generation by increasing the interval between APs (Bennett et al., 2000), including in the young dorsal striatum (DS) (Pineda et al., 1992; Bargas et al., 1999; Vilchis et al., 2000), although they have less effect in the young NAS (Ishikawa et al., 2009). As some aspects of striatal physiology can vary between young and adult animals (Belleau & Warren, 2000; Benoit-Marand & O'Donnell, 2008), we first determined the effect of SK inhibition by apamin (100 nm), a selective SK blocker (Stocker, 2004), on the ISI in adult rat MSNs, determined at the lowest current step at which a neuron fired at least two APs (Fig. 1A and B). The baseline ISI was significantly longer in latDS than in NAS neurons (Fig. 1A and B; n = 9 for both latDS and NAS neurons; region, F1,16 = 9.20, P = 0.008, post hocP < 0.001 for basal latDS vs. NAS). Furthermore, although apamin reduced the ISI in both latDS and NAS neurons (Fig. 1A and B; apamin, F1,16 = 116.3; P < 0.001), the apamin-induced reduction of the ISI was significantly greater in the latDS neurons (Fig. 1A and B; region × apamin interaction, F1,16 = 29.01, P < 0.001), indicating greater SK function in latDS neurons.
SK inhibition by apamin also produced a greater enhancement of AP firing in latDS neurons (Fig. 1A, C and D; apamin, F1,16 = 68.26, P < 0.001; region × apamin, F1,16 = 15.61, P = 0.001), determined at a current step at which three APs were generated (or four APs, if no current step produced three APs). Reduced basal firing and greater apamin enhancement of firing in latDS than in NAS neurons was further apparent in an analysis of the input–output slope, which relates AP generation to the amount of applied current (Fig. 1E and F). The basal input–output slope was significantly smaller in latDS than in NAS neurons (Fig. 1E–G; region, F1,16 = 25.17, P < 0.001), and the apamin enhancement of the input–output slope was significantly greater in latDS neurons (Fig. 1G; region × apamin, F1,16 = 9.43, P < 0.001; change in input–output slope with apamin for latDS neurons, 0.34 ± 0.03 APs per 10 pA; change in input–output slope with apamin for NAS neurons, 0.18 ± 0.04 APs per 10 pA; t16 = 3.071; P = 0.007; unpaired t-test). Thus, SKs significantly depressed depolarization-generated AP firing in latDS neurons, but had a much smaller effect in NAS neurons.
Prolonged depolarization to −70 mV can enhance calcium influx and subsequently increase calcium-activated potassium currents in DS neurons in vitro (Rutherford et al., 1988; Hernandez-Lopez et al., 1997). As suggested by several groups (Hernandez-Lopez et al., 1997; Kreitzer & Malenka, 2005), this may mimic the prolonged depolarization known as the ‘up-state’ observed in vivo, which can last hundreds of milliseconds to seconds (Wilson & Kawaguchi, 1996). Here, depolarization to −70 mV for 5 s by direct current injection significantly lengthened the ISI in both latDS and NAS neurons, as compared with holding at −90 mV (Fig. 2A and C; n = 6 and n = 9, respectively; region, F1,13 = 100.6, P < 0.001), with a significantly greater effect in latDS neurons (Fig. 2B and C; region × voltage, F1,13 = 7.64, P = 0.017). Furthermore, the apamin-induced reduction of the ISI was greatest in latDS neurons held at −70 mV (Fig. 2B and D; region × voltage, F1,13 = 8.296, P = 0.013). We should note that, in many studies of up-states, the membrane potential is typically not corrected for the liquid junction potential. Thus, the −70-mV holding potential used here represents −60 mV when not corrected for the junction potential, which is within the range observed for the up-state in vivo [e.g. a mean of approximately −54 mV in Wilson & Kawaguchi (1996)].
The time-to-peak of the afterhyperpolarization (AHP), determined relative to the AP threshold (Fig. 2E), is also strongly regulated by SKs in many cell types (Pineda et al., 1992; Bennett et al., 2000; Vilchis et al., 2000; Sah & Faber, 2002), and, accordingly, was significantly greater in latDS than in NAS neurons at both −70 and −90 mV (Fig. 2E and F; region, F1,13 = 33.62, P = 0.004; voltage, F1,13 = 33.62, P = 0.002; region × voltage, F1,13 = 4.88, P = 0.049). Furthermore, following exposure to apamin, the time-to-peak of the AHP was similar in both regions and at both holding potentials (Fig. 2E and F; region, F1,13 = 0.872, P = 0.367; voltage, F1,13 = 0.009, P = 0.924; region × voltage, F1,13 = 0.681, P = 0.424), suggesting that differences in the time-to-peak of the AHP could be attributed to differential SK function. Thus, prolonged depolarization significantly enhanced SK function, with a much greater effect in latDS than in NAS neurons, supporting the suggestion that SK function is greater in latDS MSNs than in NAS MSNs.
To further investigate the impact of SK channels on firing in latDS and NAS MSNs, we examined firing during very long (2-s) depolarizing current steps. In particular, we determined the impact of SK inhibition on accommodation, which is an increase in ISI and a decrease in instantaneous firing frequency (IFF) across an AP train (Otto et al., 2006). ISIs were determined for each AP pair in a spike train and converted to IFF values, and the IFF values across the AP train were fitted with a line. The initial and final IFF values were then derived from this line fit. Examples of firing in the second step above rheobase and the line fit of IFF values across an AP train are shown in Fig. 3A–D.
Fig. 3.
Apamin significantly enhanced the instantaneous firing frequency (IFF) in lateral dorsal striatum (latDS) neurons, but had little effect in nucleus accumbens shell (NAS) neurons. (A and B) Sample traces showing action potential (AP) firing during a 2-s depolarization at the second step above rheobase in (A) latDS neurons and (B) NAS neurons. Current steps for the sample traces are 140 and 100 pA for the latDS and NAS examples, respectively. (C and D) Graphs for the examples in A and B showing the IFF for each AP pair across the AP train in (C) latDS neurons and (D) NAS neurons. IFF values across the AP train were fitted with a line, and the initial and final IFF values from the AP train were derived from this line fit. (E and F) Grouped data for initial IFF values from the AP train before and after apamin administration in (E) latDS neurons and (F) NAS neurons. (G and H) Grouped data showing final IFF values from the AP train and initial IFF values before and after apamin administration in (G) latDS neurons and (H) NAS neurons. Data for the initial IFF values are the same as shown in E and F, and error bars are removed for clarity. *P < 0.05 for initial IFF values for basal latDS vs. latDS with apamin and basal NAS. #P < 0.05 for smaller final vs. initial IFF in NAS neurons.
For the initial IFF values, there were significant effects of both apamin and region at the first four depolarizing current steps, with a difference between latDS and NAS neurons before but not after apamin administration (except at the second current step) and an effect of apamin in latDS but not NAS neurons (except at the second current step) (Fig. 3E and F; n = 6 each for latDS and NAS neurons; see Table 1 for statistics). These data are consistent with the firing results above, and suggest that latDS neurons exhibit greater SK function than NAS neurons. In addition, there were no significant differences between the initial and final IFF in latDS neurons (initial vs. final: F1,20 = 0.193, P = 0.679) (Fig. 3G), suggesting little accommodation, which is consistent with what was previously observed in latDS MSNs from young animals (Pineda et al., 1992). Furthermore, in the top three current steps of NAS neurons, there was a significant decrease in the final IFF vs. initial IFF (initial vs. final, F1,20 = 2.935, P = 0.185; current step, F4,20 = 30.456, P < 0.001; initial vs. final × current step, F4,20 = 4.365; P = 0.021; Bonferroni P = 0.046, 0.047 and 0.017 for the top three current steps), with no effect of apamin on the final IFF in NAS neurons (Fig. 3H; Table 1). Thus, accommodation across the spike train was observed for NAS but not latDS neurons, and NAS accommodation was not related to SK function. Nonetheless, these firing rate results support our hypothesis that SK regulation of firing is greater in latDS than in NAS neurons.
Table 1.
Analyses of IFF values before and after apamin administration
| Region |
Apamin |
Region × apamin |
Bonferroni post hoc comparisons, P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Current step | F1,10 | P-value | F1,10 | P-value | F1,10 | P-value | latDS pre- vs. post-apamin |
NAS pre- vs. post- apamin |
Basal latDS vs. NAS |
Apamin latDS vs. NAS |
| Initial IFF values before and after apamin | ||||||||||
| Step 1 | 8.054 | 0.018 | 19.757 | 0.001 | 3.311 | 0.099 | 0.001 | 0.093 | 0.004 | 0.235 |
| Step 2 | 12.276 | 0.006 | 22.937 | < 0.001 | 0.635 | 0.444 | 0.003 | 0.018 | 0.004 | 0.025 |
| Step 3 | 9.186 | 0.013 | 8.003 | 0.018 | 0.995 | 0.342 | 0.022 | 0.225 | 0.007 | 0.063 |
| Step 4 | 5.710 | 0.038 | 11.873 | 0.006 | 0.926 | 0.359 | 0.011 | 0.110 | 0.022 | 0.145 |
| Step 5 | 3.238 | 0.102 | 6.611 | 0.028 | 0.590 | 0.460 | 0.040 | 0.231 | 0.071 | 0.284 |
| Final IFF values before and after apamin | ||||||||||
| Step 1 | 17.868 | 0.002 | 12.153 | 0.006 | 0.033 | 0.860 | 0.027 | 0.042 | 0.003 | 0.004 |
| Step 2 | 2.418 | 0.151 | 10.229 | 0.010 | 0.337 | 0.575 | 0.024 | 0.094 | 0.121 | 0.244 |
| Step 3 | 0.553 | 0.474 | 9.264 | 0.012 | 0.170 | 0.689 | 0.035 | 0.093 | 0.410 | 0.629 |
| Step 4 | 0.344 | 0.570 | 6.219 | 0.032 | 0.001 | 0.987 | 0.106 | 0.111 | 0.588 | 0.596 |
| Step 5 | 0.305 | 0.596 | 4.287 | 0.072 | 0.035 | 0.857 | ND | ND | ND | ND |
P-values < 0.05 are considered significant; ND, not determined; IFF, instantaneous firing frequency.
SK currents under voltage-clamp are significantly greater in latDS than in NAS neurons
To directly examine SK function, we used voltage-clamp methods to isolate SK currents. Neurons were held at −70 mV, depolarized for 400 ms in steps ranging from −60 to −10 mV (with 10 mV between steps), and then brought back to −70 mV. A tail current was evident upon return to −70 mV (Fig. 4A), which may reflect slow deactivation of a channel. Apamin blocked more than 90% of the peak tail current in both latDS and NAS neurons (Fig. 4A and B; n = 9 for latDS neurons, and n = 12 for NAS neurons), suggesting that the tail current evoked in this manner primarily reflects SK currents [see also Hopf et al. (2007)]. In addition, the peak tail current was significantly greater in latDS than in NAS neurons, when determined either as the peak tail current (Fig. 4B; t19 = 2.167, P = 0.043; unpaired t-test) or the apamin-sensitive component of the peak tail current (Fig. 4C; region, F1,32 = 6.522, P = 0.019; voltage, F1,32 = 64.33, P < 0.001; region × voltage, F2,32 = 3.13; P = 0.058). Thus, both voltage-clamp and current-clamp measures demonstrated reduced SK function in latDS neurons relative to NAS neurons. In addition, there was no difference in the decay constant τ of inactivation of the tail current (latDS, 78.1 ± 8.5 ms; NAS, 90.2 ± 9.2 ms; t19 = 0.934; P = 0.362; unpaired t-test) or in the whole-cell capacitance (latDS, 94.5 ± 10.1 pF; NAS, 89.1 ± 5.8 ms; t19 = 0.330; P = 0.745; un-paired t-test), suggesting that differences in SK function magnitude were not due to differences in these kinetic parameters.
SK currents regulate induction of synaptic plasticity in the latDS but not in the NAS
Greater SK function in the latDS may significantly modulate the induction of synaptic plasticity, as observed in other brain regions (Behnisch & Reymann, 1998; Stackman et al., 2002; Faber et al., 2005), and DS plasticity may be critical for habit and motor learning (Suri et al., 2001; Gerdeman et al., 2003). Thus, we recorded fEPSP/PSs in both latDS and NAS neurons, and examined whether SK inhibition by apamin altered the development of LTD induced by HFS (50 Hz for 2 s, repeated four times, with 15 s between initiation of trains), where 50 Hz approximates the maximal firing in the latDS during a procedural task (Barnes et al., 2005). If SKs influence plasticity, then apamin should modulate the development of plasticity in the latDS but not the NAS.
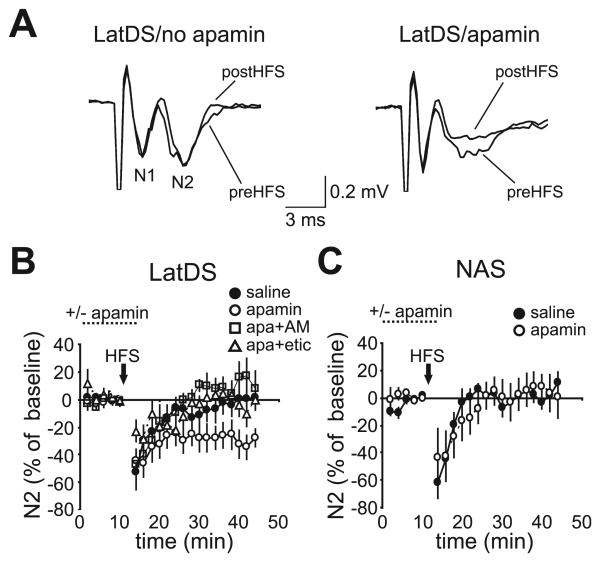
HFS induced a transient depression in the fEPSP/PS amplitude that lasted for 5–10 min in both latDS and NAS neurons (Fig. 5B and C), as has been reported in the DS (Lovinger & Choi, 1995). Exposure to apamin during HFS resulted in persisting LTD in latDS neurons (Fig. 5A and B; n = 10), relative to no LTD in the absence of apamin [Fig. 5A and B; n = 10, F3,34 = 4.324, P = 0.011; one-way anova comparing saline, apamin, apamin plus AM251, and apamin plus eticlopride; P = 0.021 (Bonferroni) for LTD with and without apamin]. In contrast, apamin did not promote LTD development in NAS neurons (Fig. 5C; n = 7 each for apamin and no apamin; t12 = 0.613, P = 0.551; unpaired t-test). Apamin had no effect on fEPSP/PS amplitude alone (latDS, 2 ± 3% change in fEPSP/PS, t9 = 0.286, P = 0.781; NAS, 1 ± 2% change in fEPSP/PS, t6 = 0.109, P = 0.917; both paired t-test), suggesting that SKs did not directly regulate glutamate release [see also Behnisch & Reymann (1998)]. Thus, apamin facilitated the induction of LTD in only latDS neurons, in agreement with greater SK regulation of firing in the latDS than in the NAS. The differential effects of apamin might also be due to different LTD induction mechanisms in the DS and the NAS (Thomas et al., 2000; Gerdeman et al., 2003). However, apamin-facilitated LTD in latDS neurons was prevented by pre-exposure to the CB1 receptor antagonist AM251 [Fig. 5B; 2 μm, n = 7, P = 0.009 (Bonferroni) vs. apamin alone] or to the D2 receptor antagonist eticlopride [Fig. 5B; 1 μm, n = 11, P = 0.020 (Bonferroni) vs. apamin alone], suggesting that apamin-facilitated LTD may be mechanistically similar to that observed in the DS by a number of groups (Gerdeman et al., 2003).
Fig. 5.
Small-conductance calcium-dependent potassium channel inhibition enhanced long-term depression (LTD) induction in lateral dorsal striatum (latDS) but not in nucleus accumbens shell (NAS) neurons. (A) Sample traces of field excitatory postsynaptic potential/population spikes (fEPSP/PSs) in latDS neurons recorded before and after high frequency stimulation (HFS) (50 Hz for 2 s, repeated four times, with 15 s between initiation of trains). N2 indicates the postsynaptic field potential, and N1 indicates the presynaptic fiber volley, which was not altered during induction of synaptic plasticity (data not shown) [see also Behnisch & Reymann (1998) and Stackman et al. (2002)]. (A–C) Apamin facilitated the development of HFS-induced LTD in latDS neurons (A and B) but not in NAS neurons (C). In addition, apamin (apa)-facilitated LTD in latDS neurons was inhibited by the CB1 receptor antagonist AM251 (AM) (B) (2 μm) and the dopamine D2 receptor antagonist eticlopride (etic) (B) (1 μm).
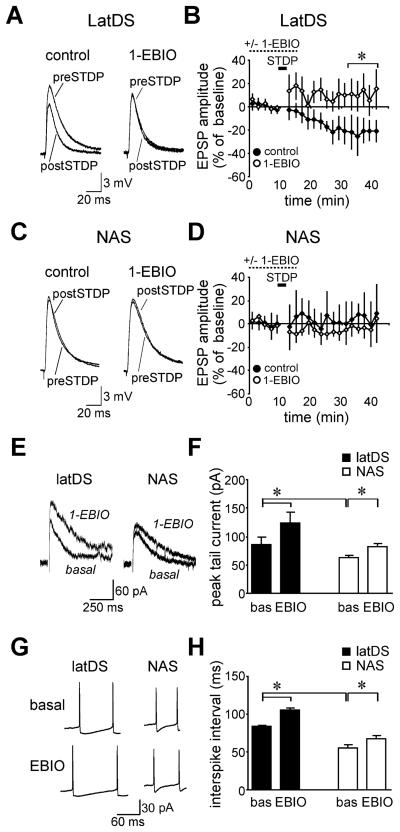
We next investigated whether activation of SKs might prevent the induction of latDS LTD. We found that 100-Hz stimulation alone was insufficient to induce LTD (data not shown), in contrast to other studies (e.g. Partridge et al., 2000), which could reflect the fact that the present experiments were performed in adult Long Evans rats, whereas other studies are generally performed in younger Sprague–Dawley rats. Instead, we found that LTD could be reliably induced in the adult latDS with use of an STDP (see legend to Fig. 6). Striatal LTD induced via an STDP can be blocked by antagonists of the D2 receptor or the CB1 receptor (Shen et al., 2008) [but see Pawlak & Kerr (2008)], suggesting that it may have some mechanistic similarities to previously described striatal LTD. Importantly, we found that pre-exposure to the SK positive modulator 1-EBIO (0.6 mm) (Pedarzani et al., 2005) prevented the induction of LTD (Fig. 6A and B; n = 7 and n = 6 for the STDP in the presence and absence of 1-EBIO; t11 = 3.241; P = 0.008; unpaired t-test). In addition, the STDP did not result in LTD in NAS neurons in the presence or absence of 1-EBIO (Fig. 6C and D; n = 4 and n = 6 in the presence and absence of 1-EBIO; t8 = 1.091; P = 0.307; unpaired t-test). Also, 1-EBIO alone did not significantly alter the EPSP amplitude in latDS neurons −5.8 ± 6.5% change in EPSP; t6 = 1.441; P = 0.200; paired t-test) or NAS neurons (6.1 ± 7.2% change in EPSP; t3 = 0.992; P = 0.499; paired t-test). Thus, LTD was not observed in NAS nerons in the presence or absence of SK activators, but activation of SKs in latDS neurons prevented the induction of LTD, supporting a role for SK in the regulation of latDS LTD.
Fig. 6.
(A and B) Examples (A) and grouped data (B) showing that long-term depression (LTD) induction in lateral dorsal striatum (latDS) neurons using a spike-timing-dependent protocol (STDP) was prevented by pre-exposure to the small-conductance calcium-dependent potassium channel (SK)-positive modulator 1-ethyl-2-benzimidazolinone (1-EBIO) (0.6 mm) (significance determined for the average percentage change across the last 10 min of the experiment). The STDP consisted of 20 bouts of excitatory postsynaptic potential (EPSP)-spike pairs delivered 5 s apart. Each bout consisted of a burst of five paired stimuli delivered at 100-ms intervals, with the onset of EPSPs following the peak of the postsynaptic action potential by 15 ms. Sufficient current was used to evoke action potentials (APs) during STDP stimulation, and this current was identical in latDS and nucleus accumbens shell (NAS) neurons. (C and D) The STDP did not induce LTD in NAS neurons in the presence or absence of 1-EBIO. (E and F) 1-EBIO increased the magnitude of the SK tail current in both latDS and NAS neurons, with a greater effect in latDS neurons. (G and H) 1-EBIO increased the magnitude of the interspike interval (ISI) in both latDS and NAS neurons, with a greater effect in latDS neurons. bas, baseline; EBIO, 1-EBIO. ISIs were determined at the current step with two APs. The sample ISI traces are for the 200-pA current step for NAS neurons and the 260-pA and 300-pA current steps for latDS neurons before and after 1-EBIO administration, respectively. *P < 0.05.
To better understand the impact of 1-EBIO on LTD generation, we examined the effect of 0.6 mm 1-EBIO on SK tail currents under voltage-clamp and on ISIs during AP firing (determined at the current step with two APs). 1-EBIO increased the peak SK tail current in both latDS and NAS neurons, with larger basal SK tail currents in latDS than in NAS neurons and a larger change in tail current with 1-EBIO in latDS neurons (Fig. 6E and F; n = 5 for latDS neurons and n = 6 for NAS neurons; region, F1,9 = 5.29, P = 0.047; apamin, F1,9 = 61.43, P < 0.001; region × apamin, F1,9 = 5.96, P = 0.037). Similar to what was observed for the tail current, 1-EBIO increased the ISI in both latDS and NAS neurons, with greater basal ISIs in latDS neurons and a greater 1-EBIO-mediated increase in ISIs in latDS neurons (Fig. 6G and H; F1,9 = 69.85, P < 0.001; apamin, F1,9 = 73.40, P < 0.001; region × apamin, F1,9 = 5.84, P = 0.039). Finally, 1-EBIO decreased AP generation to a greater extent in latDS neurons (78.3 ± 7.1% reduction in firing) than in NAS neurons (41.8 ± 5.0% reduction in firing, P = 0.002). A greater impact of SK activators in latDS neurons is in agreement with the results above showing a greater impact of SK inhibition by apamin on firing in latDS neurons, and supports the hypothesis that SK regulation of firing is greater in latDS than in NAS neurons. Thus, 1-EBIO enhanced SK function in both latDS and NAS neurons, with a greater effect in latDS neurons, and only SK activation in the latDS-modulated synaptic plasticity.
Enhanced SK regulation of firing in latDS neurons is not due to functional differences in sodium or IRK currents, or to indirect actions of apamin through cholinergic neurons
The previously discussed AP firing and LTD data suggest that SK function was significantly greater in latDS neurons than in NAS neurons. However, functional differences in other currents could also underlie the reduced AP generation in latDS neurons. In particular, AP amplitudes were significantly greater in latDS than in NAS neurons (Figs 1A and 7A and B; latDS, 88.5 ± 0.6 mV; NAS, 75.5 ± 1.0 mV; n = 52 and n = 33, respectively; t83 = 11.850; P < 0.0001; unpaired t-test), and AP thresholds were significantly hyperpolarized (latDS, −58.8 ± 0.5 mV; NAS, −51.4 ± 0.7 mV; t83 = 8.482; P < 0.001; unpaired t-test), suggesting that sodium channel function was greater in latDS than in NAS neurons (Zhang et al., 1998). In addition, partial inhibition of sodium channels with a low concentration of TTX (15–25 nm, n = 5) (D'Ascenzo et al., 2009) reduced the AP amplitude of latDS neurons to levels seen in NAS neurons [Fig. 7A and B; F2,40 = 8.365, P < 0.001, one-way anova comparing the basal NAS level and the latDS level before and after TTX administration; P = 0.164 (Bonferroni) comparing the latDS level after TTX and the basal NAS level]. TTX also elevated the latDS AP threshold (pre-TTX, −58.4 ± 0.6 mV; post-TTX, −52.9 ± 1.4 mV; t4 = 3.575, P = 0.023; paired t-test) and the latDS current required to fire APs (pre-TTX, 280 ± 17 pA; post-TTX, 320 ± 11 pA; t4 = 3.162, P = 0.034; paired t-test). However, TTX did not alter the ISI (Fig. 7C) or the input–output slope (Fig. 7D) in latDS neurons (t4 = 0.293, P = 0.784, and t4 = 1.500, P = 0.208, for ISI and input–output slope, respectively; paired t-test). These latter measures were highly sensitive to apamin (Fig. 1B and G), suggesting that greater sodium channel activity was not responsible for the greater SK function and reduced AP generation in latDS neurons.
Fig. 7.
Enhanced small-conductance calcium-dependent potassium channel (SK) regulation of firing in lateral dorsal striatum (latDS) neurons was not a consequence of greater sodium channel function. (A–D) The action potential (AP) amplitude was greater in latDS than in nucleus accumbens shell (NAS) neurons (A and B), and latDS AP amplitudes were reduced to NAS levels with low concentrations of tetrodotoxin (TTX) (15–25 nm) (A and B). However, latDS TTX administration did not alter the interspike interval (C) or input–output slope (D) in latDS neurons, indicating that the greater SK function in latDS neurons was not secondary to greater sodium channel function. (E and F) Representative examples of a phase plot of mV/ms vs. mV across the potential trajectory in latDS and NAS neurons before and after exposure to apamin. bas, baseline. *Significantly different from control latDS neurons, P < 0.05.
We also generated phase plots showing the mV/ms versus mV during the AP waveform to further compare the AP waveform in latDS and NAS neurons and to examine the impact of SK on the AP waveform. As shown in representative examples in Fig. 7E and F, NAS neurons (Fig. 7F) had a more depolarized AP threshold and a smaller AP amplitude than latDS neurons (Fig. 7E), and SK inhibition by apamin did not alter the AP waveform in either region. These data support our AP waveform analyses above suggesting that parameters previously associated with sodium channels (Zhang et al., 1998) are reduced in NAS neurons as compared with latDS neurons, and that these parameters are not related to differences in SK. These results also suggest that apamin inhibition of SK does not alter the AP waveform.
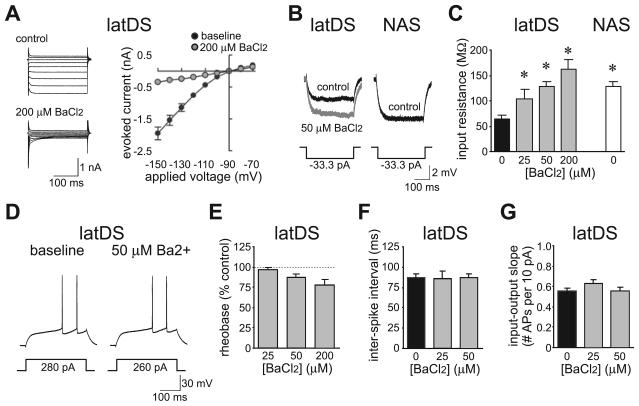
LatDS neurons also exhibited a smaller input resistance than NAS neurons, measured with a 33.3-pA hyperpolarizing step (Fig. 8B and C; latDS, 57.7 ± 3.5 mΩ; NAS, 121.6 ± 10.1 mΩ; n = 52 and n = 33; t83 = 6.977; P < 0.001; unpaired t-test), and had a higher rheobase, the minimum current required to generate an AP (latDS, 283 ± 14 pA; NAS, 239 ± 16 pA; t83 = 2.011; P = 0.048; unpaired t-test). The majority of currents activated during hyperpolarizing steps under voltage-clamp were sensitive to 200 μm BaCl2 (Fig. 8A; n = 4; t3 = 7.952; P = 0.004; paired t-test), suggesting a primary contribution of IRKs rather than potassium leak channels to the resting input resistance in latDS neurons (Nisenbaum & Wilson, 1995). Thus, we used low concentrations of BaCl2 to examine the contribution of IRKs to firing and to determine whether the greater ISI and sensitivity to apamin in latDS neurons were due to differences in IRK function. For these experiments, BaCl2 depolarized the neuron, and thus the resting membrane potential was returned to approximately −90 mV before determination of input resistance and parameters related to firing. BaCl2 dose-dependently enhanced the input resistance of latDS neurons [Fig. 8B and C; n = 5, n = 7 and n = 5 for 25, 50 and 200 μm BaCl2, respectively; F3,23 = 11.135, P < 0.001, one-way anova comparing across doses of BaCl2; P = 0.047 and P < 0.001 (Bonferroni) for 50 and 200 μm BaCl2, respectively, vs. no BaCl2], with 50 μm BaCl2 producing an input resistance in latDS neurons similar to that seen in NAS neurons (Fig. 8C). There was also a trend for a reduction in rheobase in latDS neurons with BaCl2 (Fig. 8D and E; F2,14 = 3.159, P = 0.074). However, 50 μm BaCl2 did not significantly alter the ISI (Fig. 8D and F) or the input–output slope (Fig. 8G) in latDS neurons (t6 = 0.785, P = 0.462, and t6 = 1.369, P = 0.220, for ISI and input–output slope, respectively; paired t-test). Thus, greater IRK activity in latDS neurons did increase the current required to generate APs, but the strong SK function was primarily responsible for the long ISI and low AP generation observed in latDS neurons once sufficient current was applied to generate APs.
Fig. 8.
Greater inwardly rectifying potassium channel (IRK) function in lateral dorsal striatum (latDS) neurons did not account for greater small-conductance calcium-dependent potassium channel (SK) regulation of firing. (A) Voltage-clamp experiments showing that the majority of current activated by hyperpolarization was sensitive to 200 μm BaCl2, and therefore was carried by IRKs. Neurons were held at −90 mV, and depolarized or hyperpolarized with 250-ms pulses ranging from −70 to −150 mV in 10-mV steps. (B–G) The input resistance was significantly greater in latDS than in nucleus accumbens shell (NAS) neurons (B and C), and BaCl2 dose-dependently enhanced the input resistance of latDS neurons (C), with a trend for a reduction in the current required for firing (rheobase) (D and E). However, increasing the input resistance of latDS neurons to the levels seen in NAS neurons (with 50 μm BaCl2) did not alter the interspike interval (D and F) or input–output slope (G) in latDS neurons, indicating that the greater SK function in latDS neurons was not secondary to greater IRK function. *Significantly different from control latDS, P < 0.05.
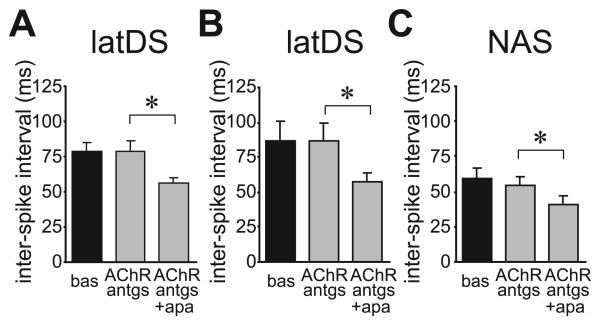
Finally, SK inhibition enhances the activity of striatal cholinergic interneurons (Bennett et al., 2000), and thus apamin might enhance MSN firing indirectly by enhancing the release of acetylcholine (ACh) and altering MSN firing through activation of nicotinic ACh receptors or muscarinic ACh receptors, respectively (e.g. Perez-Rosello et al., 2005). However, apamin reduction of latDS ISI was not prevented by pre-exposure to muscarinic ACh receptor and nicotinic ACh receptor antagonists, either with scopolamine (3 μm) and tubocurarine (50 μm) in combination (Fig. 9A; n = 5; t4 = 4.062; P = 0.015; paired t-test) or with scopolamine (3 μm), methyllycaconitine (100 nm) and dihydro-β-erythroidine (1 μm) in combination (Fig. 9B; n = 5; t4 = 2.902; P = 0.044; paired t-test). The latter cocktail also did not prevent the apamin-induced reduction in NAS ISI (Fig. 9C; n = 4; t3 = 4.587; P = 0.020; paired t-test). As these ACh receptor antagonists did not have any effects on firing alone, and did not prevent the apamin-induced reduction in ISI in latDS neurons, these results support the suggestion that apamin did not facilitate latDS firing indirectly through activation of cholinergic interneurons.
Fig. 9.
Inhibition of muscarinic acetylcholine receptors (mAChRs) and nicotinic acetylcholine receptors (nAChRs) did not prevent the apamin-dependent reduction in the interspike interval (ISI). (A) Inhibition of mAChRs and nAChRs with scopolamine (3 μm) and tubocurarine (50 μm) did not prevent the reduction in ISI upon subsequent addition of apamin. (B and C) Inhibition of mAChRs with scopolamine (3 μm) and and of nAChRs with methyllycaconitine (100 nm) and dihydro-β-erythroidine (1 μm) in a cocktail did not prevent the reduction in ISI upon subsequent addition of apamin in the lateral dorsal striatum (latDS) neurons (B) or nucleus accumbens shell (NAS) neurons (C). antgs, antagonists; apa, apamin; bas, baseline. *P < 0.05.
Discussion
The present study shows that SK channels are key modulators of AP generation and LTD in the adult rat latDS. In particular, firing rates in latDS neurons were significantly depressed by SKs, which enhanced the interval between APs, and prolonged depolarization to −70 mV dramatically enhanced SK function and reduced AP generation in the latDS. In contrast, NAS neurons exhibited less SK function, as indicated by greater basal firing rates and decreased regulation of firing by apamin or by holding at −70 mV. Direct measurement of SK function with voltage-clamp showed greater SK currents in latDS than in NAS neurons. Importantly, the development of LTD was facilitated by apamin in latDS but not in NAS neurons, with no alterations in glutamate release. In addition, SK activators produced a greater enhancement of SK tail currents and inhibition of firing in latDS neurons, and SK activation in latDS neurons prevented the development of LTD, whereas LTD was not observed in NAS neurons in the presence or absence of SK activators. Finally, differential SK function in latDS and NAS neurons could not be attributed to differences in sodium channels or IRKs, and apamin enhancement of firing was not prevented by ACh receptor antagonists, suggesting no indirect effects through cholinergic interneurons. We hypothesize that SK depression of AP generation and LTD in the latDS may represent an important cellular mechanism regulating latDS-dependent habit-based behaviors.
Greater function of SK currents in latDS neurons, indicated by greater sensitivity of latDS firing to both SK inhibitors and SK activators, is likely to be of great physiological significance, as SK currents significantly retard repetitive spike firing in many types of neuron (Bennett et al., 2000), including those in the DS but not the NAS from the young rat (Pineda et al., 1992; Vilchis et al., 2000; Ishikawa et al., 2009). Furthermore, prolonged depolarization to −70 mV greatly enhanced SK function [see also Rutherford et al. (1988)], perhaps owing to enhanced calcium current activation during holding at −70 mV (Hernandez-Lopez et al., 1997). Several groups have suggested that prolonged depolarization may mimic the depolarized up-state (Hernandez-Lopez et al., 1997; Kreitzer & Malenka, 2005), lasting hundreds of milliseconds to seconds in vivo (Wilson & Kawaguchi, 1996). Also, in response to salient cues, striatal neurons can exhibit sustained increases in firing lasting more than 1 s (Ghitza et al., 2003; Schultz et al., 2003; Nicola et al., 2004; Barnes et al., 2005; Wheeler & Carelli, 2009). Thus, our results suggest that the greater SK function in latDS neurons would be especially potent in decreasing firing rate under conditions of prolonged depolarization, for example during stimulus-related phasic firing in vivo, whereas NAS neurons would be able to fire bursts at a higher rate with less dependence on the history of previous depolarization, owing to the relative lack of SK function.
The observation of increased SK function in latDS neurons relative to NAS neurons, which has also been observed in the NAcb core relative to the NAS (Hopf et al., 2010), leaves open the question of whether greater SK function is caused increased SK function or protein levels, or by increased flux through calcium currents that activate SKs (Vilchis et al., 2000). There are three classes of SK subunit, SK1, SK2, and SK3, and the striatum contains high levels of SK3 and more moderate levels of SK1 and SK2 (Stocker, 2004). Varied SK function could be due to differential expression of these subunits among the striatal subregions, although other factors, such as differential channel distribution within a neuron (Kerr & Plenz, 2002; Day et al., 2008) or differences in calcium influx, could also contribute. The relative distribution of different calcium channels in the latDS and the NAS is more difficult to assess, as previous studies have shown that individual neurons possess a number of different calcium channel types, with some diversity among cells in the particular complement of channels, and with no clear differences between the DS and the NAS (Hoehn et al., 1993; Bargas et al., 1994; Churchill & Macvicar, 1998), although regional differences in calcium channel mRNAs have been observed (Olsen et al., 2008). Also, only certain types of calcium current activate SKs in the DS (Vilchis et al., 2000; Perez-Garci et al., 2003), although the type of calcium current that activates SKs may vary among different types of neurons (Marrion & Tavalin, 1998). Regardless of the underlying mechanisms, there were clear differences in SK currents and regulation of AP firing rates in latDS neurons relative to NAS neurons that significantly impacted on AP generation and LTD induction. In addition, there were basal differences between latDS and NAS neurons in sodium channel and IRK function, but these did not account for the greater SK regulation of firing in latDS neurons, and apamin did not alter the AP trajectory in either latDS or NAS neurons. In addition, the ability to fire at low firing frequencies seen here and the curved frequency–current relationship with higher current intensities observed by other groups (e.g. Pineda et al., 1992; Ishikawa et al., 2009) indicate that MSNs act as integrators (Izhikevich, 2000). Furthermore, accommodation during longer AP trains was observed for NAS but not latDS neurons, although this NAS accommodation was not altered by SK inhibition with apamin. Although future experiments will be required to gain further insights into differences in ion channel function and firing properties between latDS and NAS neurons, our results support the hypothesis that SKs are prominent regulators of firing in latDS but not NAS neurons.
Our results are the first to demonstrate that SK inhibition in the latDS facilitates the development of LTD, and SK activation prevents induction of latDS LTD via an STDP, suggesting that SKs can significantly regulate latDS plasticity in addition to enhancing latDS firing. In contrast, SK inhibition or activation does not enable plasticity induction in the NAS. SK regulates LTD and long-term potentiation induction in other brain regions, including the hippocampus (Behnisch & Reymann, 1998; Stackman et al., 2002; Faber et al., 2005), and SK inhibition enhances performance and learning in hippocampal-dependent tasks (Stackman et al., 2002) and other tasks (Messier et al., 1991). This raises the interesting possibility that SK currents in the latDS may modulate learning or expression of habit-based tasks. Although the behavioral role of striatal LTD has not been examined directly, NAcb LTD is required for the expression of behavioral locomotor sensization (Brebner et al., 2005). In addition, several studies have hypothesized a key role for synaptic plasticity in corticostriatal synapses for sensory, motor and habit learning (Suri et al., 2001; Gerdeman et al., 2003). However, skill learning can occur independently from the DS (Atallah et al., 2007), underscoring the potential complexity of the striatal contribution to different behaviors.
We should note that potassium channels other than SKs can also potently contribute to the regulation of striatal firing and plasticity. In this regard, Wickens et al. (1998) have demonstrated that bath application of potassium channel inhibitors can convert striatal LTD to long-term potentiation. Interestingly, intracellular perfusion of these potassium channel inhibitors significantly alters firing but does not alter plasticity. This suggests that bath-applied potassium channel blockers alter plasticity through a presynaptic mechanism, and that gross inhibition of potassium channels, which enhances neuronal firing, does not necessarily alter plasticity induction. Here, we found that SK inhibition facilitated latDS LTD induction but did not alter glutamate release, and thus that postsynaptic MSN SKs are more likely to contribute to the regulation of latDS LTD. However, the exact mechanism whereby SK inhibition facilitates LTD induction is still uncertain. SK inhibition could enhance depolarization in dendrites and thus modulate the induction of synaptic plasticity (Faber et al., 2005), and SK inhibition can also enhance endocannabinoid production through a postsynaptic mechanism (Riegel & Lupica, 2004). Although future experiments will be required to address these possibilities, which are not mutually exclusive, our studies agree with those on other brain regions suggesting that SKs can be potent regulators of synaptic plasticity.
Although we have shown significant SK regulation of firing and LTD in latDS MSNs, apamin might alter firing through targets other than MSNs. However, apamin did not directly alter fEPSP/PSs, suggesting no SK regulation of glutamate release [see also Behnisch & Reymann (1998)], from the hippocampus. In addition, antagonists of ACh receptors did not alter the apamin enhancement of latDS firing, suggesting that apamin was not acting by enhancing firing of striatal cholinergic interneurons (Bennett et al., 2000) and ACh release, and was only secondarily increasing firing in MSNs (Perez-Rosello et al., 2005).
DS MSNs are composed of two main populations (direct and indirect pathway neurons) (Gerfen, 2004; Surmeier et al., 2007), and recent studies from BAC-transgenic mice have suggested differential excitability in these two populations (Kreitzer & Malenka, 2007; Shen et al., 2007; Cepeda et al., 2008; Gertler et al., 2008). Here, measures of excitability that were strongly influenced by SK function (ISI and input–output slope; Figs 1 and 2) showed unimodal Gaussian distributions (Supporting information, Fig. S1). These results suggest that, if there were multiple populations of neurons regarding these excitability measures, then average values in the two populations would be relatively similar. Differences from previous studies could reflect species, age or subregion differences. However, our results concur with other in vitro studies suggesting that some aspects of intrinsic excitability of DS MSNs, including basal potassium channel function (Kreitzer & Malenka, 2007; Shen et al., 2007; Cepeda et al., 2008) [but see Gertler et al. (2008)] and calcium channel function (e.g. Mermelstein et al., 1998), can, on average, be similar in both cell populations.
Given that indirect and direct pathway neurons contain different complements of dopamine receptors (Gerfen, 2004; Surmeier et al., 2007), this also raises the question of how the D2 receptor antagonist could prevent apamin-induced LTD in all latDS neurons. In this regard, Wang et al. (2006) reported that LTD could be evoked in both direct and indirect pathway neurons, and LTD in both types of neurons was sensitive to D2 receptor antagonists; they attributed this to action through cholinergic interneurons. In contrast, Kreitzer & Malenka (2007) reported that LTD was predominantly generated in indirect pathway neurons. In addition, Kreitzer & Malenka (2007) suggested that the relatively moderate LTD observed in several studies, and that we observed in the present study, could reflect the fact that data from indirect and direct pathway neurons were combined. In fact, we found that LTD > 20% occurred in only six of 10 latDS neurons treated with apamin. Thus, one possible explanation for eticlopride seeming to block LTD in all neurons tested instead could be that robust LTD could be generated in only about half of the neurons treated with apamin.
Results from a number of studies have suggested that phasic firing in the DS may contribute to the initiation and execution of goal-directed and habit-based behaviors (Alexander, 1987; Schultz et al., 2003; Barnes et al., 2005). Regulation of habits by regions such as the latDS is of great interest because of the proposed dysregulation of habitual control over behavior in several important human pathological conditions, including obsessive-compulsive disorder and drug addiction (Graybiel, 1998; Berke & Hyman, 2000; Packard & Knowlton, 2002; Everitt & Robbins, 2005; Vanderschuren et al., 2005; Fuchs et al., 2006). In addition, the DS can mediate responsiveness to reward-associated cues that can drive behavior (Vanderschuren et al., 2005). The NAcb is also implicated in the processing of reward-predicting stimuli that guide learning and behavior, including addictive behavior (Cardinal et al., 2002; Kalivas & McFarland, 2003; Wheeler & Carelli, 2009), with a particular role for the NAS in processing novel stimuli and primary reinforcement (Cardinal et al., 2002). Thus, both the NAcb and the latDS can regulate goal-directed behaviors, with the latDS having a particular role in habitual behaviors, including addiction. As striatal firing could contribute to the regulation of many behaviors (Ghitza et al., 2003; Schultz et al., 2003; Nicola et al., 2004; Barnes et al., 2005), it is of great interest to determine the cellular mechanisms that control striatal AP firing.
In conclusion, SKs significantly and potently reduced AP firing in adult rat latDS neurons relative to NAS neurons. The SK-induced reduction of firing was particularly enhanced during prolonged depolarization, and may contribute to physiologically relevant sustained depolarizing episodes such as stimulus-driven phasic firing (Schultz et al., 2003; Barnes et al., 2005). Furthermore, SK inhibition in the latDS facilitated the development of LTD, and SK activation depressed latDS LTD. Thus, we speculate that SK depression of AP generation and LTD in the latDS may represent an important regulator of latDS-dependent habits, and that activation of SKs might represent a potential therapy to reduce the control of habits over behavior in conditions such as obsessive-compulsive disorder and drug addiction.
Supplementary Material
Acknowledgements
This work was supported by NIAAA RO1AA015358 (F. W. Hopf) and by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (A. Bonci). We thank D. Harada and G. Brush for custom-designed analysis software, and M. S. Bowers, S. Nicola and G. Stuber for constructive comments.
Abbreviations
- 1-EBIO
1-ethyl-2-benzimidazolinone
- ACh
acetylcholine
- ACSF
artificial cerebrospinal fluid
- AHP
afterhyperpolarization
- AP
action potential
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DC
direct current
- DS
dorsal striatum
- EPSP
excitatory postsynaptic potential
- fEPSP
field excitatory postsynaptic potential
- HFS
high-frequency stimulation
- IFF
instantaneous firing frequency
- IRK
inwardly rectifying potassium channel
- ISI
interspike interval
- latDS
lateral dorsal striatum
- LTD
long-term depression
- MSN
medium spiny neuron
- NAcb
nucleus accumbens
- NAS
nucleus accumbens shell
- PS
population spike
- SK
small-conductance calcium-dependent potassium channel
- STDP
spike-timing-dependent protocol
- TTX
tetrodotoxin
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Two measures of latDS excitability, the ISI and input–output slope, exhibited a unimodal distribution, apparent in both distributions of the number of cells and the cumulative number of cells.
Please note: As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset by Wiley-Blackwell. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Abel HJ, Lee JC, Callaway JC, Foehring RC. Relationships between intracellular calcium and after hyperpolarizations in neocortical pyramidal neurons. J. Neurophysiol. 2004;91:324–335. doi: 10.1152/jn.00583.2003. [DOI] [PubMed] [Google Scholar]
- Alexander GE. Selective neuronal discharge in monkey putamen reflects intended direction of planned limb movements. Exp. Brain Res. 1987;67:623–634. doi: 10.1007/BF00247293. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Lopez-Paniagua D, Rudy JW, O'Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat. Neurosci. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Bargas J, Howe A, Eberwine J, Cao Y, Surmeier DJ. Cellular and molecular characterization of Ca2+ currents in acutely isolated, adult rat neostriatal neurons. J. Neurosci. 1994;14:6667–6686. doi: 10.1523/JNEUROSCI.14-11-06667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargas J, Ayala GX, Vilchis C, Pineda JC, Galarraga E. Ca2+-activated outward currents in neostriatal neurons. Neuroscience. 1999;88:479–488. doi: 10.1016/s0306-4522(98)00211-5. [DOI] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Behnisch T, Reymann KG. Inhibition of apamin-sensitive calcium dependent potassium channels facilitates the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci. Lett. 1998;253:91–94. doi: 10.1016/s0304-3940(98)00612-0. [DOI] [PubMed] [Google Scholar]
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J. Neurophysiol. 2000;84:2204–2216. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J. Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, O'Donnell P. D2 dopamine modulation of corticoaccumbens synaptic responses changes during adolescence. Eur. J. Neurosci. 2008;27:1364–1372. doi: 10.1111/j.1460-9568.2008.06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri N, Stanzione P, Stefani A, Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Chandler SH, Shumate LW, Levine MS. Persistent Na+ conductance in medium-sized neostriatal neurons: characterization using infrared videomicroscopy and whole cell patch-clamp recordings. J. Neurophysiol. 1995;74:1343–1348. doi: 10.1152/jn.1995.74.3.1343. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur. J. Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Churchill D, Macvicar BA. Biophysical and pharmacological characterization of voltage-dependent Ca2+ channels in neurons isolated from rat nucleus accumbens. J. Neurophysiol. 1998;79:635–647. doi: 10.1152/jn.1998.79.2.635. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Podda MV, Fellin T, Azzena GB, Haydon P, Grassi C. Activation of mGluR5 induces spike afterdepolarization and enhanced excitability in medium spiny neurons of the nucleus accumbens by modulating persistent Na+ currents. J. Physiol. 2009;587:3233–3250. doi: 10.1113/jphysiol.2009.172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J. Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat. Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate–putamen. J. Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal ganglia. In: Paxinos G, editor. The Rat Nervous System. 3rd edn. Elsevier Academic Press; San Diego: 2004. pp. 455–508. [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J. Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J. Neurosci. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-31/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J. Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn Sinauer Associates, Inc.; Sunderland, MA: 2001. [Google Scholar]
- Hoehn K, Watson TW, MacVicar BA. Multiple types of calcium channels in acutely isolated rat neostriatal neurons. J. Neurosci. 1993;13:1244–1257. doi: 10.1523/JNEUROSCI.13-03-01244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J. Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J. Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, Cho SL, Tye K, Bonci A. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron. 2010;65:682–694. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schlüter OM, Dong Y. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J. Neurosci. 2009;29:5820–5831. doi: 10.1523/JNEUROSCI.5703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM. Neural excitability, spiking and bursting. Int. J. Bifurcat. Chaos. 2000;10:1171–1266. [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharm. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J. Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. J. Neurosci. 2002;22:1499–1512. doi: 10.1523/JNEUROSCI.22-05-01499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J. Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Choi S. Activation of adenosine A1 receptors initiates short-term synaptic depression in rat striatum. Neurosci. Lett. 1995;199:9–12. doi: 10.1016/0304-3940(95)12024-x. [DOI] [PubMed] [Google Scholar]
- Mahon S, Casassus G, Mulle C, Charpier S. Spike-dependent intrinsic plasticity increases firing probability in rat striatal neurons in vivo. J. Physiol. 2003;550:947–959. doi: 10.1113/jphysiol.2003.043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+ -activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Song WJ, Tkatch T, Yan Z, Surmeier DJ. Inwardly rectifying potassium (IRK) currents are correlated with IRK subunit expression in rat nucleus accumbens medium spiny neurons. J. Neurosci. 1998;18:6650–6661. doi: 10.1523/JNEUROSCI.18-17-06650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier C, Mourre C, Bontempi B, Sif J, Lazdunski M, Destrade C. Effect of apamin, a toxin that inhibits Ca2+ -dependent K+ channels, on learning and memory processes. Brain Res. 1991;551:322–326. doi: 10.1016/0006-8993(91)90950-z. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J. Neurophysiol. 2004;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13:135–160. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Huang Y, Goodwin S, Ciobanu DC, Lu L, Sutter TR, Winder DG. Microarray analysis reveals distinctive signaling between the bed nucleus of the stria terminalis, nucleus accumbens, and dorsal striatum. Physiol. Genomics. 2008;32:283–298. doi: 10.1152/physiolgenomics.00224.2006. [DOI] [PubMed] [Google Scholar]
- Otto JF, Yang Y, Frankel WN, White HS, Wilcox KS. A spontaneous mutation involving Kcnq2 (Kv7.2) reduces M-current density and spike frequency adaptation in mouse CA1 neurons. J. Neurosci. 2006;26:2053–2059. doi: 10.1523/JNEUROSCI.1575-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J. Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, Strobaek D, Stocker M. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current I(AHP) and modulates the firing properties of hippocampal pyramidal neurons. J. Biol. Chem. 2005;280:41404–41411. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Dolleman-Van der Weel MJ, Lopes da Silva FH. Differential membrane properties and dopamine effects in the shell and core of the rat nucleus accumbens studied in vitro. Neurosci. Lett. 1992;136:109–112. doi: 10.1016/0304-3940(92)90660-y. [DOI] [PubMed] [Google Scholar]
- Perez-Garci E, Bargas J, Galarraga E. The role of Ca2+ channels in the repetitive firing of striatal projection neurons. Neuroreport. 2003;14:1253–1256. doi: 10.1097/00001756-200307010-00013. [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E, Bargas J. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J. Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- Pineda JC, Galarraga E, Bargas J, Cristancho M, Aceves J. Charybdotoxin and apamin sensitivity of the calcium-dependent repolarization and the afterhyperpolarization in neostriatal neurons. J. Neurophysiol. 1992;68:287–294. doi: 10.1152/jn.1992.68.1.287. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J. Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford A, Garcia-Munoz M, Arbuthnott GW. An afterhyperpolarization recorded in striatal cells ‘in vitro’: effect of dopamine administration. Exp. Brain Res. 1988;71:399–405. doi: 10.1007/BF00247499. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog. Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Fredholm BB, Chergui K. NMDA depresses glutamatergic synaptic transmission in the striatum through the activation of adenosine A1 receptors: evidence from knockout mice. Neuropharmacology. 2006;51:272–282. doi: 10.1016/j.neuropharm.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat. Neurosci. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+ -activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M. Ca2+ -activated K+ channels: molecular determinants and function of the SK family. Nat. Rev. Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Suri RE, Bargas J, Arbib MA. Modeling functions of striatal dopamine modulation in learning and planning. Neuroscience. 2001;103:65–85. doi: 10.1016/s0306-4522(00)00554-6. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A. Modulation of long-term depression by dopamine in the mesolimbic system. J. Neurosci. 2000;20:5581–5586. doi: 10.1523/JNEUROSCI.20-15-05581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatch T, Baranauskas G, Surmeier DJ. Kv4.2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J. Neurosci. 2000;20:579–588. doi: 10.1523/JNEUROSCI.20-02-00579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal straitum in cue-controlled cocaine seeking. J. Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchis C, Bargas J, Ayala GX, Galvan E, Galarraga E. Ca2+ channels that activate Ca2+ -dependent K+ currents in neostriatal neurons. Neuroscience. 2000;95:745–752. doi: 10.1016/s0306-4522(99)00493-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56:149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Mckenzie D, Costanzo E, Arbuthnott GW. Effects of potassium channel blockers on synaptic plasticity in the corticostriatal pathway. Neuropharmacology. 1998;37:523–533. doi: 10.1016/s0028-3908(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Basal ganglia. In: Shepherd GM, editor. The Synaptic Organization of the Brain. 4th edn. Oxford University Press; New York: 1998. pp. 329–375. [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J. Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action–outcome contingency in instrumental conditioning. Behav. Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the ‘accumbens’ part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J. Neurosci. 1998;18:488–498. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.