Abstract
The impact of serotonergic neurotransmission on brain dopaminergic pathways has substantial relevance to many neuropsychiatric disorders. A particularly prominent role has been ascribed to the inhibitory effects of serotonin 2C receptor (5-HT2CR) activation on physiology and behavior mediated by the mesolimbic dopaminergic pathway, particularly in the terminal region of the nucleus accumbens. The influence of this receptor subtype on functions mediated by the nigrostriatal dopaminergic pathway is less clear. Here we report that a null mutation eliminating expression of 5-HT2CRs produces marked alterations in the activity and functional output of this pathway. 5-HT2CR mutant mice displayed increased activity of substantia nigra pars compacta (SNc) dopaminergic neurons, elevated baseline extracellular dopamine concentrations in the dorsal striatum (DSt), alterations in grooming behavior, and enhanced sensitivity to the stereotypic behavioral effects of d-amphetamine and GBR 12909. These psychostimulant responses occurred in the absence of phenotypic differences in drug-induced extracellular dopamine concentration, suggesting a phenotypic alteration in behavioral responses to released dopamine. This was further suggested by enhanced behavioral responses of mutant mice to the D1 receptor agonist SKF 81297. Differences in DSt D1 or D2 receptor expression were not found, nor were differences in medium spiny neuron firing patterns or intrinsic membrane properties following dopamine stimulation. We conclude that 5-HT2CRs regulate nigrostriatal dopaminergic activity and function both at SNc dopaminergic neurons and at a locus downstream of the DSt.
Introduction
The prominence of central serotonin (5-HT) and dopamine (DA) systems in the pathophysiology and treatment of neuropsychiatric disorders underscores the importance of understanding how these systems interact. It is established that the serotonin system regulates the mesoaccumbal and nigrostriatal dopaminergic pathways (Esposito, 2006; Alex and Pehek, 2007; Fink and Göthert, 2007). Serotonergic cell bodies in the raphe nuclei project to the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) and their terminal projections within the dorsal striatum (DSt) and nucleus accumbens (NAc), respectively (Bobillier et al., 1975; Fibiger and Miller, 1977; Hervé et al., 1987; Nedergaard et al., 1988). The serotonin system regulates the functional output of these pathways, but precise mechanisms remain to be clarified (Kelland et al., 1990, 1993; De Deurwaerdère et al., 1996; Saito et al., 1996).
Among the 14 serotonin receptor subtypes, the serotonin 2C receptor (5-HT2CR) is believed to play a particularly prominent role in the modulation of dopamine function (Di Matteo et al., 2002b; Esposito, 2006). 5-HT2CR mRNA is abundantly expressed on GABAergic neurons within the SNc and VTA and in the DSt and NAc (Mengod et al., 1990; Ward and Dorsa, 1996; Eberle-Wang et al., 1997). 5-HT2CRs are implicated in tonic and phasic modulation of the mesoaccumbal dopamine pathway. Pharmacological activation of 5-HT2CRs inhibits firing rates of VTA neurons and DA release within the NAc (Prisco et al., 1994; Di Matteo et al., 1998; Di Giovanni et al., 1999).
How 5-HT2CRs participate in the regulation of nigrostriatal DA system function is less clear. It has been suggested that 5-HT2CRs have less influence on the nigrostriatal than on the mesoaccumbal DA pathway (Di Matteo et al., 2001). This is based on reports that pharmacological manipulations of 5-HT2CRs produce relatively modest effects on SNc dopaminergic neuronal activity and DA release in the DSt (Kelland et al., 1990; Di Matteo et al., 1999; Di Giovanni et al., 2000). However, other studies indicate that 5-HT2CR agonist and antagonist treatments impact DA release in the DSt and NAc in a similar manner (De Deurwaerdère and Spampinato, 1999; Gobert et al., 2000; Porras et al., 2002; De Deurwaerdère et al., 2004; Navailles et al., 2004). These conflicting results may be attributable to the use of different 5-HT2CR ligands with varying specificity and modes of action.
It is possible that the impact of 5-HT2CR function on behaviors regulated by the nigrostriatal DA system may occur through the actions of these receptors within the SNc. However, 5-HT2CRs are also expressed in a number of basal ganglia structures to which the DSt projects (Wright et al., 1995; Eberle-Wang et al., 1997). 5-HT2CRs expressed in basal ganglia structures outside the DSt have previously been implicated in movement disorders such as oral dyskinesias (Eberle-Wang et al., 1996).
Here, we use a genetic approach to investigate the role of 5-HT2CRs in physiology and behaviors associated with nigrostriatal dopamine pathway function. This was pursued in studies of mice bearing a null mutation of the htr2c gene by a combination of electrophysiological, pharmacological, neurochemical, and behavioral approaches.
Materials and Methods
Animals.
Mice were produced in our rodent colony (5-HT2CR mutant and littermate wild-type controls) or were purchased from The Jackson Laboratory (wild-type C57BL/6J mice). The generation of 5-HT2CR null mutant mice has been described in detail previously (Tecott et al., 1995). The htr2c− mutation is congenic on a C57BL/6J background and maintained by mating 5-HT2CR heterozygous females and C57BL/6J males. Animals were weaned at 3 weeks and group housed in standard polypropylene cages (16 × 27 × 12 cm) with 3–5 littermates per cage. Mice had ad libitum access to water and chow (PicoLab Mouse Diet 20, Purina Mills). The housing facility was maintained at 22°C with a 12-h-on/12-h-off lighting schedule, lights on at 7:00 A.M. Unless otherwise indicated, these studies used male mice 2.5–4 months old.
Drugs.
GBR 12909 (a selective inhibitor of the dopamine reuptake transporter, Sigma-Aldrich; Tocris Bioscience) was dissolved by gentle heating and sonication. Animals received GBR 12909 doses of 0, 3, 10, and 30 mg/kg. SB 206553 (a 5-HT2CR inverse agonist, Sigma-Aldrich) at doses of 0 and 5 mg/kg were similarly prepared. d-Amphetamine (Sigma-Aldrich) was used at doses of 0, 2.5, 5, and 10 mg/kg. Quinpirole (a selective D2 receptor agonist, Tocris Bioscience) was administered at doses of 0, 0.6, 2, and 6 mg/kg. SKF 81297 (a selective D1 receptor agonist, Tocris Bioscience) was administered at 0 and 10 mg/kg. All mice received intraperitoneal doses of drug in 10 μl/g injection volumes. All vehicles used in these studies were normal saline.
Single-cell recording procedures.
Mice (n = 8 wild type, n = 8 5-HT2CR mutant) were anesthetized with chloral hydrate (400 mg/kg, i.p.) and mounted on a stereotaxic apparatus (SR-6, Narishige). Body temperature was maintained at 36–37°C. A 28 gauge needle was placed in a lateral tail vein through which additional anesthetic was administered as required. The skull overlying both the SNc and the VTA was removed. The coordinates (relative to the interaural line) for recording electrode placement were as follows: SNc, anteroposterior (AP) 0.16–0.72 mm, mediolateral (ML) 1.1–1.5 mm, dorsoventral (DV) 3.8–4.8 mm; VTA, AP 0.16–0.72 mm, ML 0.1–0.5 mm, DV 3.8–4.8 mm (Paxinos and Franklin, 2001). Extracellular recordings were obtained using single-barrel micropipettes (4–7 MΩ resistance containing 2% pontamine sky blue dye in 2 m NaCl). DA neurons were identified by standard electrophysiological criteria (Bunney et al., 1973; Grace and Bunney, 1980; Wang, 1981; Sanghera et al., 1984). Spike activity was detected using a high-impedance amplifier connected to an analog oscilloscope, audio monitor, and window discriminator. Unit activity was converted to an integrated histogram by a rate-averaging algorithm and displayed as spikes per 10 s intervals. The basal electrical activity of each DA neuron was recorded for at least 5 min to establish the baseline firing rate and bursting activity. The number of spontaneously active DA neurons within both the SNc and the VTA regions were counted by lowering the electrode through a block of tissue, which could be reproducibly located from animal to animal. Twelve electrode tracks (separated from each other by 200 μm), whose sequence was kept constant from animal to animal, were made in each region. Only cells whose electrophysiological characteristics matched those previously established for midbrain DA neurons were sampled. After each experiment, the recording site was marked by injection of pontamine sky blue dye using a −20 μA current for 10 min. Brains were removed and placed in 10% buffered formalin for 2 d before histological examination. Frozen sections were cut at 40 μm intervals and stained with neutral red. Microscopic examination of the sections was performed to verify that the electrode tip was in the SNc or the VTA.
Single-cell recording data analysis.
The mean number of cells per track was calculated by dividing the total number of DA neurons encountered by the total number of electrode tracks performed for either the SNc or the VTA. Burst analysis of DA neurons was performed using RISI (Symbolic Logic). A total of 500–1200 consecutive spikes were recorded for each neuron in both wild-type and mutant mice. Burst firing, when present, was detected using an algorithm similar to that previously described (Grace and Bunney, 1984). The mean percentage of spikes occurring in bursts was used as a measure of bursting activity. Differences in the number of cells per track, the basal firing rate, and the bursting activity between wild-type and mutant mice were analyzed by two-tailed Student's t test. All statistical analyses were performed with StatView version 5.0.1 (SAS Institute).
Microdialysis probe construction and implantation.
Probe implantation occurred 12–48 h before dialysis testing. Concentric microdialysis probes consisted of 23 gauge stainless steel and silica capillary tubing. The dialysis membrane (inner diameter 240 μm, outer diameter 290 μm, 2 mm exposed for striatum, 1 mm exposed for NAc, AN 69HF, Hospal) consisted of polyacrylonitrile/sodium methylsulfonate copolymer with an average pore size of 29 Å. Mice were anesthetized using inhalational (isoflurane or halothane, 2%, 800 ml/min O2) or injectable (ketamine 100 mg/kg–xylazine 10 mg/kg, i.p.) agents. Lidocaine was used for local anesthesia. The animals were placed in a stereotaxic frame (Kopf Instruments), and probes were inserted into the striatum (+0.7 AP, −2.0 ML, −3.0 DV for standard microdialysis; +0.5 AP, +2.0 ML, −5.0 DV for no net flux microdialysis) or NAc (−1.5 AP, +0.8 ML, −5.3 DV, only no net flux microdialysis) per established atlas coordinates (Paxinos and Franklin, 2001). Probes were secured to the skull using dental cement. Mice were then returned to a standard polycarbonate cage with sawdust bedding and allowed to regain consciousness.
Standard microdialysis procedure.
Experiments were performed 24–48 h after surgery. On the day of the experiment, the microdialysis probes were connected with flexible PEEK tubing to a microperfusion pump (Harvard Apparatus, PHD 2000) and perfused with artificial CSF (aCSF), containing 146 mm NaCl, 2.9 mm KCl, 1.2 mm CaCl2, and 1.1 mm MgCl2, at a flow rate of 1.0–1.5 μl/min (for GBR 12909 microdialysis experiments, aCSF also contained 2 mm Na2HPO4, pH 7.40). Microdialysis samples were collected at 15–20 min intervals into vials (for GBR 12909 study, containing 7.5 μl of 0.02 m formic acid). Microdialysis samples were collected by an automated fraction collector (Univentor 820) and stored at −80°C. Drug treatment (either d-amphetamine or GBR 12909) was administered immediately before collection of the sixth sample. After the experiment, mice were killed and their brains removed and incubated for 3 d in 4% paraformaldehyde. The probe position was histologically verified in coronal brain sections (Paxinos and Franklin, 2001).
No net flux microdialysis procedure.
aCSF consisted of 149 mm NaCl, 2.8 mm KCl, 1.2 mm CaCl2, 1.2 mm MgCl2, 0.25 mm ascorbic acid, and 5.4 mm d-glucose, pH 7.3. Perfusate flow rate to both probes was 0.2 μl/min for the first 10 postsurgical hours, and then increased to 0.6 μl/min 1 h before the start of dialysate collection and for the remainder of the experiment. Following equilibration, six baseline samples were collected at 10 min intervals. The perfusate was switched to aCSF containing 5 nm DA and allowed to equilibrate for 30 min followed by dialysate collection for 30 min. This same procedure was subsequently used for perfusates containing 10 and 20 nm dopamine. Following collection, dialysate samples were immediately frozen and stored at −70°C.
DA measurements.
Dialysate aliquots were injected onto a reversed phase column (3 μm C18 stationary phase, multiple vendors). Samples were eluted with a mobile phase (acetonitrile, phosphate buffer, and an ion-pairing agent, ESA) for d-amphetamine studies; NaAc buffer (4.1 g/L) with MeOH (2.5% v/v), Titriplex (150 mg/L), OSA (150 mg/L), and TMACl (150 mg/L, pH 4.1) for GBR 12909 studies, and 17% acetonitrile (v/v) in a 25 mm NaH2PO4 buffer containing 0.1 mm Na2-EDTA, 1.6 mm 1-decanesulfonic acid, and 14.4 mm triethylamine, pH 3.9, for no net flux studies. Pump flow rates were 0.25 ml/min, 0.35 ml/min, and 35 μl/min for d-amphetamine, GBR 12909, and no net flux studies, respectively. For d-amphetamine studies, DA was detected using an amperometric cell (Model 5041, ESA) at a potential of +175 mV coupled to a coulometric detector (Coulochem II, ESA). For GBR 12909 studies, DA was detected using a glassy carbon electrode at a potential of +500 mV (vs Ag/AgCl reference electrode) coupled to an Intro detector (Antec Leyden). For no net flux studies, DA was detected using a glassy carbon electrode at a potential of +700 mV (vs Ag/AgCl reference electrode) as controlled by an amperometric detector (EG&G 400, Princeton Applied Research). On-column limits of quantification were 2 fmol (d-amphetamine), 0.3 fmol (GBR 12909), and 0.5 fmol (no net flux).
For d-amphetamine and GBR 12909 studies, basal DA levels were defined as the average of the first five samples. To determine the effect of genotype on DA release after treatment, repeated-measures ANOVAs were performed on values obtained during the 180 min following drug injection (time intervals 6–14).
Syntactic grooming studies.
Syntactic grooming chains (as described by Aldridge and Berridge, 1998) were observed in cohorts of 2–3-month-old male mice (n = 9 wild type, n = 8 mutant) following gentle misting of their back flanks with room temperature tap water. Mouse grooming activity was videotaped from two directions for 10 min. All mice were tested for 10 consecutive days between 1:00 and 4:00 P.M. daily. Grooming videos were digitized to MPEG, and scored by a single investigator in a blinded manner using Observer Video-Pro 5.0 (Noldus). At least 15 min of total grooming time was evaluated for each mouse. As described, completed syntactic chains could have multiple morphologies, but all chains had to begin in phase 1, be followed by either phase 2, phase 3, or phase 2–phase 3, and then end with phase 4. Incomplete chains always began with phase 1, but did not evolve according to the above criteria. Nonsyntactic grooming states were scored for all grooming behavior not classified as either a completed or broken syntactic chain. Differences in grooming behavior properties were evaluated by Student's t test with p values adjusted for multiple comparisons using Bonferroni corrections.
Locomotor activity monitoring.
Animals were singly housed in 16 standard polypropylene cages (45 × 24 × 17 cm) surrounded by 8 × 4 photobeam activity monitoring brackets (FlexField, San Diego Instruments). Bedding was placed on the cage floor; food and water were provided ad libitum. Mice were acclimated to these home cages for 4–5 d before drug administration. Mice received intraperitoneal injections of saline vehicle between 1:00 and 3:00 P.M. each day before drug administration to habituate them to injections. At each treatment dose, repeated-measures ANOVA was conducted on values between 0 and 90 min after the injection with time as a within-subject factor and genotype and treatment as between-subject factors. In addition, univariate ANOVA was conducted on peak locomotor activity (LA) with genotype and treatment as between-subject factors.
Stereotypic behavior measurement.
The same habituation protocol for LA was followed. Home cage behavior was video recorded for 3–4 min at intervals chosen based on observations from pilot studies. These intervals were 30 and 90 min after GBR 12909 and GBR 12909/SB 206553 injection, 30 and 75 min after 2.5 and 5 mg/kg d-amphetamine injection, and 30 and 110 min after 10 mg/kg d-amphetamine injection. For SKF 81297 and quinpirole treatments, mice were videotaped for 10 min (10 min after drug injection). Videotaped behavior trials were converted to digital video and scored for stereotypy. Differences in motor and stereotypy responses between GBR 12909 and d-amphetamine have previously been noted (Hooks et al., 1994); we thus used stereotypy rating scales appropriate for each treatment. Motor stereotypy in response to GBR 12909 treatment was scored using a modified version of a previously published protocol (Creese and Iversen, 1973). Six distinct behaviors were noted: asleep (score 1), awake inactive (score 2), awake locomotion (score 3), awake “route tracing” locomotion (score 4), intermittent oral/grooming stereotypies (score 5), and continuous oral/grooming stereotypies (score 6). Motor stereotypy in response to d-amphetamine treatment was scored using a modified version of a previously published protocol (Chartoff et al., 2001). Eight distinct behaviors were noted: no movement, ambulation, oral stereotypy, sniffing, rearing, vigorous grooming, taffy pulling, and climbing. For both GBR 12909 and d-amphetamine treatment, behaviors were scored at the start and every 10 s into the trial. For SKF 81297 and quinpirole treatments, videos were scored for the entire 10 min recording period. Investigators were blinded to genotype and drug treatment status. For each treatment dose, repeated-measures ANOVA was conducted with time as a within-subject factor and genotype and treatment as between-subject factors.
Slice preparation and electrophysiology.
Male P22-P28 mice were anesthetized with halothane and decapitated, the brain was rapidly removed, and coronal slices (300 μm) were cut in 4°C aCSF using a VT1000S (Leica). Slices were recovered at 32°C in carbogen-bubbled aCSF (126 mm NaCl, 2.5 mm KCl, 1.2 mm NaH2PO4, 1.2 mm MgCl2, 2.4 mm CaCl2, 18 mm NaHCO3, and 11 mm glucose, with pH 7.2–7.4, 301–305 mOsm) for 30 min to 5 h. During experiments, slices were submerged and continuously perfused (using a peristaltic pump, ∼2 ml/min) with carbogen-bubbled aCSF warmed to 31–32°C and supplemented with CNQX (10 μm, to block AMPA-type glutamate miniature EPSPs) and picrotoxin (50 μm, to block GABAA receptors). All reagents were bath applied.
Whole-cell recordings were made from DSt medium spiny neurons (MSNs), identified as previously described (Hopf et al., 2003), using a potassium methanesulfonate-based internal solution [KOH 0.98% (v/v), methanesulfonic acid 0.76% (v/v), hydrochloric acid 0.18% (v/v), 20 mm HEPES, 0.3 mm EGTA, 2.8 mm NaCl, 2.5 mg/ml MgATP, and 0.25 mg/ml GTP, pH 7.2–7.4, 275–285 mOsm]. Current pulses were applied using Clampex 9.2 and 700A patch amplifier in current-clamp mode (Molecular Devices). Series resistance correction was 15–25 MΩ. The resting membrane potential was determined just after breaking into a neuron, and each neuron was then brought to a resting potential of approximately −80 mV by passage of DC current via the patch amplifier before collection of firing data.
To generate action potentials, neurons in current clamp were depolarized with a series of seven or eight 300 ms current pulses, with 20 pA between each current step, where the initial current step for each neuron was just subthreshold for firing. This series of current pulses was delivered every 30 s, alternating with a 33.3 pA hyperpolarizing pulse. Patch-clamp data were collected at 15 kHz and filtered at 2 kHz. All data were analyzed using Clampfit (Axon Instruments). To calculate percentage change in spiking, a current pulse was selected that exhibited two spikes at baseline, or three spikes if no current step evoked two spikes. The same current pulse was used for time points before and after drug addition. Spike firing rates during the 3 min before addition of the reagent were averaged and this value normalized to 100%. Statistical significance was determined for the average spike firing change during the last 2 min of exposure to reagents All statistics were performed using a two-tailed, unpaired Student's t test.
Receptor autoradiography.
Mice underwent rapid decapitation, brain dissection, and tissue freezing on powdered dry ice. Coronal sections (20 μm thickness) were cut on a cryostat with appropriate histological reference slides and stored desiccated at −20° until ready for tissue processing. All radioligand binding reactions occurred within 1 week of cutting the sections. Sections were preincubated in buffer (50 mm Tris-HCl, pH 7.4, 120 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, and either 40 nm ketanserin (to block nonspecific 5-HT2A and 5-HT2C receptor binding in the D1 reaction) or 0.5 μm DTG, 0.1 μm pindolol (to block nonspecific ς and 5-HT1AR receptor binding in the D2 reaction) for 1 h before indirect or direct radioligand binding. 3H-SCH23390 at 13.2 nm was used to assay for D1 receptors (run with an excess of cis-flupenthixol in the indirect reaction); 3H-YM-09151–2 at 17 μm was used to assay for D2 receptors (run with an excess of sulpiride in the indirect reaction). Following 1 h incubation on ice, sections were washed twice in chilled buffer, rinsed in chilled distilled water, and then gently dried with hot air. Sections were processed by investigators blinded to genotype and were stratified such that sections cut at similar levels were processed in the same batch. Slides were then placed in autoradiography cassettes reserved for tritium use, tritiated standards were placed with each film, and exposures were taken at 4°C for 4 weeks onto Kodak tritium hyperfilm. Films were developed by hand, scanned to computer, and analyzed using the public domain NIH Image program (developed at the United States National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/). Landmarks for the DSt and NAc were taken from a standard mouse brain atlas (Paxinos and Franklin, 2001).
Results
SNc dopaminergic neurons display increased basal firing rates and bursting activity in 5-HT2CR mutant mice
Extracellular microelectrode recordings were obtained to assess how the htr2c− mutation influenced baseline electrophysiological properties of midbrain dopaminergic neurons. No significant differences in the number of spontaneously active DA neurons were found between wild-type and mutant mice, either in the SNc or the VTA (Fig. 1A). At the SNc, statistically significant increases in dopaminergic neuron tonic firing rate (Fig. 1B) and percentage bursting activity (Fig. 1C) were noted in 5-HT2CR mutant mice compared with wild types. Basal activity of SNc DA neurons in mutant mice increased by 20%, whereas bursting activity of these neurons increased by almost 50%. Interspike interval histograms revealed a skew toward lower-frequency activity in 5-HT2CR mutant mice compared with wild types (Fig. 1D,E). This probably occurs as a result of the increased bursting behavior, leading to an increase in long interspike intervals between the bursts, although the mean frequency is increased. No statistically significant differences in either basal or percentage bursting activity were observed between 5-HT2CR mutant and wild-type mice at the VTA (Fig. 1B,C).
Figure 1.
5-HT2CR mutant mice display increased SNc dopaminergic neuronal activity. A, Numbers of spontaneously active dopaminergic neurons encountered while recording from SNc and VTA revealed no phenotypic difference. B, 5-HT2CR mutant mice exhibit increased dopaminergic cell firing in SNc but not in VTA (5.38 ± 0.23 vs 4.49 ± 0.22 spikes/s for SNc, p < 0.01; 4.55 ± 0.21 vs 4.60 ± 0.16 spikes/s for VTA, NS). C, 5-HT2CR mutant mice exhibit increased dopaminergic cell burst firing in SNc but not VTA (16.2 ± 2.5% vs 8.6 ± 1.9% cells bursting for SNc, p < 0.05; 16.6 ± 2.5% vs 17.5 ± 2% cells bursting for VTA, NS). D, E, Interspike interval histograms for SNc neurons recorded from wild-type (D) and 5-HT2CR mutant (E) mice demonstrate that SNc neurons from mutant mice have overall higher-frequency activity spike trains than those seen in wild-type mice. Data are represented as mean ± SEM. n = 8 per genotype. *p < 0.05; **p < 0.01.
Extracellular dopamine is increased in the DSt and NAc of 5-HT2CR mutant mice
To determine whether alterations in tonic and phasic SNc dopaminergic neuron activity were reflected at the dopaminergic terminal fields, we performed no net flux microdialysis to measure DSt and NAc baseline extracellular dopamine concentrations in awake, behaving mice. Estimates of extracellular DA concentrations in each brain region were calculated from the mean of three samples at each perfusate concentration (0, 5, 10, and 20 nm DA). First-order regressions were used to obtain slope and intercept values, which were solved for the point of no net flux (zero intercept on the y-axis) (Parsons and Justice, 1994).
Figure 2, A and C, depicts data where the microdialysis probe was placed within the DSt. DSt extracellular dopamine concentrations were nearly twice as large in mutant mice compared with wild types. Extracellular dopamine concentrations in the NAc were modestly increased in mutant mice compared with wild types (Fig. 2B,C).
Figure 2.
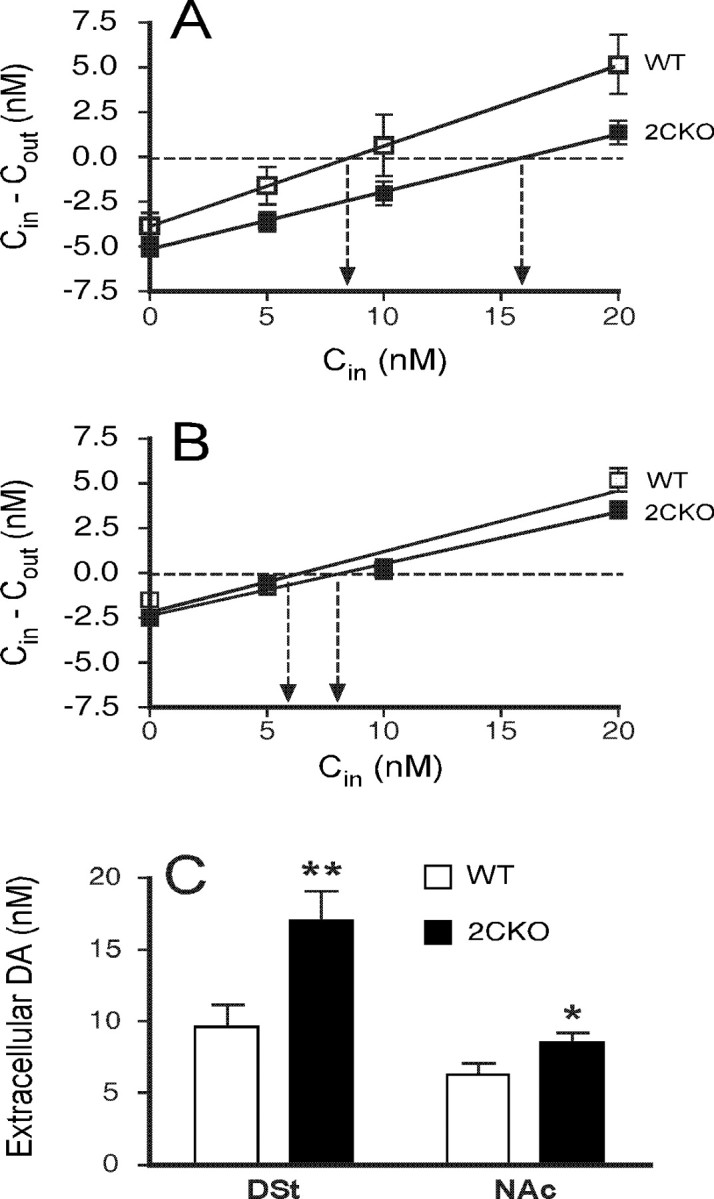
5-HT2CR mutant mice display elevated striatal extracellular dopamine levels. A, B, No net flux plots in DSt (A) and NAc (B) of DA from 5-HT2CR mutant and wild-type mice. The y-axis indicates DA concentration differences between aCSF entering the probe (Cin) and aCSF exiting the probe (Cout). The point of no net flux (y = 0 nm; horizontal dotted line) corresponds to DA equilibrium between tissue and microdialysis probe revealing extracellular DA concentration (arrows). Estimates of in vivo DA recovery in the DSt, as indicated by the slope of the no net flux curve, did not differ between 5-HT2CR mutant mice (0.32 ± 0.03) and wild types (0.45 ± 0.07, F(1,14) = 3.537, NS). Similarly, estimates of in vivo DA recovery in the NAc did not differ between groups (5-HT2CR mutant mice, 0.29 ± 0.04; wild types, 0.34 ± 0.03, F(1,14) = 0.881, NS). C, 5-HT2CR mutant mice display elevated mean basal dopamine concentration in the DSt (17 ± 2.1 nm vs 9.6 ± 1.6 nm in wild types, F(1,15) = 8.924, p < 0.01) and NAc (8.5 ± 0.7 nm vs 6.3 ± 0.8 nm in wild types, F(1,15) = 4.887, p < 0.05). Data are represented as mean ± SEM. n = 8 per genotype. *p < 0.05; **p < 0.01.
Increased syntactic grooming chain failures and altered grooming behaviors in 5-HT2CR mutant mice
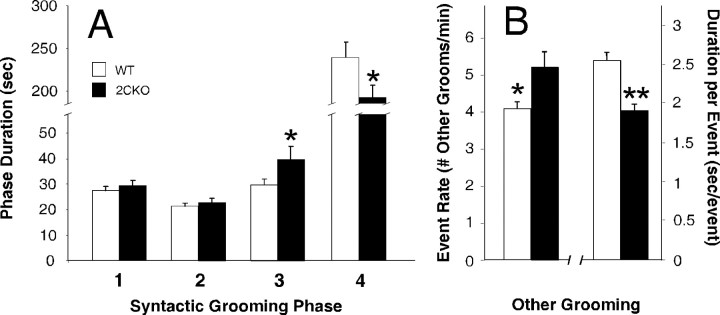
Given the above electrophysiological and neurochemical evidence that 5-HT2CR loss increases nigrostriatal dopaminergic activity, we proceeded to determine whether these changes were accompanied by altered DSt-organized behaviors. One such behavior is syntactic grooming. More than 15 min of overall grooming activity (pooled over the 10 d of observation) were obtained for all mice studied. In general, the majority of observed grooming behavior was nonsyntactic. The percentage of grooming activity occurring within syntactic chains (5%, total observation) was similar to that reported by other groups (Aldridge and Berridge, 1998). No statistically significant phenotypic differences in syntactic grooming initiation rate, syntactic grooming frequency, or latency to first syntactic grooming episode were observed. However, 5-HT2CR mutant mice spent significantly more time in syntactic phase 3 and significantly less time in syntactic phase 4 (Fig. 3A).
Figure 3.
5-HT2CR mutant mice exhibit altered syntactic grooming chains. A, Increased duration of phase 3 and decreased duration of phase 4 syntactic grooming in 5-HT2CR mutant mice. Mean phase 3 duration was 29.8 ± 2.2 s in wild-type mice, 39.8 ± 5 s in 5-HT2CR mutant mice (p < 0.05); mean phase 4 duration was 239.2 ± 17.9 s in wild-type mice, 192.0 ± 14.2 s in 5-HT2CR mutant mice (p < 0.03). B, Alteration of nonsyntactic grooming properties in 5-HT2CR mutant mice. 5-HT2CR mutant mice exhibit increased nonsyntactic grooming event initiation (5.20 ± 0.4 grooming events per minute vs 4.07 ± 0.16 in wild types; p < 0.015) and reduced nonsyntactic grooming duration (1.89 ± 0.1 vs 2.55 ± 0.11 s in wild types, p < 0.0007). Data are represented as mean ± SEM. n = 8 per genotype. *p < 0.05; **p < 0.001.
5-HT2CR mutant mice also demonstrated gross differences in nonsyntactic grooming behavior compared with wild-type mice (Fig. 3B). While there were no significant differences in overall grooming duration between wild-type and 5-HT2CR mutant mice, 5-HT2CR mutant mice had a significantly higher nonsyntactic grooming initiation rate and a correspondingly significant decrease in nonsyntactic grooming episode duration. No differences in latency to nonsyntactic grooming episodes were noted.
5-HT2CR mutant mice perform more orofacial motor stereotypies following d-amphetamine treatment
We evaluated LA and stereotypy responses to d-amphetamine treatment in wild-type and 5-HT2CR mutant mice. LA in response to 2.5, 5, and 10 mg/kg d-amphetamine is depicted in Figure 4A–C. Characteristic dose- and time-dependent locomotor responses to d-amphetamine were observed. A 2.5 mg/kg dose of d-amphetamine resulted in a robust locomotor response that reached peak values at 40 min, then declined, reaching control values at 110 min after injection (Fig. 4A). After 5 mg/kg d-amphetamine, locomotion increased more rapidly, peaking 30 min after injection, declining at a slower rate, and finally attaining baseline values 180 min after injection (Fig. 4B). An early but smaller peak in locomotion (10 min after injection) was observed after 10 mg/kg dose of d-amphetamine, but locomotion was rapidly suppressed, remaining at minimal levels until 110–120 min after injection, followed by another rise in locomotion lasting until >210 min after injection (Fig. 4C). Overall, 5-HT2CR mutant mice showed less LA response to d-amphetamine.
Figure 4.
5-HT2CR mutant mice display altered motor responses to d-amphetamine. ○, d-Amphetamine-treated wild-type mice; ●, d-amphetamine-treated 5-HT2CR mutant mice; □, saline-treated wild-type mice; ■, saline-treated 5-HT2CR mutant mice. A, Locomotor activity following 2.5 mg/kg d-amphetamine. Repeated-measures ANOVA on locomotor distance traveled between 0 and 90 min after injection revealed significant effects of treatment (F(1,27) = 131.14; p < 0.001) and treatment × time interaction (F(1,27) = 11.69; p < 0.01). Inset, ANOVA conducted on peak activity showed significant effects of treatment (F(1,26) = 175.69; p < 0.001) and treatment × genotype interaction (F(1,26) = 5.79; p < 0.05). B, Locomotor activity following 5 mg/kg d-amphetamine. Repeated-measures ANOVA on locomotor distance traveled between 0 and 90 min after injection revealed significant effects of time (F(1,26) = 9.24; p < 0.01), treatment (F(1,26) = 301.19; p < 0.001), and treatment × genotype interaction (F(1,26) = 6.94; p < 0.05). Inset, ANOVA conducted on peak activity showed a significant effect of treatment (F(1,26) = 196.26; p < 0.001). C, Locomotor activity following 10 mg/kg d-amphetamine. Repeated-measures ANOVA on locomotor distance traveled between 0 and 90 min after injection showed significant effects of genotype (F(1,26) = 6.04, p < 0.05), treatment (F(1,26) = 41.31, p < 0.001), and time (F(1,26) = 215.61, p < 0.001), as well as interactions of time × genotype (F(1,26) = 4.93; p < 0.05), treatment × genotype (F(1,26) = 12.85; p < 0.01), and time × treatment (F(1,26) = 47.82; p < 0.001). The tertiary interaction of time × treatment × genotype was also significant (F(1,26) = 10.25; p < 0.01). Inset, ANOVA conducted on peak activity showed significant effects of treatment (F(1,26) = 63.44; p < 0.001) and treatment × genotype (F(1,26) = 5.76; p < 0.05). D, Repeated-measures ANOVA conducted on percentage time spent in stereotypy following 2.5 mg/kg d-amphetamine revealed no significant phenotypic differences. E, 5-HT2CR mutant mice display greater focused stereotypy following 5 mg/kg d-amphetamine (F(1,11) = 5.14, p < 0.05). F, No phenotypic difference in focused stereotypy following 10 mg/kg d-amphetamine. Data are represented as mean ± SEM. n = 7–8 per genotype per treatment.
The transient decrease in d-amphetamine-induced LA has been proposed to result from an increase in focused stereotypies (Yates et al., 2007). Our data indicate that the expression of these stereotypies may be enhanced in 5-HT2CR mutant mice. To test this hypothesis, we measured motor stereotypy in response to 2.5, 5, and 10 mg/kg d-amphetamine in wild-type and 5-HT2CR mutant mice. After 2.5 mg/kg d-amphetamine, there were no significant phenotypic differences in stereotypic activity (Fig. 4D). However, detailed analysis of stereotypic behaviors revealed that 5-HT2CR mutant mice spent significantly more time in rearing and jumping (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Following 5 mg/kg d-amphetamine, 5-HT2CR mutant mice spent significantly more time in total stereotypy than did wild type (Fig. 4E). At the highest dose of 10 mg/kg, mice were almost fully engaged in orofacial stereotypy, but there was no significant phenotypic difference (Fig. 4F).
5-HT2CR mutant mice demonstrate increased stereotypic behaviors following selective DAT blockade
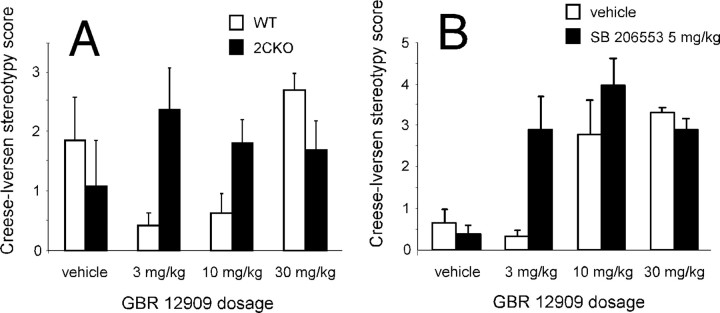
We then examined stereotypic behavioral responses to the selective DAT antagonist GBR 12909 (Heikkila and Manzino, 1984) in 5-HT2CR mutant and wild-type mice. We used a modified Creese-Iversen scale to score stereotyped behaviors in wild-type and 5-HT2CR mutant mice treated with GBR 12909 (Fig. 5A). We also examined the impact of 5-HT2CR pharmacological blockade (using SB 206553) on stereotypic behavioral responses to GBR 12909 (Fig. 5B). 5-HT2CR mutant mice displayed increased stereotypic behaviors in response to intermediate doses of GBR 12909. Similarly, mice receiving both SB 206553 and intermediate doses of GBR 12909 displayed greater stereotypic behaviors compared with wild-type mice receiving GBR 12909.
Figure 5.
Enhanced sensitivity of 5-HT2CR mutant mice to induction of motor stereotypy by dopamine reuptake blockade. A, Increased GBR 12909-evoked stereotypy observed in 5-HT2CR mutant mice compared with wild types (F(3,56) = 4.017, p < 0.012 for genotype × dose interaction). Behavior evaluated 90 min after drug administration. B, Increased GBR 12909-evoked stereotypy observed in C57BL/6J mice following treatment with the 5-HT2CR inverse agonist SB 206553 (F(3,55) = 3.764, p < 0.016 for SB 206553 × GBR 12909 dose interaction). In animals receiving both GBR 12909 and SB 206553, drugs were administered simultaneously. Behavior evaluated 90 min after drug administration. Data are represented as mean ± SEM. n = 7 per genotype per treatment.
No phenotypic difference in striatal DA release after d-amphetamine or selective DAT blockade
Given the phenotypic differences we observed in locomotor activity and stereotypy following psychostimulant administration, we hypothesized that we would find phenotypic differences in psychostimulant-evoked DSt dopamine extracellular concentrations. We examined dopamine release in the DSt of 5-HT2CR mutant and wild-type mice in response to 2.5 and 5 mg/kg d-amphetamine (Fig. 6A,B). A dose of 2.5 mg/kg d-amphetamine induced a sharp increase in DA release, which peaked 40 min after the injection (883.7 ± 114.13 and 731 ± 92.7% increase for wild-type and 5-HT2CR mutant mice respectively, NS). Similarly, a 5 mg/kg d-amphetamine dose induced a sharp increase in DA release, which peaked 40 min after injection (1072.35 ± 140.3 and 1242.6 ± 190.7% increases in wild-type and 5-HT2CR mutant mice respectively, NS). However, neither genotype nor any of its relevant interactions were found to be significant by repeated-measures ANOVA. Thus, we were unable to detect any phenotypic differences in d-amphetamine-evoked DSt extracellular dopamine concentrations.
Figure 6.
No phenotypic effect on DSt extracellular dopamine concentrations after treatment with d-amphetamine or GBR 12909. ○, Wild-type mice; ●, 5-HT2CR mutant mice. A–D, DSt extracellular dopamine following d-amphetamine 2.5 mg/kg (A), d-amphetamine 5 mg/kg (B), GBR 12909 3 mg/kg (C), and GBR 12909 10 mg/kg (D). For A–D, no phenotypic differences were noted between drug- and vehicle-treated groups. Arrows indicate the time of drug administration. Data are represented as mean ± SEM. n = 5–8 per genotype per treatment.
We then examined dopamine release in the DSt of 5-HT2CR mutant and wild-type mice in response to 3 and 10 mg/kg GBR 12909 administration (Fig. 6C,D). We observed an overall increase in DSt extracellular dopamine concentration following drug administration. However, neither genotype nor any genotype interactions were found to be significant by repeated-measures ANOVA. Thus, we observe that differences in locomotor and stereotypical behavior following psychostimulant administration are not accompanied by phenotypic differences in DSt extracellular dopamine concentrations.
5-HT2CR mutant mice display enhanced D1 receptor agonist-induced stereotypy
The above findings suggest that 5-HT2CRs influence behavioral responses to dopamine release. This raises the possibility that 5-HT2CR mutant mice have enhanced behavioral responses to DA receptor activation. Figure 7 displays LA in response to 10 mg/kg injection of the D1 receptor agonist SKF 81297. SKF 81297 increased LA in both wild-type and 5-HT2CR mutant mice relative to saline-injected controls. However, the magnitude of the increase was diminished in 5-HT2CR mutant mice. Stereotypy observed during these trials was mainly orofacial in nature, with 5-HT2CR mutant mice displaying a robust enhancement of stereotypy relative to the wild-type mice (mutant mice spent 86.91 ± 6.92% time in focused stereotypy compared with 21.29 ± 6.18 for wild-type mice, F(1,6) = 49.98, p < 0.001). We further examined the effect of quinpirole (0.6, 2, and 6 mg/kg) on LA and stereotypy. Quinpirole treatment evoked no phenotypic effects on LA or stereotypy.
Figure 7.
5-HT2CR mutant mice display decreased locomotion in response to systemic treatment with the D1R agonist SKF 81297. Repeated-measures ANOVA on locomotor distance traveled between 0 and 90 min after injection revealed significant effects of time (F(1,25) = 39.1, p < 0.001) and time × genotype (F(1,25) = 4.65, p < 0.05) and time × treatment (F(2,25) = 5.89, p < 0.01) interactions. There was also a significant time × genotype × treatment interaction (F(2,25) = 10.69, p < 0.001). ANOVA conducted on peak activity showed significant effects of genotype (F(1,25) = 23.49, p < 0.001), treatment (F(2,25) = 60.46, p < 0.001), and genotype × treatment interaction (F(2,25) = 22.06, p < 0.001). Data are represented as mean ± SEM. n = 6–7 per group.
Absence of phenotypic difference in striatal D1 and D2 receptor binding
The enhanced responses of 5-HT2CR mutant mice to D1 receptor stimulation may reflect a phenotypic difference in D1 receptor expression. To test this hypothesis, 3H receptor-ligand-binding autoradiography was used to assess D1 and D2 receptor binding in DSt and NAc. No phenotypic differences in receptor binding in DSt and NAc were observed in either of these regions when comparing wild-type to 5-HT2CR mutant mice (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
Absence of phenotypic difference in D1 receptor activation of DSt MSNs
Brain slices were used to examine the firing properties of DSt MSNs. Neurons were brought to approximately −80 mV by passage of DC current via the patch amplifier, and then a series of 300 ms current pulses was delivered every 30 s. Dopamine, acting via the D1 receptor, can enhance action potential firing in the DSt (Surmeier et al., 1995; Hernández-López et al., 1997). In agreement, the selective D1 receptor agonist SKF 81297 dose-dependently enhanced firing in wild-type and 5-HT2CR mutant mice, with significant enhancement of action potential generation at 10 μm (supplemental Fig. 3A, available at www.jneurosci.org as supplemental material) but not 3 μm (supplemental Fig. 3C, available at www.jneurosci.org as supplemental material). However, there were no phenotypic differences in firing enhancement. SKF 81297 did not alter the input resistance, measured using a 33.3 pA hyperpolarizing pulse, suggesting that there was no effect of D1 receptor activation on inwardly rectifying potassium channels (supplemental Fig. 3B,D, available at www.jneurosci.org as supplemental material). Finally, as shown in supplemental Table 1 (available at www.jneurosci.org as supplemental material), there were no differences in a number of basic firing parameters related to action potential waveform and input resistance (n = 14 cells from six wild-type mice, n = 14 cells from five 5-HT2CR mice), suggesting that 5-HT2CR deletion did not alter the basal function of several channels active during firing.
Discussion
These studies suggest that 5-HT2CRs have a significant role in controlling nigrostriatal physiology and behavior. We demonstrate that 5-HT2CR loss increases SNc dopaminergic neuron firing rates and DSt extracellular dopamine concentrations and enhances behaviors associated with DSt activation. Loss of 5-HT2CR function enhances psychostimulant-induced stereotypy. This enhancement occurs without phenotypic differences in the elevation of psychostimulant-induced striatal extracellular dopamine concentrations. Thus, loss of 5-HT2CR function may be accompanied by enhanced behavioral responses to released dopamine. Phenotypic differences in stereotypic behavior following selective D1 receptor agonist stimulation support this hypothesis. These differences were not attributable to increases in DSt D1 receptor expression or MSN response to dopaminergic stimulation. Thus, these findings suggest that 5-HT2CRs inhibit behaviors associated with nigrostriatal function at multiple loci.
5-HT2CR loss evokes a tonic activation of nigrostriatal dopaminergic neurotransmission
In mice lacking 5-HT2CRs, SNc dopaminergic neurons displayed elevated tonic firing rates and an increased percentage of burst-firing cells. 5-HT2CRs are expressed on GABAergic neuronal subpopulations (Di Giovanni et al., 2001) of the SNc [by in situ hybridization (Eberle-Wang et al., 1997)] and VTA [by immunocytochemistry (Bubar and Cunningham, 2007)]. Thus, loss of 5-HT2CR-evoked activity within these GABAergic cells could diminish inhibitory drive onto SNc neurons. This is consistent with pharmacological studies suggesting that 5-HT2CRs suppress SNc neuronal firing [Di Giovanni et al. (1999) and Porras et al. (2002), using SB-206553] and burst firing [Blackburn et al. (2006), using SB-200646A].
Surprisingly, our data demonstrate that the htr2c− null mutation has a greater impact on SNc cell firing rate and bursting properties than on those of VTA dopaminergic neurons. Previous studies clearly demonstrate that pharmacological 5-HT2CR activation/inhibition evokes a corresponding inhibition/activation of VTA neuronal firing (Di Giovanni et al., 1999, 2000; Di Matteo et al., 1999, 2000); (for review, see Giorgetti and Tecott, 2004). Many of these studies suggest that systemic modulation of 5-HT2CR activity alters VTA neuronal activity to a greater extent than SNc neuronal activity (Di Matteo et al., 2001, 2002a; Blackburn et al., 2002). However, a number of factors complicate this interpretation. Studies that use different 5-HT2CR antagonists have yielded disparate results. For example, previous reports suggest that 5-HT2CR antagonism increases (Di Giovanni et al., 1999) or has no effect on (Di Matteo et al., 1999) SNc dopaminergic neuronal firing rates. Some of these issues may relate to differences in the extent to which antagonist compounds block 5-HT2CR constitutive activity (De Deurwaerdère et al., 2004).
Increases in SNc neuron firing rate and bursting were accompanied by increases in extracellular DSt dopamine concentration as determined by no net flux methods. This finding is also concordant with previous studies demonstrating increased striatal dopamine concentrations following treatment with 5-HT2CR antagonists (Navailles et al., 2004). Conversely, extracellular striatal dopamine concentrations were decreased after systemic 5-HT2CR agonism (Di Matteo et al., 2004). Moreover, constitutive 5-HT2CR activity in absence of bound ligand has been proposed to decrease striatal dopamine concentrations (De Deurwaerdère et al., 2004).
In accord with the observed changes in baseline SNc neuronal firing properties and DSt extracellular DA concentrations, we observed altered grooming behavior in 5-HT2CR mutant mice. Increases in total grooming have previously been observed following treatment with dopamine D1 receptor agonists (Berridge and Aldridge, 2000) and in mice expressing a hypoactive dopamine reuptake transporter (Berridge et al., 2005). Like 5-HT2CR mutant mice, the dopamine reuptake transporter hypomorphic mice displayed elevated nonsyntactic grooming event rates. 5-HT2CR mutant mice also demonstrate a dysregulation of syntactic grooming, a behavior specifically linked to DSt function (Cromwell and Berridge, 1996; Aldridge and Berridge, 1998; Aldridge et al., 2004). Similar to what is observed following D1 receptor agonist treatment, 5-HT2CR mutant mice spend less time in the final phase (phase 4) of syntactic grooming chains compared with wild-type mice (Matell et al., 2006). Although these findings are consistent with an enhancement of nigrostriatal dopamine system activity, it is also possible that the absence of 5-HT2CRs from basal ganglia structures could contribute to the observed grooming phenotypes.
5-HT2CR loss enhances psychostimulant-induced motor activity
Both locomotor stereotypies and focused stereotypies (such as grooming and gnawing) are prominent behaviors associated with psychostimulants. Typically, locomotor stereotypic behaviors are observed at lower psychostimulant doses, while focused stereotypies predominate with higher psychostimulant doses. In 5-HT2CR mutant mice, focused stereotypies were the prevalent behavior following d-amphetamine treatment. In contrast, locomotor stereotypies were more prevalent in wild-type mice except at the highest tested d-amphetamine dosage. In mutant mice, behavioral responses were characterized by greater duration of focused stereotypy and greater sensitivity to focused stereotypy. Because d-amphetamine nonspecifically influences monoamine release and uptake, we examined the consequences of selective DA transporter blockade using GBR 12909. Similarly, focused stereotypies were observed more frequently in 5-HT2CR mutant mice than in wild-type littermates following GBR 12909 treatment.
Unexpectedly, we did not observe phenotypic differences in DSt extracellular dopamine concentrations following administration of either GBR 12909 or d-amphetamine. Nevertheless, we observed enhanced focused stereotypies in 5-HT2CR mutant mice. The finding of phenotypic differences in psychostimulant-evoked nigrostriatal behaviors in the absence of differences in nigrostriatal extracellular dopamine concentration raised the possibility that behavioral responses to released dopamine are enhanced by the htr2c− mutation.
5-HT2CR loss enhances behavioral sensitivity to D1 receptor activation
Phenotypic differences in the response to dopamine release could occur through changes within signaling pathways activated by D1-like and/or D2-like receptors. 5-HT2CR mutant mice demonstrated enhanced sensitivity to the behavioral effects of the D1 receptor agonist SKF 81297, while no phenotypic differences were noted in behavioral responses to the D2 receptor agonist quinpirole. Thus, D1 receptor signaling pathways may contribute substantially to the enhanced behavioral responses of 5-HT2CR mutant mice to psychostimulants.
In light of these findings and the known expression of 5-HT2CRs within the DSt (Alex et al., 2005), we evaluated D1 receptor expression and function in wild-type and mutant mice. We found no phenotypic difference in DSt D1 receptor expression by autoradiography. We then examined DSt MSN responses to D1 receptor stimulation. Electrophysiological studies revealed enhancement of firing after D1 receptor activation, as has been described (Hernández-López et al., 1997). However, no significant phenotypic differences in the D1 receptor firing enhancement were noted. Additionally, we did not observe gross phenotypic differences in MSN action potential waveform or other basic parameters. Thus, we did not detect significant phenotypic differences in intrinsic striatal function.
Within the basal ganglia, information from the “direct” [consisting of striatal to substantia nigra reticulata/globus pallidus interna (SNr/GPi) projections] and “indirect” [consisting of striatal to globus pallidus externa (GPe) to subthalamic nucleus (STN) projections] pathways is integrated to produce an inhibitory output that is fed back to thalamocortical motor centers (Graybiel, 2004). In addition to its expression in the SNc and DSt components of these circuits, 5-HT2CRs are highly expressed in the STN and SNr/GPi (Pompeiano et al., 1994; Wright et al., 1995; Eberle-Wang et al., 1997). Serotonin applied to STN neurons in slice preparations evokes a large inward current that can be blocked by 5-HT2CR antagonists (Shen et al., 2007). This is accompanied by increases in cell firing (Fox et al., 1998; Stanford et al., 2005; Invernizzi et al., 2007; Shen et al., 2007). Furthermore, serotonin applied to SNr neurons in slice preparations evokes an excitatory inward current that is blocked by 5-HT2CR antagonists (Stanford and Lacey, 1996). Therefore, decreases in 5-HT2CR function could decrease SNr activity. The extent to which functional perturbations within the STN and GPi contribute to the phenotypic abnormalities observed here warrants further investigation.
We conclude that 5-HT2CRs have a substantial role controlling the nigrostriatal dopaminergic system and the behaviors it regulates. Previous studies have already shown that 5-HT2CRs have a role in mesoaccumbal dopaminergic system physiology. 5-HT2CRs are thus key regulators of the two major CNS ascending dopaminergic systems, and may play an important role integrating serotonergic and dopaminergic signaling. 5-HT2CRs may thus prove to be a clinically relevant target for the development of drugs to treat CNS disorders in which dopaminergic systems have been implicated (Di Matteo et al., 1999), including schizophrenia, substance abuse, attention-deficit/hyperactivity disorder, Parkinson's disease, and drug-induced movement disorders.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant MH019552-12 (L.A.); NIH Grant MH065983 and the Brookdale National Fellowship Program (S.J.B.); NIH Grants AA012294, AA012294, AA014619, and DA019962 (L.H.P., L.O'D.); funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (A.B., F.W.H.); and NIH Grant DA11177 and National Alliance for Research on Schizophrenia and Depression (L.H.T.).
References
- Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: a “natural action” approach to movement sequence. J Neurosci. 1998;18:2777–2787. doi: 10.1523/JNEUROSCI.18-07-02777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JW, Berridge KC, Rosen AR. Basal ganglia neural mechanisms of natural movement sequences. Can J Physiol Pharmacol. 2004;82:732–739. doi: 10.1139/y04-061. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex KD, Yavanian GJ, McFarlane HG, Pluto CP, Pehek EA. Modulation of dopamine release by striatal 5-HT2C receptors. Synapse. 2005;55:242–251. doi: 10.1002/syn.20109. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW. Super-stereotypy II: enhancement of a complex movement sequence by intraventricular dopamine D1 agonists. Synapse. 2000;37:205–215. doi: 10.1002/1098-2396(20000901)37:3<205::AID-SYN4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW, Houchard KR, Zhuang X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive compulsive disorder and Tourette's. BMC Biol. 2005;3:4. doi: 10.1186/1741-7007-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn TP, Minabe Y, Middlemiss DN, Shirayama Y, Hashimoto K, Ashby CR., Jr Effect of acute and chronic administration of the selective 5-HT2C receptor antagonist SB-243213 on midbrain dopamine neurons in the rat: an in vivo extracellular single cell study. Synapse. 2002;46:129–139. doi: 10.1002/syn.10116. [DOI] [PubMed] [Google Scholar]
- Blackburn TP, Suzuki K, Ashby CR., Jr The acute and chronic administration of the 5-HT(2B/2C) receptor antagonist SB-200646A significantly alters the activity of spontaneously active midbrain dopamine neurons in the rat: An in vivo extracellular single cell study. Synapse. 2006;59:502–512. doi: 10.1002/syn.20263. [DOI] [PubMed] [Google Scholar]
- Bobillier P, Pettijean F, Salvert D, Ligier M, Seguin S. Differential projections of the nucleus raphe dorsalis and nucleus raphe centralis as revealed by autoradiography. Brain Res. 1975;85:205–210. doi: 10.1016/0006-8993(75)90071-2. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146:286–297. doi: 10.1016/j.neuroscience.2006.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effects of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Chartoff EH, Marck BT, Matsumoto AM, Dorsa DM, Palmiter RD. Induction of stereotypy in dopamine-deficient mice requires striatal D1 receptor activation. Proc Natl Acad Sci U S A. 2001;98:10451–10456. doi: 10.1073/pnas.181356498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Iversen SD. Blockage of amphetamine induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res. 1973;55:369–382. doi: 10.1016/0006-8993(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdère P, Bonhomme N, Lucas G, Le Moal M, Spampinato U. Serotonin enhances striatal dopamine outflow in vivo through dopamine uptake sites. J Neurochem. 1996;66:210–215. doi: 10.1046/j.1471-4159.1996.66010210.x. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, Spampinato U. Role of serotonin2A and serotonin2B/2C receptor subtypes in the control of accumbal and striatal dopamine release elicited in vivo by dorsal raphe nucleus electrical stimulation. J Neurochem. 1999;73:1033–1042. doi: 10.1046/j.1471-4159.1999.0731033.x. [DOI] [PubMed] [Google Scholar]
- De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24:3235–3241. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni G, De Deurwaerdére P, Di Mascio M, Di Matteo V, Esposito E, Spampinato U. Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience. 1999;91:587–597. doi: 10.1016/s0306-4522(98)00655-1. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E. Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin2C/2B receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse. 2000;35:53–61. doi: 10.1002/(SICI)1098-2396(200001)35:1<53::AID-SYN7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, La Grutta V, Esposito E. m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience. 2001;103:111–116. doi: 10.1016/s0306-4522(00)00561-3. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Selective blockade of serotonin2C/2B receptors enhances dopamine release in the rat nucleus accumbens. Neuropharmacology. 1998;37:265–272. doi: 10.1016/s0028-3908(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin 2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Biochemical and electrophysiological evidence that RO 60–0175 inhibits mesolimbic dopaminergic function through serotonin2C receptors. Brain Res. 2000;865:85–90. doi: 10.1016/s0006-8993(00)02246-0. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT2C receptors in the control of central dopamine function. Trends Pharmacol Sci. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Cacchio M, Di Giulio C, Di Giovanni G, Esposito E. Biochemical evidence that the atypical antipsychotic drugs clozapine and risperidone block 5-HT(2C) receptors in vivo. Pharmacol Biochem Behav. 2002a;71:607–613. doi: 10.1016/s0091-3057(01)00714-6. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Cacchio M, Di Giulio C, Esposito E. Role of serotonin2C receptors in the control of brain dopaminergic function. Pharmacol Biochem Behav. 2002b;71:727–734. doi: 10.1016/s0091-3057(01)00705-5. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Esposito E. Selective stimulation of serotonin2C receptors blocks the enhancement of striatal and accumbal dopamine release induced by nicotine administration. J Neurochem. 2004;89:418–429. doi: 10.1111/j.1471-4159.2004.02337.x. [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Lucki I, Chesselet MF. A role for the subthalamic nucleus in 5-HT2C-induced oral dyskinesia. Neuroscience. 1996;72:117–128. doi: 10.1016/0306-4522(95)00548-x. [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF. Pattern of expression of the serotonin 2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol. 1997;384:233–247. [PubMed] [Google Scholar]
- Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7:177–185. doi: 10.2174/138945006775515455. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Miller JJ. An anatomical and electrophysiological investigation of the serotonergic projection from the dorsal raphe nucleus to the substantia nigra in the rat. Neuroscience. 1977;2:975–987. [Google Scholar]
- Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Fox SH, Moser B, Brotchie JM. Behavioral effects of 5-HT2C receptor antagonism in the substantia nigra zona reticulata of the 6-hydroxydopamine-lesioned rat model of Parkinson's disease. Exp Neurol. 1998;151:35–49. doi: 10.1006/exnr.1998.6792. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Tecott LH. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur J Pharmacol. 2004;488:1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Nigral dopamine neurons: intracellular recording and identification with L-DOPA injection and histofluorescence. Science. 1980;210:654–656. doi: 10.1126/science.7433992. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Network-level neuroplasticity in cortico-basal ganglia pathways. Parkinsonism Relat Disord. 2004;10:293–296. doi: 10.1016/j.parkreldis.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L. Behavioral properties of GBR 12909, GBR 13069 and GBR 13098: specific inhibitors of dopamine uptake. Eur J Pharmacol. 1984;103:241–248. doi: 10.1016/0014-2999(84)90483-7. [DOI] [PubMed] [Google Scholar]
- Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones DN, Holtzman SG, Juncos JL, Kalivas PW, Justice JB., Jr Individual differences in behavior following amphetamine, GBR-12909, or apomorphine but not SKF-38393 or quinpirole. Psychopharmacology (Berl) 1994;116:217–225. doi: 10.1007/BF02245065. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein βγ subunits. J Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi RW, Pierucci M, Calcagno E, Di Giovanni G, Di Matteo V, Benigno A, Esposito E. Selective activation of 5-HT(2C) receptors stimulates GABA-ergic function in the rat substantia nigra pars reticulata: a combined in vivo electrophysiological and neurochemical study. Neuroscience. 2007;144:1523–1535. doi: 10.1016/j.neuroscience.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Kelland MD, Freeman AS, Chiodo LA. Serotonergic afferent regulation of the basic physiology and pharmacological responsiveness of nigrostriatal dopamine neurons. J Pharmacol Exp Ther. 1990;253:803–811. [PubMed] [Google Scholar]
- Kelland MD, Freeman AS, Rubin J, Chiodo LA. Ascending afferent regulation of rat midbrain dopamine neurons. Brain Res Bull. 1993;31:539–546. doi: 10.1016/0361-9230(93)90121-q. [DOI] [PubMed] [Google Scholar]
- Matell MS, Berridge KC, Wayne Aldridge J. Dopamine D1 activation shortens the duration of phases in stereotyped grooming sequences. Behav Processes 71:241–249. e mesoaccumbens circuit by serotonin 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Neurosci. 2006;21:7781–7787. doi: 10.1016/j.beproc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Mengod G, Nguyen H, Le H, Waeber C, Lübbert H, Palacios JM. The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry. Comparison with receptor binding distribution. Neuroscience. 1990;35:577–591. doi: 10.1016/0306-4522(90)90330-7. [DOI] [PubMed] [Google Scholar]
- Navailles S, De Deurwaerdère P, Porras G, Spampinato U. In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2004;29:319–326. doi: 10.1038/sj.npp.1300329. [DOI] [PubMed] [Google Scholar]
- Nedergaard S, Bolam JP, Greenfield SA. Facilitation of a dendritic calcium conductance by 5-hydroxytryptamine in the substantia nigra. Nature. 1988;333:174–177. doi: 10.1038/333174a0. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Quantitative approaches to in vivo brain microdialysis. Crit Rev Neurobiol. 1994;8:189–220. [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. San Diego: Academic; 2001. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdère P, Caccia S, Esposito E, Spampinato U. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Prisco S, Pagannone S, Esposito E. Serotonin-dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J Pharmacol Exp Ther. 1994;271:83–90. [PubMed] [Google Scholar]
- Saito H, Matsumoto M, Togashi H, Yoshioka M. Functional interactions between serotonin and other neuronal systems: focus on in vivo microdialysis studies. Jpn J Pharmacol. 1996;70:203–225. doi: 10.1254/jjp.70.203. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Trulson ME, German DC. Electrophysiological properties of mouse dopamine neurons: in vivo and in vitro studies. Neuroscience. 1984;12:793–801. doi: 10.1016/0306-4522(84)90171-4. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Kozell LB, Johnson SW. Multiple conductances are modulated by 5-HT receptor subtypes in rat subthalamic nucleus neurons. Neuroscience. 2007;148:996–1003. doi: 10.1016/j.neuroscience.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Kantaria MA, Chahal HS, Loucif KC, Wilson CL. 5-Hydroxytryptamine induced excitation and inhibition in the subthalamic nucleus: action at 5-HT(2C), 5-HT(4) and 5-HT(1A) receptors. Neuropharmacology. 2005;49:1228–1234. doi: 10.1016/j.neuropharm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Wang RY. Dopaminergic neurons in the rat ventral tegmental area. I. Identification and characterization. Behav Res Rev. 1981;3:123–140. [Google Scholar]
- Ward RP, Dorsa DM. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol. 1996;370:405–414. doi: 10.1002/(SICI)1096-9861(19960701)370:3<405::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci Lett. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]