Abstract
A 5-year-old Thoroughbred-cross mare was diagnosed with insulin-dependent diabetes mellitus. Partial glycemic control and clinical improvement were achieved with daily insulin administration for 18 mo. The mare subsequently developed evidence of hypoadrenocorticism and died. Necropsy findings included lymphocytic infiltration of the pancreas, adrenal cortex, adrenal medulla, and thyroid glands, suggestive of an immune-mediated polyendocrinopathy.
Résumé
Diabète insulino-dépendant associé à un syndrome pluri-endocrinien auto-immun présumé chez une jument. Une jument Thoroughbred croisée âgée de 5 ans a été diagnostiquée avec le diabète insulino-dépendant. Le contrôle glycémique partiel et l’amélioration clinique ont été obtenus avec l’administration quotidienne d’insuline pendant 18 mois. La jument a ensuite développé des signes d’hypoadrénocorticisme et est morte. Les constatations à la nécropsie incluaient l’infiltration lymphocytaire du pancréas, du cortex surrénalien, de la médullosurrénale et des glandes thyroïdiennes, ce qui suggère une poly-endocrinopathie à médiation immunitaire.
(Traduit par Isabelle Vallières)
Diabetes mellitus (DM) is associated with persistent hyperglycemia due to deficient production of or reduced sensitivity to insulin (1). Diabetes is classified into insulin-dependent diabetes mellitus (IDDM or type 1) and noninsulin-dependent diabetes mellitus (NIDDM or type 2). Other categories in humans include secondary diabetes mellitus (type S), impaired glucose tolerance (IGT), and gestational diabetes (1,2). Diabetes mellitus is uncommon in horses and the few reported cases have included insulin resistant and type S DM, secondary to pituitary-dependent hyperadrenocorticism (1,3–11). Other reported causes of diabetes in horses include chronic pancreatitis, granulosa cell ovarian tumor, and pregnancy (1,6,8,11). Type I diabetes is even less common in horses (3,6). Johnson et al (3) recently reported a case of IDDM in a Spanish mustang mare, diagnosed with an abnormally low serum insulin concentration [21 pmol/L, reference interval (RI): 30 to 300 pmol/L] in the face of persistent hyperglycemia (20.2 mmol/L, RI: 4.0 to 6.3 mmol/L) (3). Insulin-dependent diabetes mellitus occurs as a result of insulin deficiency due to destruction of pancreatic beta cells; in humans this usually progresses to a state of absolute insulin deficiency (2). In most human patients, an autoimmune process is thought to play a significant role in the development of IDDM (2).
Insulin-dependent diabetes mellitus is successfully managed in several species, most notably humans, dogs, and cats. Of the many challenges in the treatment of IDDM in horses, among the most significant is the paucity of clinical and reported experience with long-term administration of insulin, the absence of monitoring tools, and little experience regarding dietary and exercise management.
Case description
A 470-kg, 5-year-old Thoroughbred-Percheron cross mare was presented to the Veterinary Medical Teaching Hospital (VMTH), University of California, for a 6-week history of weight loss despite polyphagia, as well as polydipsia and polyuria. The mare had been consuming approximately 170 L/d (RI: 17.6 ± 0.998 to 31.4 ± 1.05 L/d for 384 to 474 kg horses on varying diets) (12). The mare also had a 3-day history of pyrexia, cough, nasal discharge, and decreased appetite.
One week prior to presentation, a serum chemistry panel and complete blood (cell) count (CBC) were performed. These revealed marked hyperglycemia (16.0 mmol/L, RI: 4.4 to 5.9 mmol/L), increased liver enzymes (AST 940 IU/L, RI: 168 to 494 IU/L; GGT 45 U/L, RI: 8 to 22 IU/L), increased anion gap (AG 21, RI: 9 to 17); hypochloremia (84 mEq/L, RI: 91 to 104 mEq/L), and a mild anemia (hematocrit = 27.3%, RI: 30% to 44%). One day prior to presentation, a repeat CBC and serum biochemistry panel revealed more pronounced hyperglycemia (17.6 mmol/L), further increased liver and muscle enzymes (AST 1422 IU/L, GGT 56 IU/L, CK 361 IU/L), higher anion gap (AG 23), and persistent hypochloremia (81 mEq/L) and anemia (Hct = 25%).
Physical examination abnormalities included lethargy, a body condition score (BCS) of 3/9, injected and icteric sclera, mild bilateral nasal discharge, a grade III/VI diastolic murmur on the left and a III/VI holosystolic murmur on the right, and increased digital pulses. Mild crackles were ausculted with a rebreathing bag in the right ventral lung field. Differential diagnosis for the hyperglycemia included pars intermedia dysfunction (Cushing’s disease), metabolic syndrome or insulin resistance, pancreatitis, granulosa cell tumor, alimentary lymphoma, liver disease, neoplasia, and primary diabetes mellitus.
Additional diagnostic tests included measurement of serum amylase, lipase, triglyceride, and bile acid, blood ammonia, and plasma endogenous ACTH concentrations. Tests to further evaluate the hyperglycemia included endogenous insulin, fructosamine, and glycosylated hemoglobin concentrations. Diagnostics to rule out a granulosa cell ovarian tumor included measurement of serum inhibin, testosterone and progesterone concentrations. The amylase, lipase, bile acids, endogenous ACTH concentrations, and granulosa cell tumor panel results were within normal limits. The mare’s serum was grossly lipemic and the triglyceride concentration was markedly increased (34.1 mmol/L, RI: 0.02 to 0.46 mmol/L). The endogenous insulin concentration was below the level of detection (< 9 pmol/L, RI: 29 to 179 pmol/L). The fructosamine concentration was increased (559 μmol/L, RI: 316 to 402 μmol/L, based on concentrations found in 3 clinically normal age-matched horses), as was glycosylated hemoglobin (Hb A1c) (24.3%, RI: 2.5% to 5.0%). A urinalysis revealed marked glucosuria, mild to moderate ketonuria, and trace hematuria. The urine specific gravity was 1.035, although this was likely increased due to the presence of glucose and ketones. Due to the mare’s debilitated condition, increased digital pulses, and persistent hyperglycemia, a dexamethasone suppression test was not performed.
Additional diagnostics for the acute signs of fever, cough, and nasal discharge included thoracic and abdominal ultrasound. Cardiac ultrasonographic findings were unremarkable and mild pleural irregularities (“comet tails”) were present on the right cranioventral thorax. Abdominal ultrasound revealed mild hepatomegaly with increased echogenicity, consistent with hepatic lipidosis or fibrosis. No parasites were detected on fecal flotation. Results from a clotting panel were within normal limits. A liver biopsy revealed hepatic lipidosis.
A diagnosis of IDDM was made based on the clinical signs, persistent hyperglycemia, low insulin, and high glycosylated hemoglobin and fructosamine concentrations. The hypertriglyceridemia and polyuria/polydipsia were attributed to IDDM. The mare was also diagnosed with presumptive mild pneumonia based on the recent onset of fever, cough and nasal discharge as well as the thoracic ultrasound findings.
The mare was administered a balanced electrolyte solution (Normosol R; Abbott Laboratories, North Chicago, Illinois, USA), 10 mL/kg over 1 h, and was started on regular insulin (Humulin® R; Eli Lilly, Indianapolis, Indiana, USA), 0.1 U/kg, IM, q12h. Blood glucose concentrations were monitored every 2 h using a bedside glucometer (Accu-Check Instant, Boehringer Mannheim, Indianapolis, Indiana, USA). The horse was initially fed approximately 3 to 4 kg each of alfalfa and oat hay, 2.4 kg of a complete pelleted feed (Equine Senior; Purina Mills LLC, St. Louis, Missouri, USA), a small amount of wheat bran mash, 237 mL corn oil twice a day, and 3000 IU vitamin E daily for the first week. The diet fed longer term is described below. Flunixin meglumine (Banamine; Intervet/Schering-Plough Animal Health, Elkhorn Nebraska, USA), 0.5 mg/kg, IV, q12h was also administered. Trimethoprim sulfamethoxazole (Sulfamethoxazole and Trimethoprim Double Strength; Amneao Pharmaceuticals, Glasgow, Kentucky, USA), 30 mg/kg PO, q12h was empirically administered for possible mild pneumonia. Unfortunately, a transtracheal wash was not performed. The glucose concentrations remained increased, and the frequency of insulin administration was increased to every 6 h. This improved blood glucose values, but nadir values were still increased (peak 17.8 mmol/L, nadir 13.1 mmol/L).
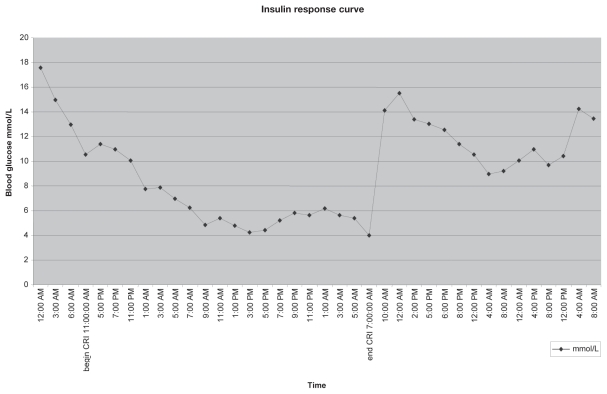
A constant rate infusion (CRI) of regular insulin (Humulin® R; Eli Lilly), 0.1 U/kg/h was initiated to improve glucose control. Blood glucose concentrations decreased and were within the normal range by 14 h post-initiation of the infusion (Figure 1). The mare’s appetite and mentation improved concurrently. Triglyceride concentrations markedly improved, but remained mildly increased (0.81 mmol/L). The insulin CRI was discontinued after 40 h. A 5-day course of larvicidal-dose fenbendazole (10 mg/kg, PO, q24h) (Panacur PowerPac; Intervet Ln. Millsboro, Delaware, USA) was administered to treat for possible pancreatitis induced by aberrant parasite migration, as has been previously reported (8).
Figure 1.
Blood glucose concentrations in response to a continuous infusion of regular insulin.
A variety of insulin formulations and doses were tried for long-term management. The goal of insulin therapy was to maintain blood glucose concentrations < 11 mmol/L. Initially the mare was treated with Ultralente insulin (0.3 to 0.4 U/kg IM, q24h; Humilin® U Ultralente®; Eli Lilly) and metformin (Glucophage® XR®; Bristol-Myers Squibb, New York, New York, USA), 1 mg/kg PO, q24h. The mare was discharged after 1 wk, when the blood glucose concentrations were ranging from 9.3 to 13 mmol/L and she had gained 10 kg. The respiratory signs (nasal discharge, cough, fever) had improved. The ongoing care was managed by the owner, a veterinary student.
After 2 wk of therapy with ultralente insulin and metformin, blood glucose concentrations increased and ranged from 13.9 to 22.2 mmol/L. The insulin dose was reduced to 0.04 U/kg because of concerns over possible Somogyi effect as the cause of the hyperglycemia. After 10 d at this reduced insulin dose, the mare became increasingly lethargic and anorexic, the serum became grossly lipemic, hyperglycemia persisted, and further weight loss was noted. Subsequently, the dose of ultralente insulin was again increased to 0.4 U/kg q24h as a Somogyi effect was now considered unlikely. Metformin was increased to 1 mg/kg PO, q12h, followed by 2 mg/kg q12h after 1 wk and glyburide was added (Micronase; Pfizer, Morris Plains, New Jersey, USA), 0.01 mg/kg PO, q24h, then 0.02 mg/kg q12h after 1 wk due to persistent hyperglycemia.
Insulin glargine (Lantus®; Aventis, Bridgewater New Jersey, USA), 0.2 U/kg SQ, q24h, another long acting insulin, was started because of poor glucose control with ultralente insulin. Glargine is an insulin analogue that has the advantage of continuous activity for 24 h, without major fluctuations in plasma concentrations. Although the mare remained bright and alert, she appeared to lose weight and remained hyperglycemic. The dose was gradually increased to 0.4 U/kg, which resulted in moderate improvement; blood glucose concentrations ranged from 11.8 to 16.9 mmol/L. Although good clinical control was achieved with 0.4 U/kg of glargine, an alternative type of insulin was sought due to financial constraints. Treatment with an intermediate-acting insulin, NPH (Humulin® N; Eli Lilly), 0.4 U/kg q24h was initiated. Within 3 wk of instituting this therapy the mare had gained weight (520 kg). Three months into therapy the mare’s water consumption was recorded to be 75 L/day, less than half of what it had been prior to treatment. This dose and form of insulin was continued for 58 wk. At that time the mare weighed 535 kg, had a body condition score of 5/9 and was returned to her previous level of work as a riding horse. A blood glucose curve obtained while on the NPH insulin showed an 18-h period during which blood glucose was maintained < 11 mmol/L. By the 24-h mark post-injection, the blood glucose ranged from 11.1 to 13.9 mmol/L.
Upon discharge and for longer term management, the mare was fed Orchard grass hay free choice, along with 1.4 kg complete feed pellets (Equine Senior Purina Mills LLC; St. Louis, Missouri, USA), 237 mL corn oil, and 0.7 kg alfalfa pellets twice a day along with a vitamin/mineral supplement (Red Cell; Farnam Companies, Phoenix, Arizona, USA). After 1 y, the hay was changed to alfalfa hay and the hay pellets to grass hay due to availability.
Thirteen months into therapy, fructosamine remained elevated at 606 mmol/L, while glycosylated hemoglobin (Hb A1c) values had dropped to 11.3%. The decrease in glycosylated hemoglobin percentage was consistent with improvement in long-term hyperglycemia, while the high fructosamine concentrations and 18-h duration of glucose control reflected only partial control.
Upon return to full exercise, an increased sensitivity to insulin and occasional post-exercise hypoglycemia (2.2 to 3.3 mmol/L) were measured. Clinical signs of hypoglycemia were not noted by the owner. For a period of 48 to 72 h after the hypoglycemic episodes, the mare was consistently hyperglycemic, possibly representing a Somogyi effect. These post-exercise hypoglycemic and subsequent hyperglycemic episodes led to a more regular schedule of riding or lunging for 20 to 45 min, 5 to 6 days a week. The mare was exercised in the morning before feeding, and prior to insulin administration.
Eighteen months into therapy, the mare developed persistent, marked hyperglycemia (22.7 mmol/L) and was increasingly polyuric and polydipsic. The insulin dose was increased (0.5 U/kg IM q24h) without notable effect. The mare’s vital signs and a CBC were unremarkable, but she was lethargic. A chemistry panel indicated marked hyponatremia (104 mEq/L), hypochloremia (70 mEq/L), and hyperkalemia (7.0 mEq/L) without azotemia. The serum triglyceride concentration (3.0 mmol/L) was also increased. The mare subsequently died prior to treatment.
A necropsy revealed a small pancreas at 0.22 kg (reference = 0.35 kg) with marked, segmental parenchymal collapse, and bilaterally enlarged adrenal glands (right = 31.52 g, left = 27.04 g, RI: 15 to 17 g) with marked cortical atrophy (13,14). Urine obtained from the bladder contained 55.5 mmol/L of glucose (Chemstrip; Roche Diagnostics, Palo Alto, California, USA). The thyroid glands were diffusely pale red-brown. The pituitary gland was grossly normal.
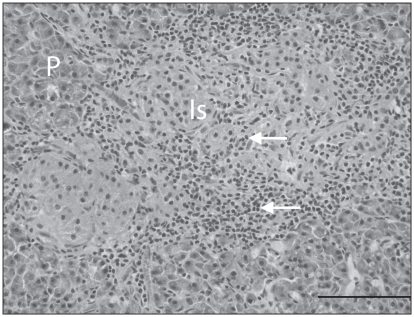
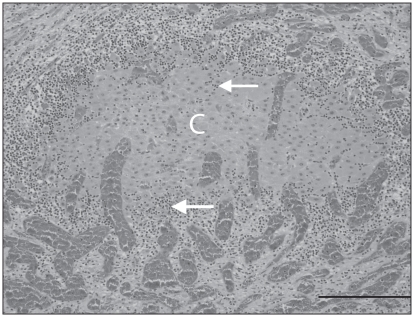
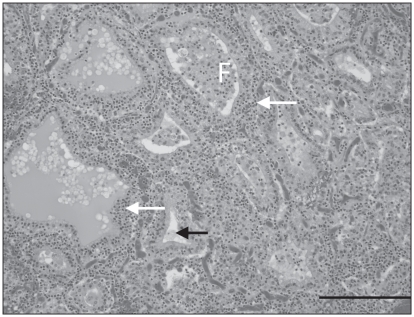
Histologically, multiple pancreatic lobules were partially to completely effaced by a moderately to markedly cellular inflammatory infiltrate of small lymphocytes and rare plasma cells. These cells dissected between the acini and acinar cells of the exocrine pancreas, resulting in mild disruption of lobular architecture to complete lobular collapse. Islets of the endocrine pancreas were infiltrated by a similar population of lymphocytes (Figure 2). There was diffuse congestion of the pancreatic vessels and occasional multifocal intralobular hemorrhage. The adrenal cortex and medulla were effaced by a markedly cellular lymphocytic infiltrate with rare plasma cells, similar to that in the pancreas, with segmental loss of cells in the zona glomerulosa, zona fasiculata, and zona reticularis, resulting in atrophy of the adrenal cortex. Remaining clusters of adrenal cortical cells were rimmed by lymphocytes and plasma cells (Figure 3) that also multifocally infiltrated the capsule. The adrenal medulla was expanded and the architecture disrupted by large numbers of lymphocytes and rare plasma cells that dissected between, separated and replaced chromaffin cells. Beneath the adrenal capsule, there were multifocal aggregates of hemosiderin laden macrophages, indicating previous hemorrhage. The thyroid glands were diffusely infiltrated by a markedly cellular infiltrate of lymphocytes and rare plasma cells, similar to that previously described that dissected between, frequently separated and occasionally transmigrated the walls of follicles, which had markedly depleted colloid (Figure 4). There were no histologic abnormalities in the pituitary gland.
Figure 2.
Chronic lymphocytic pancreatic isletitis with β-cell depletion. Centrally, there are multiple pancreatic islets (Is) surrounded and infiltrated by numerous small lymphocytes (white arrows) resulting in β-cell depletion (identification of β-cells by immunohistochemistry not shown). Peripherally, there is a small amount of lymphocytic inflammation infiltrating the exocrine pancreas (P). Hematoxylin and eosin (H&E). Bar =100 μm.
Figure 3.
Chronic lymphocytic adrenalitis with cortical atrophy. Remnant adrenal cortical cells (C) are surrounded and infiltrated by abundant small lymphocytes (white arrows) and there is loss of surrounding adrenal cortical cells with collapse of the parenchyma (atrophy). Cortical vessels are congested. H&E. Bar = 200 μm.
Figure 4.
Chronic lymphocytic thyroiditis with colloid depletion. Large numbers of small lymphocytes (white arrows) infiltrate and disrupt normal thyroid follicular (F) architecture, and infiltrate and expand the interfollicular space. Colloid is depleted within some follicles (black arrow). H&E. Bar = 200 μm.
Immunohistochemical staining of the pancreatic islets with antibodies to insulin (polyclonal guinea pig; Dako, Carpinteria, California, USA) and glucagon (polyclonal guinea pig; Linco Reseach, St. Charles, Missouri, USA) demonstrated marked β-cell depletion in the presence of a normal α-cell density. Immunophenotyping of the lymphocytic infiltrate in the pancreas, adrenal and thyroid glands demonstrated large numbers of lymphocytes with strong immunoreactivity to CD3 antibody (monoclonal rat, clone CD3-12; Moore, PF, University of California, Davis, California, USA), indicating the majority of lymphocytes were T-lymphocytes, and small numbers of lymphocytes with immunoreactivity to CD79a (monoclonal mouse, clone HM57; Dako) and CD20 (polyclonal rabbit; Thermo Scientific, Fremont, California, USA) antibodies, indicating there were small numbers of B-lymphocytes and/or plasma cells. The rearrangement of the variable region of the antigen receptor genes of the T- and B-lymphocyte populations was examined by PCR (Moore PF, unpublished data). The T-cell receptor γ genes were polyclonal. The B-cell immunoglobulin heavy chain genes were oligoclonal with 4 distinct clones in the adrenal gland.
The following morphologic diagnoses were made: 1) pancreas (endocrine): mild multifocal lymphocytic isletitis (insulitis) with severe diffuse β-cell depletion; 2) pancreas (exocrine): severe regionally extensive lymphocytic pancreatitis with segmental lobular atrophy; 3) adrenal cortex: severe diffuse bilateral lymphocytic adrenalitis with marked segmental cortical atrophy; 4) adrenal medulla: severe diffuse bilateral lymphocytic adrenalitis; 5) thyroid: severe diffuse bilateral lymphocytic thyroiditis with colloid depletion.
Discussion
To the authors’ knowledge, long-term therapy of IDDM in the horse has not been described in the peer-reviewed literature. Previously, a pony with diabetes secondary to pancreatitis had been satisfactorily controlled using protamine zinc insulin 0.5 U/kg intramuscularly q12h for 42 d (6). In previously described cases of equine diabetes, when the disorder was secondary to pituitary dysfunction, the animals were reportedly markedly insulin-resistant and refractory to successful management (1). The mare of our report had no evidence of pituitary dysfunction; antemortem clinical evidence of the underlying polyendocrine disease diagnosed at necropsy was IDDM due to insulin deficiency and terminal electrolyte abnormalities consistent with adrenal insufficiency. The electrolyte abnormalities and death of the horse may have been associated with adrenal insufficiency, as the adrenal gland also exhibited lymphocytic inflammation with effacement of the adrenal cortex.
Good clinical results, including weight gain, return to exercise, improvement in polyuria/polydipsia, and partial glycemic control were achieved with insulin administered once daily. Long-term glycemic control is monitored with serial glycosylated hemoglobin samples in other species (15). Glycosylated hemoglobin (HbA1c) is less affected by daily or weekly variations in glucose concentrations than is fructosamine or single plasma glucose values (2). This minimizes the over-interpretation of transient hyperglycemia. In the mare of our report, HbA1c dropped to less than half of its original value, consistent with at least partial response to insulin treatment.
Johnson et al (3) used a combination therapy of metformin and glyburide, which was helpful in achieving short-term glycemic control in a diabetic mare; however, the horse in that report was euthanized after 6 wk (3). Metformin is used to decrease hepatic glucose production, decrease intestinal absorption of glucose, reduce fatty acid oxidation, and improve tissue insulin sensitivity in type-2 diabetics (16,17). Many of the metabolic alterations brought about by insulin resistance are ameliorated by metformin, making this a front line medication for the treatment of type-2 diabetes and metabolic syndrome in other species (16). Recently, a study showed low bioavailability of metformin in horses; an effective dosing regimen remains to be developed (18). Glyburide stimulates the release of endogenous insulin from beta-cells; as such, it is dependent on functional beta-cells. The lack of response of the mare of our report to glyburide is consistent with IDDM rather than type-2 diabetes (19).
Dietary management is an important component of diabetes in other species. High fiber, low glycemic index foods with healthy fats are recommended for humans with diabetes mellitus. Dietary recommendations for diabetes mellitus in small animals include frequent, small feedings of a high fiber, moderate fat, and low simple carbohydrate diet (20). In horses, the optimal diet is unknown, but it is likely that a high fiber, low nonstructural carbohydrate diet would be optimal, with added calories in the form of fat. Blood glucose and insulin concentrations have been shown to vary minimally after a meal of hay, whereas after 1.5 to 2 kg of a high starch concentrate blood glucose peaked at 5.5 to 11.1 mmol/L within 60 to 90 min in normal horses; insulin peaks of 190 IU/mL occurred at 90 to 120 min (21). In this same study, concentrate feeds containing high fat (6% to 10%), fiber [> 20% neutral detergent factor (NDF)], or both resulted in lower glycemic responses and may be better suited for use in diabetic horses (21). In retrospect, the mare’s initial diet over the first week could have been improved in that oat hay has a high carbohydrate load. However, at that point she was partially anorexic and was encouraged to eat by using a variety of feeds. The alfalfa, grass hay, alfalfa pellets, and wheat bran fed were appropriate for a horse with DM. The complete senior feed used in this case has a glycemic index similar to oats (22). Feeds with lower indices, therefore, would have been better. At the time this case was managed there were no commercially available low glycemic feeds. Currently, there are commercially available feeds that have a low glycemic index, with low to no starch and sugars that would make an ideal diet for diabetic horses. Examples of such diets include WellSolve L/S (Purina Mills LLC, St. Louis, Missouri, USA) and Safe Choice (Nutrena Animal Feeds, Elk River, Minnesota, USA). Feeds with low glycemic indices for horses include alfalfa, some grass hays, rice bran, beet pulp (without molasses), and soybean hulls; wheat bran has a moderate index (23).
Exercise has numerous therapeutic benefits in diabetic patients. Despite this, exercise can result in hypoglycemia in type-1 diabetic patients as it did in the mare of this report. A relative excess of insulin during exercise, due to increased muscular sensitivity to insulin, is an important mechanism for causing hypoglycemia (24). Limits in the duration and intensity of exercise, as well as adjustments in insulin dose, were used in the management of the mare of our report. Acute exercise increases the sensitivity of muscle to insulin and the receptor affinity for insulin in horses (22–28). Powell et al (29) found that insulin sensitivity was improved by 60% after 7 consecutive days of exercise in obese mares compared with obese sedentary mares, and 48% in lean exercised mares compared with lean sedentary mares (29). In addition to increased sensitivity to insulin, acute bouts of exercise increase the utilization of glucose and therefore result in decreases in blood glucose concentration (21).
Based on the presence of massive lymphocytic infiltrates in multiple endocrine organs and 2 clinically manifested endocrine disorders, a presumptive diagnosis of autoimmune polyendocrine syndrome (APS), type II was made. This syndrome is defined in humans by the presence of Addison’s disease (AD), IDDM, and chronic thyroiditis (30). In humans, APS is hypothesized to occur secondary to loss of immune tolerance to a peptide(s) in a molecule of the target organ. This loss of self recognition is followed by clonal expansion of CD4 T-cells that recognize the peptide(s), promote release of pro-inflammatory cytokines, and stimulate autoantibody production by B-cells (30). The diagnosis of APS in humans is made antemortem based on characteristic biochemical abnormalities associated with loss of endocrine function and assays for various organ specific autoantibodies (30,31). The diagnosis in this horse was made based on biochemical abnormalities and histology. This horse had terminal electrolyte abnormalities consistent with AD and had IDDM. Histologically, there was chronic lymphocytic adrenalitis, pancreatitis, and thyroiditis. Alone, each of these conditions is rare in horses; combined in 1 horse, they make a compelling case for APS Type II.
Hypoadrenocorticism is rare in horses with 1 report in a neonatal foal, and 1 in an adult horse secondary to exogenous steroid administration (32,33). Adrenal insufficiency in this case was secondary to lymphocytic adrenalitis and loss of steroidogenic adrenal cortical cells. Hypoadrenocorticism causing electrolyte derangements, hypovolemic shock, and vascular collapse is the presumed cause of death. The autoimmune nature of the inflammation in this horse was presumed. In humans, 60% to 70% of cases of AD are autoimmune (34). Definitive diagnosis of autoimmune AD is based on the presence of autoantibodies to enzymes in the cortisol synthesis pathway such as 21-hydroxylase, 17α-hydroxylase, and P450-side chain cleavage enzyme; tests for these enzymes are not available for horses (35).
There was similar lymphocytic inflammation in the pancreatic islets and β-cell depletion in the presence of a normal α-cell density, which suggests the lymphocytic infiltrate was directed at the β-cells. The lymphocytic infiltrate in the exocrine pancreas could be an extension from the islets or due to a cross reaction with an antigen similar to β-cell antigens. In humans with IDDM, the autoimmune attack is directed against multiple β-cell antigens, including glutamic acid decarboxylase.
Immune-mediated (Hashimoto-like) thyroiditis is rare in horses with only a single case series reported (36). In that study, lymphocytic thyroiditis diagnosed histologically was interpreted as autoimmune based on the presence of increased serum concentrations of thyroglobulin and thyroperoxidase autoantibody (36). In this horse, there were no clinical signs associated with the thyroiditis and the autoimmune nature of the lymphocytic infiltrate was presumed.
Immunophenotyping of the lymphocytic infiltrate in the pancreas, adrenal, and thyroid demonstrated a predominance of T-cells with rare B-cells. The T-cell population in the adrenal, pancreas, and thyroid was polyclonal, ruling out disseminated lymphoma. The B-cell population in the adrenal was oligoclonal with 4 distinct clones suggesting there was restricted diversity of the B-cell lymphocytic infiltrate and clonal expansion of cells responding to specific antigens.
In conclusion, this is the first report of long-term (> 6 wk) treatment of IDDM in a horse. In this mare, the inciting cause of IDDM was a polyendocrinopathy, suspected to be autoimmune, which has not been previously described in horses. Though complete control of hyperglycemia was not achieved, partial control led to clinical improvement with return of the horse to previous athletic function. Further study of long-term control of diabetes mellitus and hyperglycemia, as well as the pathophysiology of immune-mediated polyendocrinopathy, in horses is warranted.
Acknowledgement
The authors acknowledge Dr. Jaromir Benak for his contribution to the necropsy and postmortem findings. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Stogdale L. Definition of diabetes mellitus. Cornell Vet. 1986;76:156–174. [PubMed] [Google Scholar]
- 2.Kuzuya T, Nakagawa S, Satoh J, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 3.Johnson PJ, Scotty NC, Wiedmeyer C, Messer NT, Kreeger JM. Diabetes mellitus in a domesticated Spanish Mustang. J AmVet Med Assoc. 2005;226:584–588. doi: 10.2460/javma.2005.226.584. [DOI] [PubMed] [Google Scholar]
- 4.King JM, Kavanaugh JF, Bentinck-Smith J. Diabetes mellitus with pituitary neoplasms in a horse and dog. Cornell Vet. 1962;52:133–145. [PubMed] [Google Scholar]
- 5.Tasker JB, Whiteman CE, Martin BR. Diabetes mellitus in the Horse. J Am Vet Med Assoc. 1996;49:393–399. [PubMed] [Google Scholar]
- 6.Jeffery JR. Diabetes Mellitus secondary to chronic pancreatitis in a pony. J Am Vet Med Assoc. 1968;153:1168–1175. [PubMed] [Google Scholar]
- 7.Baker JR, Ritchie HE. Diabetes mellitus in the horse: A case report and review of the literature. Equine Vet J. 1974;6:7–11. doi: 10.1111/j.2042-3306.1974.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 8.Bulgin MS, Anderson BC. Verminous arteritis and pancreatic necrosis with diabetes mellitus in a pony. Compend Contin Educ Pract Vet. 1983;5:S482–S485. [Google Scholar]
- 9.Ruoff WW, Baker DC, Morgan SJ, Abbitt B. Type II diabetes mellitus in a horse. Equine Vet J. 1986;18:143–144. doi: 10.1111/j.2042-3306.1986.tb03571.x. [DOI] [PubMed] [Google Scholar]
- 10.Muylle E, Van Den Hende C, Deprez P, Nuytten J, Oyaert W. Non-insulin dependent diabetes mellitus in a horse. Equine Vet J. 1986;18:145–146. doi: 10.1111/j.2042-3306.1986.tb03572.x. [DOI] [PubMed] [Google Scholar]
- 11.McCoy DJ. Diabetes mellitus associated with bilateral granulosa cell tumors in a mare. J Am Vet Med Assoc. 1986;188:733–735. [PubMed] [Google Scholar]
- 12.Fonnesbeck PV. Consumption and excretion of water by horses receiving all hay and hay-grain diets. J Anim Sci. 1968;27:1350–1356. [Google Scholar]
- 13.Sisson S. Sisson and Grossman’s The Anatomy of Domestic Animals. 5th ed. Philadelphia: WB Saunders; 1975. Digestive system; p. 491. [Google Scholar]
- 14.Dybdal NO. Large Animal Internal Medicine. 3rd ed. St. Louis, Missouri: Mosby; 2002. Endocrine and metabolic disorders; p. 1235. [Google Scholar]
- 15.Scroggie DA, Albright A, Harris MD. The effect of glucosamine- chondroitin supplementation on glycosylated hemoglobin levels in patients with type 2 diabetes mellitus, a placebo-controlled, double- blinded, randomized clinical trial. Arch Intern Med. 2003;163:1587–1590. doi: 10.1001/archinte.163.13.1587. [DOI] [PubMed] [Google Scholar]
- 16.Giannarelli R, Aragona M, Coppelli A, Del Prato S. Reducing insulin resistance with metformin: The evidence today. Diabet Metab. 2003;29:6, S28–6S, 35. doi: 10.1016/s1262-3636(03)72785-2. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti P, Del Guerra S, Marselli L, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrin Metab. 2004;89:5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- 18.Hustace JL, Firshman AM, Mata JE. Pharmacokinetics and bioavailability of metformin in horses. Am J Vet Res. 2009;70:665–668. doi: 10.2460/ajvr.70.5.665. [DOI] [PubMed] [Google Scholar]
- 19.Strowig SM, Raskin P. Combination therapy using metformin or thiazolidinediones and insulin in the treatment of diabetes mellitus. Diab Obesity Metab. 2005;7:633–641. doi: 10.1111/j.1463-1326.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- 20.Nelson RW, Couto CG. Small Animal Internal Medicine. 3rd ed. St. Louis, Missouri: Mosby; 2003. pp. 792–794. [Google Scholar]
- 21.Ralston SL. Insulin and glucose regulation. Vet Clin North Am Equine Pract. 2002;18:295–304. doi: 10.1016/s0749-0739(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 22.Pagan JD. Nutritional management of metabolic disorders. Kentucky Equine Research, Inc.; Versailles, Kentucky: [Google Scholar]
- 23.Rodiek AV, Stull CL. Glycemic index of ten common horse feeds. J Eq Vet Sci. 2007;27:205–211. [Google Scholar]
- 24.Sandoval DA, Aftab Guy DL, Richardson MA, Ertl AC, Davis SN. Effects of low and moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes. Diabetes. 2004;53:1798–1806. doi: 10.2337/diabetes.53.7.1798. [DOI] [PubMed] [Google Scholar]
- 25.Duncan GE, Hutson AD, Perri MG, Eckel RH, Theriaque DW, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003;26:557–562. doi: 10.2337/diacare.26.3.557. [DOI] [PubMed] [Google Scholar]
- 26.Lacombe VA, Hinchcliff KW, Devor ST. Effects of exercise and glucose administration on content of insulin-sensitive glucose transporter in equine skeletal muscle. Am J Vet Res. 2003;64:1500–1506. doi: 10.2460/ajvr.2003.64.1500. [DOI] [PubMed] [Google Scholar]
- 27.Nout YS, Hinchcliff KW, Jose-Cullineras E, Dearth LR, Sivko GS, DeWille JW. Effect of moderate exercise immediately followed by induced hyperglycemia on gene expression and content of the glucose transporter-4 protein in skeletal muscle of horses. Am J Vet Res. 2003;64:1401–1408. doi: 10.2460/ajvr.2003.64.1401. [DOI] [PubMed] [Google Scholar]
- 28.Di Loreto C, Ranchelli A, Fanelli C, et al. Make your diabetic patients walk, long term impact of different amounts of physical activity on type 2 diabetes. Diabetes Care. 2005;28:1295–1302. doi: 10.2337/diacare.28.6.1295. [DOI] [PubMed] [Google Scholar]
- 29.Powell DM, Reedy SE, Sessions DR, Fitzgerald BP. Effect of short-term exercise training on insulin sensitivity in obese and lean mares. Equine Vet J, Supplemental. 2002;34:81–84. doi: 10.1111/j.2042-3306.2002.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 30.Eiesenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Eng J Med. 2004;350:2068–2079. doi: 10.1056/NEJMra030158. [DOI] [PubMed] [Google Scholar]
- 31.Barker JM. Clinical review: Type 1 diabetes-associated autoimmunity: Natural history, genetic associations, and screening. J Clin Endocrinol Metab. 2006;91:1210–1217. doi: 10.1210/jc.2005-1679. [DOI] [PubMed] [Google Scholar]
- 32.Couetil LL, Hoffman AM. Adrenal insufficiency in a neonatal foal. J Am Vet Med Assoc. 1998;212:1594–1596. [PubMed] [Google Scholar]
- 33.Dowling PM, Williams MA, Clark TP. Adrenal insufficiency associated with long-term anabolic steroid administration in a horse. J Am Vet Med Assoc. 1993;203:1166–1169. [PubMed] [Google Scholar]
- 34.Peterson P, Uibo R, Krohn KJ. Adrenal autoimmunity: Results and developments. Trends in Endocrinol Metab. 2000;11:285–290. doi: 10.1016/s1043-2760(00)00283-6. [DOI] [PubMed] [Google Scholar]
- 35.Falorni A, Laureti S, Santeusanio F. Autoantibodies in autoimmune polyendocrine syndrome type II. Endocr Metab Clin N Am. 2002;31:369–89. vii. doi: 10.1016/s0889-8529(01)00010-x. [DOI] [PubMed] [Google Scholar]
- 36.Perillo A, Passantino G, Passantino L, et al. First observation of an Hashimoto thyroiditis-like disease in horses from Eastern Europe: Histopathological and immunological findings. Immunopharm Immunotox. 2002;27:241–253. doi: 10.1081/iph-200067743. [DOI] [PubMed] [Google Scholar]