Abstract
We describe a case of proximal mandibular nerve block with ropivacaine, using electrolocation, for perioperative pain management in a geriatric dog undergoing rostral mandibulectomy. The patient did not require intraoperative analgesia or analgesic supplementation for 8 h after the end of the surgery.
Résumé
Bloc du nerf mandibulaire proximal, en utilisant l’électrolocation, pour une mandibulectomie rostrale chez un chien gériatrique. Nous décrivons un cas de bloc du nerf mandibulaire proximal avec de la ropivacaïne, en utilisant l’électrolocation, pour la gestion périopératoire de la douleur chez un chien gériatrique subissant une mandibulectomie rostrale. La patient n’a pas nécessité d’analgésie peropératoire ni d’apport complémentaire d’analgésiques pendant 8 heures après la fin de la chirurgie.
(Traduit par Isabelle Vallières)
Introduction
Loco-regional techniques are becoming an important part of pain management of patients undergoing surgery of the oral cavity (1). These techniques, usually performed under general anesthesia, can reduce the dosage of simultaneously administered inhaled or injectable anesthetics, minimizing their depressive effects on cardiovascular and respiratory functions (2). Loco-regional blocks, combined with general anesthesia, allow reduction of the amount of opioid administered, decreasing their potential side effects such as excitement, vomiting, sedation, and respiratory depression (2,3). It is well-recognized that, whenever possible, local and regional anesthesia should be used for geriatric patients (4). In dogs, use of a mandibular nerve block was reported on the inferior (distal) alveolar branch of the mandibular nerve as it enters the mandibular canal at the mandibular foramen (1,2,5). A bilateral proximal mandibular nerve block with ropivacaine, using electrolocation, was used herein to obtain adequate perioperative analgesia in a geriatric dog undergoing rostral mandibulectomy for an oral cavity malignant melanoma.
Case description
A 14-year-old female, mixed-breed dog, weighing 9 kg, was referred for en bloc oral cavity surgery applied to a malignant melanoma localized in the rostral portion of the right mandible. The melanoma was classified as T2N0M0 following the WHO staging system (6). No physical abnormalities were found during a complete physical examination and thorax radiography that were performed before surgery. A complete blood (cell) count (CBC) and routine biochemical analyses were found to be within normal ranges. The dog was fasted for approximatively 8 h before anesthesia, and water was withdrawn 2 h before induction. The preanesthetic protocol consisted of acepromazine (Prequillan; Fatro, Ozzano Emilia, Bologna, Italy), 0.02 mg/kg body weight (BW) administered intramuscularly (IM). An 18-gauge catheter was placed in the right saphenous vein. Anesthesia was induced 30 min later by a total of 40 mg propofol (PropoVet; Esteve, Bologna, Italy) administered intravenously (IV).
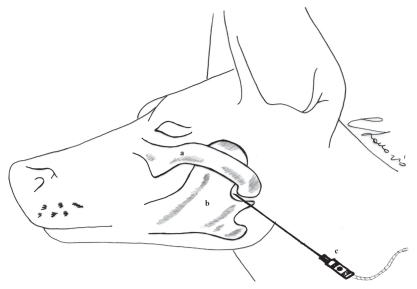
Following tracheal intubation, anesthesia was maintained with isoflurane (IsoFlo; Esteve, Bologna, Italy) delivered in oxygen (100%). Lactated Ringer’s solution (SALF, Bergamo, Italy) was infused throughout the procedure at a rate of 5 mL/kg/h. Cephazoline (Dorom, Milano, Italy), 25 mg/kg BW, was administered IV preoperatively. After induction, right and left proximal mandibular nerve blocks were performed with the dog in lateral recumbency using an extra-oral approach. The block was carried out after aseptical scrubbing of the lateral aspect of the face and the neck. The nerve was located with the aid of a nerve stimulator (Plexygon Nerve Stimulator; Vygon Italia, Padova, Italy) with an atraumatic needle (21-gauge, 50 mm). The positive red electrode of the nerve stimulator was attached to the skin of the neck of the dog and the negative black electrode to the stimulating needle connected to the syringe with an extension tube. In order to recognize the presence of the nerve, the stimulating current was initially set at 1 mA, 1 Hz, and 300 μs, gradually decreasing this value until the muscle contractions were still present with 0.5 mA. The location of the nerve was recognized by the absence of muscle contraction at 0.2 mA (7). The anatomic landmarks for the mandibular branch of the trigeminal nerve were the caudo-ventral aspect of the zygomatic arch and the temporomandibular joint (Figure 1).
Figure 1.
Anatomic landmarks for the proximal mandibular nerve block. a — zygomatic arch, b — mandible, and c — stimulating needle.
The stimulating needle was inserted at the level of the temporomandibular joint in a caudo-rostral and latero-medial direction until there was evidence of contraction of the digastricus, pterigoideus medialis and lateralis, and masseter muscles, resulting in movements of the jaw and movements of the auricular pinna in a rostral manner. Continuous negative pressure was applied to the syringe during electrolocation to avoid intravascular injection. The total volume of local anesthetic solution consisted of 1.8 mL (2 mg/kg BW) of 1% ropivacaine (Naropina; Astra Zeneca, Basiglio, Milano, Italy) diluted with saline solution (SALF, Bergamo, Italy) to obtain a concentration of 0.75% (2.4 mL) and divided into 2 doses (1.2 mL/site) for the left and right mandibular branches. During the intraoperative period, end tidal isoflurane concentration (Et-ISF), heart rate (HR), respiratory rate (RR), non-invasive arterial blood pressure (NIBP), hemoglobin oxygen saturation (SpO2), end tidal carbon dioxide (Et CO2), and rectal temperature (T°) were monitored. Eventual cardiovascular complications such as disturbance of pacemaker activity, excitability and conduction, induced by the local anesthetic were recorded through an electrocardiogram (ECG). Possible neurological signs, hematomas, or direct nerve damage were also recorded during follow-up observations (8).
Ropivacaine was used as the sole preemptive analgesic treatment applied during surgery and at 8 h after extubation. In order to evaluate the analgesic efficacy of this locoregional nerve block, we decided not to administer any other analgesic. Considering the absence of systemic analgesia during the perioperative period, signs of intraoperative and postoperative pain were strictly monitored, as previously reported by Wenger et al (9). During surgery, acute increases in heart rate (20% or more) or blood pressure (20% or more) would have been treated with fentanyl (Fentanest; Pfizer Italia, Milano, Italy), 0.01 mg/kg BW, considered as a rescue analgesic drug. Postoperatively, a multiparametric scale was used to assess the presence of pain at 0 (extubation time) 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h (9). The parameters evaluated in the postoperative setting were: overall subjective evaluation of pain, overall and interactive behaviors, increase in heart rate above baseline, reaction to wound palpation, and intensity of these reactions with a score from 0 to 3 points for each parameter. The total score was assigned to 1 of 4 levels: no pain (0 point), slight pain (from 1 to 5), moderate pain (from 6 to 10), and severe pain (from 11 to 18). If the dog had a pain score > 5 during postoperative observation, methadone (Molteni, Scandicci, Firenze, Italy), 0.2 mg/kg BW could have been used (9). At 8 h from the extubation time we administered analgesics consisting of carprofen (Rimadyl; Pfizer Animal Health, Milano, Italy), 4 mg/kg BW, IV and tramadol (Altadol; Formevet, Milano, Italy), 4 mg/kg BW, IV before hospitalization, independent of the results achieved from the nerve block procedure.
The time between administration of the nerve block and surgery was 30 min. During surgery, end tidal isoflurane ranged from 1.5% to 1.0%. The HR varied between 85 and 90 beats/min and RR between 12 and 14 breaths/min. Mean NIBP ranged from 65 to 75 mmHg. SpO2 remained above 98% and Et CO2 was between 39 and 44 mmHg. Rectal temperature ranged from 38.5°C to 36.7°C. Total anesthesia time was 155 min from the time of the nerve block administration to the time of tracheal extubation.
The dog did not require analgesic supplementation during surgery. No cardiovascular complications were detected. The patient, extubated 2 min after the end of the surgery, was standing 6 min later and walking 25 min after the end of the surgical procedure. The dog had a total pain score of 0 (no pain) for the first 4 h after tracheal extubation and of 1 (slight pain) at 5, 6, 7, 8 h after extubation. Recovery was uneventful and no rescue analgesia was needed during the postoperative period. The dog was hospitalized after an observation period that lasted 8 h from the end of the surgery and 10.5 h from the block procedure. At the end of the hospitalization, the patient showed no signs of pain and recovery was considered good. At that time the dog received the analgesics according to the protocol previously described. The day after, the dog was discharged. Tramadol was continued by intramuscular injection every 12 h for 3 d.
The dog was able to eat the morning of the day after surgery. Two days after discharge, the dog was reevaluated and was alert and active with a slight lingual ptosis at the site of mandibulectomy. There were no adverse effects associated with the peripheral nerve block, such as mechanical trauma to the nerve, hematomas, or neurological signs, during a follow-up period of 5 months.
Discussion
Bilateral proximal mandibular nerve block, using electrolocation, was applied to a geriatric dog undergoing aggressive oncologic surgery involving the most rostral part of the mandible and the mandibular symphysis. This local anesthetic technique, using ropivacaine, provided adequate pain relief for the intraoperative and postoperative periods without any complications and without the need of rescue analgesic agents.
Rostral mandibulectomy represents a common aggressive surgical treatment for oral cavity malignant neoplasia (10,11). Several analgesic protocols, such as 1 or more regional nerve blocks, have been reported in canine and feline oral cavity surgery to ensure satisfactory pain relief during the perioperative period (1,5,11). The use of electrolocation, for the location of the nerve trunks, is widely reported in dogs, for forelimb and hind limb locoregional anesthesia (9,12–16). Few studies have investigated the use of a nerve stimulator in the region innervated by the trigeminal nerve (17–19). In this case, we used the nerve stimulator to ensure the correct location of the nerve in order to obtain a complete nerve block. The trigeminal nerve (5th cranial nerve) has both motor and sensory components. It divides into 3 branches and, since the mandibular branch is the only sensory and motor branch of the trigeminal nerve (20), it can be located directly using the nerve stimulator. The proximal approach used in this patient could be done without electrolocation but since the anatomic landmarks for peripheral nerve block of the proximal mandibular branch of the trigeminal nerve are not well established in dogs, a nerve stimulator was used as precision of the administration of the local anesthetic is directly related to the accuracy of the location of the nerve (16). Because skulls differ more in size and shape among domestic dogs than in other mammalian species (21), we used electrolocation rather than a blind technique to achieve an accurate local anesthetic block. This proximal mandibular nerve block was used to ensure pain relief in a larger area such as the most caudal part of the mandible or the entire mandible.
Performing a right and a left proximal mandibular nerve block was designed to also provide adequate analgesia on the contralateral mandibular branch, although there was no literature to support this proximal bilateral approach.
An initial stimulating current of 1 mA and 1 Hz, as suggested by Futema et al (12), minimized the pain caused by contraction of the muscles and the gradual reduction of this value to 0.2 mA avoided accidental intraneural injection (7,12). In accordance with the literature, a short atraumatic stimulating needle was used, which is preferable to avoid neural damage (8). The extension tube connected to the needle allowed constant aspiration and gave more stability while moving the needle during electrolocation (8).
The nerve block was performed with ropivacaine. This local anesthetic was chosen due to its low toxic potential for the central nervous and cardiovascular systems and its long-lasting action (up to 8 h) (22,23). Satisfactory analgesic effects of ropivacaine have been demonstrated after various loco-regional blocks in human patients (24–26) and in dogs (27,28). The ropivacaine dose in this dog was 2 mg/kg BW. The low dosage, well below the cardiovascular (41.6 mg/kg BW, IV) (29) and convulsant (4.88 mg/kg BW, IV) toxic levels (30), and the avoidance of intravascular injection during electrolocation resulted in an absence of cardiovascular complications or neurological signs during the intraoperative and follow-up periods.
The stability of the monitored parameters during the surgery may be attributable to the low minimum alveolar concentration (MAC) of isoflurane needed to maintain anesthesia and to the adequate analgesic management used. Similarly, the fast functional recovery from anesthesia could be due to the low depression induced by this anesthetic protocol. These conditions should be achieved in geriatric patients undergoing general anesthesia because, in most cases, these patients show physiological decline in organ functions or in their mechanisms of compensation (4).
The dog, at the beginning of hospitalization, did not show signs of pain, and only a low pain score was detected for the entire 8 h of postoperative observation. This satisfactory result could be attributed to the preemptive analgesia performed by means of the bilateral proximal mandibular nerve block that, in this case, extended the analgesic effect of ropivacaine at 635 min rather than the range values normally considered in the dog (180 to 480 min) (23). The pain control achieved with this nerve block procedure seemed to be clinically relevant, but caution should be exercised as this is the first time this procedure has been performed. The pain scale that was used (9) was previously employed in a larger sample population undergoing nerve block, using electrolocation and a long-lasting local anesthetic, before surgery.
The decision to stop pain assessment 8 h after extubation time was taken arbitrarily and was not based on the expected duration of action of ropivacaine. In future, we would like to observe the duration of analgesic efficacy of this nerve block procedure for 24 h.
In conclusion, a proximal mandibular nerve block with ropivacaine, using electrolocation, seemed to be useful in a dog that underwent oncologic oral cavity surgery. The protocol was assessed to be easy, harmless, and effective in obtaining adequate intraoperative and postoperative analgesia. This locoregional block represents a novel approach used in addition to systemic analgesia, was easy to learn, and was associated with a minimal risk of direct trauma to the nerve (8) and a reduced level of general anesthetic and analgesic agents. We believe that the added costs for this technique are justified by the extreme versatility of this procedure since locoregional anesthesia can be performed in almost the entire body. In fact this guided technique could be applied to other nerves of the head as well as for the brachial and lumbosacral plexus block, to provide analgesia and to contribute to a more balanced anesthetic approach.
In this case report, we highlight the analgesic efficacy of a locoregional nerve block achieved with the aid of a nerve stimulator to obtain an accurate local anesthetic block without comparing this new approach with the traditional technique of blocking the mandibular nerve. We suggest that future clinical investigation include a larger number of patients, a longer period of pain assessment to evaluate the duration of this kind of block, and comparison of this block with other well-accepted techniques.
Acknowledgments
The authors acknowledge Dr. Paola Valenti and Dr. Andrea Jacchetti for editorial and linguistic revision of the manuscript. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Woodward TM. Pain management and regional anesthesia for the dental patient. Top Companion Anim Med. 2008;23:106–114. doi: 10.1053/j.tcam.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Skarda RT, Tranquilli WJ. Local and regional anesthetic and analgesic techniques: Dogs. In: Tranquilli WJ, Thurmon JC, Grimm KA, editors. Lumb and Jones’ Veterinary Anesthesia and Analgesia. 4th ed. Oxford: Blackwell Publishing; 2007. pp. 561–593. [Google Scholar]
- 3.Haskins SC. Postoperative analgesia. Vet Clin North Am Small Anim Pract. 1992;2:89–96. doi: 10.1016/s0195-5616(92)50632-5. [DOI] [PubMed] [Google Scholar]
- 4.Pettifer GR, Grubb TL. Neonatal and geriatric patients. In: Tranquilli WJ, Thurmon JC, Grimm KA, editors. Lumb and Jones’ Veterinary Anesthesia and Analgesia. 4th ed. Oxford: Blackwell Publishing; 2007. pp. 985–991. [Google Scholar]
- 5.Duke T. Local and regional analgesic techniques in the dog and cat: Part II, infiltration and nerve blocks. Can Vet J. 2000;41:949–952. [PMC free article] [PubMed] [Google Scholar]
- 6.Owen LN. TNM classification of tumors in domestic animals. Geneva: World Health Organization; 1980. [Google Scholar]
- 7.Clark L. The use of nerve stimulators in forelimb regional anaesthesia in the dog. Proc Autumn AVA Meet; Barcelona. 2008. pp. 16–21. [Google Scholar]
- 8.Clark L. Fundamentals of nerve stimulator guidance in peripheral nerve blockade. Proc Autumn AVA Meet Barcelona. 2008:9–15. [Google Scholar]
- 9.Wenger S, Moens Y, Jäggin N, Schatzmann U. Evaluation of the analgesic effect of lidocaine and bupivacaine used to provide a brachial plexus block for forelimb surgery in 10 dogs. Vet Rec. 2005;156:639–642. doi: 10.1136/vr.156.20.639. [DOI] [PubMed] [Google Scholar]
- 10.Birchard S, Carothers M. Aggressive surgery in the management of oral neoplasia. Vet Clin North Am Small Anim Pract. 1990;20:1117–1140. doi: 10.1016/s0195-5616(90)50088-1. [DOI] [PubMed] [Google Scholar]
- 11.Verstraete FJ. Mandibulectomy and maxillectomy. Vet Clin North Am Small Anim Pract. 2005;35:1009–1039. doi: 10.1016/j.cvsm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Futema F, Fantoni TD, Costa Auler JO, Gaido Cortopassi SR, Acaui A, Stopiglia AJ. A new brachial plexus block technique in dogs. Vet Anaesth Analg. 2002;29:133–139. doi: 10.1046/j.1467-2995.2002.00082.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahler SP, Adogwa AO. Anatomical and experimental studies of brachial plexus sciatic, and femoral nerve-location using peripheral nerve stimulation in the dog. Vet Anaesth Analg. 2008;35:80–89. doi: 10.1111/j.1467-2995.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 14.Lamont LA, Lemke KA. The effects of medetomidine on radial nerve blockade with mepivacaine in dogs. Vet Anaesth Analg. 2008;35:62–68. doi: 10.1111/j.1467-2995.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 15.Wenger S. Brachial plexus block using electrolocation for pancarpal arthrodesis in a dog. Vet Anaesth Analg. 2004;31:272–275. doi: 10.1111/j.1467-2995.2004.00167.x. [DOI] [PubMed] [Google Scholar]
- 16.Mahler SP, Reece JLM. Electrical nerve stimulation to facilitate placement of an indwelling catheter for repeated brachial plexus block in a traumatized dog. Vet Anaesth Analg. 2007;34:365–370. doi: 10.1111/j.1467-2995.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 17.Bernard JM, Péréon Y. Nerve stimulator for regional anesthesia of the face: Use of the blink reflex to confirm the localization of the trigeminal nerve. Anesth Analg. 2005;101:589–591. doi: 10.1213/01.ANE.0000155287.54904.EB. [DOI] [PubMed] [Google Scholar]
- 18.Wellehean JF, Gunkel CI, Kledzik D, Robertson SA, Heard DJ. Use of a nerve locator to facilitate administration of mandibular nerve blocks in crocodilians. J Zoo Wildl Med. 2006;37:405–408. doi: 10.1638/05-047.1. [DOI] [PubMed] [Google Scholar]
- 19.Ravasio G, Carotenuto AM, Gobbi R, et al. Analgesic effects of trigeminal/facial nerve blockade by ropivacaine or bupivacaine in dogs undergoing total ear canal ablation/lateral bull osteotomy: Comparison with those obtained by morphine systemic use. Proc Autumn AVA Meeting; Barcelona. 2008. p. 68. [Google Scholar]
- 20.Evans HE, Kitchell RL. Miller’s Anatomy of the dog. 3th ed. Philadelphia: WB Saunders; 1993. Cranial nerves and cutaneus innervations of the head; pp. 962–980. [Google Scholar]
- 21.Evans HE, Kitchell RL. Miller’s Anatomy of the Dog. 3th ed. Philadelphia: WB Saunders; 1993. The skeleton; pp. 122–218. [Google Scholar]
- 22.Leone S, Di Cianni S, Casati A, Fanelli G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008;79:92–105. [PubMed] [Google Scholar]
- 23.Skarda RT, Tranquilli WJ. Local anesthetics. In: Tranquilli WJ, Thurmon JC, Grimm KA, editors. Lumb and Jones’ Veterinary Anesthesia and Analgesia. 4th ed. Oxford: Blackwell Publishing; 2007. pp. 395–418. [Google Scholar]
- 24.Fanelli G, Casati A, Beccaria P, et al. A double-blinded comparison of ropivacaine, bupivacaine and mepivacaine during sciatic and femoral nerve blockade. Anesth Analg. 1998;87:597–600. doi: 10.1097/00000539-199809000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Casati A, Fanelli G, Magistris L, Beccaria P, Berti M, Torri G. Minimum local anaesthetic volume blocking the femoral nerve in 50% of cases: A double blinded comparison between 0.5% ropivacaine and 0.5% bupivacaine. Anesth Analg. 2001;92:205–208. doi: 10.1097/00000539-200101000-00039. [DOI] [PubMed] [Google Scholar]
- 26.Casati A, Fanelli G, Albertin A, et al. Interscalene brachial plexus anesthesia with either 0.5% ropivacaine or 0.5% bupivacaine. Minerva Anestesiol. 2000;66:34–44. [PubMed] [Google Scholar]
- 27.Duke T, Caulkett NA, Ball SD, Remedios AM. Comparative analgesic and cardiopulmonary effects of bupivacaine and ropivacaine in the epidural space of the conscious dog. Vet Anesth Analg. 2000;27:13–21. doi: 10.1046/j.1467-2995.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 28.Dumas MPY, Ravasio G, Carotenuto AM, et al. Post-operative effects, after orthopaedic surgery in the dog, of loco-regional ropivacaine and bupivacaine blockade using the nerve locator technique: 159 cases. Vet Res Commun. 2008;32 (Suppl 1):283–286. doi: 10.1007/s11259-008-9129-8. [DOI] [PubMed] [Google Scholar]
- 29.Groban L, Dwight DD, Vernon JC, James RL, Buttherworth J. Cardiac resuscitation after incremental overdosage with lidocaine, bupivacaine, levobupivacaine and ropivacaine in anesthetized dogs. Anesth Analg. 2001;92:37–43. doi: 10.1097/00000539-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Feldman HS, Arthur GR, Covino BG. Comparative systemic toxicity of convulsivant and supraconvulsivant doses of intravenous ropivacaine, bupivacaine, and lidocaine in the conscious dog. Anesth Analg. 1989;69:794–801. [PubMed] [Google Scholar]